Processed Pearl Millet Improves the Morphology and Gut Microbiota in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing of Millet Grains and Flour Preparation
2.2. Experimental Design
2.3. Hemoglobin Measurement
2.4. Bristol Scale and Feces Color
2.5. Fecal pH
2.6. DNA Extraction, Sequencing and Data Analysis
2.7. Short-Chain Fatty Acids (SCFA) Concentration
2.8. Histomorphometry Analysis of the Colon
2.9. Correlation Analysis of Gut Microbiota and SCFA Concentration with Markers of Inflammation and Oxidative Stress
2.10. In Silico Docking
2.11. Statistical Analysis
3. Results
3.1. Effect of Processed Pearl Millet on Food Intake, Body Weight, Hemoglobin Levels, Cecum Weight and Intestinal Transit in Rats
3.2. Effect of Processed Pearl Millet on Fecal pH and Short-Chain Fatty Acids Concentration
3.3. Effects of Processed Pearl Millet on Microbial Community Diversity
3.4. Effects of Processed Pearl Millet on Intestinal Morphology
3.5. Correlation of Gut Microbiota and SCFA Concentration with Inflammation and Oxidative Stress Markers
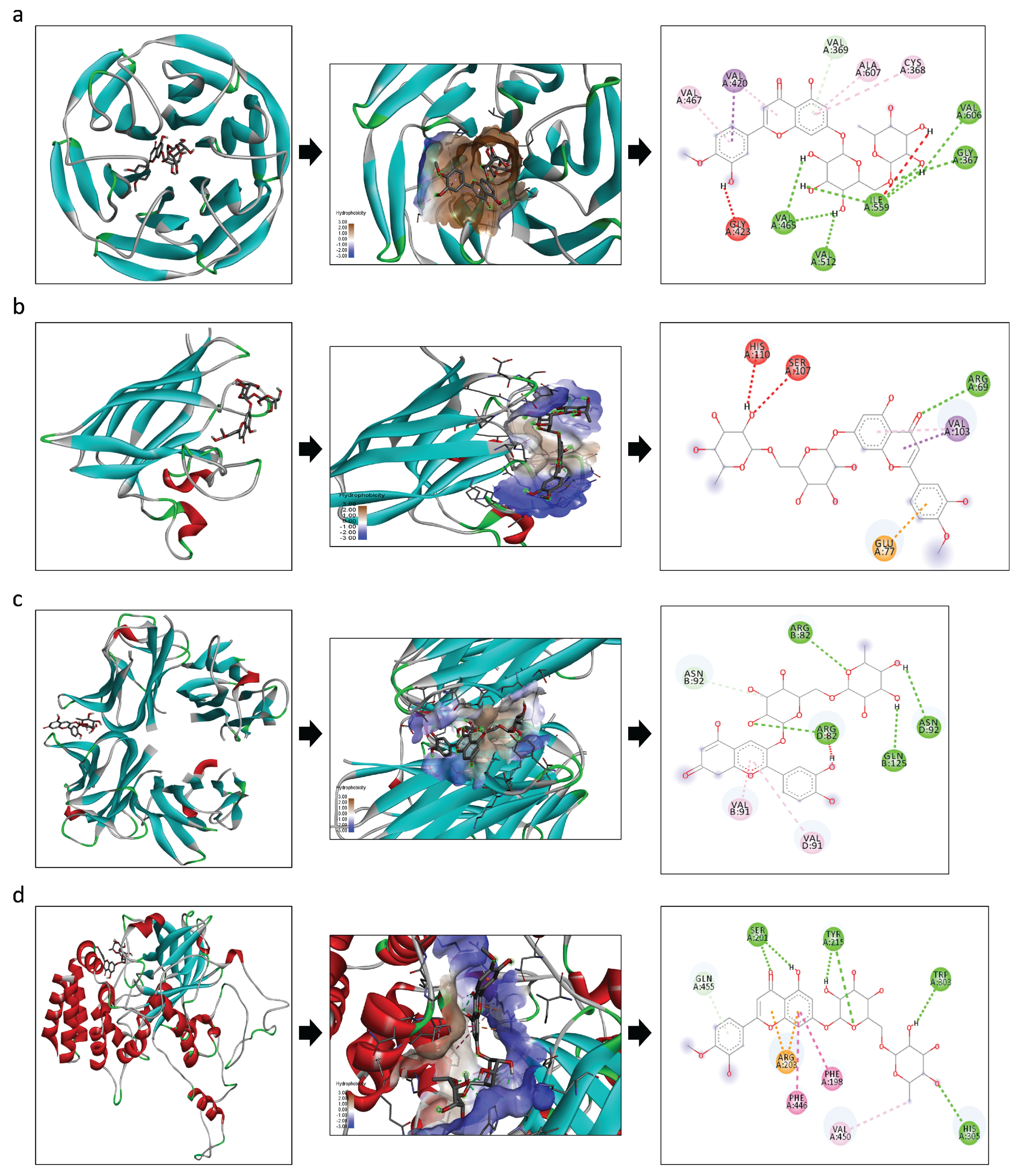
3.6. In Silico Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SD + FS | standard diet + ferrous sulfate |
| SD + NGOPCMF | standard diet + non-germinated open-pan cooked millet flour |
| SD + GOPCMF | standard diet + germinated open-pan cooked millet flour |
| SD + ECMF | standard diet + extrusion-cooked millet flour |
| SCFAs | short-chain fatty acids |
References
- Parmanand, B.A.; Kellingray, L.; Le Gall, G.; Basit, A.W.; Fairweather-Tait, S.; Narbad, A. A Decrease in Iron Availability to Human Gut Microbiome Reduces the Growth of Potentially Pathogenic Gut Bacteria; an in Vitro Colonic Fermentation Study. J. Nutr. Biochem. 2019, 67, 20–27. [Google Scholar] [CrossRef]
- Botta, A.; Barra, N.G.; Lam, N.H.; Chow, S.; Pantopoulos, K.; Schertzer, J.D.; Sweeney, G. Iron Reshapes the Gut Microbiome and Host Metabolism. J. Lipid Atheroscler. 2021, 10, 160–183. [Google Scholar] [CrossRef]
- Phipps, O.; Al-Hassi, H.O.; Quraishi, M.N.; Kumar, A.; Brookes, M.J. Influence of Iron on the Gut Microbiota in Colorectal Cancer. Nutrients 2020, 12, 2512. [Google Scholar] [CrossRef]
- Auerbach, M.; Schrier, S. Treatment of Iron Deficiency Is Getting Trendy. Lancet Haematol. 2017, 4, e500–e501. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Bartkowiak-Wieczorek, J.; Winkler-Galicki, J.; Nowicka, A.; Dzięciołowska, D.; Błaszczyk, M.; Gajniak, P.; Słowińska, K.; Niepolski, L.; Walkowiak, J.; et al. The Dark Side of Iron: The Relationship Between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia—A Narrative Review. Nutrients 2022, 14, 3478. [Google Scholar] [CrossRef]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef]
- Anitha, S.; Kane-Potaka, J.; Botha, R.; Givens, D.I.; Sulaiman, N.L.B.; Upadhyay, S.; Vetriventhan, M.; Tsusaka, T.W.; Parasannanavar, D.J.; Longvah, T.; et al. Millets Can Have a Major Impact on Improving Iron Status, Hemoglobin Level, and in Reducing Iron Deficiency Anemia–A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 712. [Google Scholar] [CrossRef]
- Vetriventhan, M.; Upadhyaya, H.D.; Azevedo, V.C.R.; Allan, V.; Anitha, S. Variability and Trait-Specific Accessions for Grain Yield and Nutritional Traits in Germplasm of Little Millet (Panicum Sumatrense Roth. Ex. Roem. & Schult.). Crop. Sci. 2021, 61, 2658–2679. [Google Scholar] [CrossRef]
- Satyavathi, C.T.; Ambawat, S.; Khandelwal, V.; Srivastava, R.K. Pearl Millet: A Climate-Resilient Nutricereal for Mitigating Hidden Hunger and Provide Nutritional Security. Front. Plant Sci. 2021, 12, 659938. [Google Scholar] [CrossRef]
- Gannon, B.M.; Glahn, R.P.; Mehta, S. Iron Bioavailability from Multiple Biofortified Foods Using an in vitro Digestion, Caco-2 Assay for Optimizing a Cyclical Menu for a Randomized Efficacy Trial. Curr. Dev. Nutr. 2021, 5, nzab111. [Google Scholar] [CrossRef] [PubMed]
- Dias-Martins, A.M.; Pessanha, K.L.F.; Pacheco, S.; Rodrigues, J.A.S.; Carvalho, C.W.P. Potential Use of Pearl Millet (Pennisetum glaucum (L.) R. Br.) in Brazil: Food Security, Processing, Health Benefits and Nutritional Products. Food Res. Int. 2018, 109, 175–186. [Google Scholar] [CrossRef]
- Theodoro, J.M.V.; Grancieri, M.; Oliveira, L.A.; Lucia, C.M.D.; de Carvalho, I.M.M.; Bragagnolo, F.S.; Rostagno, M.A.; Glahn, R.P.; Carvalho, C.W.P.; da Silva, B.P.; et al. Chemical Composition and in vitro Iron Bioavailability of Extruded and Open-Pan Cooked Germinated and Ungerminated Pearl Whole Millet “Pennisetum glaucum (L.) R. Br.”. Food Chem. 2024, 457, 140170. [Google Scholar] [CrossRef]
- Theodoro, J.M.V.; Martinez, O.D.M.; Grancieri, M.; Toledo, R.C.L.; Dias Martins, A.M.; Dias, D.M.; Carvalho, C.W.P.; Martino, H.S.D. Germinated Millet Flour (Pennisetum glaucum (L.) R. Br.) Reduces Inflammation, Oxidative Stress, and Liver Steatosis in Rats Fed with High-Fat High-Fructose Diet. J. Cereal Sci. 2021, 99, 103207. [Google Scholar] [CrossRef]
- Li, S.; Yu, W.; Guan, X.; Huang, K.; Liu, J.; Liu, D.; Duan, R. Effects of Millet Whole Grain Supplementation on the Lipid Profile and Gut Bacteria in Rats Fed with High-Fat Diet. J. Funct. Foods 2019, 59, 49–59. [Google Scholar] [CrossRef]
- Sarma, S.M.; Khare, P.; Jagtap, S.; Singh, D.P.; Baboota, R.K.; Podili, K.; Boparai, R.K.; Kaur, J.; Bhutani, K.K.; Bishnoi, M.; et al. Kodo Millet Whole Grain and Bran Supplementation Prevents High-Fat Diet Induced Derangements in a Lipid Profile, Inflammatory Status and Gut Bacteria in Mice. Food Funct. 2017, 8, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yin, R.; Hou, D.; Xue, Y.; Zhang, M.; Diao, X.; Zhang, Y.; Wu, J.; Hu, J.; Hu, X.; et al. The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial. Nutrients 2018, 10, 1509. [Google Scholar] [CrossRef]
- Owheruo, J.O.; Ifesan, B.O.T.; Kolawole, A.O. Physicochemical Properties of Malted Finger Millet (Eleusine coracana) and Pearl Millet (Pennisetum glaucum). Food Sci. Nutr. 2019, 7, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Pessanha, K.L.F.; Santos, M.B.; de Grandi Castro Freitas-Sá, D.; Takeiti, C.Y.; Carvalho, C.W.P. Impact of Whole Millet Extruded Flour on the Physicochemical Properties and Antihyperglycemic Activity of Gluten Free Pasta. J. Food Sci. Technol. 2023, 60, 1738–1748. [Google Scholar] [CrossRef]
- Magalhães, T.L.S.; Machado, A.M.; da Silva, L.A.; José, V.P.B.d.S.; Lúcio, H.G.; Fortini, T.V.L.; Carvalho, C.W.P.; da Silva, B.P.; Martino, H.S.D. Effects of Acute Consumption of a Beverage Based on Extruded Whole-Grain Pearl Millet Flour on Glycemic and Insulinemic Control, Food Intake, and Appetite Sensation in Eutrophic Adults: A Randomized Cross-over Clinical Trial. Nutrition 2024, 126, 112506. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- da Silva, B.P.; Matyelka, J.C.D.S.; Moreira, M.E.D.C.; Toledo, R.C.L.; Della Lucia, C.M.; Pinheiro-Sant’Ana, H.M.; Martino, H.S.D. A High Fat Diet Does Not Affect the Iron Bioavailability in Wistar Rats Fed with Chia and Increases Gene Expression of Iron Metabolism Proteins. Food Funct. 2016, 7, 4861–4868. [Google Scholar] [CrossRef]
- Martinez, A.P.; Azevedo, G.R. Tradução, Adaptação Cultural e Validação Da Bristol Stool Form Scale Para a População Brasileira. Rev. Lat. Am. Enferm. 2012, 20, 583–589. [Google Scholar] [CrossRef]
- Silveira Júnior, A.O. O Exame Coprológico e as Funçoes Digestivas, 1st ed.; Santos: São Paulo, Brazil, 1988. [Google Scholar]
- Grancieri, M.; Costa, N.M.B.; Vaz Tostes, M.d.G.; de Oliveira, D.S.; Nunes, L.d.C.; Marcon, L.d.N.; Veridiano, T.A.; Viana, M.L. Yacon Flour (Smallanthus sonchifolius) Attenuates Intestinal Morbidity in Rats with Colon Cancer. J. Funct. Foods 2017, 37, 666–675. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A Web-Based Tool for Comprehensive Statistical, Visual and Meta-Analysis of Microbiome Data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Siegfried, B.R.; Ruckemann, H.S.G. Method for the Determination of Organic Acids in Silage by High Performance Liquid Chromatography. Landwirtsch. Forsch. 1984, 37, 298. [Google Scholar]
- Gomes, M.J.C.; Silva, J.S.d.; Alves, N.E.G.; Assis, A.d.; Mejía, E.G.d.; Mantovani, H.C.; Martino, H.S.D. Cooked Common Bean Flour, but Not Its Protein Hydrolysate, Has the Potential to Improve Gut Microbiota Composition and Function in BALB/c Mice Fed a High-Fat Diet Added with 6-Propyl-2-Thiouracil. J. Nutr. Biochem. 2022, 106, 109022. [Google Scholar] [CrossRef]
- Theodoro, J.M.V.; da Silva, B.P.; Toledo, R.C.L.; Grancieri, M.; Prado, P.V.C.d.; de Carvalho, I.M.M.; Carvalho, C.W.P.; Martino, H.S.D. Conventional and Germinated Pearl Millet Flour (Pennisetum glaucum (L.) R. Br.) Improves Iron Metabolism and Antioxidant Capacity in Wistar Rats. J. Cereal Sci. 2024, 116, 103840. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Albarracín, M.; Dyner, L.; Giacomino, M.S.; Weisstaub, A.; Zuleta, A.; Drago, S.R. Modification of Nutritional Properties of Whole Rice Flours (Oryza sativa L.) by Soaking, Germination, and Extrusion. J. Food Biochem. 2019, 43, e12854. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.C.; da Silva, J.S.; Assis, A.; de Mejia, E.G.; Mantovani, H.C.; Martino, H.S.D. Common Bean (Phaseolus vulgaris L.) Flour Can Improve the Gut Microbiota Composition and Function in Mice Fed a High-Fat Diet. Curr. Dev. Nutr. 2021, 5, 1159. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Zheng, P.; He, J.; Chen, D. Influences of Dietary Starch Structure on Intestinal Morphology, Barrier Functions, and Epithelium Apoptosis in Weaned Pigs. Food Funct. 2020, 11, 4446–4455. [Google Scholar] [CrossRef]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of Dietary Fiber on Intestinal Microorganisms and Its Effects on Animal Health. Anim. Nutr. 2023, 14, 356–369. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as Modifiers of Gut Microbial Communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- da Silva, B.P.; Dias, D.M.; de Castro Moreira, M.E.; Toledo, R.C.L.; da Matta, S.L.P.; Lucia, C.M.D.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chia Seed Shows Good Protein Quality, Hypoglycemic Effect and Improves the Lipid Profile and Liver and Intestinal Morphology of Wistar Rats. Plant Foods Hum. Nutr. 2016, 71, 225–230. [Google Scholar] [CrossRef]
- Theodoro, J.M.V.; Grancieri, M.; Gomes, M.J.C.; Toledo, R.C.L.; de São José, V.P.B.; Mantovani, H.C.; Carvalho, C.W.P.; da Silva, B.P.; Martino, H.S.D. Germinated Millet (Pennisetum glaucum (L.) R. Br.) Flour Improved the Gut Function and Its Microbiota Composition in Rats Fed with High-Fat High-Fructose Diet. Int. J. Environ. Res. Public Health 2022, 19, 15217. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of Gut Microbiota in Infectious and Inflammatory Diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, J. The Role of Goblet Cells in Crohn’ s Disease. Cell Biosci. 2024, 14, 43. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Bulut, N.; Chambry, E.; Ruthes, A.; Iacomini, M.; Keshavarzian, A.; Johnson, T.A.; Hamaker, B.R. Dietary Fiber Hierarchical Specificity: The Missing Link for Predictable and Strong Shifts in Gut Bacterial Communities. mBio 2021, 12, e0102821. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. New View on Dietary Fiber Selection for Predictable Shifts in Gut Microbiota. mBio 2020, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Muñoz, M.; Guerrero-Araya, E.; Cortés-Tapia, C.; Plaza-Garrido, Á.; Lawley, T.D.; Paredes-Sabja, D. Comprehensive Genome Analyses of Sellimonas Intestinalis, a Potential Biomarker of Homeostasis Gut Recovery. bioRxiv 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Li, Y.K.; Xiao, C.L.; Ren, H.; Li, W.R.; Guo, Z.; Luo, J.Q. Comparison of the Effectiveness of Probiotic Supplementation in Glucose Metabolism, Lipid Profile, Inflammation and Oxidative Stress in Pregnant Women. Food Funct. 2024, 15, 3479–3495. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Paganini, D.; Zimmermann, M.B. The Effects of Iron Fortification and Supplementation on the Gut Microbiome and Diarrhea in Infants and Children: A Review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef] [PubMed]


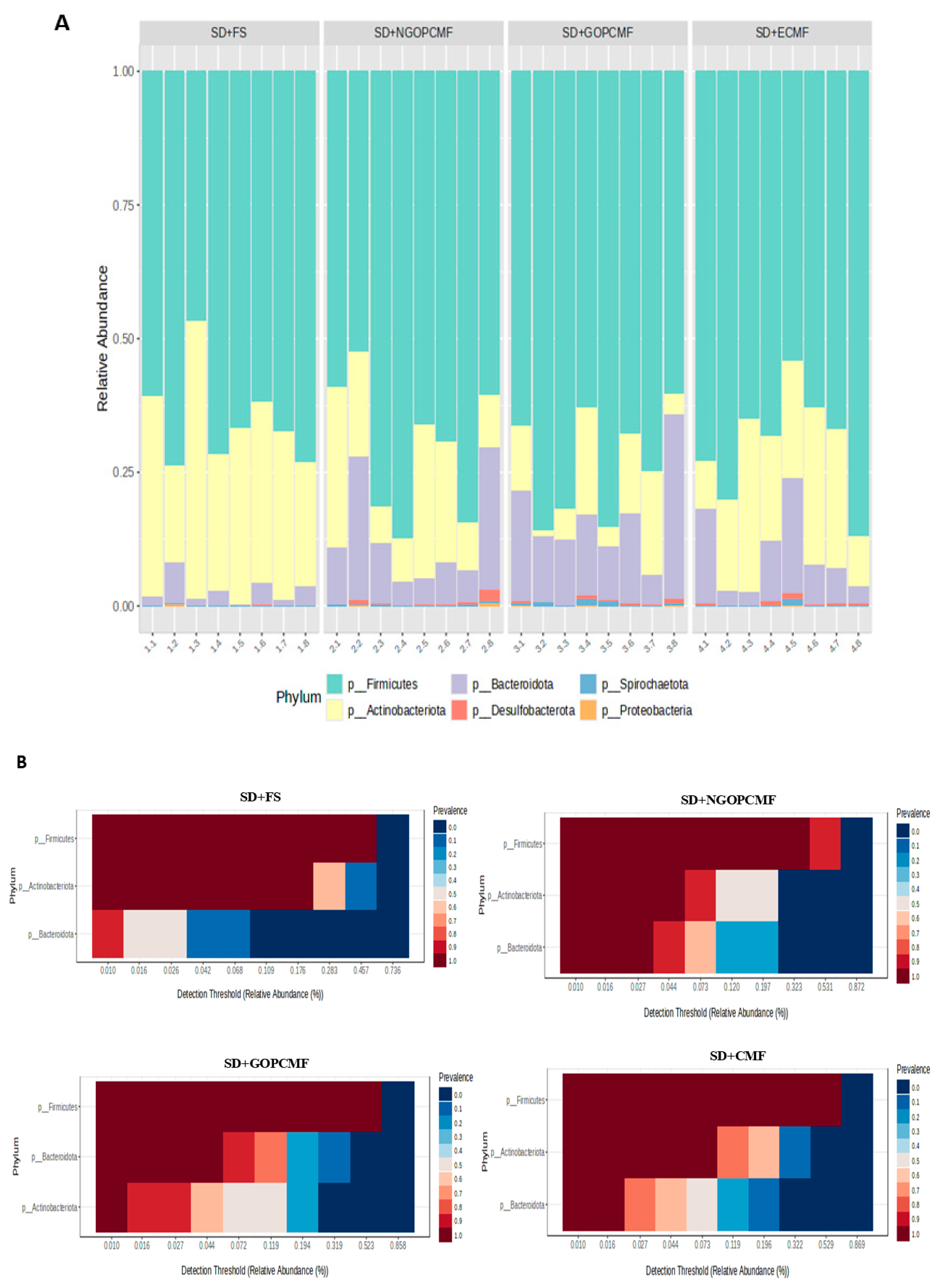
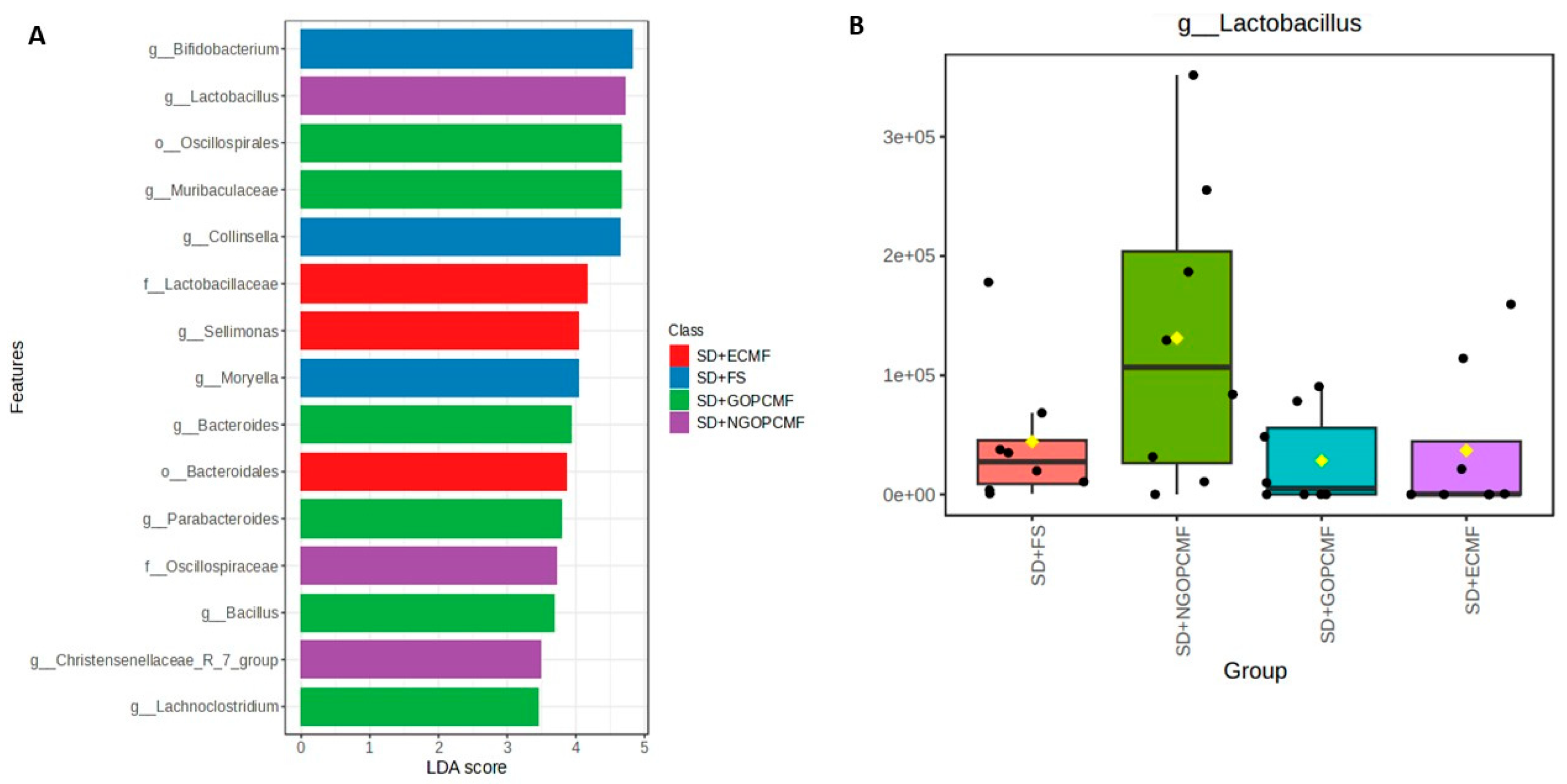
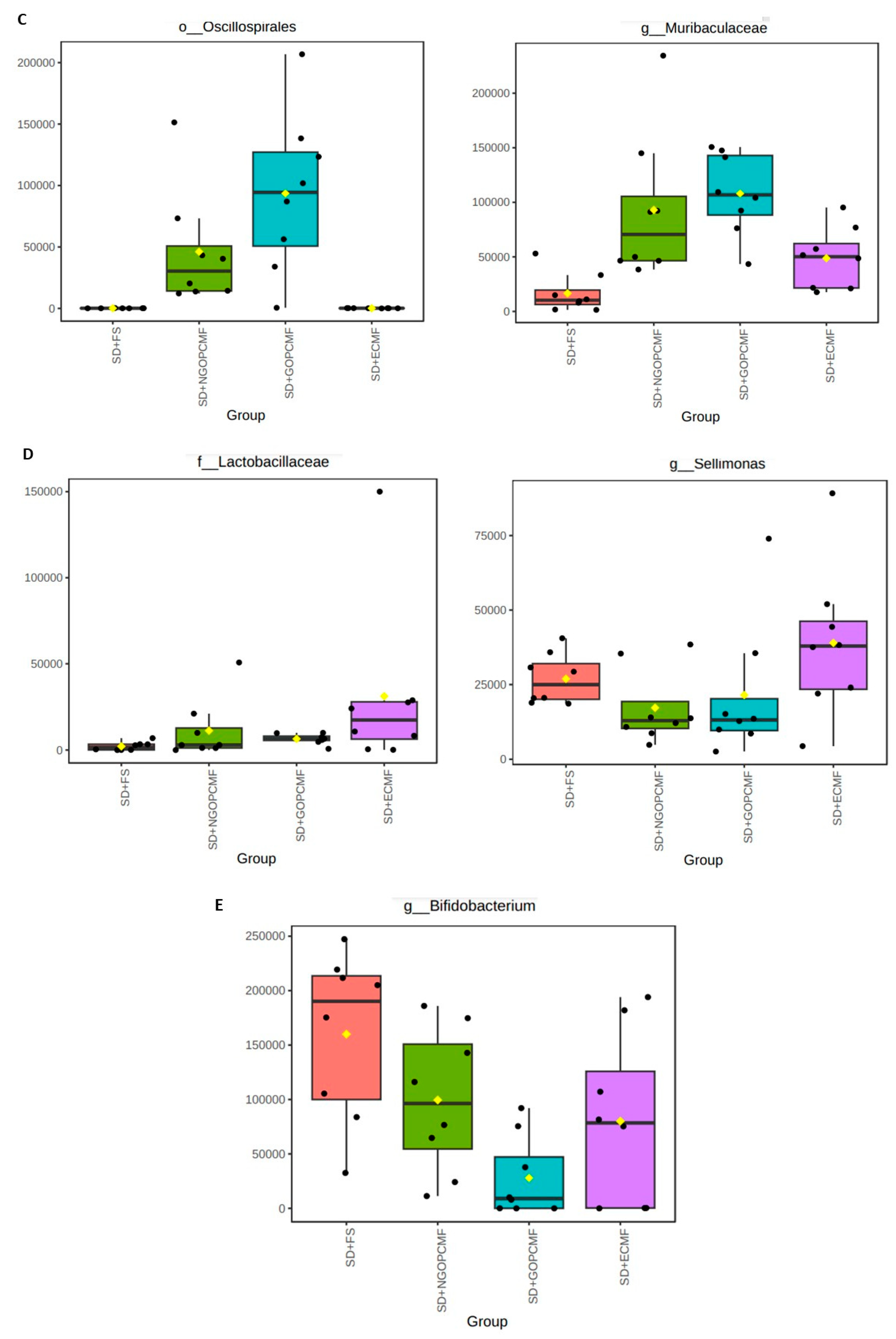


| Group | Feces Color | Feces Consistency |
|---|---|---|
| SD + FS | Light brown-colored feces | Similarly to a sausage, but with a crack on the surface |
| SD + NGOPCMF | Dark brown-colored feces | Like sausage or snake, smooth and soft |
| SD + GOPCMF | Dark brown-colored feces | Like sausage or snake, smooth and soft |
| SD + ECMF | Light brown-colored feces | Like sausage or snake, smooth and soft |
| Variables | SD + FS | SD + NGOPCMF | SD + GOPCMF | SD + ECMF |
|---|---|---|---|---|
| Fecal pH | 8.57 ± 0.18 a | 8.07 ± 0.35 b | 7.71 ± 0.24 c | 8.37 + 0.11 a |
| Short-chain fatty acids (mM) | ||||
| Acetic acid (mM) | 2.78 ± 0.50 a | 3.23 ± 0.84 a | 3.24 ± 0.69 a | 2.85 ± 0.43 a |
| Propionic acid (mM) | 1.69 ± 0.76 a | 1.73 ± 0.56 a | 1.37 ± 0.29 a | 1.12 ± 0.22 a |
| Butyric acid (mM) | 0.77 ± 0.14 a | 0.72 ± 0.14 a | 0.75 ± 0.19 a | 0.67 ± 0.08 a |
| Valeric acid (mM) | 0.20 ± 0.05 a | 0.21 ± 0.03 a | 0.19 ± 0.00 a | 0.18 ± 0.03 a |
| Variables | SD + FS | SD + NGOPCMF | SD + GOPCMF | SD + ECMF |
|---|---|---|---|---|
| Number of goblet cells | 9.12 ± 0.86 b | 12.03 ± 1.47 a | 12.32 ± 1.62 a | 10.96 ± 0.74 a |
| Goblet cell area | 101.40 ± 3.64 c | 143.70 ± 0.86 a | 146.40 ± 3.87 a | 113.30 ± 3.65 b |
| Crypts depth (µM) | 121.40 ± 1.91 b | 131.10 ± 1.75 a | 132.80 ± 1.77 a | 121.50 ± 1.03 b |
| Crypts thickness (µM) | 22.54 ± 0.93 b | 24.67 ± 0.93 a | 24.52 ± 0.77 a | 23.56 ± 0.76 ab |
| Longitudinal mucus layer width (µM) | 38.56 ± 6.68 c | 50.55 ± 7.45 b | 73.71 ± 4.39 a | 48.28 ± 5.94 b |
| Circular mucus layer width (µM) | 124.10 ± 14.46 b | 147.40 ± 16.48 a | 161.30 ± 13.32 a | 126.10 ± 16.89 b |
| Compounds | Diosmin | Cyanidin 3-O-Rutinoside Betaine | ||
|---|---|---|---|---|
| EFE | Interacting AA Residues | EFE | Interacting AA Residues | |
| Nrf2 | −11.4 | VAL A: 467; VAL A: 420; VAL A: 369; ALA A: 607; CYS A: 368; VAL A: 606; GLY A: 367; ILE A: 559; VAL A: 465; VAL A: 512; GLY A: 423. | −9.9 | SER A: 363; SER A: 602; ARG A: 415; GLY A: 509; ALA A: 556; VAL A: 604 |
| SOD | −7.9 | HIS A: 110; SER A: 107; ARG A: 69; VAL A: 103; GLU A: 77 | −7.4 | SER A: 102; SER A: 105; GLN A: 22; SER A: 25; HIS A: 110; VAL A: 103; LEU A: 67 |
| TNF-alpha | −7.9 | LEU A: 36; TYR A: 59; TYR A: 151 | −9.6 | ARG A: 82; ASN B: 92; ASN D: 92; GLN B: 125; ARG D: 82; VAL B: 91; VAL D: 91 |
| Catalase | −9.2 | GLN A: 455; SER A: 201; TYR A: 215; TRP A: 303; ARG A: 203; PHE A: 446; PHE A: 198; VAL A: 450; HIS A: 305 | −9.0 | ARG A: 203; TYR A: 215; PRO A: 151; PHE A: 198; ASN A: 149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoro, J.M.V.; da Silva, L.A.; de São José, V.P.B.; Willis, N.B.; Toledo, R.C.L.; Grancieri, M.; Carvalho, C.W.P.; Pierre, J.F.; da Silva, B.P.; Martino, H.S.D. Processed Pearl Millet Improves the Morphology and Gut Microbiota in Wistar Rats. Foods 2025, 14, 2752. https://doi.org/10.3390/foods14152752
Theodoro JMV, da Silva LA, de São José VPB, Willis NB, Toledo RCL, Grancieri M, Carvalho CWP, Pierre JF, da Silva BP, Martino HSD. Processed Pearl Millet Improves the Morphology and Gut Microbiota in Wistar Rats. Foods. 2025; 14(15):2752. https://doi.org/10.3390/foods14152752
Chicago/Turabian StyleTheodoro, Jaqueline Maciel Vieira, Lucimar Aguiar da Silva, Vinícius Parzanini Brilhante de São José, Nathaniel Baldwin Willis, Renata Celi Lopes Toledo, Mariana Grancieri, Carlos Wanderlei Piler Carvalho, Joseph Francis Pierre, Bárbara Pereira da Silva, and Hércia Stampini Duarte Martino. 2025. "Processed Pearl Millet Improves the Morphology and Gut Microbiota in Wistar Rats" Foods 14, no. 15: 2752. https://doi.org/10.3390/foods14152752
APA StyleTheodoro, J. M. V., da Silva, L. A., de São José, V. P. B., Willis, N. B., Toledo, R. C. L., Grancieri, M., Carvalho, C. W. P., Pierre, J. F., da Silva, B. P., & Martino, H. S. D. (2025). Processed Pearl Millet Improves the Morphology and Gut Microbiota in Wistar Rats. Foods, 14(15), 2752. https://doi.org/10.3390/foods14152752








