Constructing Stable Emulsion Gel from Soy Protein Isolate and Konjac Glucomannan as Pork Fat Substitute: Effect of Oil Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Emulsion Gels
2.3. Rheological Properties
2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5. X-Ray Diffraction (XRD)
2.6. Scanning Electron Microscope (SEM)
2.7. Confocal Laser Scanning Microscopy (CLSM)
2.8. Water Holding Capacity (WHC) and Oil Binding Capacity (OHC)
2.9. Appearance and Color Determination
2.10. Texture Profile Analysis (TPA)
2.11. Nutrient Content Determination and Energy Values
2.12. Statistical Analysis
3. Results and Discussion
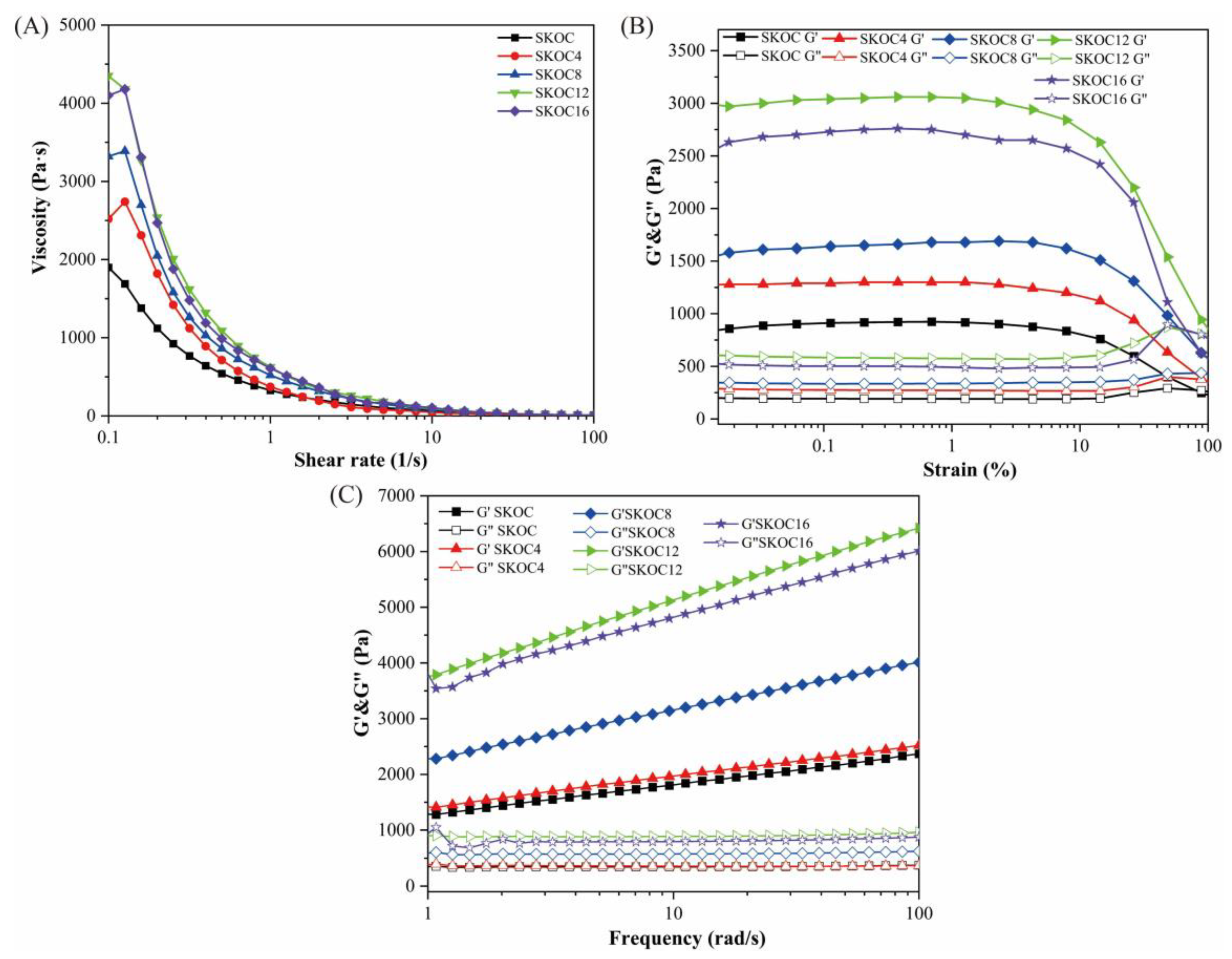
3.1. Rheological Properties of Emulsion Gels
3.2. FT-IR Analysis of Emulsion Gels
3.3. XRD Analysis of Emulsion Gels
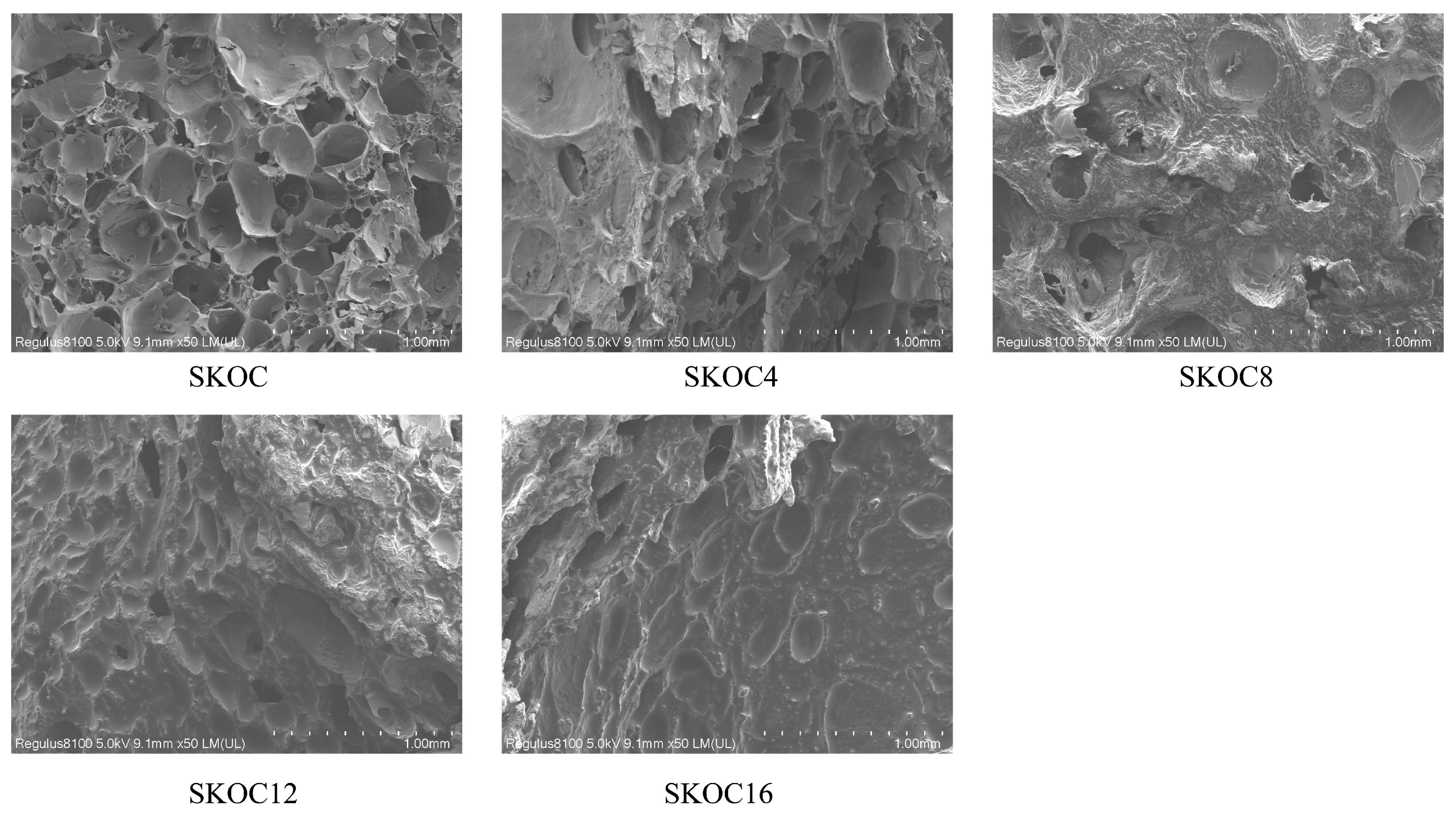
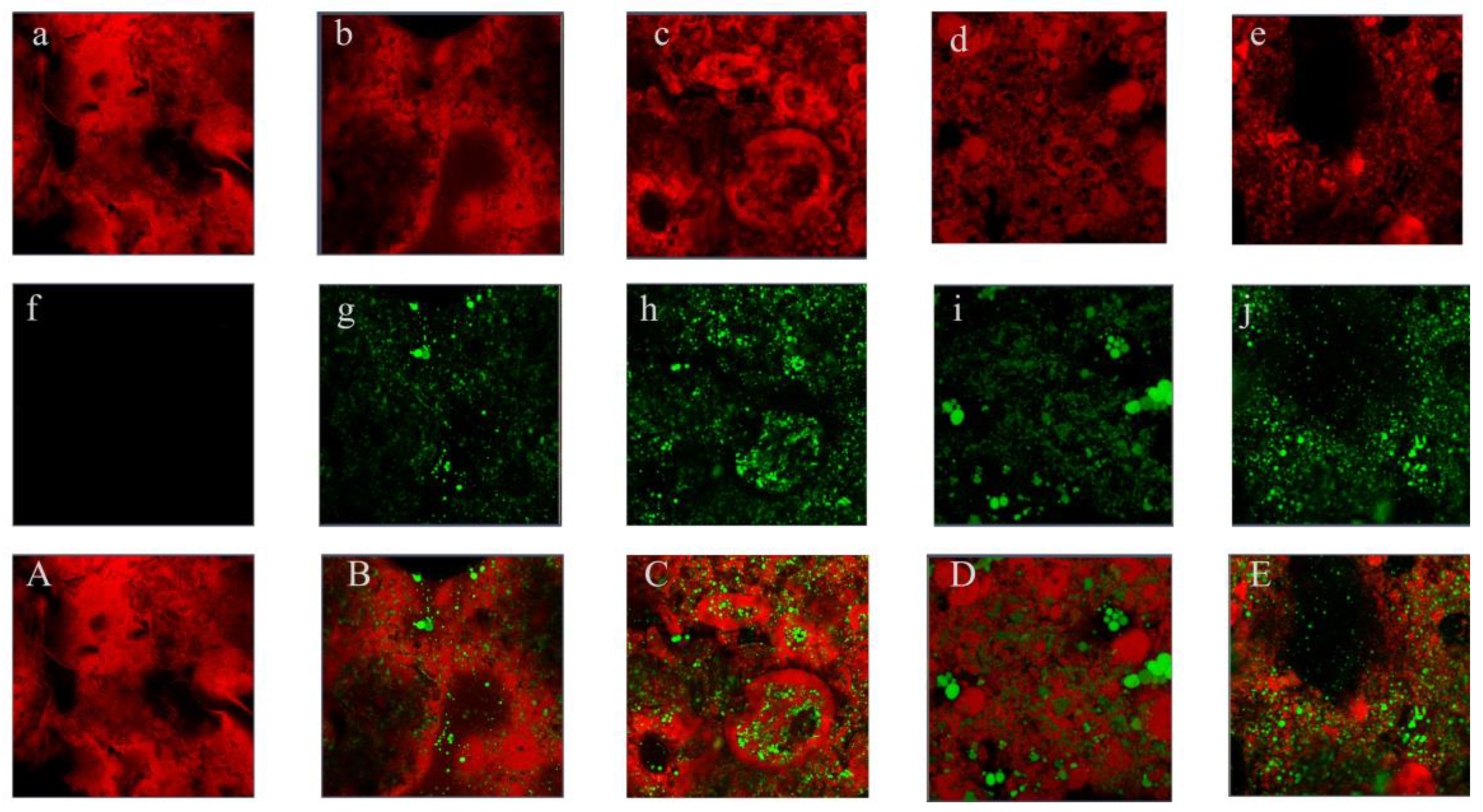
3.4. The Microstructure of Emulsion Gels
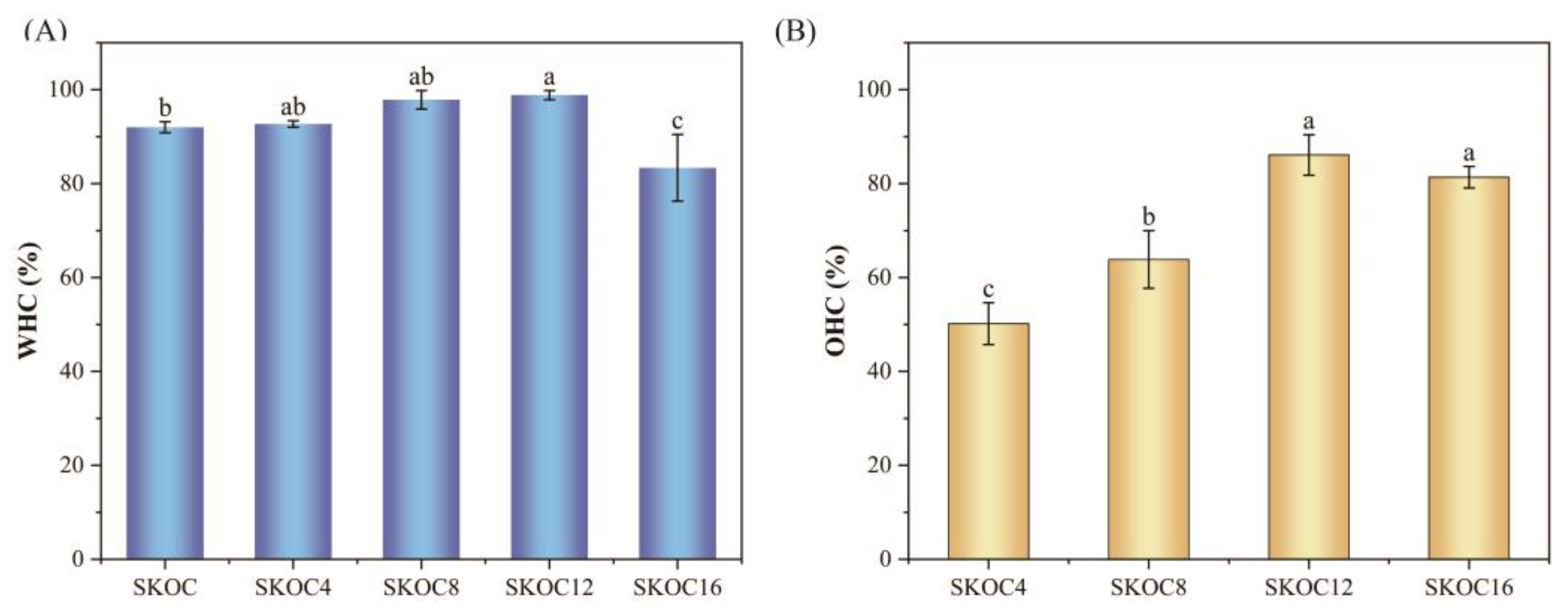
3.5. WHC and OHC of Gels
3.6. Appearance and Color Analysis of Emulsion Gels
3.7. TPA of Emulsion Gels
3.8. Nutritional Content and Energy Value of Emulsion Gels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho Barros, J.; Munekata, P.E.S.; de Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of Tiger Nut (Cyperus esculentus L.) Oil Emulsion as Animal Fat Replacement in Beef Burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef]
- Lee, J.; Wi, G.; Choi, M.-J. The rheological properties and stability of gelled emulsions applying to κ-carrageenan and methyl cellulose as an animal fat replacement. Food Hydrocoll. 2023, 136, 108243. [Google Scholar] [CrossRef]
- Tian, T.; Chen, L.; Cao, X.; Ren, K.; Zheng, L.; Tong, X.; Tian, M.; Wang, H.; Huang, Z.; Jiang, L. Effect of different protein ratios on in vitro digestive properties of soybean-wheat co-precipitated protein high-moisture extrudates. Food Chem. 2025, 478, 143354. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Fernández-Martín, F.; Rodríguez-Salas, L.; Ruiz-Capillas, C. Konjac gel fat analogue for use in meat products: Comparison with pork fats. Food Hydrocoll. 2012, 26, 63–72. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Choi, J.-H.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Kim, H.-W.; Jeong, J.-Y.; Kim, C.-J. Characteristics of low-fat meat emulsion systems with pork fat replaced by vegetable oils and rice bran fiber. Meat Sci. 2009, 82, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Advances in microencapsulation of polyunsaturated fatty acids (PUFAs)-rich plant oils using complex coacervation: A review. Food Hydrocoll. 2017, 69, 369–381. [Google Scholar] [CrossRef]
- Ren, Y.; Ye, X.; Wei, L.; Li, H.; Cao, J.; Safdar, B.; Liu, X. Influence of variation in phase ratio and protein content on physicochemical properties and structure of soy protein isolate-konjac glucomannan double emulsion gels applicable as solid cubic fat substitutes. Food Chem. 2025, 465, 142023. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, L.; Hao Yoong, J.; Miao, Z.; Li, H.; Cao, J.; Liu, X. Effect of variation in basic emulsion structure and polysaccharide content on the physicochemical properties and structure of composite-based emulsion gels as cube fat mimetics. Food Chem. 2024, 434, 137450. [Google Scholar] [CrossRef]
- Feng, L.; Jia, X.; Zhu, Q.; Liu, Y.; Li, J.; Yin, L. Investigation of the mechanical, rheological and microstructural properties of sugar beet pectin /soy protein isolate-based emulsion-filled gels. Food Hydrocoll. 2019, 89, 813–820. [Google Scholar] [CrossRef]
- Zhao, J.; Chang, B.; Wen, J.; Fu, Y.; Luo, Y.; Wang, J.; Zhang, Y.; Sui, X. Fabrication of soy protein isolate-konjac glucomannan emulsion gels to mimic the texture, rheological behavior and in vitro digestion of pork fat. Food Chem. 2025, 468, 142462. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ren, Y.; Li, H.; Zhang, Q.; Wang, Y.; Cao, J.; Liu, X. Create Fat Substitute From Soybean Protein Isolate/Konjac Glucomannan: The Impact of the Protein and Polysaccharide Concentrations Formulations. Front. Nutr. 2022, 9, 843832. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ren, Y.; Huang, L.; Ye, X.; Li, H.; Li, J.; Cao, J.; Liu, X. Quality, Thermo-Rheology, and Microstructure Characteristics of Cubic Fat Substituted Pork Patties with Composite Emulsion Gel Composed of Konjac Glucomannan and Soy Protein Isolate. Gels 2024, 10, 111. [Google Scholar] [CrossRef]
- Li, D.; Zhong, W.; Li, L.; Tong, C.; Yu, S.; Duan, M.; Xu, J.; Liu, X.; Pang, J.; Wu, C. Effect of chitin nanowhiskers on structural and physical properties of konjac glucomannan hydrogels nanocomposites. Food Hydrocoll. 2023, 141, 108731. [Google Scholar] [CrossRef]
- Bu, N.; Sun, R.; Huang, L.; Lin, H.; Pang, J.; Wang, L.; Mu, R. Chitosan films with tunable droplet size of Pickering emulsions stabilized by amphiphilic konjac glucomannan network. Int. J. Biol. Macromol. 2022, 220, 1072–1083. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, H.; Zhu, D.; Liu, J.; Zhang, C.; Li, C.; Han, J. Influence of Soybean Dietary Fiber on the properties of Konjac Glucomannan/κ-Carrageenan Corn Oil Composite Gel. Food Hydrocoll. 2022, 129, 107602. [Google Scholar] [CrossRef]
- Zhao, S.; Yuan, X.; Yang, L.; Zhu, M.; Ma, H.; Zhao, Y. The effects of modified quinoa protein emulsion as fat substitutes in frankfurters. Meat Sci. 2023, 202, 109215. [Google Scholar] [CrossRef]
- Kang, Z.-L.; Wang, T.-t.; Li, Y.-p.; Li, K.; Ma, H.-j. Effect of sodium alginate on physical-chemical, protein conformation and sensory of low-fat frankfurters. Meat Sci. 2020, 162, 108043. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Han, L.; Xu, Q.; Li, S.; Prakash, S.; Dong, X. Enhanced rheological and 3D-printing properties of high internal phase emulsions stabilized by egg white microgels synergized with konjac gum/xanthan gum. Food Hydrocoll. 2024, 153, 109981. [Google Scholar] [CrossRef]
- Yu, X.; Han, L.; Liu, J.; Jiang, W.; Pan, J.; Yu, C.; Dong, X. Preparation and 3D printing of high internal-phase Pickering emulsions stabilized by chicken egg white microgel. Food Hydrocoll. 2024, 147, 109393. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Chen, X.; Tang, Z.; Yang, J.; Jiang, X.; Fang, Y.; Ding, J. Fabrication of strong and elastic HIPPEs gel by rigid-flexible double network structure as a novel adipose tissue substitutes. Food Hydrocoll. 2024, 156, 110367. [Google Scholar] [CrossRef]
- Foudazi, R.; Qavi, S.; Masalova, I.; Malkin, A.Y. Physical chemistry of highly concentrated emulsions. Adv. Colloid Interface Sci. 2015, 220, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wei, S.; Zhang, M.; Song, Y.; Pang, J.; Zhang, H. Decoding protein structure effects: Konjac glucomannan mediated oleogel networks with different structural properties via emulsion interface design. Food Chem. 2025, 491, 145220. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, D.; Xie, F. The role of alginate in starch nanocrystals-stabilized Pickering emulsions: From physical stability and microstructure to rheology behavior. Food Chem. 2024, 431, 137017. [Google Scholar] [CrossRef]
- Su, C.-y.; Li, D.; Wang, L.-j.; Wang, Y. Development of corn starch-sodium alginate emulsion gels as animal fat substitute: Effect of oil concentration. Food Hydrocoll. 2024, 157, 110439. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Li, C.; Fu, X.; Huang, Q. Pickering emulsion gel stabilized by octenylsuccinate quinoa starch granule as lutein carrier: Role of the gel network. Food Chem. 2020, 305, 125476. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, S.; Du, L.; Liu, Y.; Meng, Z. Soft κ-carrageenan microgels stabilized pickering emulsion gels: Compact interfacial layer construction and particle-dominated emulsion gelation. J. Colloid Interface Sci. 2021, 602, 822–833. [Google Scholar] [CrossRef]
- Gan, J.; Sun, L.; Guan, C.; Ren, T.; Zhang, Q.; Pan, S.; Zhang, Q.; Chen, H. Preparation and Properties of Salecan–Soy Protein Isolate Composite Hydrogel Induced by Thermal Treatment and Transglutaminase. Int. J. Mol. Sci. 2022, 23, 9383. [Google Scholar] [CrossRef]
- He, M.; Zhang, M.; Gao, T.; Chen, L.; Liu, Y.; Huang, Y.; Teng, F.; Li, Y. Assembly of soy protein-corn starch composite gels by thermal induction: Structure, and properties. Food Chem. 2024, 434, 137433. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, L.; Chen, F.; Zhang, P.; Lv, D.; Zhu, T.; Duan, X. Effect of soybean oil content on textural, rheological, and microstructural properties of WBAXs-SPI emulsion-filled gels. J. Texture Stud. 2021, 52, 251–259. [Google Scholar] [CrossRef]
- Hao, T.; Xia, S.; Song, J.; Ma, C.; Xue, C.; Jiang, X. Comprehensive investigation into the effects of yeast dietary fiber and temperature on konjac glucomannan/kappa-carrageenan for the development of fat analogs. Int. J. Biol. Macromol. 2024, 254, 127459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, C.; Liu, Y.; Xu, D.; Cui, B. The influence of a hydroxypropyl-beta-cyclodextrin composite on the gelation of kappa-carrageenan. Food Hydrocoll. 2019, 90, 276–284. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Tang, J.; Huang, C.; Liu, W.; Zeng, X.; Zhang, J.; Liu, W.; Pang, J.; Wu, C. Preparation and characterization of a konjac glucomannan-based bio-nanocomposite film and its application in cherry tomato preservation. Food Hydrocoll. 2025, 159, 110689. [Google Scholar] [CrossRef]
- Tong, C.; Jiang, S.; Ye, D.; Li, K.; Liu, J.; Zeng, X.; Wu, C.; Pang, J. Enhanced mechanical property and freeze-thaw stability of alkali-induced heat-set konjac glucomannan hydrogel through anchoring interface effects of carboxylated cellulose nanocrystals. Food Hydrocoll. 2023, 142, 108812. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, Y.L.; Chen, J. Role of disulphide linkages between protein-coated lipid droplets and the protein matrix in the rheological properties of porcine myofibrillar protein–peanut oil emulsion composite gels. Meat Sci. 2011, 88, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Liang, R.; Sun, H.; Wu, L.; Yang, C.; Liu, Y. Development and characterization of ultrastable emulsion gels based on synergistic interactions of xanthan and sodium stearoyl lactylate. Food Chem. 2023, 400, 133957. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, Y.; Zhao, H.; He, X.; Li, X.; Yi, S.; Li, J. Diacylglycerol pre-emulsion prepared through ultrasound improves the gel properties of golden thread surimi. Ultrason. Sonochem. 2022, 82, 105915. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pan, S.; Regenstein, J.M.; Huang, J.; Wang, L. Characteristics of composite gels composed of citrus insoluble nanofiber and amylose and their potential to be used as fat replacers. Food Chem. 2023, 409, 135269. [Google Scholar] [CrossRef]
- Lan, M.; Li, L.; Li, T.; Wang, S.; Yang, T.; Luo, S.; Zhang, X.; Xing, H.; Chen, J.; Li, B. Gel properties and in vitro digestion of sea bass (Lateolabrax japonicus) myofibrillar protein—Lipid composite gels: Focus on lipid type and content. LWT 2024, 191, 115561. [Google Scholar] [CrossRef]
- Cui, B.; Mao, Y.; Liang, H.; Li, Y.; Li, J.; Ye, S.; Chen, W.; Li, B. Properties of soybean protein isolate/curdlan based emulsion gel for fat analogue: Comparison with pork backfat. Int. J. Biol. Macromol. 2022, 206, 481–488. [Google Scholar] [CrossRef]
- Liu, W.; He, K.; McClements, D.J.; Jin, Z.; Chen, L. Pea starch-based oleogels based on capillary water crosslinking: Physicochemical properties and 3D printing performance. Food Hydrocoll. 2024, 154, 110161. [Google Scholar] [CrossRef]
- Sun, C.; Wei, Z.; Xue, C. Construction of foam-templated Antarctic krill oil oleogel based on pea protein fibril and ι-carrageenan. Carbohydr. Polym. 2025, 347, 122729. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; McClements, D.J. Preparation of plant-based meat analogs using emulsion gels: Lipid-filled RuBisCo protein hydrogels. Food Res. Int. 2023, 167, 112708. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, N.; van Dommelen, J.A.W.; Geers, M.G.D. Intrinsic mechanical properties of food in relation to texture parameters. Mech. Time-Depend. Mater. 2021, 26, 323–346. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Li, H.; Yoong, J.H.; Miao, Z.; Cao, J.; Liu, X. Effect of palm oil content and melting point on the freeze-thaw stability of fat substitutes. LWT 2023, 188, 115452. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Tam, B.; Bhandari, B.; Prakash, S. Effect of different types and concentrations of fat on the physico-chemical properties of soy protein isolate gel. Food Hydrocoll. 2021, 111, 106226. [Google Scholar] [CrossRef]
- Katan, M.B.; Mozaffarian, D.; Micha, R.; Wallace, S. Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.; Jo, Y.-J.; Choi, M.-J. Thermo-irreversible emulsion gels based on deacetylated konjac glucomannan and methylcellulose as animal fat analogs. Food Hydrocoll. 2023, 137, 108407. [Google Scholar] [CrossRef]




| L* | A* | B* | Whiteness | |
|---|---|---|---|---|
| SKOC | 26.99 ± 0.18 e | −1.19 ± 0.06 d | 8.27 ± 0.27 d | 26.48 ± 0.15 c |
| SKOC4 | 29.95 ± 2.23 d | −1.21 ± 0.16 d | 12.17 ± 0.35 c | 28.88 ± 2.14 c |
| SKOC8 | 35.71 ± 0.43 c | 0.61 ± 0.07 c | 12.55 ± 0.51 c | 34.50 ± 0.41 b |
| SKOC12 | 46.74 ± 1.74 ab | 1.75 ± 0.19 b | 14.62 ± 0.52 b | 44.74 ± 1.66 a |
| SKOC16 | 48.57 ± 1.68 a | 1.88 ± 0.12 ab | 17.39 ± 0.56 a | 45.67 ± 1.41 a |
| PBF | 45.34 ± 2.24 b | 2.01 ± 0.16 a | 4.58 ± 0.54 e | 45.13 ± 2.19 a |
| Scheme. | Hardness (N) | Springiness (mm) | Cohesiveness (−) | Chewiness (mJ) |
|---|---|---|---|---|
| SKOC | 545.29 ± 6.84 d | 0.96 ± 0.01 a | 0.86 ± 0.03 a | 460.27 ± 5.22 e |
| SKOC4 | 626.53 ± 39.19 c | 0.96 ± 0.02 a | 0.85 ± 0.03 a | 504.23 ± 17.47 d |
| SKOC8 | 676.83 ± 29.09 b | 0.96 ± 0.02 a | 0.85 ± 0.04 a | 549.27 ± 3.84 c |
| SKOC12 | 768.07 ± 19.65 a | 0.98 ± 0.01 a | 0.86 ± 0.04 a | 684.38 ± 42.47 a |
| SKOC16 | 746.53 ± 17.24 a | 0.98 ± 0.02 a | 0.87 ± 0.06 a | 613.12 ± 8.15 b |
| PBF | 761.13 ± 19.67 a | 0.98 ± 0.01 a | 0.84 ± 0.04 a | 686.16 ± 23.37 a |
| PBF | SKOC12 | |
|---|---|---|
| Fat (%) | 88.33 ± 0.08 | 12.43 ± 0.09 |
| Moisture (%) | 7.50 ± 0.01 | 69.67 ± 0.21 |
| Protein (%) | 3.99 ± 0.05 | 10.94 ± 0.06 |
| Ash (%) | 0.11 ± 0.00 | 1.24 ± 0.01 |
| Energy (kcal/100 g) | 820.47 ± 0.46 | 181.41 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Li, D.; Zhang, J.; Xie, X.; Shi, S.; Shi, J.; Li, Y.; Pang, J.; Wu, C. Constructing Stable Emulsion Gel from Soy Protein Isolate and Konjac Glucomannan as Pork Fat Substitute: Effect of Oil Concentration. Foods 2025, 14, 2760. https://doi.org/10.3390/foods14162760
Tang J, Li D, Zhang J, Xie X, Shi S, Shi J, Li Y, Pang J, Wu C. Constructing Stable Emulsion Gel from Soy Protein Isolate and Konjac Glucomannan as Pork Fat Substitute: Effect of Oil Concentration. Foods. 2025; 14(16):2760. https://doi.org/10.3390/foods14162760
Chicago/Turabian StyleTang, Junjie, Danjie Li, Jianxi Zhang, Xianyang Xie, Si Shi, Jie Shi, Yuanzhao Li, Jie Pang, and Chunhua Wu. 2025. "Constructing Stable Emulsion Gel from Soy Protein Isolate and Konjac Glucomannan as Pork Fat Substitute: Effect of Oil Concentration" Foods 14, no. 16: 2760. https://doi.org/10.3390/foods14162760
APA StyleTang, J., Li, D., Zhang, J., Xie, X., Shi, S., Shi, J., Li, Y., Pang, J., & Wu, C. (2025). Constructing Stable Emulsion Gel from Soy Protein Isolate and Konjac Glucomannan as Pork Fat Substitute: Effect of Oil Concentration. Foods, 14(16), 2760. https://doi.org/10.3390/foods14162760





