Chemometric Approach for Discriminating the Volatile Profile of Cooked Glutinous and Normal-Amylose Rice Cultivars from Representative Japanese Production Areas Using GC × GC-TOFMS
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Volatile Extraction Using MonoTrap
2.3. GC × GC-TOFMS Analysis
2.4. Chromatographic and Statistical Analysis
3. Results and Discussion
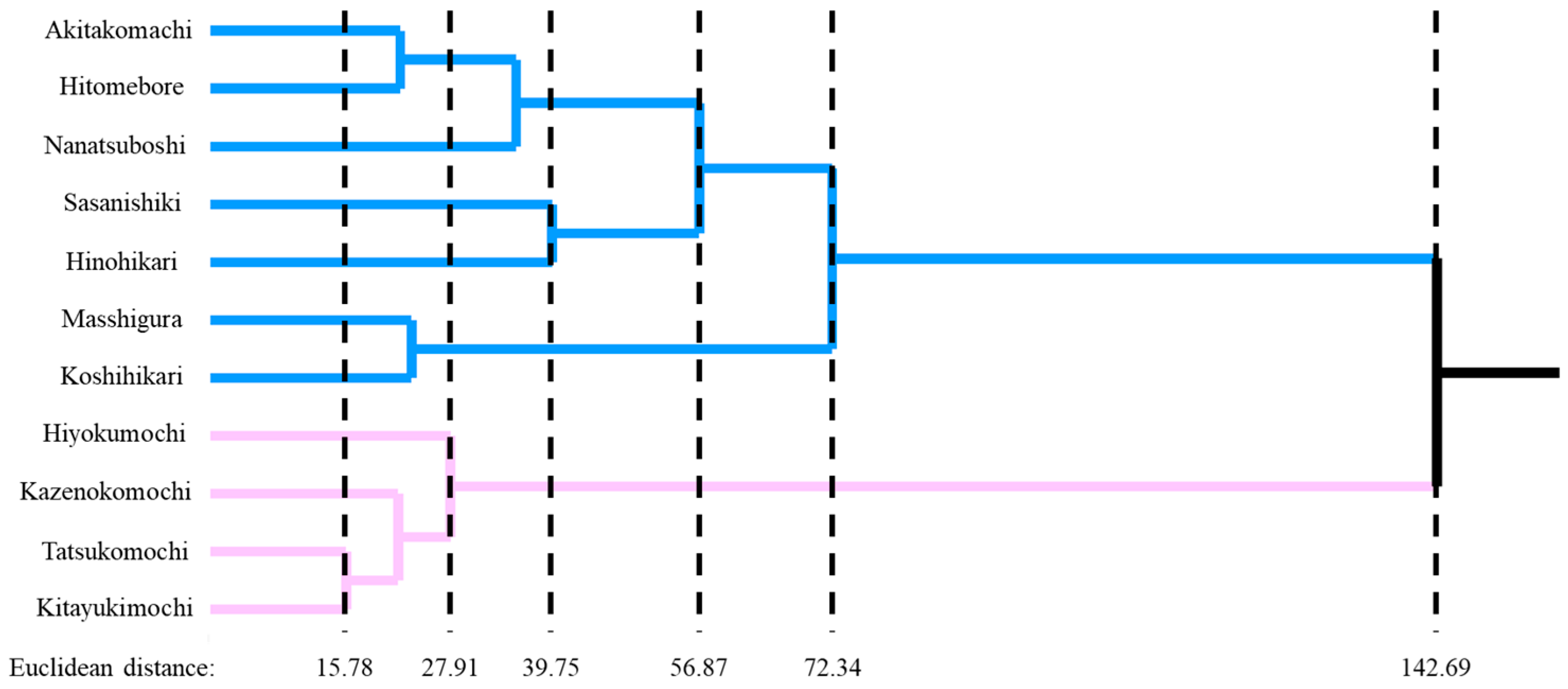
3.1. HCA Analysis
3.2. PCA Analysis
3.3. Heatmap Analysis
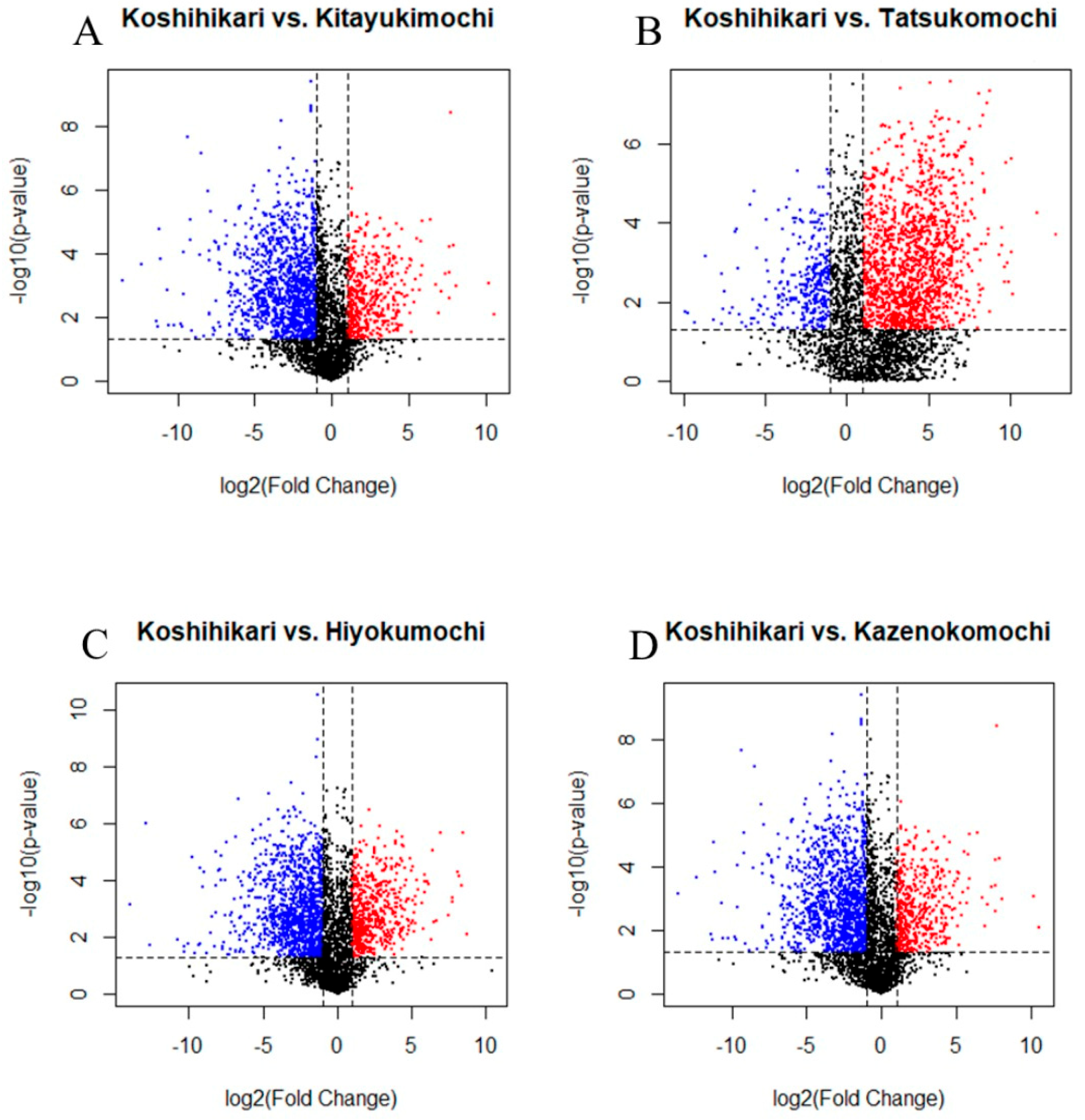
3.4. Volcano Plot Analysis
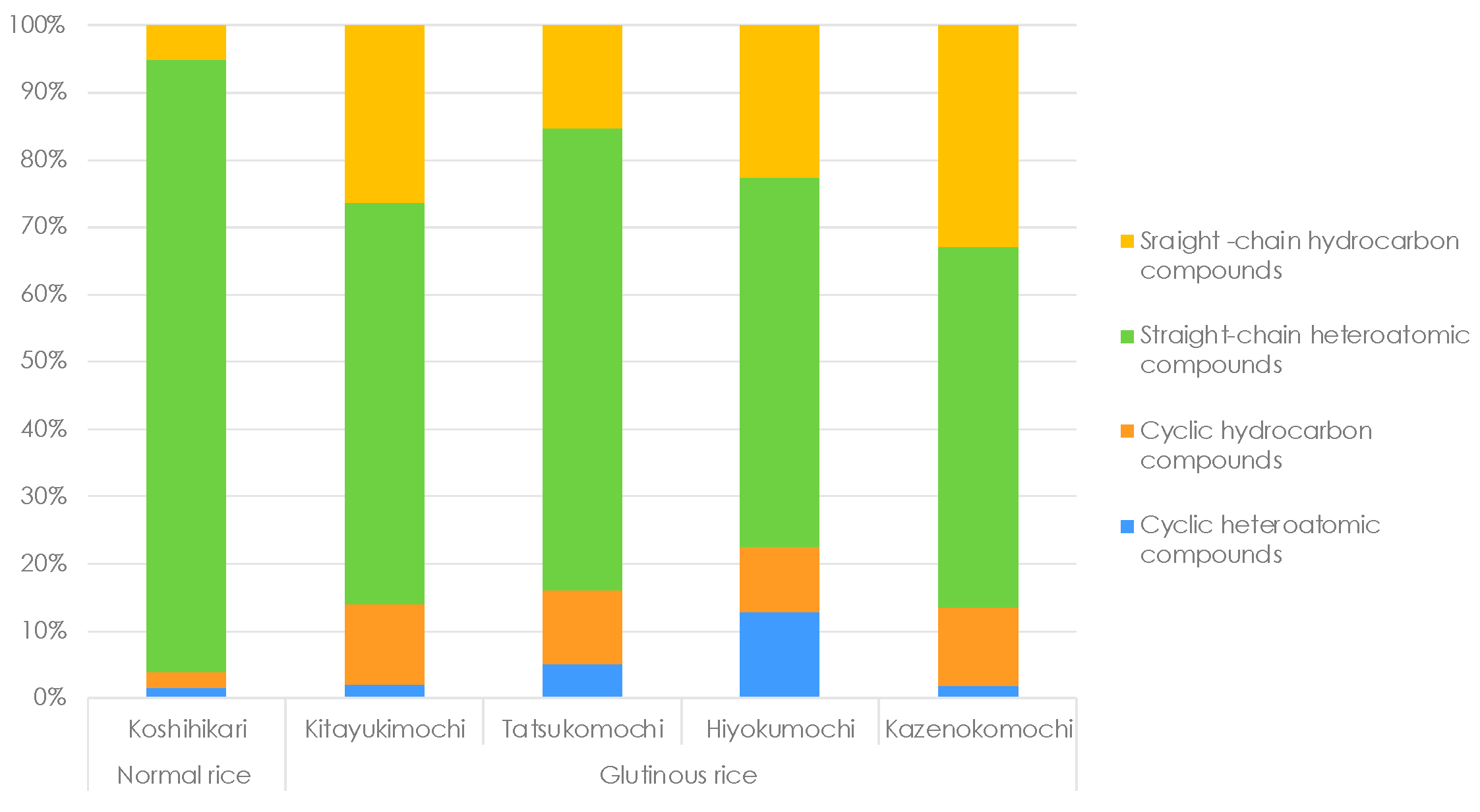
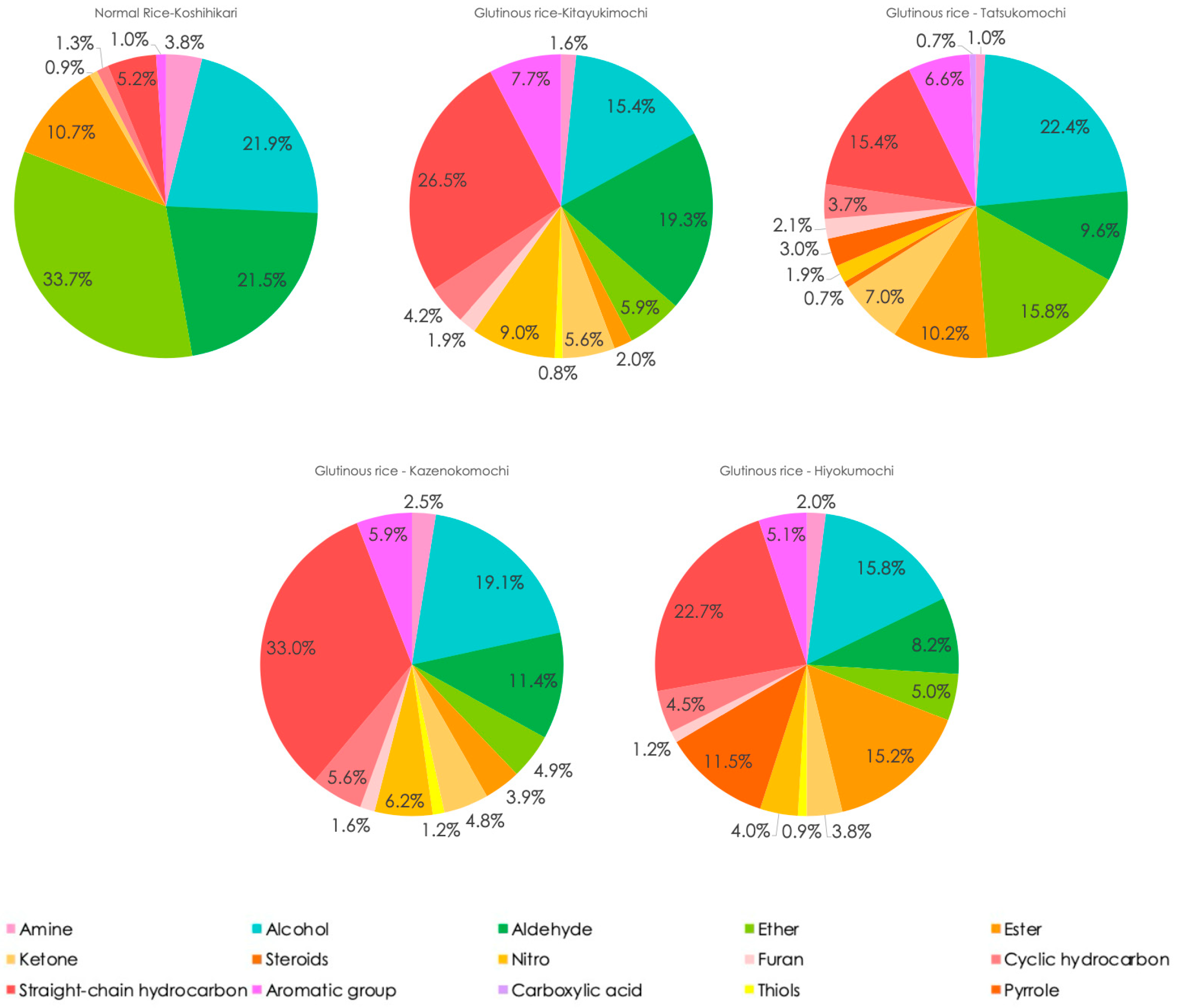
3.5. Functional Groups Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Name | Formula | Average/ Similarity | Average/F-Ratio | Koshihikari_01 | Koshihikari_02 | Koshihikari_03 | RSD Value |
|---|---|---|---|---|---|---|---|
| 3-exo-Chloro-6-endo-nitrobicyclo[2.2.1]heptan-2-one | C7H8ClNO3 | 832 | 392.86 | 4.66 × 107 | 4.63 × 107 | 4.89 × 107 | 3.04 |
| 2,2-Bis(ethylsulfonyl)propane | C16H14Cl2O2 | 562 | 23.22 | 3.21 × 104 | 6.56 × 104 | 3.23 × 104 | 44.49 |
| p-Toluic acid, 3-pentadecyl ester | C16H26 | 776 | 6.04 | 3.22 × 102 | 2.51 × 102 | 2.83 × 102 | 12.41 |
| Cyclohexanone, 2-(2-nitro-2-propenyl)- | C11H20O | 668 | 50.46 | 1.90 × 106 | 2.12 × 106 | 2.09 × 106 | 5.83 |
| Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester | C15H18N4O3S3 | 551 | 38.27 | 6.43 × 106 | 6.51 × 106 | 6.88 × 106 | 3.66 |
| Tetraborane(10) | C30H52O2 | 634.5 | 27.31 | 1.88 × 104 | 3.23 × 104 | 1.15 × 104 | 50.56 |
| Formic acid, hexyl ester | C5H6O | 771 | 91.00 | 1.36 × 106 | 1.57 × 106 | 1.50 × 106 | 7.38 |
| 1-[trans-4-(2-Iodoethyl)cyclohexyl]-trans-4-pentylcyclohexane | C21H42 | 791 | 21.19 | 1.52 × 105 | 2.43 × 105 | 1.38 × 105 | 32.10 |
| 3,6,9,12-Tetraoxatetradecan-1-ol, 14-[4-(1,1,3,3-tetramethylbutyl)phenoxy]- | C15H17ClN2O3 | 550 | 8.66 | 7.88 × 102 | 1.14 × 103 | 1.82 × 103 | 42.07 |
| Furan, 2-pentyl- | C27H50O6 | 726.666667 | 36.74 | 6.76 × 104 | 9.86 × 104 | 1.34 × 105 | 33.28 |
| Isoamylalcohol | C20H30O2 | 696 | 34.76 | 6.38 × 103 | 9.99 × 103 | 6.44 × 103 | 27.18 |
| 1H-Fluorene, dodecahydro- | C9H6O2 | 652 | 20.39 | 2.56 × 106 | 1.88 × 106 | 2.82 × 106 | 20.07 |
| Mannosamine | C16H32O3 | 722.75 | 49.03 | 2.82 × 107 | 2.24 × 107 | 3.41 × 107 | 20.70 |
| 1,3,5-Trioxane, 2,4,6-trimethyl- | C23H46O3 | 664 | 20.46 | 2.27 × 105 | 2.70 × 105 | 2.37 × 105 | 9.17 |
| 1,1-D2-2-(d3-methyl)-4-methyl-1-pentene | C4Cl3F7 | 619 | 33.71 | 8.56 × 102 | 3.47 × 103 | 5.97 × 102 | 96.89 |
| 1-Hexadecanol | C19H30 | 576 | 10.36 | 4.32 × 105 | 4.50 × 105 | 6.10 × 105 | 19.71 |
| Benzenemethanol | C7H5NS | 863 | 24.39 | 4.29 × 106 | 7.36 × 106 | 5.52 × 106 | 26.96 |
| Bicyclo[7.1.0]dec-2-yne | C4H8O2 | 868 | 52.29 | 3.48 × 106 | 3.57 × 106 | 3.94 × 106 | 6.68 |
| 2,3-Octanedione | C16H24O2 | 612.5 | 14.41 | 1.12 × 106 | 1.17 × 106 | 1.18 × 106 | 3.15 |
| Nonane, 3-methyl- | C39H80O2 | 573 | 19.60 | 1.97 × 106 | 2.13 × 106 | 1.91 × 106 | 5.56 |
| (2R,3R)-2-(benzoxymethyl)-3-(chloromethyl)oxirane | C19H40 | 668 | 18.34 | 3.83 × 104 | 3.48 × 104 | 2.65 × 104 | 18.26 |
| Acetamide, N-(2,3-dichlorophenyl)-2-[(1-naphthalenylmethyl)amino]- | C20H32O3 | 613 | 18.00 | 8.03 × 103 | 1.53 × 104 | 8.81 × 103 | 37.45 |
| 1-Hepten-3-ol | C9H9N | 680 | 32.75 | 3.45 × 103 | 8.53 × 103 | 3.42 × 103 | 57.39 |
| 3-Amino-2-oxazolidinone | C8H18O | 576 | 29.99 | 2.78 × 105 | 4.04 × 105 | 2.80 × 105 | 22.60 |
| 1-deoxy-D-ribitol | C31H64O | 820.65 | 20.86 | 1.90 × 107 | 2.87 × 107 | 1.96 × 107 | 24.15 |
| 2-Nonanone | C3H5FO | 636 | 134.53 | 3.65 × 106 | 3.63 × 106 | 4.08 × 106 | 6.61 |
| Cyclohexane, 1,2,4,5-tetramethyl-, (1alpha±,2alpha±,4alpha±,5alpha±)- | C13H24 | 762 | 15.99 | 3.33 × 103 | 1.01 × 104 | 5.56 × 103 | 54.45 |
| 2-Butyl-1-decene | C15H22O | 731 | 24.60 | 8.48 × 104 | 1.19 × 105 | 7.21 × 104 | 26.12 |
| Lauric acid, 2-methylbutyl ester | C19H34O2 | 575 | 35.43 | 1.66 × 107 | 1.69 × 107 | 1.63 × 107 | 1.77 |
| 4-Methyl-hexadecahydro-pyrene | C20H38 | 682 | 7.47 | 2.34 × 103 | 4.22 × 103 | 3.43 × 103 | 28.26 |
| 1,2,4-Butanetriol | C20H32 | 724 | 109.54 | 3.52 × 107 | 3.63 × 107 | 3.42 × 107 | 2.98 |
| Cyclohexane, 1,2,3,5-tetraisopropyl- | C19H34 | 753.333333 | 28.80 | 1.10 × 107 | 1.29 × 107 | 9.98 × 106 | 13.24 |
| 1,2-Dihydrothieno[3,4-d]pyridazine | C9H16 | 727 | 28.63 | 2.55 × 105 | 2.72 × 105 | 2.90 × 105 | 6.38 |
| Benzenepropanenitrile <4-ethyl-, alpha,alpha-, dimethyl-> | C15H30O2 | 785 | 10.40 | 2.20 × 105 | 1.75 × 105 | 2.52 × 105 | 17.82 |
| Hexanal | ClHO | 756.375 | 413.64 | 1.07 × 109 | 1.07 × 109 | 1.10 × 109 | 1.50 |
| 15-Bromo-2-pentadecanone | C14H31N | 691 | 80.56 | 9.49 × 104 | 6.96 × 104 | 6.29 × 104 | 22.28 |
| Isoamyl laurate | C26H44O4S | 672 | 138.56 | 1.75 × 105 | 4.09 × 105 | 2.10 × 105 | 47.60 |
| 4,6-Dimethylundecane | C9H16O | 916 | 18.08 | 6.04 × 102 | 1.10 × 103 | 1.65 × 103 | 46.76 |
| Glycine, N-benzoyl- | C40H82O2 | 710 | 17.64 | 4.48 × 103 | 1.64 × 104 | 4.90 × 103 | 78.67 |
| 1-Decene, 3,4-dimethyl- | C8H18O | 799.857143 | 27.82 | 1.06 × 109 | 1.26 × 109 | 1.24 × 109 | 9.26 |
| 1-Dodecanamine, N,N-dimethyl- | C12H20 | 594 | 107.94 | 2.25 × 107 | 2.22 × 107 | 2.25 × 107 | 0.68 |
| Acetamide, N-(6-acetylaminobenzothiazol-2-yl)-2-(adamantan-1-yl)- | C14H24 | 652 | 15.30 | 1.16 × 102 | 2.58 × 102 | 0 | 103.61 |
| 1-Dodecanol | C19H30 | 576 | 10.36 | 4.32 × 105 | 4.50 × 105 | 6.10 × 105 | 19.71 |
| 3-Nonyn-1-ol | C15H12BrN3O2 | 639.285714 | 138.63 | 1.01 × 107 | 1.27 × 107 | 1.25 × 107 | 12.22 |
| Octanal | C17H36O | 712 | 145.31 | 4.20 × 107 | 4.42 × 107 | 3.95 × 107 | 5.58 |
| Dodecanoic acid, 1,1-dimethylpropyl ester | C18H38O2 | 695 | 40.70 | 2.99 × 105 | 3.43 × 105 | 3.28 × 105 | 6.83 |
| Benzene, hexyl- | C4H8O | 695.4 | 37.23 | 4.09 × 106 | 4.19 × 106 | 4.46 × 106 | 4.55 |
| Hexane, 1-nitro- | C27H30O9 | 570 | 27.63 | 1.84 × 104 | 7.17 × 104 | 4.97 × 104 | 57.48 |
| 1-Pentanamine | C27H32ClNO2 | 611 | 188.34 | 2.40 × 106 | 2.39 × 106 | 2.65 × 106 | 5.84 |
| Butanal, 3-methyl- | C12H24 | 550 | 135.20 | 3.29 × 105 | 3.88 × 105 | 3.94 × 105 | 9.64 |
| No. | Name | Formula | m/z | Area | RT1 | RT2 | −log10(p Value) | log2(FC) |
|---|---|---|---|---|---|---|---|---|
| 1 | 3-exo-Chloro-6-endo-nitrobicyclo[2.2.1]heptan-2-one | C7H8ClNO3 | 189.02 | 4.04 × 106 | 423.5339 | 0.8867 | 5.61 | 2.70 |
| 2 | 2,2-Bis(ethylsulfonyl)propane | C7H16O4S2 | 228.33 | 9.52 × 107 | 544.5436 | 0.8334 | 5.17 | 2.51 |
| 3 | p-Toluic acid, 3-pentadecyl ester | C23H38O2 | 346.50 | 8.58 × 105 | 539.0431 | 1.7335 | 4.75 | 2.76 |
| 4 | Cyclohexanone, 2-(2-nitro-2-propenyl)- | C9H13NO3 | 183.20 | 2.58 × 105 | 467.5374 | 1.8568 | 4.62 | 2.45 |
| 5 | Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester | C12H24O3 | 216.32 | 4.9 × 106 | 1138.5911 | 1.3934 | 4.41 | 3.01 |
| 6 | Tetraborane(10) | B4H10 | 53.32 | 1.71 × 105 | 126.5101 | 3.3336 | 4.37 | 5.55 |
| 7 | Formic acid, hexyl ester | C7H14O2 | 130.18 | 5.74 × 104 | 555.5444 | 2.4202 | 4.35 | 2.13 |
| 8 | 1-[trans-4-(2-Iodoethyl)cyclohexyl]-trans-4-pentylcyclohexane | C19H35I | 390.39 | 1.84 × 106 | 709.5568 | 4.7470 | 4.23 | 8.69 |
| 9 | 3,6,9,12-Tetraoxatetradecan-1-ol, 14-[4-(1,1,3,3-tetramethylbutyl)phenoxy]- | C24H42O6 | 426.60 | 5.02 × 106 | 1039.5832 | 1.0001 | 4.22 | 4.97 |
| 10 | Furan, 2-pentyl- | C9H14O | 138.21 | 3.24 × 103 | 445.5356 | 1.3818 | 4.20 | 2.62 |
| 11 | Isoamylalcohol | C5H12O | 88.15 | 1.23 × 105 | 456.5365 | 0.9934 | 4.19 | 3.44 |
| 12 | 1H-Fluorene, dodecahydro- | C13H22 | 178.31 | 1.59 × 106 | 676.5541 | 2.8936 | 4.12 | 2.46 |
| 13 | Mannosamine | C6H13NO5 | 179.17 | 1.2 × 105 | 143.0114 | 3.6536 | 4.11 | 4.85 |
| 14 | 1,3,5-Trioxane, 2,4,6-trimethyl- | C6H12O3 | 132.16 | 4.26 × 103 | 242.0194 | 2.3335 | 4.10 | 3.83 |
| 15 | 1,1-D2-2-(d3-methyl)-4-methyl-1-pentene | C7H9D5 | 103.14 | 1.02 × 109 | 242.0194 | 1.4201 | 4.09 | 3.73 |
| 16 | 1-Hexadecanol | C16H34O | 242.44 | 9.06 × 107 | 1462.2003 | 2.2402 | 4.04 | 2.95 |
| 17 | Benzenemethanol | C7H8O | 108.14 | 5.65 × 106 | 1133.0906 | 0.8734 | 4.04 | 4.81 |
| 18 | Bicyclo[7.1.0]dec-2-yne | C10H14 | 134.22 | 1.03 × 103 | 1303.6043 | 2.9602 | 4.00 | 2.48 |
| 19 | 2,3-Octanedione | C8H14O2 | 142.20 | 9.13 × 105 | 517.0414 | 1.1934 | 3.99 | 2.47 |
| 20 | Nonane, 3-methyl- | C10H22 | 142.28 | 3.68 × 104 | 1078.0862 | 3.7336 | 3.92 | 3.96 |
| 21 | (2R,3R)-2-(benzoxymethyl)-3-(chloromethyl)oxirane | C11H13ClO2 | 212.67 | 3.54 × 105 | 940.5752 | 5.1737 | 3.88 | 2.95 |
| 22 | Acetamide, N-(2,3-dichlorophenyl)-2-[(1-naphthalenylmethyl)amino]- | C19H16Cl2N2O | 359.25 | 7.76 × 107 | 1276.1021 | 1.3601 | 3.84 | 4.83 |
| 23 | 1-Hepten-3-ol | C7H14O | 114.19 | 2.61 × 104 | 643.5515 | 2.5002 | 3.81 | 4.85 |
| 24 | 3-Amino-2-oxazolidinone | C3H6N2O2 | 102.09 | 2.71 × 104 | 269.5216 | 3.8003 | 3.80 | 3.95 |
| 25 | 1-deoxy-D-ribitol | C5H12O4 | 136.15 | 1.22 × 106 | 236.5189 | 0.8401 | 3.78 | 2.34 |
| 26 | 2-Nonanone | C9H18O | 142.24 | 7.18 × 107 | 583.0466 | 1.3934 | 3.76 | 2.87 |
| 27 | Cyclohexane, 1,2,4,5-tetramethyl-, (1alpha±,2alpha±,4alpha±,5alpha±)- | C10H20 | 140.27 | 2.62 × 105 | 280.5224 | 1.7735 | 3.74 | 3.22 |
| 28 | 2-Butyl-1-decene | C14H28 | 196.37 | 2.31 × 106 | 588.5471 | 3.6603 | 3.74 | 2.24 |
| 29 | Lauric acid, 2-methylbutyl ester | C17H34O2 | 270.45 | 1.18 × 107 | 1390.2362 | 2.8736 | 3.74 | 6.53 |
| 30 | 4-Methyl-hexadecahydro-pyrene | C17H28 | 232.40 | 1.73 × 106 | 1298.1038 | 3.0069 | 3.72 | 2.10 |
| 31 | 1,2,4-Butanetriol | C4H10O3 | 106.12 | 3.69 × 103 | 299.7740 | 3.3536 | 3.69 | 4.29 |
| 32 | Cyclohexane, 1,2,3,5-tetraisopropyl- | C18H36 | 252.50 | 6.42 × 103 | 778.3123 | 5.1004 | 3.66 | 8.80 |
| 33 | 1,2-Dihydrothieno[3,4-d]pyridazine | C6H6N2S | 138.19 | 7.39 × 107 | 429.0343 | 2.0402 | 3.66 | 2.34 |
| 34 | Benzenepropanenitrile <4-ethyl-, alpha,alpha-, dimethyl-> | C13H17N | 187.28 | 2.69 × 106 | 2334.0200 | 1.1334 | 3.64 | 3.52 |
| 35 | Hexanal | C6H12O | 100.16 | 3.47 × 104 | 368.5295 | 1.2157 | 3.63 | 4.57 |
| 36 | 15-Bromo-2-pentadecanone | C15H29BrO | 304.14 | 1.99 × 105 | 1556.6245 | 2.3269 | 3.63 | 7.97 |
| 37 | Isoamyl laurate | C17H34O2 | 270.50 | 1.76 × 108 | 1393.4448 | 2.8513 | 3.60 | 6.34 |
| 38 | 4,6-Dimethylundecane | C13H28 | 184.36 | 2.38 × 105 | 330.0264 | 3.3269 | 3.57 | 3.56 |
| 39 | Glycine, N-benzoyl- | C9H9NO3 | 179.17 | 2.83 × 106 | 1727.1382 | 0.7934 | 3.55 | 4.17 |
| 40 | 1-Decene, 3,4-dimethyl- | C12H24 | 168.32 | 1.64 × 104 | 445.5356 | 2.9802 | 3.54 | 2.93 |
| 41 | 1-Dodecanamine, N,N-dimethyl- | C14H31N | 213.40 | 3.35 × 105 | 836.0669 | 4.5270 | 3.54 | 2.76 |
| 42 | Acetamide, N-(6-acetylaminobenzothiazol-2-yl)-2-(adamantan-1-yl)- | C21H25N3O2S | 383.50 | 1.52 × 107 | 610.5488 | 0.8867 | 3.50 | 2.26 |
| 43 | 1-Dodecanol | C12H26O | 186.33 | 1.03 × 108 | 1190.8453 | 1.8379 | 3.48 | 4.29 |
| 44 | 3-Nonyn-1-ol | C9H16O | 140.22 | 1.31 × 105 | 467.5374 | 1.5935 | 3.46 | 3.40 |
| 45 | Octanal | C8H16O | 128.21 | 8.47 × 108 | 485.4138 | 1.4384 | 3.43 | 2.40 |
| 46 | Dodecanoic acid, 1,1-dimethylpropyl ester | C17H34O2 | 270.50 | 5.73 × 103 | 1386.1109 | 3.7003 | 3.42 | 7.66 |
| 47 | Benzene, hexyl- | C12H18 | 162.27 | 2.52 × 103 | 731.5585 | 1.8535 | 3.42 | 3.89 |
| 48 | Hexane, 1-nitro- | C6H13NO2 | 131.17 | 5.3 × 106 | 693.0554 | 1.1468 | 3.41 | 3.86 |
| 49 | 1-Pentanamine | C5H13N | 87.16 | 1.02 × 107 | 511.5409 | 0.9334 | 3.39 | 2.92 |
| 50 | Butanal, 3-methyl- | C5H10O | 86.13 | 2.19 × 104 | 253.0202 | 1.2368 | 3.37 | 2.52 |
| No. | Name | Formula | m/z | Area | RT1 | RT2 | −log10(p Value) | log2(FC) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2,3-dihydro-2(S),5-dimethyl-1,5-benzoxazepin-4(5H)-thione | C11H13NOS | 207.07 | 3.89 × 104 | 1529.1223 | 4.6537 | 5.42 | −4.61 |
| 2 | N,N-Dimethyl-1-decanamine | C12H27N | 185.35 | 2.58 × 103 | 819.5656 | 3.8970 | 4.81 | −4.40 |
| 3 | Undecane, 4-ethyl- | C13H28 | 184.36 | 5.73 × 102 | 654.5524 | 4.4004 | 4.40 | −6.62 |
| 4 | 1-Heptadecanol | C17H36O | 256.50 | 2.54 × 102 | 621.5497 | 3.8536 | 4.32 | −6.51 |
| 5 | Methyl 2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)propanoate | C12H11NO4 | 233.22 | 4.29 × 105 | 1688.6351 | 3.1936 | 4.31 | −4.86 |
| 6 | Methanediamine, N,N,N’,N’-tetrabutyl- | C17H38N2 | 270.50 | 9.70 × 105 | 308.0246 | 1.5646 | 4.24 | −4.91 |
| 7 | Benzoic acid, tetradecyl ester | C21H34O2 | 318.50 | 8.28 × 104 | 2040.6632 | 2.1113 | 4.16 | −4.13 |
| 8 | (1R,3aS,8aS)-7-Isopropyl-1,4-dimethyl-1,2,3,3a,6,8a-hexahydroazulene | C15H24 | 204.35 | 9.26 × 106 | 1045.0836 | 2.8136 | 4.13 | −5.91 |
| 9 | (3E)-3-Icosene | C20H40 | 280.50 | 1.82 × 106 | 693.0554 | 4.6070 | 4.11 | −3.47 |
| 10 | 1,7,7-trimethylbicyclo[2.2.1]heptan-2-one | C10H16O | 152.23 | 6.82 × 101 | 665.5532 | 1.2201 | 4.00 | −6.22 |
| 11 | 2-Methyl-E,E-3,13-octadecadien-1-ol | C19H36O | 280.50 | 1.58 × 104 | 2057.1646 | 2.0802 | 4.00 | −4.31 |
| 12 | Benzoic acid, tridecyl ester | C20H32O2 | 304.50 | 1.17 × 104 | 2046.1637 | 2.1135 | 3.98 | −4.50 |
| 13 | Propane, 2,2’-[ethylidenebis(oxy)]bis- | C8H18O2 | 146.23 | 1.20 × 103 | 2541.2033 | 2.8869 | 3.97 | −3.88 |
| 14 | Dichloroacetic acid, 2-tridecyl ester | C15H28Cl2O2 | 311.30 | 5.86 × 105 | 1358.6087 | 1.3134 | 3.83 | −4.31 |
| 15 | 3-(3-(Naphthalen-2-yl)propa-1,2-dienyl)thiophene | C17H12S | 248.07 | 2.83 × 104 | 2010.4108 | 2.2668 | 3.78 | −4.21 |
| 16 | 2,5-Furandione, 3-dodecyl- | C16H26O3 | 266.38 | 7.88 × 101 | 1331.1065 | 5.1737 | 3.78 | −8.29 |
| 17 | Dichloroacetic acid, 3-tetradecyl ester | C16H30Cl2O2 | 325.30 | 1.09 × 107 | 682.0546 | 5.1971 | 3.66 | −4.11 |
| 18 | Heneicosane, 5-methyl- | C22H46 | 310.60 | 3.16 × 102 | 1289.8532 | 5.2138 | 3.62 | −3.67 |
| 19 | Sulfurous acid, butyl cyclohexylmethyl ester | C11H22O3S | 234.36 | 7.58 × 101 | 885.5708 | 4.3203 | 3.36 | −4.98 |
| 20 | Benzo[k]fluoranthene-2,3-dione | C20H10O2 | 282.30 | 6.29 × 104 | 2700.7160 | 4.1403 | 3.31 | −5.72 |
| 21 | Cyclohexane, undecyl- | C17H34 | 238.50 | 5.23 × 102 | 1287.1030 | 4.2470 | 3.30 | −3.06 |
| 22 | (9E,11E)-Octadecadienoic acid | C18H32O2 | 280.40 | 2.18 × 105 | 2640.2112 | 1.5468 | 3.28 | −3.64 |
| 23 | Tetracosane <n-> | C24H50 | 338.70 | 9.57 × 103 | 1168.2935 | 0.5854 | 3.20 | −3.89 |
| 24 | (3R,13R)-3,13-Dimethylheptadecane | C19H40 | 268.50 | 1.92 × 105 | 819.5656 | 0.3867 | 3.16 | −11.61 |
| 25 | 1,3,3-Trimethyl-2-(2-methyl-cyclopropyl)-cyclohexene | C13H22 | 178.31 | 7.54 × 105 | 610.5488 | 2.6869 | 3.14 | −2.58 |
| 26 | 1-Methyl-2-(4-methylpentyl)cyclopentane | C12H24 | 168.32 | 1.19 × 105 | 418.0334 | 2.5269 | 3.08 | −2.79 |
| 27 | cis-4a-Methyl-decahydronaphthalene | C11H20 | 152.28 | 6.45 × 102 | 786.5629 | 2.8036 | 3.08 | −5.63 |
| 28 | Ginsenol | C15H26O | 222.37 | 2.71 × 105 | 759.0607 | 3.6536 | 3.04 | −6.47 |
| 29 | Benzene, 1,3-bis(2-ethoxypropyl)- | C16H26O2 | 250.38 | 4.60 × 104 | 2805.2244 | 3.9737 | 2.98 | −2.29 |
| 30 | Cyclododecylamine | C12H25N | 183.33 | 3.70 × 106 | 984.5788 | 3.0936 | 2.97 | −4.02 |
| 31 | Tetracosane | C24H50 | 338.70 | 1.38 × 103 | 1136.7576 | 1.7312 | 2.96 | −4.04 |
| 32 | 3-Ethyl-3-methylnonadecane | C22H46 | 310.60 | 1.2 × 108 | 891.0713 | 5.3604 | 2.92 | −3.43 |
| 33 | 2-Propanol, 1-propoxy- | C6H14O2 | 118.17 | 5.52 × 104 | 1694.1355 | 1.6001 | 2.90 | −3.38 |
| 34 | 1,3,12-Nonadecatriene | C19H34 | 262.50 | 1.41 × 106 | 1182.5946 | 4.0603 | 2.89 | −4.68 |
| 35 | Methyl 2,2-dimethyldecanoate | C13H26O2 | 214.34 | 2.51 × 101 | 1424.6140 | 0.8867 | 2.84 | −8.44 |
| 36 | Isochiapin B | C19H22O6 | 346.38 | 3.90 × 103 | 561.0449 | 3.3003 | 2.83 | −3.57 |
| 37 | Cyclododecanepentanoic acid, 1-nitro-beta,2-dioxo-, phenylmethyl ester | C24H33NO6 | 431.23 | 1.20 × 106 | 1369.6096 | 1.0734 | 2.81 | −3.43 |
| 38 | 6-Dodecene, (Z)- | C12H24 | 168.32 | 6.87 × 103 | 434.5348 | 2.4535 | 2.76 | −6.26 |
| 39 | D:A-Friedooleanan-7-ol, (7alpha±)- | C30H52O | 428.70 | 2.66 × 103 | 1474.1179 | 4.3804 | 2.76 | −3.58 |
| 40 | Propane, 2-bromo-1-chloro- | C3H6BrCl | 157.44 | 6.35 × 102 | 643.5515 | 4.9337 | 2.73 | −4.24 |
| 41 | Tetracontane | C40H82 | 563.10 | 1.65 × 102 | 1424.6140 | 0.9267 | 2.73 | −5.66 |
| 42 | 2,6-Di-tert-butyl-4-nitrophenol | C14H21NO3 | 251.32 | 5.08 × 103 | 2046.1637 | 1.2734 | 2.70 | −3.70 |
| 43 | 2,10-Phenanthrenediol, 4b,5,6,7,8,8a,9,10-octahydro-4b,8,8-trimethyl-1-(1-methylethyl)-, [4bS-(4balpha±,8abeta,10beta)]- | C20H30O2 | 302.46 | 7.10 × 102 | 2332.1866 | 2.0335 | 2.70 | −4.07 |
| 44 | 1-Nonanol | C9H20O | 144.25 | 9.94 × 106 | 913.0730 | 1.1268 | 2.60 | −3.77 |
| 45 | 8-endo-Methylbicyclo[4.2.0]oct-2-ene | C9H14 | 122.21 | 5.72 × 103 | 995.5796 | 4.8337 | 2.59 | −3.04 |
| 46 | 5-Amino-2-methyl-2-phenyl-2,3-dihydro[1,2,4]triazolo[1,5-a][1,3,5]triazine | C11H12N6 | 228.11 | 2.23 × 103 | 2128.6703 | 1.7801 | 2.58 | −3.29 |
| 47 | (3E)-1,4-diphenyl-3-buten-2-ol | C16H16O | 224.30 | 5.21 × 105 | 2095.6676 | 1.8135 | 2.56 | −2.89 |
| 48 | 3,4-Dimethylbenzophenone | C15H14O | 210.27 | 8.06 × 103 | 2079.1663 | 1.2468 | 2.50 | −3.27 |
| 49 | Butanoic acid, 4-methoxy- | C5H10O3 | 118.13 | 8.16 × 106 | 2612.7090 | 0.3867 | 2.50 | −5.84 |
| 50 | 16-Hentriacontanone | C31H62O | 450.80 | 8.30 × 106 | 2497.1998 | 4.5248 | 2.49 | −5.57 |
References
- Singh, N.K.; Narang, M.K.; Thakur, S.S.; Singh, M.; Singh, S.K.; Prakash, A. Influence of transplanting techniques and age of wash root type seedlings on planting attributes of paddy rice. Cogent Food Agric. 2023, 9, 2176978. [Google Scholar] [CrossRef]
- Hu, X.; Lu, L.; Guo, Z.; Zhu, Z. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Champagne, E.T. Rice Aroma and Flavor: A Literature Review. Cereal Chem. 2008, 85, 445–454. [Google Scholar] [CrossRef]
- Grimm, C.; Champagne, E.T.; Ohtsubo, K. Analysis of volatile compounds in the headspace of rice using SPME/GC/MS. In Flavor, Fragrance, and Odor Analysis; Marsili, R., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 229–248. [Google Scholar]
- Kim, H.-R.; Kim, K.M.; Woo, K.; Jeong, H.S.; Kim, K.O. Changes in volatile compounds of waxy rice and gangjeong (a traditional Korean oil-puffed snack) under different steeping conditions. Food Sci. Biotechnol. 2015, 24, 1565–1572. [Google Scholar] [CrossRef]
- Feng, Y.; Cao, H.; Song, H.; Huang, K.; Zhang, Y.; Zhang, Y.; Li, S.; Li, Y.; Lu, J.; Guan, X. The formation mechanism, analysis strategies and regulation measures of cereal aroma: A review. Trends Food Sci. Technol. 2024, 147, 104452. [Google Scholar] [CrossRef]
- Xia, Q.; Mei, J.; Yu, W.; Li, Y. High hydrostatic pressure treatments enhance volatile components of pre-germinated brown rice revealed by aromatic fingerprinting based on HS-SPME/GC–MS and chemometric methods. Food Res. Int. 2017, 91, 103–114. [Google Scholar] [CrossRef]
- Panić, O.; Górecki, T. Comprehensive two-dimensional gas chromatography (GC×GC) in environmental analysis and monitoring. Anal. Bioanal. Chem. 2006, 386, 1013–1023. [Google Scholar] [CrossRef]
- Górecki, T.; Harynuk, J.; Panić, O. The evolution of comprehensive two-dimensional gas chromatography (GC×GC). J. Sep. Sci. 2004, 27, 359–379. [Google Scholar] [CrossRef]
- Dias, R.P.; Johnson, T.A.; Ferrão, L.F.V.; Munoz, P.R.; De La Mata, A.P.; Harynuk, J.J. Improved sample storage, preparation and extraction of blueberry aroma volatile organic compounds for gas chromatography. J. Chromatogr. Open 2023, 3, 100075. [Google Scholar] [CrossRef]
- Nam, S.; De La Mata, A.; Harynuk, J. Automated Screening and Filtering Scripts for GC×GC-TOFMS Metabolomics Data. Separations 2021, 8, 84. [Google Scholar] [CrossRef]
- Sorochan Armstrong, M.D.; Arredondo Campos, O.R.; Bannon, C.C.; De La Mata, A.P.; Case, R.J.; Harynuk, J.J. Global metabolome analysis of Dunaliella tertiolecta, Phaeobacter italicus R11 Co-cultures using thermal desorption—Comprehensive two-dimensional gas chromatography—Time-of-flight mass spectrometry (TD-GC×GC-TOFMS). Phytochemistry 2022, 195, 113052. [Google Scholar] [CrossRef]
- Tarazona Carrillo, K.; Béziat, N.S.; Cebrián-Torrejón, G.; Gros, O.; De La Mata, A.P.; Harynuk, J.J. Metabolomic analysis of secondary metabolites from Caribbean crab gills using comprehensive two-dimensional gas chromatography—Time-of-flight mass spectrometry—New inputs for a better understanding of symbiotic associations in crustaceans. J. Chromatogr. Open 2022, 2, 100069. [Google Scholar] [CrossRef]
- InertCap 5MS/NP. GL Science. 2021. Available online: https://www.glsciences.eu/gc-columns/InertCap_5MS-NP_Technical_Information.pdf?utm_source (accessed on 20 June 2025).
- InertCap WAX-HT|Products|GL Sciences. Glsciences.com. 2025. Available online: https://www.glsciences.com/product/gc_capillary_columns/inertcap/00142.html?utm_source (accessed on 20 June 2025).
- Jezussek, M.; Juliano, B.O.; Schieberle, P. Comparison of Key Aroma Compounds in Cooked Brown Rice Varieties Based on Aroma Extract Dilution Analyses. J. Agric. Food Chem. 2002, 50, 1101–1105. [Google Scholar] [CrossRef]
- Yang, D.S.; Lee, K.; Kim, K.; Kays, S.J. Site of Origin of Volatile Compounds in Cooked Rice. Cereal Chem. 2008, 85, 591–598. [Google Scholar] [CrossRef]
- Yang, D.S.; Lee, K.-S.; Jeong, O.-Y.; Kim, K.-J.; Kays, S.J. Characterization of Volatile Aroma Compounds in Cooked Black Rice. J. Agric. Food Chem. 2008, 56, 235–240. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, H.; Zhang, T.; Tamogami, S.; Chen, J.Y. Analysis of flavor volatiles of glutinous rice during cooking by combined gas chromatography–mass spectrometry with modified headspace solid-phase microextraction method. J. Food Compos. Anal. 2009, 22, 347–353. [Google Scholar] [CrossRef]
- Hu, X.; Fang, C.; Zhang, W.; Lu, L.; Guo, Z.; Li, S.; Chen, M. Change in volatiles, soluble sugars and fatty acids of glutinous rice, japonica rice and indica rice during storage. LWT 2023, 174, 114416. [Google Scholar] [CrossRef]
- Yamashita, K.; Kato, N.; Sakakibara, K.; Seguchi, A.; Kobayashi, A.; Miyagawa, S.; Uchimura, T. Characterization of Cooked Nonglutinous Rice Cultivars Based on Flavor Volatiles and Their Change during Storage. ACS Omega 2023, 8, 14823–14829. [Google Scholar] [CrossRef]
- Ajarayasiri, J.; Chaiseri, S. Comparative Study on Aroma-Active Compounds in Thai, Black and White Glutinous Rice Varieties. Agric. Nat. Resour. 2008, 42, 715–722. [Google Scholar]
- Fukuda, T.; Takeda, T.; Yoshida, S. Comparison of Volatiles in Cooked Rice with Various Amylose Contents. Food Sci. Technol. Res. 2014, 20, 1251–1259. [Google Scholar] [CrossRef]
- Hu, X.; Fang, C.; Lu, L.; Hu, Z.; Zhang, W.; Chen, M. Dynamic Changes in Volatiles, Soluble Sugars, and Fatty Acids in Glutinous Rice during Cooking. Foods 2023, 12, 1700. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xi, J.; Xu, D.; Jin, Y.; Wu, F.; Tong, Q.; Yin, Y.; Xu, X. A comparative HS-SPME/GC-MS-based metabolomics approach for discriminating selected japonica rice varieties from different regions of China in raw and cooked form. Food Chem. 2022, 385, 132701. [Google Scholar] [CrossRef]
- Zhao, Q.; Xi, J.; Xu, X.; Yin, Y.; Xu, D.; Jin, Y.; Tong, Q.; Dong, L.; Wu, F. Volatile fingerprints and biomarkers of Chinese fragrant and non-fragrant japonica rice before and after cooking obtained by untargeted GC/MS-based metabolomics. Food Biosci. 2022, 47, 101764. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, Y.; Shen, Q. Changes in the major aroma-active compounds and taste components of Jasmine rice during storage. Food Res. Int. 2020, 133, 109160. [Google Scholar] [CrossRef]
- Yang, D.S.; Lee, K.-S.; Kays, S.J. Characterization and discrimination of premium-quality, waxy, and black-pigmented rice based on odor-active compounds. J. Sci. Food Agric. 2010, 90, 2595–2601. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Yang, M.; Shi, X.; Mei, Y.; Li, J.; Yang, C.; Pu, S.; Wen, J. Analysis of the Differences in Volatile Organic Compounds in Different Rice Varieties Based on GC-IMS Technology Combined with Multivariate Statistical Modelling. Molecules 2023, 28, 7566. [Google Scholar] [CrossRef]
- Ramtekey, V.; Cherukuri, S.; Modha, K.G.; Kumar, A.; Kethineni, U.B.; Pal, G.; Singh, A.N.; Kumar, S. Extraction, characterization, quantification, and application of volatile aromatic compounds from Asian rice cultivars. Rev. Anal. Chem. 2021, 40, 272–292. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, H.; Chen, J.Y.; Zhang, T.; Matsunaga, R. Flavor Volatiles of Rice During Cooking Analyzed by Modified Headspace SPME/GC-MS. Cereal Chem. 2008, 85, 140–145. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, S.; Wei, J.; Chen, X.; Zhu, S.; Zhou, X. Systematical construction of rice flavor types based on HS-SPME-GC–MS and sensory evaluation. Food Chem. 2023, 413, 135604. [Google Scholar] [CrossRef]



| No. | Cultivar | Amylose Content % | Area | Producing Year |
|---|---|---|---|---|
| 1 | Akitakomachi | 20.0 | Akita | 2021 |
| 2 | Masshigura | 19.6 | Aomori | 2021 |
| 3 | Nanatsuboshi | 19.8 | Hokkaido | 2021 |
| 4 | Hitomebore | 20.1 | Iwate | 2021 |
| 5 | Sasanishiki | 19.7 | Miyagi | 2022 |
| 6 | Hinohikari | 19.4 | Ooita | 2022 |
| 7 | Koshihikari | 20 | Uonuma, Niigata | 2022 |
| 8 | KitayukiMochi | 0 | Hokkaido | 2021 |
| 9 | Tatsukomochi | 0 | Akita | 2021 |
| 10 | Hiyokumochi | 0 | Saga | 2021 |
| 11 | KazenokoMochi | 0 | Hokkaido | 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Zhang, J.; Isoya, S.; Maeda, T.; Hasegawa, K.; Araki, T. Chemometric Approach for Discriminating the Volatile Profile of Cooked Glutinous and Normal-Amylose Rice Cultivars from Representative Japanese Production Areas Using GC × GC-TOFMS. Foods 2025, 14, 2751. https://doi.org/10.3390/foods14152751
Tanaka T, Zhang J, Isoya S, Maeda T, Hasegawa K, Araki T. Chemometric Approach for Discriminating the Volatile Profile of Cooked Glutinous and Normal-Amylose Rice Cultivars from Representative Japanese Production Areas Using GC × GC-TOFMS. Foods. 2025; 14(15):2751. https://doi.org/10.3390/foods14152751
Chicago/Turabian StyleTanaka, Takayoshi, Junhan Zhang, Shuntaro Isoya, Tatsuro Maeda, Kazuya Hasegawa, and Tetsuya Araki. 2025. "Chemometric Approach for Discriminating the Volatile Profile of Cooked Glutinous and Normal-Amylose Rice Cultivars from Representative Japanese Production Areas Using GC × GC-TOFMS" Foods 14, no. 15: 2751. https://doi.org/10.3390/foods14152751
APA StyleTanaka, T., Zhang, J., Isoya, S., Maeda, T., Hasegawa, K., & Araki, T. (2025). Chemometric Approach for Discriminating the Volatile Profile of Cooked Glutinous and Normal-Amylose Rice Cultivars from Representative Japanese Production Areas Using GC × GC-TOFMS. Foods, 14(15), 2751. https://doi.org/10.3390/foods14152751







