Effects of Different Drying Methods on the Quality of Forest Ginseng Revealed Based on Metabolomics and Enzyme Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instrument
2.2. Sample Collection and Drying Treatment
2.3. Enzyme Activity Analyses [14]
2.4. Widely Targeted Metabolomic Analyses [15]
2.5. Data Analysis
3. Results
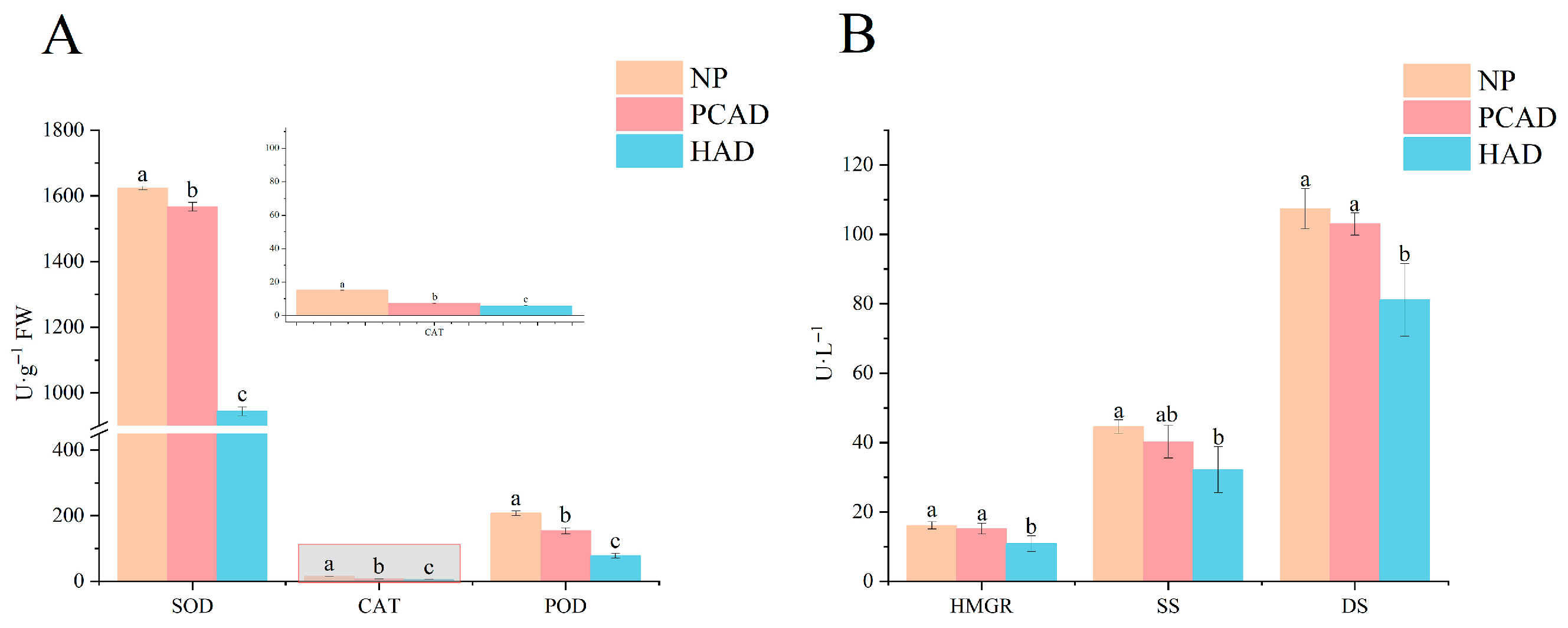
3.1. Enzyme Activity
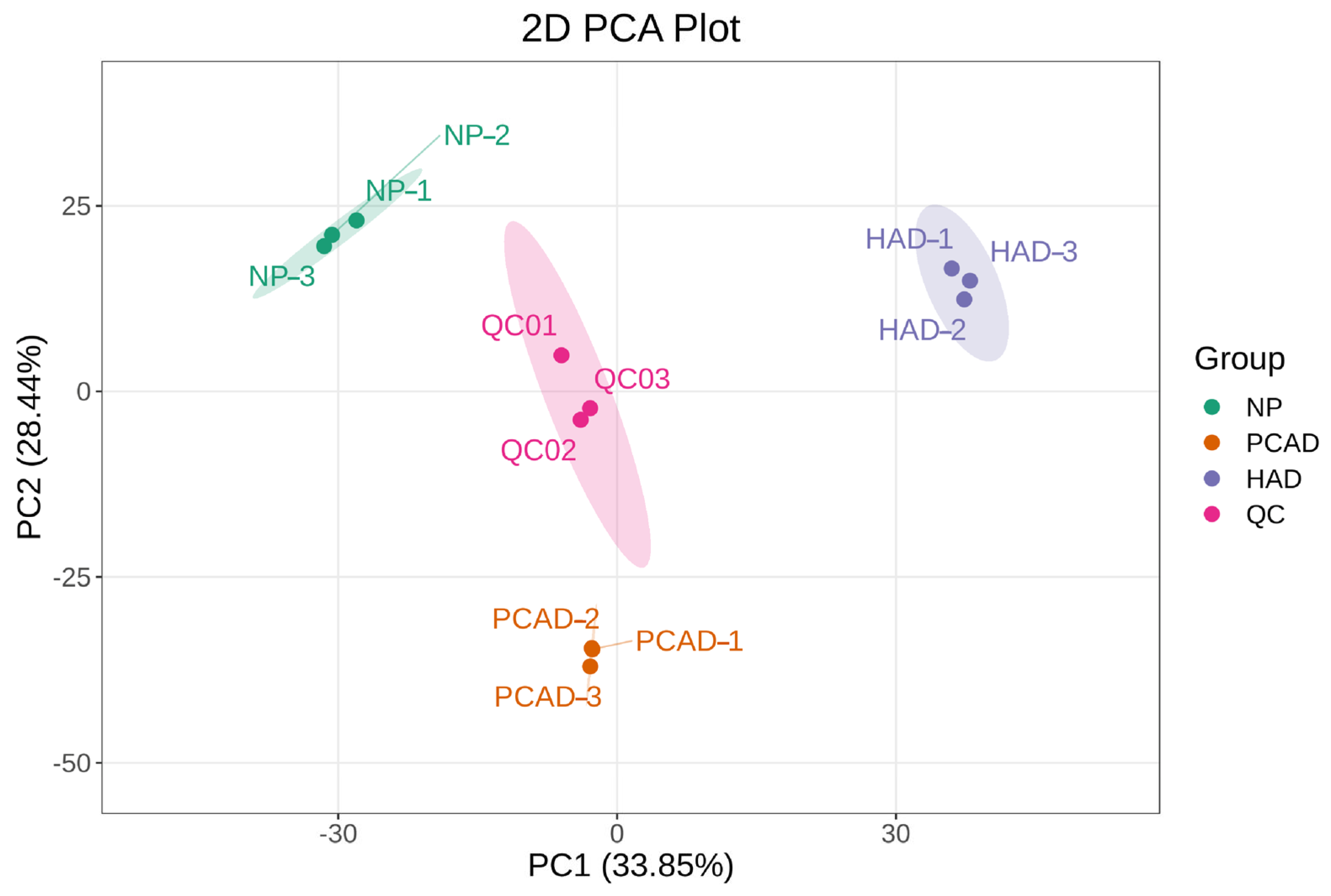
3.2. Metabolome Analysis
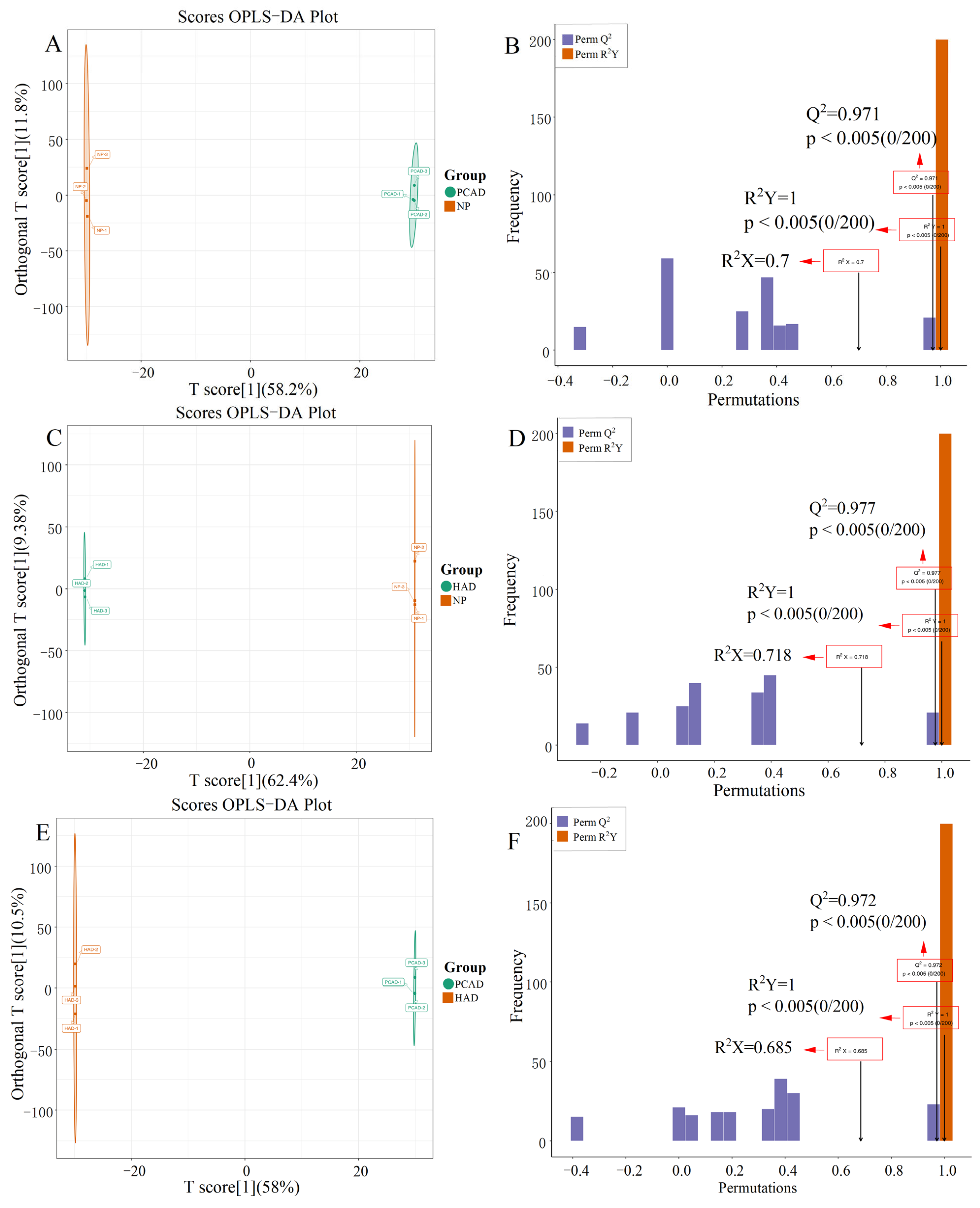
3.2.1. PCA and OPLS-DA Analysis Results
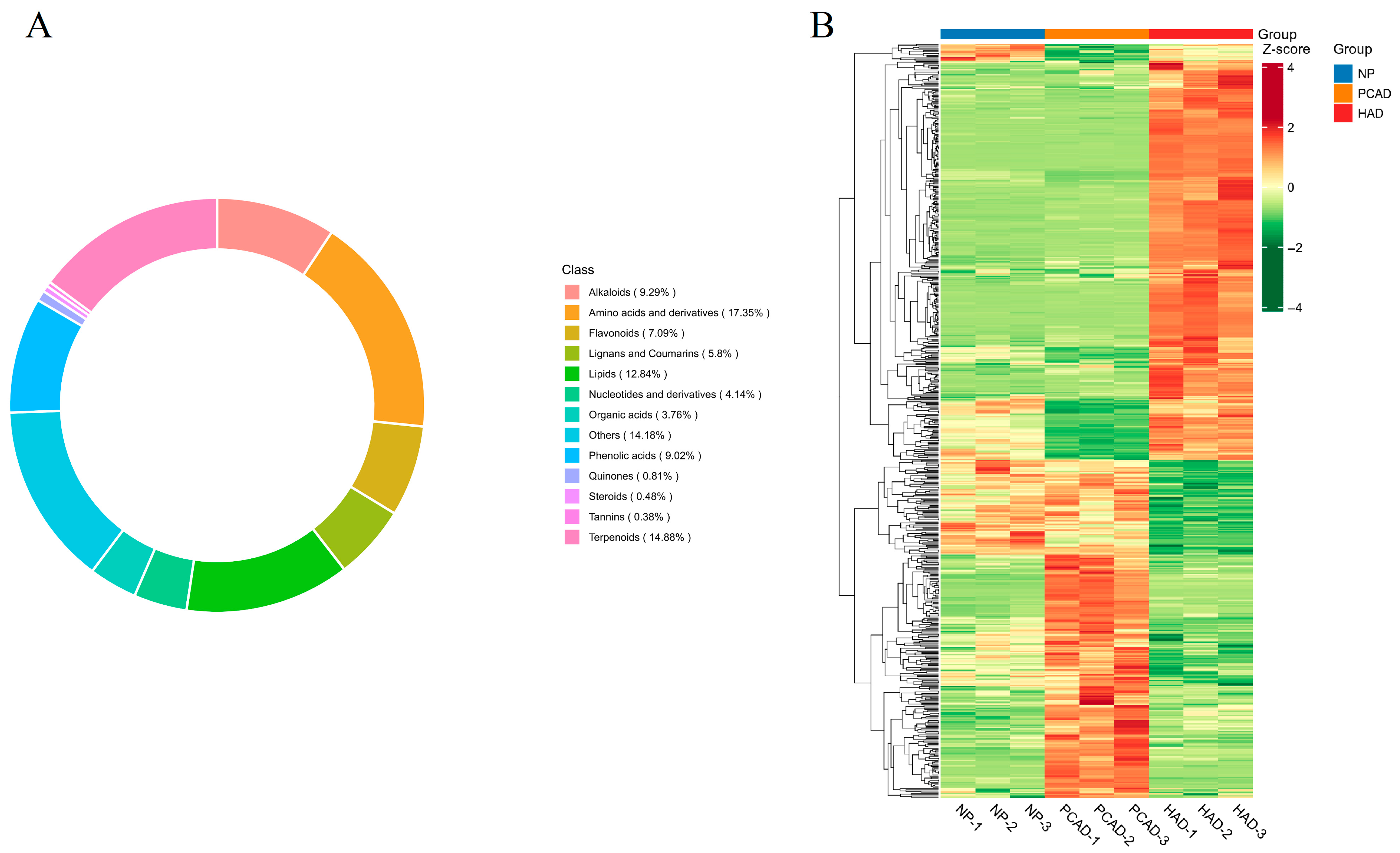
3.2.2. Composition and Classification of Metabolites
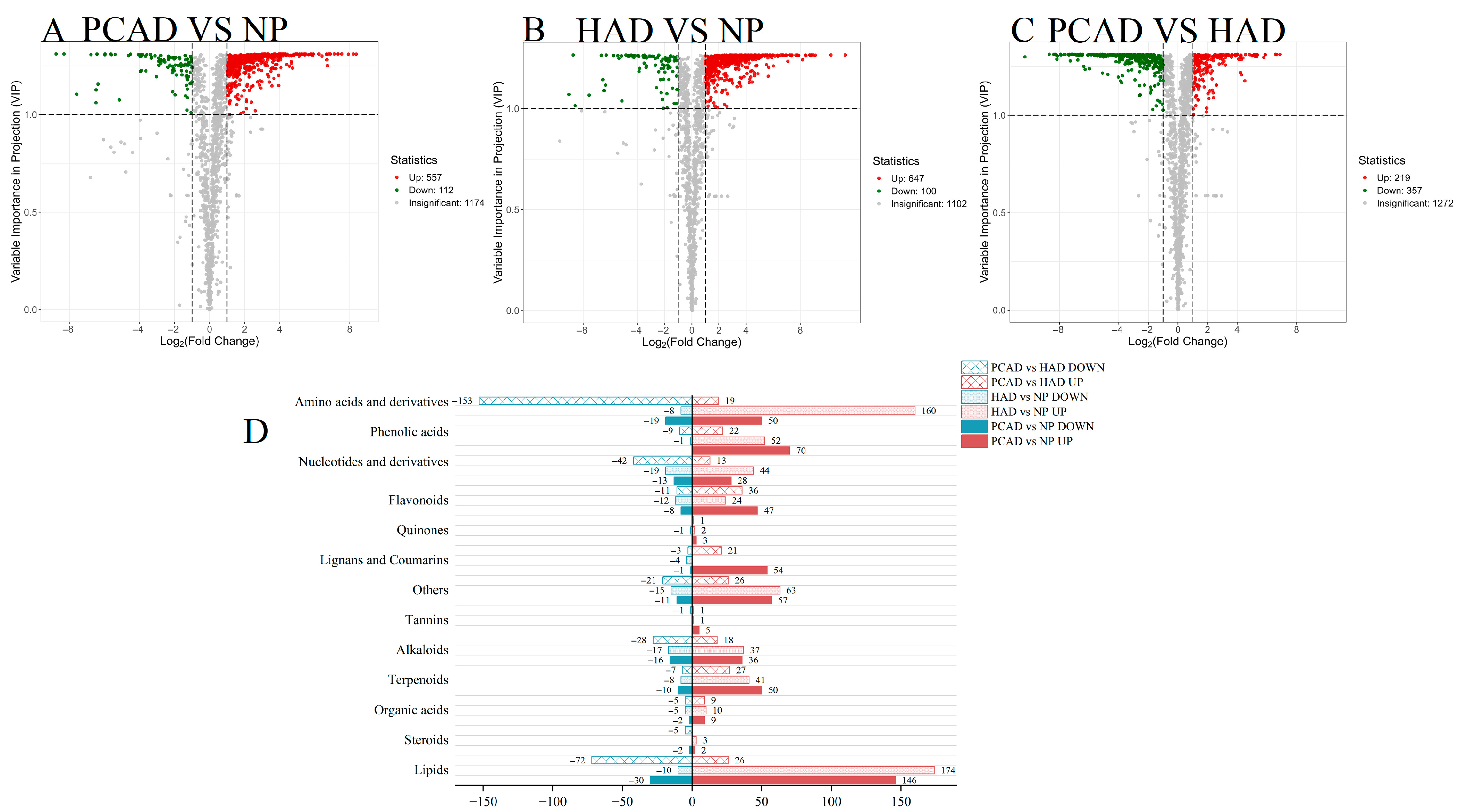
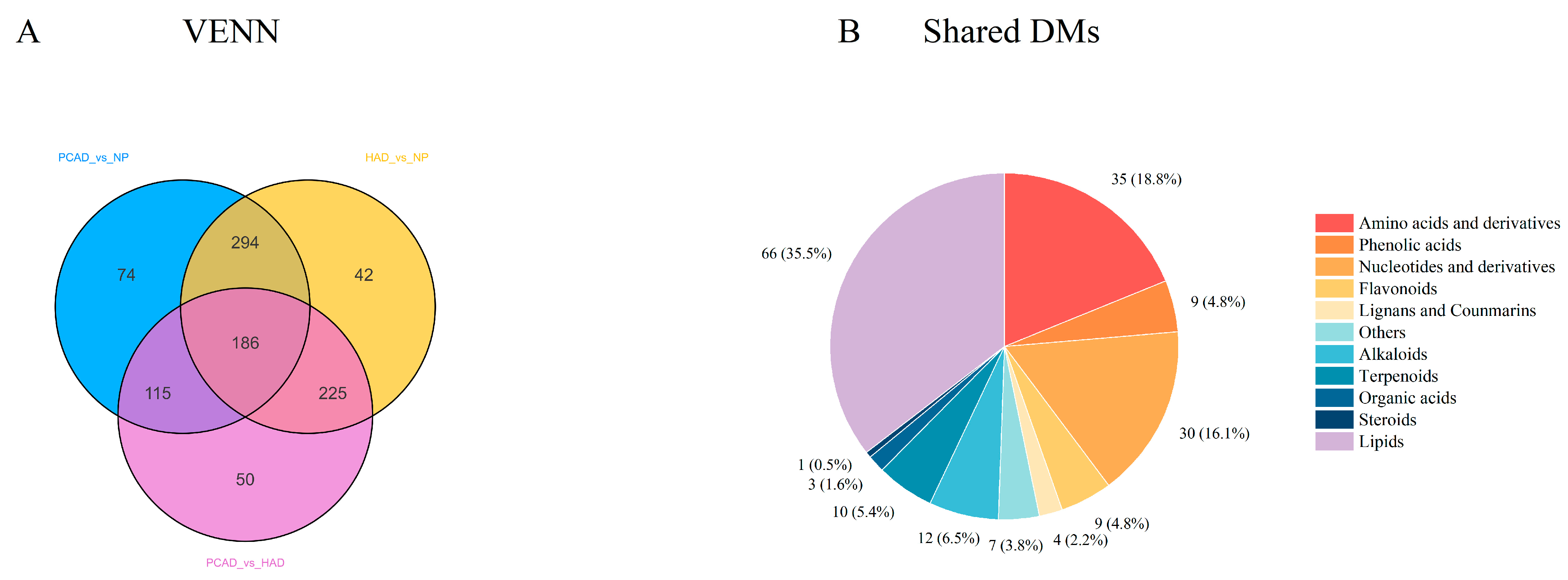
3.2.3. Differential Metabolites (DMs)
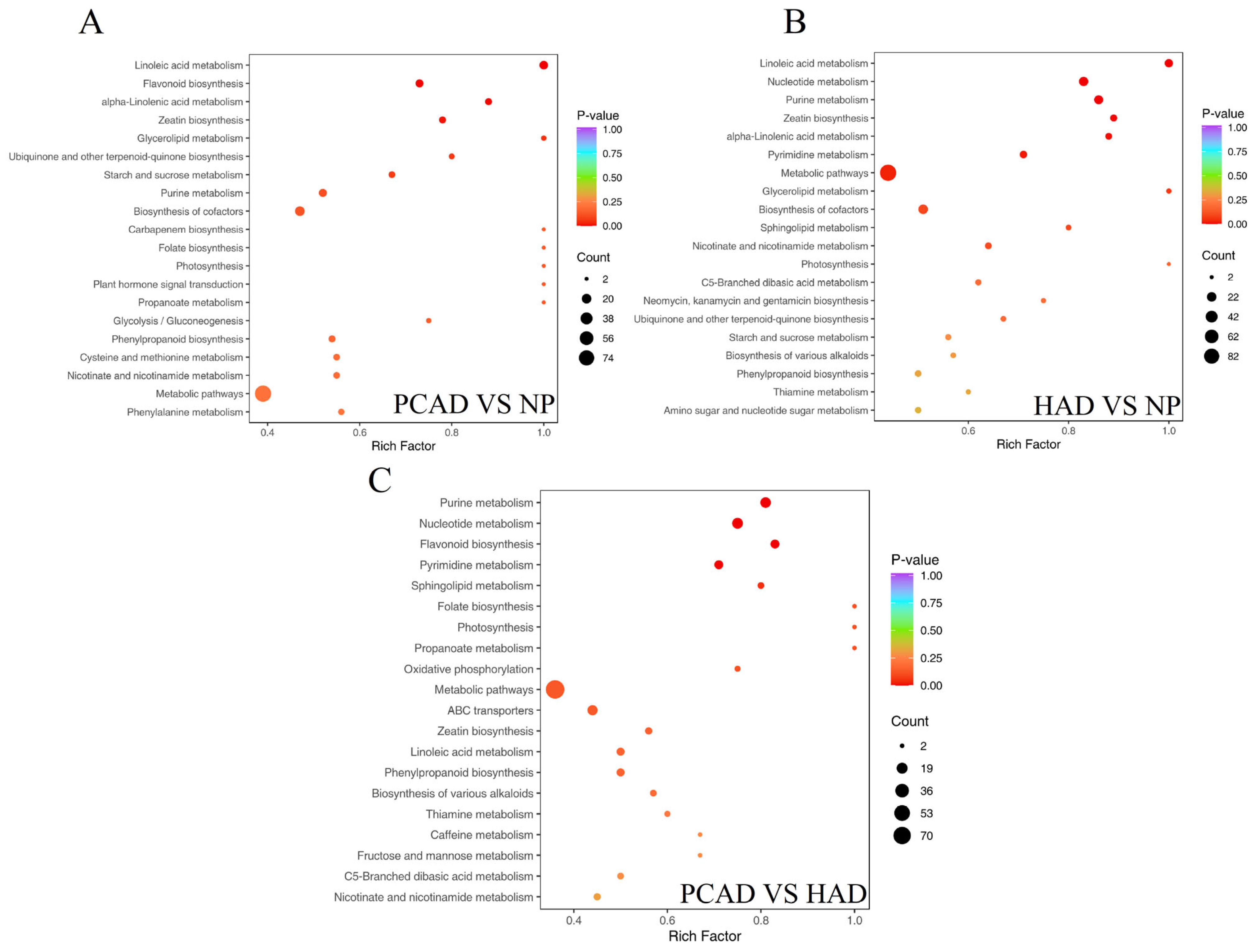
3.2.4. Metabolic Pathway Analysis
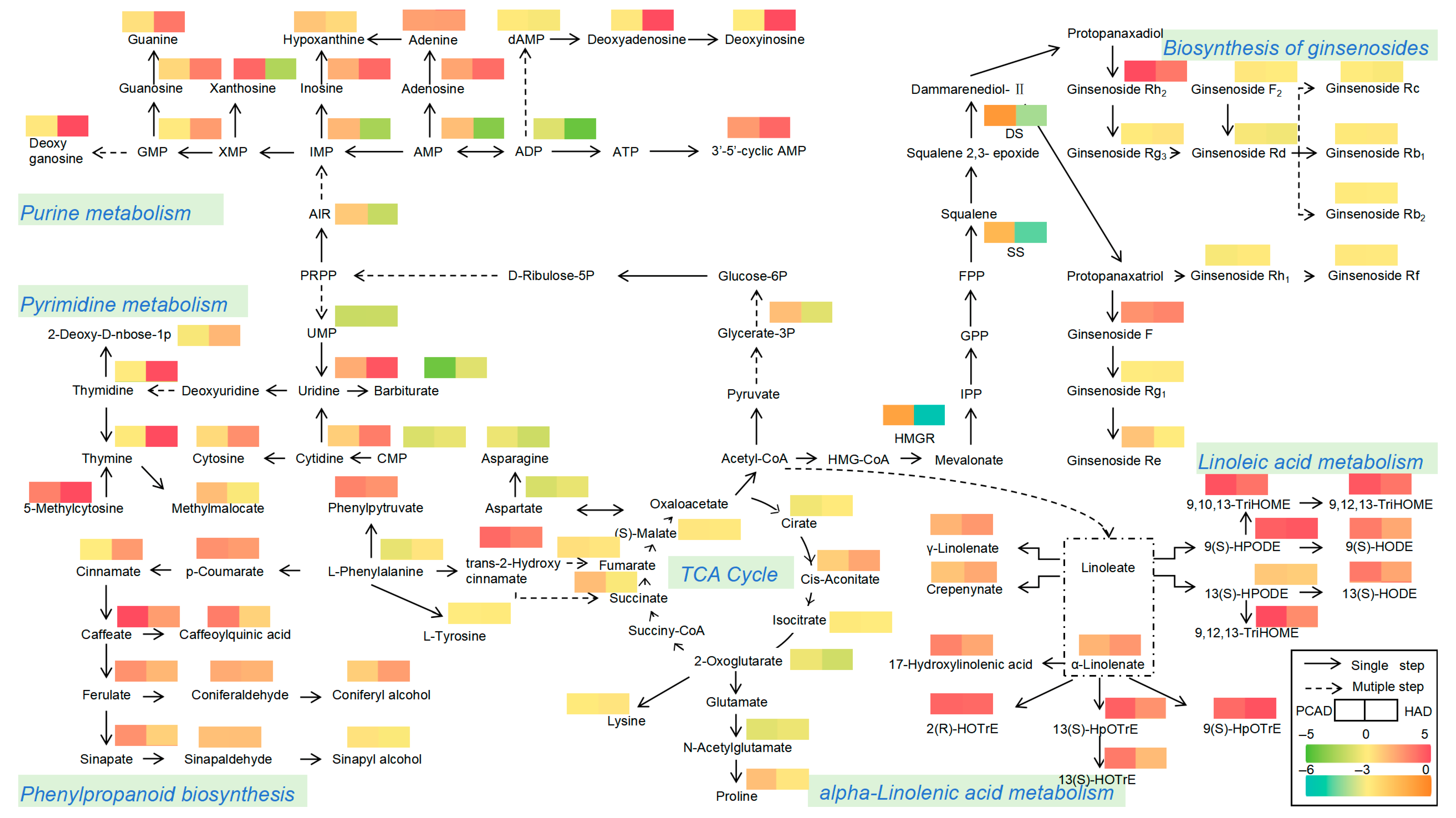
3.2.5. Analysis of Metabolic Pathways of Representative Bioactive Ingredients
4. Discussion
4.1. Effect of Drying on Enzyme Activity
4.2. Effects of Drying on Metabolome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, X.-F.; Cheng, X.-L.; Lin, Q.-H.; Li, S.-S.; Jia, Z.; Han, T.; Lin, R.-C.; Wang, D.; Wei, F.; Li, X.-R. Identification of mountain-cultivated ginseng and cultivated ginseng using UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy. J. Ginseng Res. 2016, 40, 344–350. [Google Scholar] [CrossRef]
- Zhu, L.L.; Luan, X.N.; Yuan, Y.; Dou, D.Q.; Huang, L.Q. The characteristics of ginsenosides and oligosaccharides in mountain- and garden-cultivated ginseng. J. Sci. Food Agric. 2021, 101, 1491–1498. [Google Scholar] [CrossRef]
- Xu, X.; Yu, S.; Dou, D. Comparison of the effects of garden ginseng on immune function in mice. Ginseng Res. 2014, 4, 2–4. [Google Scholar]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Y.; Xu, H.; Sun, S.; Wang, Z. Differentiation of the Root of Cultivated Ginseng, Mountain Cultivated Ginseng and Mountain Wild Ginseng Using FT-IR and Two-Dimensional Correlation IR Spectroscopy. J. Mol. Struct. 2008, 883, 228–235. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. 2020, 61, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Arora, A. Alternate microwave and convective hot air application for rapid mushroom drying. J. Food Eng. 2018, 223, 208–219. [Google Scholar] [CrossRef]
- Jalali, N.; Goli, M.; Sheikhan, N.; Shahi, S.; Kermani, S. Effect of Calcium Chloride Extracted from Poultry Eggshells on the Physicochemical Properties of Iranian High-Fat White Cheese. Iran. J. Chem. Chem. Eng. 2024, 42, 4443–4455. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Q.Y.; Niu, L.C.; Yuan, H.B.; Shan, X.J.; Hua, J.J.; Chen, L.; Zhang, Q.T.; Feng, Y.N.; Yu, X.L.; et al. Integration of intelligent sensory evaluation, metabolomics, quantification, and enzyme activity analysis to elucidate the influence of first-drying methods on the flavor formation of congou black tea and its underlying mechanism. Food Chem. 2025, 480, 143858. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Su, H.; Zhao, S.; Jin, R.; Cheng, H.; Xu, A.; Lai, W.; Yin, X.; Wang, Y. Transcriptome and Phytochemical Analysis Reveals the Alteration of Plant Hormones, Characteristic Metabolites, and Related Gene Expression in Tea (Camellia sinensis L.) Leaves During Withering. Plants 2020, 9, 204. [Google Scholar] [CrossRef]
- Zhang, H.F.; Lu, Q.; Liu, R. Widely targeted metabolomics reveals the effect of fermentation on the chemical composition of bee pollen. Food Chem. 2022, 375, 131908. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J.; Su, L.; Sun, K.; Li, D.; Liu, Y.; Wang, H.; Chen, Z.; Guo, T. Metabolic profile analysis and identification of key metabolites during rice seed germination under low-temperature stress. Plant Sci. 2019, 289, 110282. [Google Scholar] [CrossRef]
- Cai, E.; Du, R.; Han, J.; Dang, W.; Zhao, Y.; Xue, L. A Kind of Negative Pressure Dry Processing Internal Circulation System. China ZL 202420876517.6, 6 December 2024. [Google Scholar]
- Ma, R.; Sun, L.; Chen, X.; Mei, B.; Chang, G.; Wang, M.; Zhao, D. Proteomic analyses provide novel Insights into plant growth and ginsenoside biosynthesis in forest cultivated Panax ginseng (F. Ginseng). Front. Plant Sci. 2016, 7, 1. [Google Scholar] [CrossRef]
- Xiao, J.Q.; Gu, C.Q.; He, S.; Zhu, D.X.; Huang, Y.K.; Zhou, Q.Q. Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Res. Int. 2021, 141, 110128. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Li, L.; Aghdam, M.; Wei, X.; Liu, J.; Xu, Y.; Luo, Z. Elevated CO2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chem. 2019, 285, 163–170. [Google Scholar] [CrossRef]
- Li, T.; Shi, D.; Wu, Q.; Zhang, Z.; Qu, H.; Jiang, Y. Sodium para-aminosalicylate delays pericarp browning of litchi fruit by inhibiting ROS-mediated senescence during postharvest storage. Food Chem. 2019, 278, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Pratama, B.P.; Pranoto, Y.; Supriyadi, S.; Swasono, R.T. Effect of drying time and temperature to the chemical properties and enzymatic activities related to the β-ocimene production in syzygium polyanthum leaves. Trends Sci. 2022, 19, 1526. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, H.; Hua, J.; Jiang, Y.; Dong, C.; Deng, Y.; Yang, Y. Effects of second-drying process parameters on the hot-air drying characteristics and quality of congou black tea. Transact. Chin. Soc. Agr. Eng. 2020, 36, 287–296. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, Z.; Cui, F.; Zhang, Q.; Song, P.; Li, B.; Tang, Z.; Hu, F.; Shi, X. Characterization of chemical composition variations in raw and processed Codonopsis Radix by integrating metabolomics and glycomics based on multiple chromatography-mass spectrometry technology. J. Sep. Sci. 2022, 45, 2375–2393. [Google Scholar] [CrossRef]
- Hyun, S.H.; Bhilare, K.D.; In, G.; Park, C.K.; Kim, J.H. Effects of Panax ginseng and ginsenosides on oxidative stress and cardiovascular diseases: Pharmacological and therapeutic roles. J. Ginseng Res. 2022, 46, 33–38. [Google Scholar] [CrossRef]
- Hao, J. Quality Evaluation and Influence Factors of Ginseng Product. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2012. [Google Scholar]
- Yao, F.; Li, X.; Sun, J.; Cao, X.X.; Liu, M.M.; Li, Y.H.; Liu, Y.J. Thermal transformation of polar into less-polar ginsenosides through demalonylation and deglycosylation in extracts from ginseng pulp. Sci. Rep. 2021, 11, 1513. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Xiao, W.; Cheng, S.; Liu, F.; Huang, L. Determination of drying kinetics and quality changes of Panax quinquefolium L. dried in hot-blast air. LWT 2019, 116, 108563. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, Q.; Jiang, L.; Chen, C.; Huang, X.; Zhu, X.; Zhou, T.; Chen, J.; Yan, J.; Wen, F.; et al. Metabolomics analysis reveals metabolite changes during freeze-drying and oven-drying of Angelica dahurica. Sci. Rep. 2023, 13, 6022. [Google Scholar] [CrossRef] [PubMed]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wen, P.P.; Yuan, Y.M.; Yu, X.F.; Chen, Y.J.; Xu, H.Q.; Cui, G.Y.; Wang, J. Effect of roasting temperature on lipid and protein oxidation and amino acid residue side chain modification of beef patties. RSC Adv. 2021, 35, 21629–21641. [Google Scholar] [CrossRef]
- Witte, C.; Herde, M. Nucleotide metabolism in plants. Plant Physiol. 2022, 182, 63–78. [Google Scholar] [CrossRef]
| Component | Average Relative Content | |
|---|---|---|
| PCAD | HAD | |
| Ginseng Rh4 | 2.11 × 105 | 2.22 × 105 |
| Ginseng Rg7 | 5.21 × 105 | 6.17 × 105 |
| Ginseng Rc | 2.32 × 105 | 1.80 × 105 |
| Ginseng Rh14 | 1.15 × 106 | 9.70 × 105 |
| Ginseng Rk1 | 3.20 × 105 | 4.52 × 105 |
| Ginseng F4 | 3.20 × 105 | 4.52 × 105 |
| Ginseng F3 | 2.05 × 104 | 2.68 × 104 |
| Pseudo ginsenoside F11 | 6.67 × 104 | 5.51 × 104 |
| Ginseng Re5 | 3.26 × 105 | 3.11 × 105 |
| Ginseng Rg6 | 7.56 × 105 | 7.25 × 105 |
| Ginseng Rd | 1.66 × 105 | 1.51 × 105 |
| Malonyl ginsenoside Re | 1.71 × 105 | 6.69 × 104 |
| Ginseng Rh15 | 4.92 × 105 | 4.70 × 105 |
| Ginseng Rh8 | 1.57 × 105 | 2.28 × 105 |
| Malonyl ginsenoside Rb1 | 6.75 × 105 | 8.65 × 105 |
| Ginseng Rh19 | 8.13 × 104 | 1.17 × 105 |
| Ginseng Rd2 | 5.58 × 104 | 3.58 × 104 |
| Ginseng Mx | 4.88 × 105 | 4.35 × 105 |
| Ginseng Rf1 | 1.66 × 104 | 1.10 × 104 |
| Ginseng Rh2 | 1.15 × 105 | 1.94 × 104 |
| Ginseng Rs1 | 1.32 × 104 | 1.96 × 104 |
| Malonyl ginsenoside Rd | 2.33 × 105 | 1.84 × 105 |
| Ginsenoside Rk2-acetyl | 2.78 × 104 | 3.69 × 104 |
| Ginseng ST2 | 1.08 × 104 | 1.17 × 103 |
| Ginseng Ro | 1.61 × 106 | 1.69 × 106 |
| Ginseng Rb1 | 1.35 × 105 | 1.85 × 105 |
| Ginseng Rg2 | 1.53 × 105 | 1.61 × 105 |
| Ginseng F2 | 1.53 × 104 | 1.30 × 104 |
| Ginseng Rf | 9.93 × 105 | 9.84 × 105 |
| 6′-O-acetyl-Ginsenoside Rg1 | 3.55 × 106 | 3.33 × 106 |
| 5,6-didehydroginsenoside Rd | 4.32 × 105 | 3.54 × 105 |
| Ginseng Rg1 | 2.06 × 106 | 2.17 × 106 |
| Ginseng La | 1.15 × 106 | 9.70 × 105 |
| Ginseng Re | 1.41 × 105 | 1.03 × 105 |
| Ginseng Rb2 | 1.03 × 105 | 1.19 × 105 |
| Ginseng Rg3 | 5.31 × 103 | 7.32 × 103 |
| Ginseng Rh1 | 8.79 × 103 | 1.02 × 104 |
| Ginseng F1 | 3.81 × 103 | 5.06 × 103 |
| Total ginsenosides | 1.72 × 107 | 1.68 × 107 |
| Component | Average Relative Content | |
|---|---|---|
| PCAD | HAD | |
| JiangxiBaiyingsu I | 3.42 × 105 | 5.27 × 105 |
| 3,4-Dihydroxy-7,8-dihydro-β-ionone-4-O-β-D-glucoside | 8.74 × 105 | 6.49 × 105 |
| polygodial | 1.58 × 105 | 1.46 × 105 |
| 1,6-Dihydroxy-4(14)-eudesmene | 5.13 × 104 | 4.85 × 104 |
| β-Eudesmol | 1.30 × 104 | 1.54 × 104 |
| 3,7,11-trimethyldodeca-3,7-diene-1,10,11-triol 1-O-(beta-D-Xylopyranosyl)-beta-D-glucopyranoside | 2.14 × 105 | 1.89 × 105 |
| Humulene epoxide II | 1.75 × 105 | 1.58 × 105 |
| Cyclocolorenone | 9.96 × 104 | 6.86 × 104 |
| Anhydro-β-rotunol | 3.62 × 105 | 2.97 × 105 |
| Septemlobin D | 6.29 × 104 | 7.75 × 104 |
| 6α,10α-Dihydroxy-1-oxoeremophila-7(11),8(9)- dien-12,8-olide | 8.87 × 106 | 8.79 × 106 |
| Nootkatone | 7.58 × 104 | 8.53 × 104 |
| 3-Hydroxy-3,7,11-trimethyldodeca-1,6E,10-trien- 9-yl isobutyrate | 2.40 × 106 | 2.30 × 106 |
| 13-Hydroxygermacrone | 1.23 × 105 | 1.07 × 105 |
| Aristolone | 6.45 × 104 | 3.10 × 104 |
| 3,7,11-trimethyldodeca-3,7-diene-1,10,11-triol 10-O-(beta-D-Xylopyranosyl)-beta-D-glucopyranoside | 1.16 × 105 | 1.73 × 105 |
| Sessilifol O | 1.84 × 104 | 2.33 × 104 |
| Leucodin | 6.45 × 104 | 3.47 × 104 |
| (6R,9R)-3-Oxo-α-ionol-β-D-malonyl-glucoside | 5.43 × 104 | 2.04 × 104 |
| Micheliolide | 1.81 × 104 | 1.01 × 104 |
| Septemlobin E | 4.28 × 104 | 4.54 × 104 |
| 3-Oxo-Alpha-Ionol diglucoside | 7.64 × 104 | 6.31 × 104 |
| solajiangxins H | 4.47 × 104 | 3.07 × 104 |
| Santalol A | 3.71 × 105 | 3.25 × 105 |
| 8-methoxy-3,4,5-trimethyl-5,6,7,8-tetrahydrobenzo[f][1]benzofuran | 1.30 × 105 | 6.23 × 104 |
| 11,12-O-Isopropyfidenesolajiangxin F | 5.03 × 104 | 4.99 × 104 |
| Blumenol B malonyl Glucoside | 6.03 × 104 | 1.60 × 104 |
| Blumenol C malonyl Glucoside | 2.16 × 104 | 1.90 × 104 |
| Dendronobilin I | 4.58 × 105 | 8.93 × 104 |
| 10α-Hydroperoxy-guaia-1,11-diene | 3.71 × 104 | 2.64 × 104 |
| Icariside B2 | 1.19 × 105 | 9.97 × 104 |
| 1,1′-(6-hydroxy-2,3-dihydro-1H-indene- 2,5-diyl)bis(ethan-1-one) | 1.30 × 105 | 1.11 × 105 |
| Glucosyl dihydroroseoside | 3.13 × 104 | 2.06 × 104 |
| 15-Hydroxysessilifol F | 2.89 × 104 | 3.56 × 104 |
| (2E)-3-(1-Hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexen-1-yl)-2-propenoic acid | 5.39 × 104 | 2.27 × 104 |
| Ampelopsisionoside | 8.56 × 105 | 5.14 × 105 |
| Dihydrophaseic acid | 4.70 × 104 | 1.14 × 104 |
| Cryptomeridiol | 8.21 × 104 | 9.27 × 104 |
| 5-Hydroxylbisabolon-9-one | 5.16 × 104 | 4.53 × 104 |
| Glucosyl 10,11-dihydroxy-2,6-farneSadienoate | 2.08 × 105 | 1.83 × 105 |
| patrinioside | 2.74 × 106 | 2.17 × 106 |
| 6′-O-Glucosylaucubin | 1.26 × 105 | 2.35 × 105 |
| Dihydrovomifoliol-O-β-D-glucoside | 8.74 × 105 | 6.49 × 105 |
| Abscisic acid | 1.31 × 104 | 7.61 × 103 |
| Total sesquiterpenoids | 2.08 × 107 | 1.87 × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, J.; Li, X.; Dang, W.; Yang, L.; Zhang, L.; Li, W.; Zhao, Y.; Han, J.; Cai, E. Effects of Different Drying Methods on the Quality of Forest Ginseng Revealed Based on Metabolomics and Enzyme Activity. Foods 2025, 14, 2753. https://doi.org/10.3390/foods14152753
Xing J, Li X, Dang W, Yang L, Zhang L, Li W, Zhao Y, Han J, Cai E. Effects of Different Drying Methods on the Quality of Forest Ginseng Revealed Based on Metabolomics and Enzyme Activity. Foods. 2025; 14(15):2753. https://doi.org/10.3390/foods14152753
Chicago/Turabian StyleXing, Junjia, Xue Li, Wenyu Dang, Limin Yang, Lianxue Zhang, Wei Li, Yan Zhao, Jiahong Han, and Enbo Cai. 2025. "Effects of Different Drying Methods on the Quality of Forest Ginseng Revealed Based on Metabolomics and Enzyme Activity" Foods 14, no. 15: 2753. https://doi.org/10.3390/foods14152753
APA StyleXing, J., Li, X., Dang, W., Yang, L., Zhang, L., Li, W., Zhao, Y., Han, J., & Cai, E. (2025). Effects of Different Drying Methods on the Quality of Forest Ginseng Revealed Based on Metabolomics and Enzyme Activity. Foods, 14(15), 2753. https://doi.org/10.3390/foods14152753







