Development and Validation of QuEChERS Extraction Coupled with Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry for the Detection of Nine Macrolides in Fish Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Ethics Statement
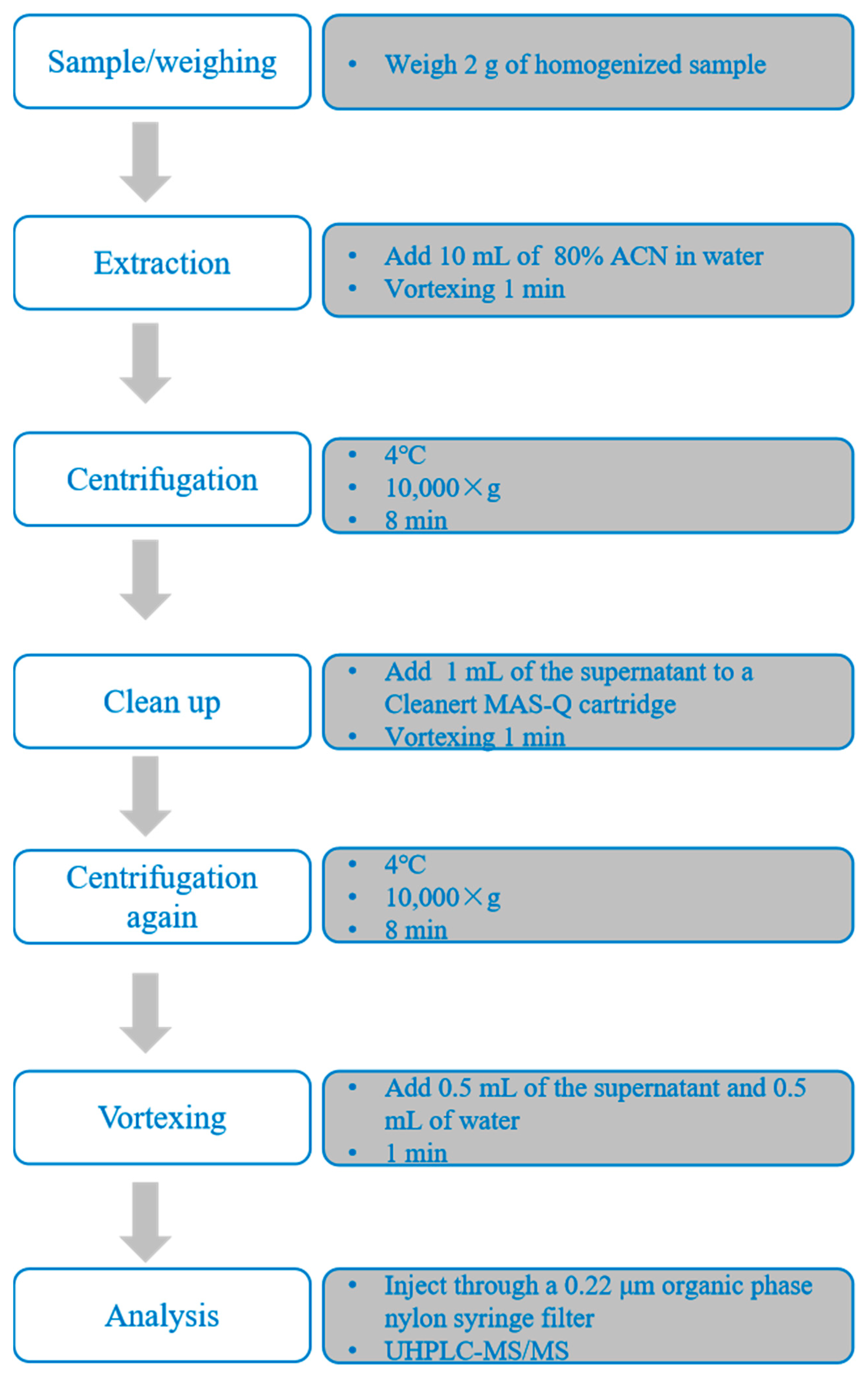
2.3. Sample Preparation
2.4. UHPLC-MS/MS Analysis
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
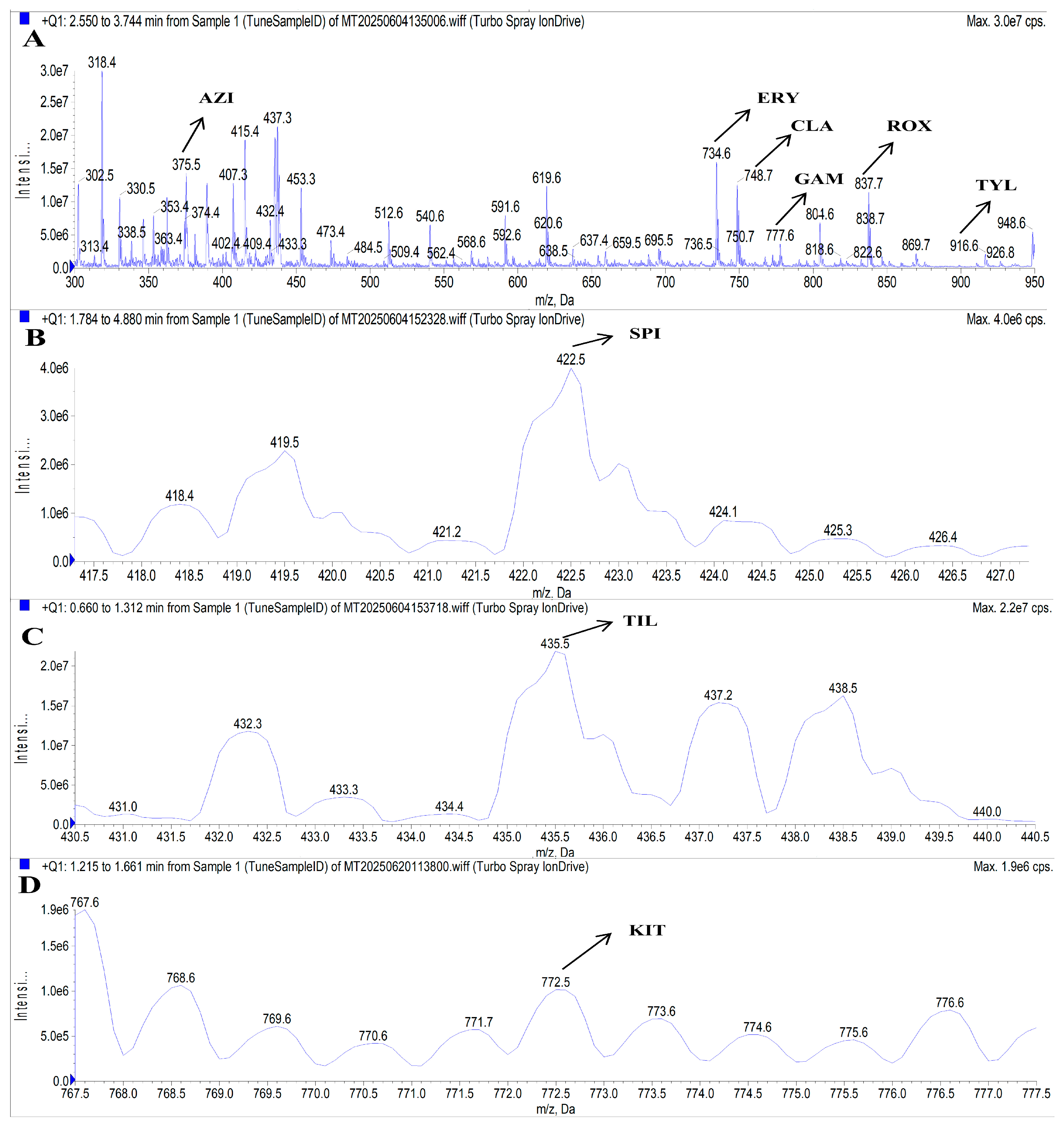
3.1. Optimization of the UHPLC-MS/MS Conditions
3.2. Sample Clean-Up
3.3. Matrix Effect (ME) Evaluation
3.4. Method Validation Results
3.4.1. Specificity and Linearity
3.4.2. LOD, LOQ, CCα, and CCβ
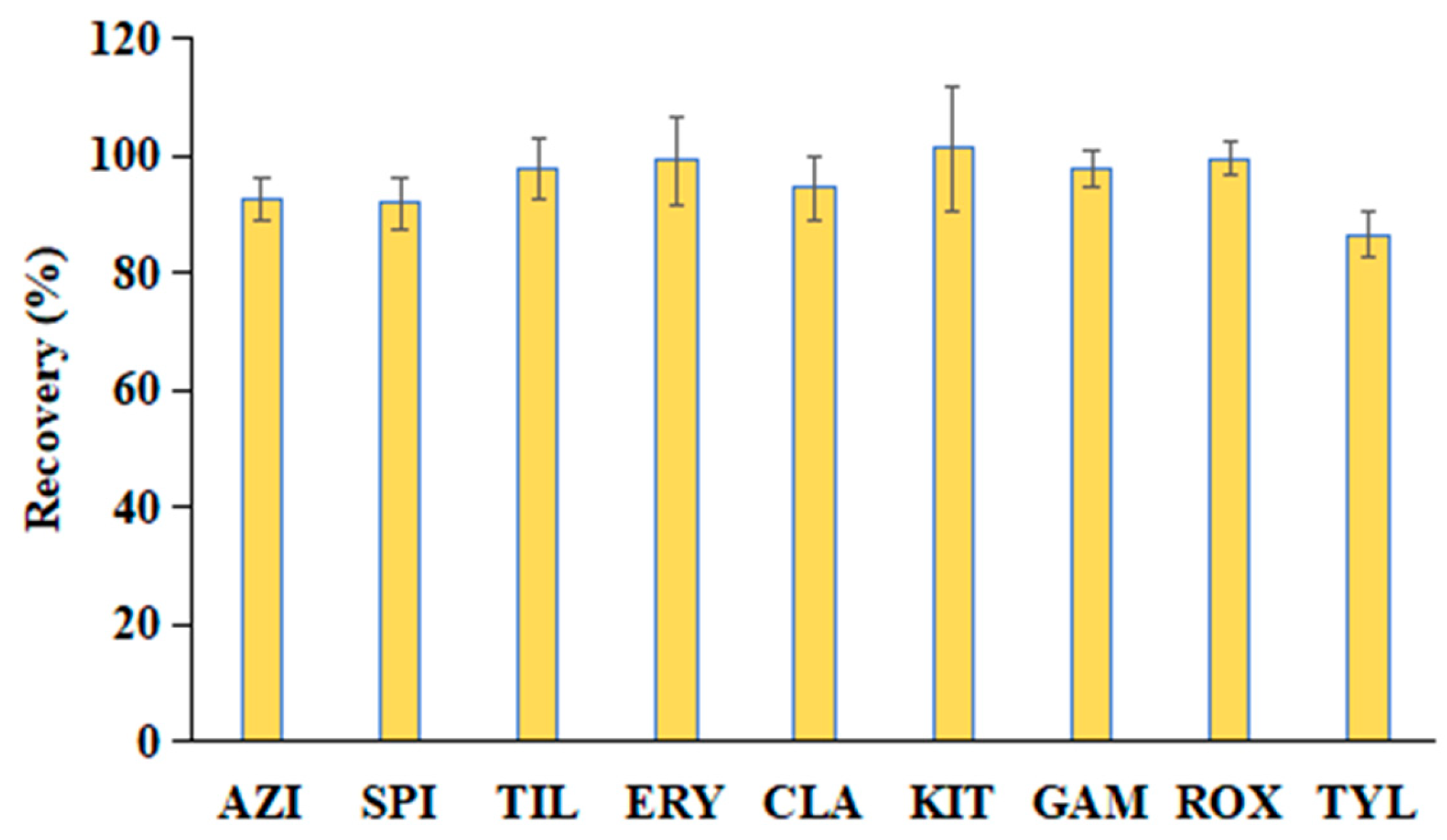
3.4.3. Recovery and Precision
3.5. Comparison with Other Methods
3.6. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.; Fernandes, C.; Silva, L.H.; Gloria, M.B. A simple, fast and sensitive screening LC-ESI-MS/MS method for antibiotics in fish. Talanta 2017, 163, 85–93. [Google Scholar] [CrossRef]
- Wang, B.; Xie, K.; Lee, K. Veterinary drug residues in animal-derived foods: Sample preparation and analytical methods. Foods 2021, 10, 555. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Zhou, Y.; Liu, Z.F.; Meng, Q.; Feng, X.S. A review of pretreatment and analysis of macrolides in food (Update Since 2010). J. Chromatogr. A 2020, 1634, 461662. [Google Scholar] [CrossRef]
- Tao, Y.; Yu, G.; Chen, D.; Pan, Y.; Liu, Z.; Wei, H.; Peng, D.; Huang, L.; Wang, Y.; Yuan, Z. Determination of 17 macrolide antibiotics and avermectins residues in meat with accelerated solvent extraction by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2012, 897, 64–71. [Google Scholar] [CrossRef]
- Kaiser, G. Protein synthesis inhibitors: Macrolides mechanism of action animation. In Classification of Agents Pharmamotion; The Community College of Baltimore County: Baltimore, MD, USA, 2009. [Google Scholar]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic residues in food: Extraction, analysis, and human health concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Beyene, T. Veterinary drug residues in food-animal products: Its risk factors and potential effects on public health. J. Vet. Sci. Technol. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Boobis, A.; Cerniglia, C.; Chicoine, A.; Fattori, V.; Lipp, M.; Reuss, R.; Verger, P.; Tritscher, A. Characterizing chronic and acute health risks of residues of veterinary drugs in food: Latest methodological developments by the joint FAO/WHO expert committee on food additives. Crit. Rev. Toxicol. 2017, 47, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU). No.37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union. 2010, 15, 1–72. [Google Scholar]
- GB 31650-2019; Chinese Ministry of Agriculture and Rural Affairs. National food safety standard-Maximum residue limits for veterinary drugs in foods. Chinese Ministry of Agriculture and Rural Affairs: Beijing, China, 2019.
- US Food and Drug Administration. CFR-Code of Federal Regulations Title 21 Part 556 Tolerances for Residues of New Animal Drugs in Food; US Food and Drug Administration: Silver Spring, MD, USA, 2014. [Google Scholar]
- Shaaban, H.; Mostafa, A. Simultaneous determination of antibiotics residues in edible fish muscle using eco-friendly SPE-UPLC-MS/MS: Occurrence, human dietary exposure and health risk assessment for consumer safety. Toxicol. Rep. 2023, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mei, G.; Zhang, X.; Huang, D.; He, P.; Xu, D. Dispersive solid-phase extraction and ultra-performance liquid chromatography-tandem mass spectrometry-a rapid and accurate method for detecting 10 macrolide residues in aquatic products. Foods 2024, 13, 866. [Google Scholar] [CrossRef]
- Zhou, W.; Ling, Y.; Liu, T.; Zhang, Y.; Li, J.; Li, H.; Wu, W.; Jiang, S.; Feng, F.; Yuan, F.; et al. Simultaneous determination of 16 macrolide antibiotics and 4 metabolites in milk by using Quick, Easy, Cheap, Effective, Rugged, and Safe extraction (QuEChERS) and high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B. 2017, 1061–1062, 411–420. [Google Scholar] [CrossRef]
- Xu, J.; Yang, M.; Wang, Y.; Yang, Y.; Tu, F.; Yi, J.; Hou, J.; Lu, H.; Jiang, X.; Chen, D. Multiresidue analysis of 15 antibiotics in honey using modified QuEChERS and high performance liquid chromatography-tandem mass spectrometry. J. Food Compos. Anal. 2021, 103, 104120. [Google Scholar] [CrossRef]
- Song, X.Q.; Zhou, T.; Li, J.F.; Su, Y.J.; Xie, J.M.; He, L.M. Determination of macrolide antibiotics residues in pork using molecularly imprinted dispersive solid-phase extraction coupled with LC-MS/MS. J. Sep. Sci. 2018, 41, 1138–1148. [Google Scholar] [CrossRef]
- Song, X.Q.; Zhou, T.; Zhang, J.H.; Su, Y.J.; Zhou, H.; He, L.M. Preparation and application of molecularly imprinted monolithic extraction column for the selective microextraction of multiple macrolide antibiotics from animal muscles. Polymers 2019, 11, 1109. [Google Scholar] [CrossRef]
- Campanharo, S.C.; da Silva, A.F.B.; Bleuzen, A.; da Silva, J.J.M.; de Freitas, L.V.P.; Assane, I.M.; Pilarski, F.; Paschoal, J.A.R. The association of modified QuEChERS and DLLME to offer high analytical detectability to assess residual depletion profile of erythromycin in fish. Food Chem. 2023, 405, 134852. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, S.; Zhu, Y.; Zhang, H.; Xiong, D.; Guan, T.; Zheng, X.; Yang, Z.; Zhang, T.; Zhang, G.; et al. Determination of erythromycin, clarithromycin and N-desmethyl-erythromycin A residues in pork, beef and lamb based on a simple and fast extraction procedure followed by ultrahigh-performance liquid chromatography with triple quadrupole/linear ion trap mass spectrometry. J. Food Compos. Anal. 2024, 129, 106093. [Google Scholar] [CrossRef]
- Chen, Y.; Schwack, W. High-performance thin-layer chromatography screening of multi class antibiotics in animal food by bioluminescent bioautography and electrospray ionization mass spectrometry. J. Chromatogr. A 2014, 1356, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sharkawi, M.M.; Safwat, M.T.; Abdelaleem, E.A.; Abdelwahab, N.S. TLC densitometric analysis of triple antibiotic therapy; Erythromycin, Sulfadiazine and Trimethoprim in different edible chicken tissues. J. Liq. Chromatogr. Relat. Technol. 2023, 46, 79–88. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yang, Q.X.; Chen, X.T.; Song, Y.M.; Wu, Q.H.; Yang, Y.Y.; He, L.P. Sensitive analysis of trace macrolide antibiotics in complex food samples by ambient mass spectrometry with molecularly imprinted polymer-coated wooden tips. Talanta 2019, 204, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Shu, H.; Tian, X.; Ren, J.; Cui, X.; Bai, H.; Xiao, X.C.; Wang, Y.D. Electrochemical sensor based on molecularly imprinted poly-arginine for highly sensitive and selective erythromycin determination. J. Mater. Sci. Mater. Electron. 2023, 34, 6. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, L.Q.; Kuang, H.; Cui, G.; Xu, C.L. A paper-based colorimetric assay for rapid detection of four macrolides in milk. Mater. Chem. Front. 2019, 3, 2175–2183. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.Q.; Hou, R.; Wang, Y.L.; Wang, X.; Xie, C.Q.; Chen, Y.S.; Wu, S.M.; Peng, D.P. Single-chain variable fragment antibody-based ic-ELISA for rapid detection of macrolides in porcine muscle and computational simulation of its interaction mechanism. Food Control. 2022, 133, 108571. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Dong, W.; Hu, Q.; Li, Y.; Shuang, S.; Dong, C.; Cai, L.; Gong, X. Azithromycin detection in cells and tablets by N,S co-doped carbon quantum dots. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2021, 252, 119506. [Google Scholar] [CrossRef]
- Hamidian, K.; Amini, M.; Samadi, N. Consistency evaluation between matrix components ratio and microbiological potency of tylosin major components. DARU J. Pharm. Sci. 2018, 26, 155–164. [Google Scholar] [CrossRef]
- Cañadas, R.; Martínez, R.G.; González, G.P.; Hernando, P.F. Development of a molecularly imprinted polymeric membrane for determination of macrolide antibiotics from cow milk. Polymer 2022, 249, 124843. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharyya, A.; Shinde, R.; Dhanshetty, M.; Elliott, C.T.; Banerjee, K. Development and validation of a multiresidue method for pesticides and selected veterinary drugs in animal feed using liquid- and gas chromatography with tandem mass spectrometry. J. Chromatogr. A 2020, 1627, 461416. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Moltó, J.C.; Mañes, J.; Font, G. Determination of macrolide and lincosamide antibiotics by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in meat and milk. Food Control 2010, 21, 1703–1709. [Google Scholar] [CrossRef]
- Jo, M.R.; Lee, H.J.; Lee, T.S.; Park, K.; Oh, E.G.; Kim, P.H.; Lee, D.S.; Horie, M. Simultaneous determination of macrolide residues in fish and shrimp by liquid chromatography-tandem mass spectrometry. Food Sci. Biotechnol. 2011, 20, 823–827. [Google Scholar] [CrossRef]
- Du, J.; Li, X.; Tian, L.; Li, J.; Wang, C.; Ye, D.; Zhao, L.; Liu, S.; Xu, J.; Xia, X. Determination of macrolides in animal tissues and egg by multi-walled carbon nanotube-based dispersive solid-phase extraction and ultra-high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2021, 365, 130502. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Y.; Liu, S.; Zhang, H.; Guan, T.; Xu, X.; Zheng, X.; Yang, Z.; Zhang, T.; Zhang, G.; et al. Quantitative analysis of erythromycin, its major metabolite and clarithromycin in chicken tissues and eggs via QuEChERS extraction coupled with ultrahigh-performance liquid chromatography-tandem mass spectrometry. Food Chem. X 2024, 22, 101468. [Google Scholar] [CrossRef] [PubMed]
- The European Communities. Commission decision 2002/657/EC of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–36. [Google Scholar]
- European Commission. 2021/808/EC Commission implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Communities 2021, L180, 1–31. [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration; Center for Drug Evaluation and Research; Center for Veterinary Medicine. Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar]
- Matuszewski, B.K.; Constanzer, M.; Chavez-Eng, C. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Nováková, L.; Matysová, L.; Solich, P. Advantages of application of UPLC in pharmaceutical analysis. Talanta 2006, 68, 908–918. [Google Scholar] [CrossRef]
- Griboff, J.; Carrizo, J.C.; Bonansea, R.I.; Valdés, M.E.; Wunderlin, D.A.; Amé, M.V. Multiantibiotic residues in commercial fish from Argentina. The presence of mixtures of antibiotics in edible fish, a challenge to health risk assessment. Food Chem. 2020, 332, 127380. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analysis of macrolide antibiotics, using liquid chromatography-mass spectrometry, in food, biological and environmental matrices. Mass. Spectrom. Rev. 2009, 28, 50–92. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, R.; Dzingelevičienė, R.; Abbasi, S.; Szultka-Młyńska, M.; Buszewski, B. Determination of 15 human pharmaceutical residues in fish and shrimp tissues by high-performance liquid chromatography-tandem mass spectrometry. Environ. Monit. Assess. 2022, 194, 325. [Google Scholar] [CrossRef] [PubMed]
- Susakate, S.; Poapolathep, S.; Chokejaroenrat, C.; Tanhan, P.; Hajslova, J.; Giorgi, M.; Saimek, K.; Zhang, Z.; Poapolathep, A. Multiclass analysis of antimicrobial drugs in shrimp muscle by ultra high performance liquid chromatography-tandem mass spectrometry. J. Food Drug Anal. 2019, 27, 118–134. [Google Scholar] [CrossRef]
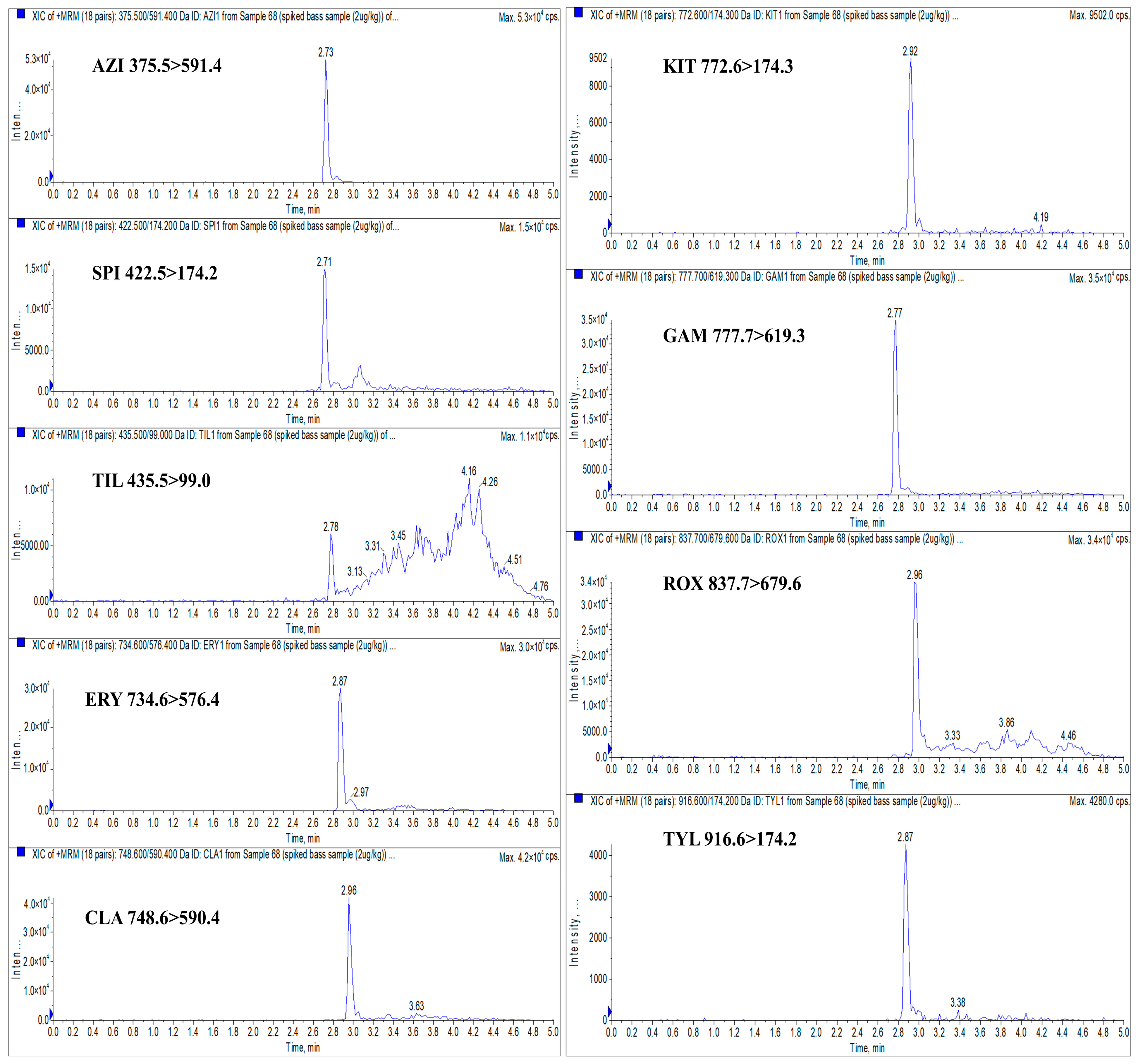
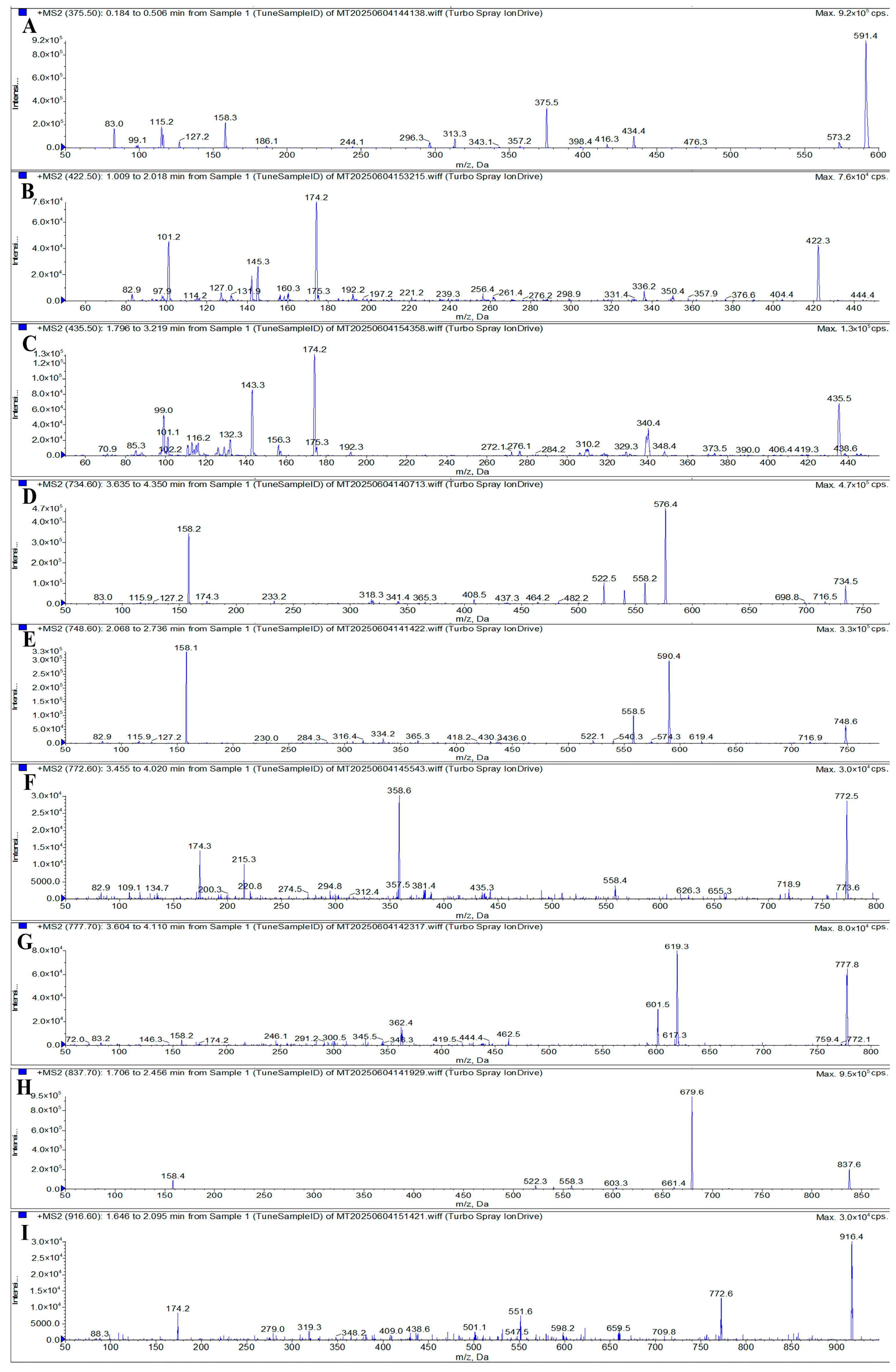
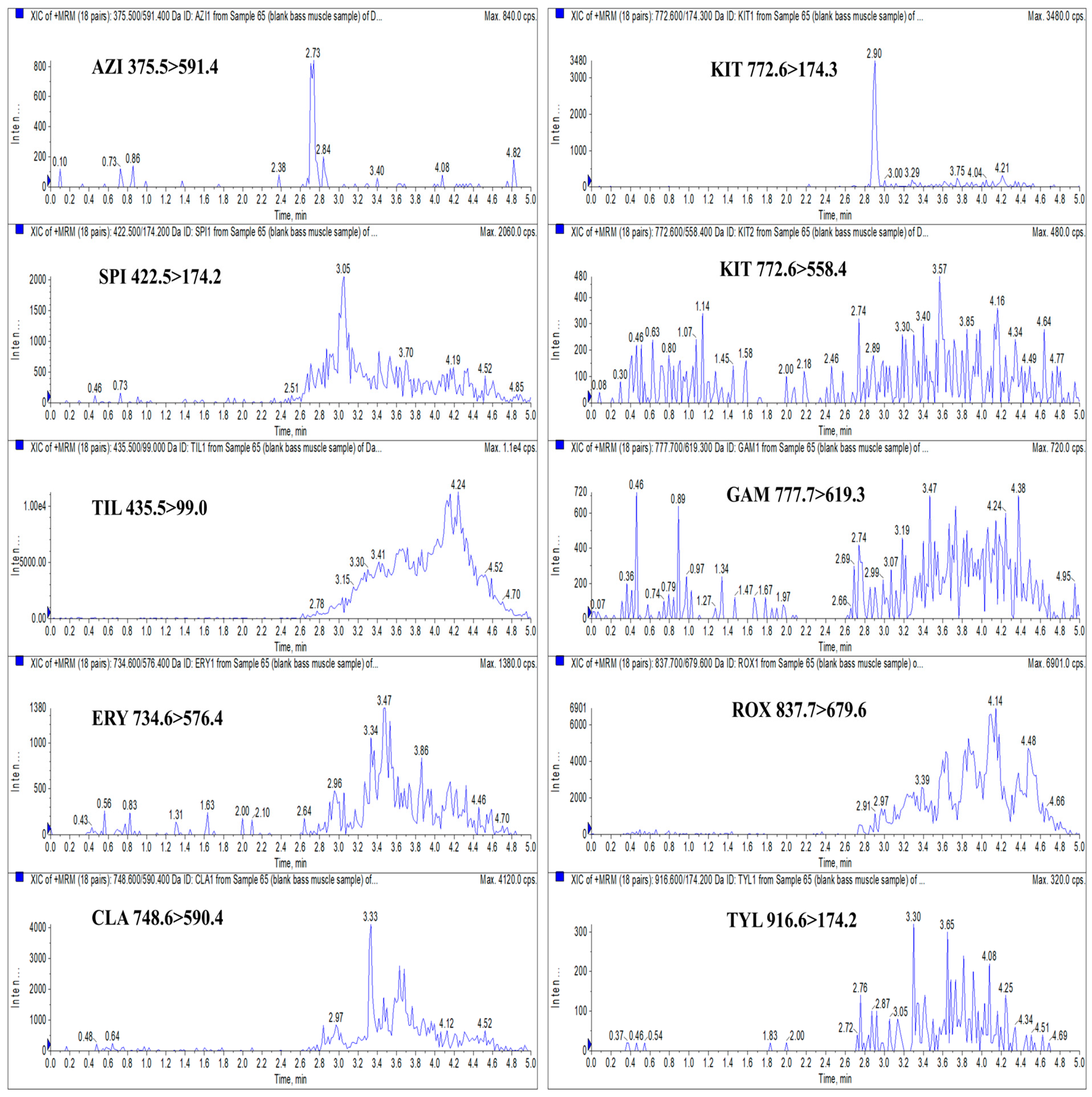
| Analyte | Retention Time (min) | Molecular Weight (g/mol) | Molecular Formula | Precursor Ion (m/z) | Product Ion (m/z) | Declustering Potential (V) | Collision Energy (eV) |
|---|---|---|---|---|---|---|---|
| AZI | 2.73 | 749.0 | C38H72N2O12 | 375.5 | 591.4 * 83.0 | 73 | 23 22 |
| SPI | 2.71 | 843.1 | C43H74N2O14 | 422.5 | 174.2 * 101.2 | 68 | 31 25 |
| TIL | 2.78 | 869.1 | C46H80N2O13 | 435.5 | 99.0 * 174.2 | 95 | 26 34 |
| ERY | 2.87 | 733.9 | C37H67NO13 | 734.6 | 576.4 * 158.2 | 90 | 25 37 |
| CLA | 2.96 | 748.0 | C38H69NO13 | 748.6 | 590.4 * 158.1 | 95 | 29 39 |
| KIT | 2.92 | 771.9 | C39H65NO14 | 772.6 | 174.3 * 558.4 | 75 | 43 37 |
| GAM | 2.77 | 777.0 | C40H76N2O12 | 777.7 | 619.3 * 601.5 | 112 | 46 49 |
| ROX | 2.96 | 837.0 | C41H76N2O15 | 837.7 | 679.6 * 158.2 | 110 | 33 37 |
| TYL | 2.87 | 916.1 | C46H77NO17 | 916.6 | 174.2 * 772.6 | 88 | 46 44 |
| Analyte | Regression Equation | Determination Coefficient (R2) | CCα (μg/kg) | CCβ (μg/kg) | ME |
|---|---|---|---|---|---|
| AZI | y = 444,404x + 1794.9 | 0.9999 | 2.21 | 2.37 | 1.0 |
| SPI | y = 153,120x + 4212.1 | 0.9998 | 208.45 | 216.89 | 1.0 |
| TIL | y = 64,969x − 4049.5 | 0.9997 | 57.89 | 65.78 | 1.1 |
| ERY | y = 452,604x + 45,786 | 0.9980 | 211.73 | 223.45 | 1.0 |
| CLA | y = 508,239x + 84,309 | 0.9998 | 2.16 | 2.27 | 0.8 |
| KIT | y = 92,904x + 7733.7 | 0.9998 | 215.71 | 231.42 | 0.8 |
| GAM | y = 352,943x + 143,383 | 0.9981 | 162.43 | 174.86 | 0.9 |
| ROX | y = 478,430x + 13,071 | 0.9996 | 2.13 | 2.22 | 0.9 |
| TYL | y = 59,211x + 2240.8 | 0.9998 | 110.22 | 120.43 | 0.8 |
| Analyte | Added Level (μg/kg) | Recovery (%) (n = 6) | RSD (%) (n = 6) | Intraday RSD (%) (n = 6) | Interday RSD (%) (n = 18) |
|---|---|---|---|---|---|
| AZI | 2 | 104.3 ± 5.4 | 5.2 | 7.1 | 9.2 |
| 20 | 91.1 ± 2.8 | 3.1 | 9.0 | 6.3 | |
| 200 | 93.6 ± 3.8 | 4.1 | 6.4 | 5.7 | |
| SPI | 2 | 92.8 ± 4.3 | 4.6 | 4.2 | 4.2 |
| 20 | 93.7 ± 4.4 | 4.7 | 7.9 | 7.0 | |
| 200 | 94.3 ± 4.4 | 4.7 | 6.8 | 5.4 | |
| TIL | 2 | 91.8 ± 6.3 | 6.9 | 8.2 | 7.3 |
| 20 | 90.3 ± 4.6 | 5.1 | 5.9 | 5.3 | |
| 200 | 95.8 ± 8.1 | 8.5 | 8.8 | 8.8 | |
| ERY | 2 | 90.4 ± 5.8 | 6.4 | 4.7 | 5.0 |
| 20 | 97.7 ± 5.6 | 5.7 | 2.9 | 5.8 | |
| 200 | 100.0 ± 6.0 | 6.0 | 4.8 | 7.5 | |
| CLA | 2 | 89.3 ± 6.8 | 7.6 | 4.1 | 6.7 |
| 20 | 100.7 ± 7.6 | 7.5 | 8.0 | 8.7 | |
| 200 | 94.0 ± 8.1 | 8.6 | 9.5 | 11.2 | |
| KIT | 2 | 108.4 ± 5.3 | 4.9 | 6.1 | 5.8 |
| 20 | 101.8 ± 8.3 | 8.2 | 8.5 | 7.8 | |
| 200 | 107.9 ± 10.3 | 9.5 | 9.8 | 9.0 | |
| GAM | 2 | 107.5 ± 9.3 | 8.7 | 7.3 | 11.7 |
| 20 | 101.2 ± 8.5 | 8.4 | 7.7 | 7.4 | |
| 200 | 96.6 ± 8.6 | 8.9 | 7.5 | 7.8 | |
| ROX | 2 | 92.3 ± 5.6 | 6.1 | 5.8 | 7.4 |
| 20 | 97.3 ± 11.4 | 11.7 | 5.9 | 11.6 | |
| 200 | 97.3 ± 10.8 | 11.1 | 11.6 | 12.5 | |
| TYL | 2 | 92.5 ± 4.1 | 4.4 | 5.4 | 6.3 |
| 20 | 91.3 ± 3.3 | 3.6 | 4.1 | 4.1 | |
| 200 | 89.4 ± 4.0 | 4.5 | 7.8 | 7.1 |
| Detection Method | Sample Preparation Method | Animal-Derived Food | Analyte | Recovery (%) | LOD (μg/kg) | LOQ (μg/kg) | Detection Time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|
| HPLC-MS/MS | SPE | Fish and shrimp | AZI, ERY, and CLA | 92.0–99.2 | 0.053–0.417 | 0.159–1.251 | 18 | [41] |
| UHPLC-MS/MS | LLE | Shrimp | SPI, ERY, TIL, TYL, and JOS | 74.3–111.1 | 2.0 | 5.0 | 15 | [42] |
| HPLC-MS/MS | QuEChERS-DLLME | Fish | ERY | 103–110 | 0.1 | 1 | 7 | [18] |
| UHPLC-MS/MS | d-SPE | Fish, shrimp, crab, and shellfish | AZI, SPI, TIL, ERY, CLA, KIT, ROX, TYL, OLE, and JOS | 83.1–116.6 | 0.25–0.50 | 0.50–1.00 | 10 | [13] |
| UHPLC-MS/MS | QuEChERS | Fish | AZI, SPI, TIL, ERY, CLA, KIT, GAM, ROX, and TYL | 89.3–108.4 | 0.4 | 2.0 | 5 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Ma, Y.; Yang, J.; Lu, X.; Wang, S.; Zheng, X.; Yang, Z.; Xu, L.; Wang, B. Development and Validation of QuEChERS Extraction Coupled with Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry for the Detection of Nine Macrolides in Fish Products. Foods 2025, 14, 2768. https://doi.org/10.3390/foods14162768
Sun C, Ma Y, Yang J, Lu X, Wang S, Zheng X, Yang Z, Xu L, Wang B. Development and Validation of QuEChERS Extraction Coupled with Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry for the Detection of Nine Macrolides in Fish Products. Foods. 2025; 14(16):2768. https://doi.org/10.3390/foods14162768
Chicago/Turabian StyleSun, Changhua, Yue Ma, Jia Yang, Xubin Lu, Shuai Wang, Xiangfeng Zheng, Zhenquan Yang, Li Xu, and Bo Wang. 2025. "Development and Validation of QuEChERS Extraction Coupled with Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry for the Detection of Nine Macrolides in Fish Products" Foods 14, no. 16: 2768. https://doi.org/10.3390/foods14162768
APA StyleSun, C., Ma, Y., Yang, J., Lu, X., Wang, S., Zheng, X., Yang, Z., Xu, L., & Wang, B. (2025). Development and Validation of QuEChERS Extraction Coupled with Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry for the Detection of Nine Macrolides in Fish Products. Foods, 14(16), 2768. https://doi.org/10.3390/foods14162768







