Unveiling the Nutritional Quality of the Sicilian Strawberry Tree (Arbutus unedo L.), a Neglected Fruit Species
Abstract
1. Introduction
2. Materials and Methods
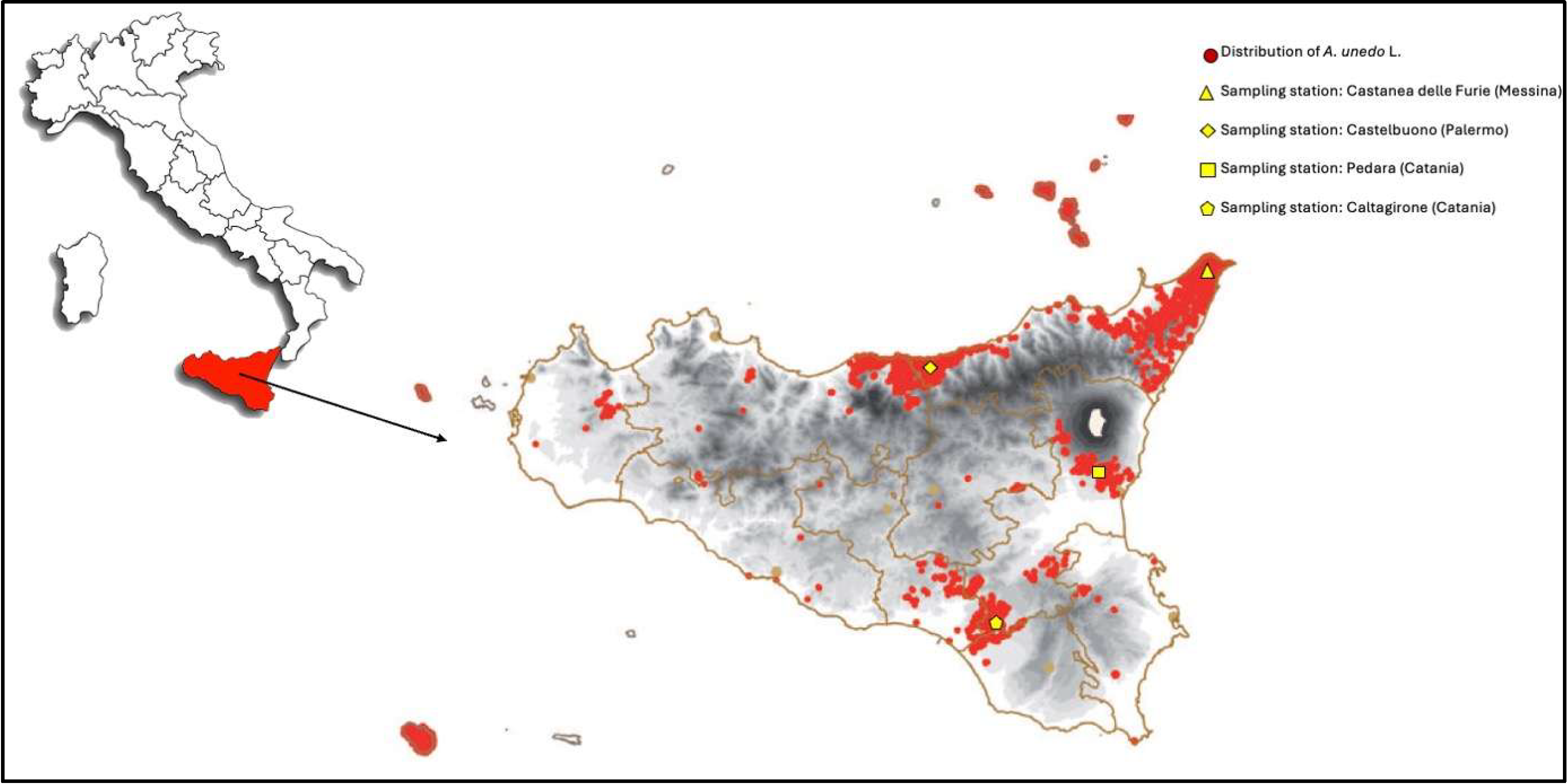
2.1. Samples
2.2. Materials and Reagents
2.3. Proximate Composition
2.4. Sugars
2.5. Fatty Acid (FA) Profile
2.6. Tocopherols
2.7. Total Carotenoids and Total Polyphenols
2.8. Inorganic Elements
2.9. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Sugars
3.3. Fatty Acid (FA) Profile
3.4. Tocopherols
3.5. Total Carotenoids and Total Polyphenols
3.6. Inorganic Elements
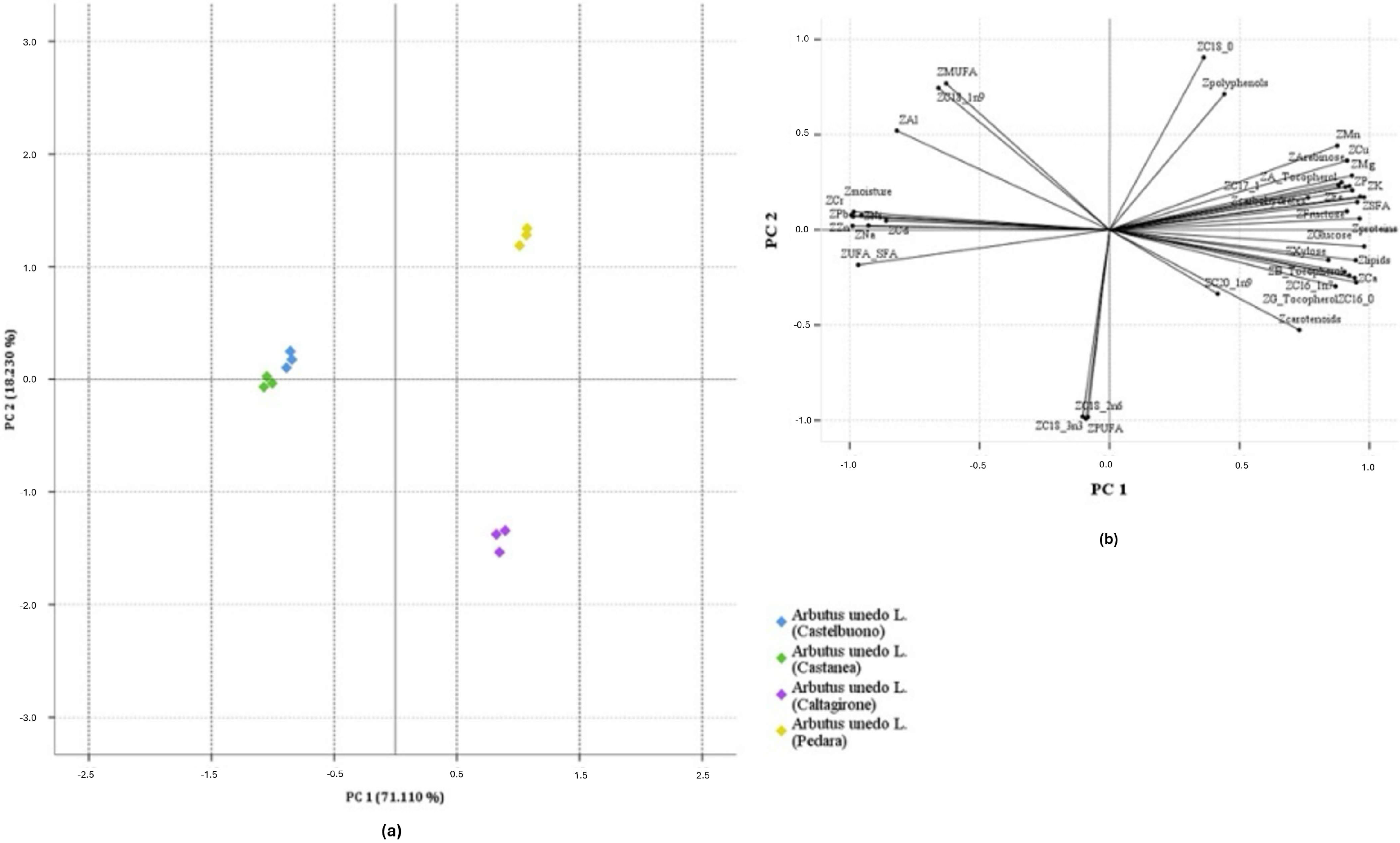
3.7. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almeida, A.M.; Martins, M.J.; Campagnolo, M.L.; Fernandez, P.; Albuquerque, T.; Gerassis, S.; Gonçalves, J.C.; Ribeiro, M.M. Prediction scenarios of past, present, and future environmental suitability for the Mediterranean species Arbutus unedo L. Sci. Rep. 2022, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Amar, Y.M.B.; Potortì, A.G.; Albergamo, A.; Litrenta, F.; Rando, R.; Mouad, L.B.; Brigui, J.; Chouaibi, N.; Di Bella, G. Study of the lipid fraction of Moroccan and Italian carobs (Ceratonia siliqua L.). Eur. J. Lipid Sci. Technol. 2024, 12, 2400036. [Google Scholar] [CrossRef]
- Slimani, W.A.; Albergamo, A.; Rando, R.; Nava, V.; Safi, M.O.; Bensenane, S.M.B.; Lo Turco, V.; Benmahioul, B.; Di Bella, G. Preliminary evaluation of the effect of domestication on the marketable and nutritional quality of B. aegyptiaca (L.) Delile oil from Algeria. Foods 2024, 13, 2752. [Google Scholar] [CrossRef] [PubMed]
- FAO. Promoting Value Chains of Neglected and Underutilized Species for Pro-Poor Growth and Biodiversity Conservation. Guidelines and Good Practices. Available online: https://www.fao.org/plant-treaty/tools/toolbox-for-sustainable-use/details/en/c/1293822/ (accessed on 9 April 2025).
- Pinheiro, M.N.C.; Gomes, F.; Botelho, G.; Rodrigues, I.; Mariychuk, R.; Symochko, L. Exploring the multifaceted aspects of strawberry tree (Arbutus unedo L.) forests in Portugal. Land 2025, 14, 468. [Google Scholar] [CrossRef]
- El Haouari, M.; Assem, N.; Changan, S.; Kumar, M.; Da¸stan, S.D.; Rajkovic, J.; Taheri, Y.; Sharifi-Rad, J.; Kabra, A. An insight into phytochemical, pharmacological, and nutritional properties of Arbutus unedo L. from Morocco. Evid.-Based Complement. Altern. Med. 2021, 2021, 1794621. [Google Scholar] [CrossRef]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef]
- Oliveira, I.; Coelho, V.; Baltasar, R.; Pereira, J.A.; Baptista, P. Scavenging capacity of strawberry tree (Arbutus unedo L.) leaves on free radicals. Food Chem. Toxicol. 2009, 47, 1507–1511. [Google Scholar] [CrossRef]
- Malheiro, R.; Sá, O.; Pereira, E.; Aguiar, C.; Baptista, P.; Pereira, J.A. Arbutus unedo L. leaves as source of phytochemicals with bioactive properties. Ind. Crops Prod. 2012, 37, 473–478. [Google Scholar] [CrossRef]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Doudach, L.; Mrabti, H.N.; Al-Mijalli, S.H.; Kachmar, M.R.; Benrahou, K.; Assaggaf, H.; Qasem, A.; Abdallah, E.M.; Rajab, B.S.; Harraqui, K.; et al. Phytochemical, antidiabetic, antioxidant, antibacterial, acute and sub-chronic toxicity of moroccan Arbutus unedo leaves. J. Pharmacopunct. 2023, 26, 27–37. [Google Scholar] [CrossRef]
- Kachkoul, R.; Squalli Housseini, T.; Mohim, M.; El Habbani, R.; Miyah, Y.; Lahrichi, A. Chemical compounds as well as antioxidant and litholytic activities of Arbutus unedo L. leaves against calcium oxalate stones. J. Integr. Med. 2019, 17, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; Esposito, E.; Di Paola, R.; Ciampa, A.; Mazzon, E.; de Prati, A.C.; Darra, E.; Vincenzi, S.; Cucinotta, G.; Caminiti, R. Protective effect of Arbutus unedo aqueous extract in carrageenan-induced lung inflammation in mice. Pharmacol. Res. 2008, 57, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Habachi, E.; Rebey, I.B.; Dakhlaoui, S.; Hammami, M.; Sawsen, S.; Msaada, K.; Merah, O.; Bourgou, S. Arbutus unedo: Innovative source of antioxidant, anti-inflammatory and anti-tyrosinase phenolics for novel cosmeceuticals. Cosmetics 2022, 9, 143. [Google Scholar] [CrossRef]
- Courtin, O.; Weber, S.; Blanchet, N. Cosmetic Use of an Arbutus unedo Fruit Extract. WO2018041936A1, 8 March 2018. [Google Scholar]
- Scarano, P.; Guida, R.; Zuzolo, D.; Tartaglia, M.; Prigioniero, A.; Postiglione, A.; Pinto, G.; Illiano, A.; Amoresano, A.; Schicchi, R.; et al. An endemic plant of the mediterranean area: Phytochemical characterization of strawberry tree (Arbutus unedo L.) fruits extracts at different ripening stages. Front. Nutr. 2022, 9, 915994. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Skendrović Babojelić, M.; Duralija, B.; Šic Žlabur, J.; Putnik, P.; Bursać Kovačević, D. Strawberry tree fruits and leaves (Arbutus unedo L.) as raw material for sustainable functional food processing: A Review. Horticulturae 2022, 8, 881. [Google Scholar] [CrossRef]
- Masmoudi, M.; Ammar, I.; Ghribi, H.; Attia, H. Physicochemical, radical scavenging activity and sensory properties of a soft cheese fortified with Arbutus unedo L. extract. Food Biosci. 2020, 35, 100579. [Google Scholar] [CrossRef]
- Celikel, G.; Demirsoy, L.; Demirsoy, H. The strawberry tree (Arbutus unedo L.) selection in Turkey. Sci. Hortic. 2008, 118, 115–119. [Google Scholar] [CrossRef]
- Gündoğdu, M.; Ercisli, S.; Canan, I.; Orman, E.; Sameeullah, M.; Naeem, M.; Ayed, R.B. Diversity in phenolic compounds, biochemical and pomological characteristics of Arbutus unedo fruits. Folia Hortic. 2018, 30, 139. [Google Scholar] [CrossRef]
- Sagbas, H.I.; Ilhan, G.; Zitouni, H.; Anjum, M.A.; Hanine, H.; Necas, T.; Ondrasek, I.; Ercisli, S. Morphological and biochemical characterization of diverse strawberry tree (Arbutus unedo L.) genotypes from northern Turkey. Agronomy 2020, 10, 1581. [Google Scholar] [CrossRef]
- Zitouni, H.; Hssaini, L.; Ouaabou, R.; Viuda-Martos, M.; Hernández, F.; Ercisli, S.; Ennahli, S.; Messaoudi, Z.; Hanine, H. Exploring antioxidant activity, organic acid, and phenolic composition in strawberry tree fruits (Arbutus unedo L.) growing in Morocco. Plants 2020, 9, 1677. [Google Scholar] [CrossRef]
- Zitouni, H.; Fauconnier, M.L.; Hssaini, L.; Ouaabou, R.; Viuda-Martos, M.; Hernández, F.; Ercisli, S.; Haddou, L.A.; Messaoudi, Z.; Hanine, H. Phenols, volatile compounds, organic acids and antioxidant activity of strawberry tree (Arbutus unedo L.) fruits belonging to five genotypes growing in Morocco. Int. J. Fruit Sci. 2022, 22, 414–437. [Google Scholar] [CrossRef]
- Ait Lhaj, Z.; Bchitou, R.; Gaboun, F.; Abdelwahd, R.; Benabdelouahab, T.; Kabbour, M.R.; Pare, P.; Diria, G.; Bakhy, K. Moroccan strawberry tree (Arbutus unedo L.) fruits: Nutritional value and mineral composition. Foods 2021, 10, 2263. [Google Scholar] [CrossRef]
- El Cadi, H.; El Cadi, A.; Kounnoun, A.; El Majdoub, Y.O.; Lovillo, M.P.; Brigui, J.; Dugo, P.; Mondello, L.; Cacciola, F. Wild strawberry (Arbutus unedo): Phytochemical screening and antioxidant properties of fruits collected in northern Morocco. Arab. J. Chem. 2020, 13, 6299–6311. [Google Scholar] [CrossRef]
- Salem, I.B.; Ouesleti, S.; Mabrouk, Y.; Landolsi, A.; Saidi, M.; Boulilla, A. Exploring the nutraceutical potential and biological activities of Arbutus unedo L.(Ericaceae) fruits. Ind. Crops Prod. 2018, 122, 726–731. [Google Scholar] [CrossRef]
- Boussalah, N.; Boussalah, D.; Cebadera-Miranda, L.; Fernández-Ruiz, V.; Barros, L.; Ferreira, I.C.; Mata, M.C.; Madani, K. Nutrient composition of Algerian strawberry-tree fruits (Arbutus unedo L.). Fruits 2018, 73, 283–297. [Google Scholar] [CrossRef]
- Asmaa, N.; Abdelaziz, G.; Boulanouar, B.; Carbonell-Barrachina, Á.A.; Cano-Lamadrid, M.; Noguera-Artiaga, L. Chemical composition, antioxidant activity and mineral content of Arbutus unedo (leaves and fruits). J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1335. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Carotenoid composition of strawberry tree (Arbutus unedo L.) fruits. Food Chem. 2016, 199, 165–175. [Google Scholar] [CrossRef]
- Izcara, S.; Morante-Zarcero, S.; Casado, N.; Sierra, I. Study of the phenolic compound profile of Arbutus unedo L. fruits at different ripening stages by HPLC-TQ-MS/MS. Appl. Sci. 2021, 11, 11616. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Bogdanović, S.; Voća, S.; Skendrović Babojelić, M. Biological potential of fruit and leaves of strawberry tree (Arbutus unedo L.) from Croatia. Molecules 2020, 25, 5102. [Google Scholar] [CrossRef]
- Brčić Karačonji, I.; Jurica, K.; Gašić, U.; Dramićanin, A.; Tešić, Ž.; Milojković Opsenica, D. Comparative study on the phenolic fingerprint and antioxidant activity of strawberry tree (Arbutus unedo L.) leaves and fruits. Plants 2021, 11, 25. [Google Scholar] [CrossRef]
- Fonseca, D.F.; Salvador, Â.C.; Santos, S.A.; Vilela, C.; Freire, C.S.; Silvestre, A.J.; Rocha, S.M. Bioactive phytochemicals from wild Arbutus unedo L. berries from different locations in Portugal: Quantification of lipophilic components. Int. J. Mol. Sci. 2015, 16, 14194–14209. [Google Scholar] [CrossRef]
- Marques, M.P.; Martin, D.; Bosch, M.; Martins, J.; Biswal, A.K.; Zuzarte, M.; de Carvalho, L.B.; Canhoto, J.; da Costa, R. Unveiling the compositional remodelling of Arbutus unedo L. fruits during ripening. Sci. Hortic. 2022, 303, 111248. [Google Scholar] [CrossRef]
- Macchioni, V.; Santarelli, V.; Carbone, K. Phytochemical profile, antiradical capacity and α-glucosidase inhibitory potential of wild Arbutus unedo L. fruits from central Italy: A chemometric approach. Plants 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, M.; Scarano, P.; Prigioniero, A.; Zuzolo, D.; Postiglione, A.; Falzarano, A.; Amoresano, A.; Illiano, A.; Pinto, G.; Schicchi, R.; et al. Multi-omic characterisation as a tool to improve knowledge, valorisation and conservation of wild fruit genetic resources: The case of Arbutus unedo L. Front. Plant Sci. 2023, 14, 1195673. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G. AOAC Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists Arlington: Gaithersburg, MD, USA, 2012. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Amar, Y.M.B.; Nava, V.; Mouad, L.B.; Brigui, J.; Chouaibi, N.; Potortì, A.G.; Litrenta, F.; Albergamo, A.; Di Bella, G. Proximate composition and mineral profile of Moroccan and Italian carobs. J. Food Comp. Anal. 2025, 143, 107628. [Google Scholar]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Amor, N.B.; Lo Vecchio, G.; Nava, V.; Rando, R.; Ben Mansour, H.; Lo Turco, V. Chemical characterization of different products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef]
- Di Bella, G.; Vecchio, G.L.; Albergamo, A.; Nava, V.; Bartolomeo, G.; Macrì, A.; Bacchetta, L.; Turco, V.L.; Potortì, A.G. Chemical characterization of Sicilian dried nopal [Opuntia ficus-indica (L.) Mill.]. J. Food Comp. Anal. 2022, 106, 104307. [Google Scholar] [CrossRef]
- Lo Turco, V.; Sgrò, B.; Albergamo, A.; Nava, V.; Rando, R.; Potortì, A.G.; Di Bella, G. Assessment of the accuracy of nutrition label and chemical composition of plant-based milks available on the Italian market. Foods 2023, 12, 3207. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Lo Turco, V.; Litrenta, F.; Nava, V.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Potortì, A.G.; Di Bella, G. Effect of filtration process on oxidative stability and minor compounds of the cold-pressed hempseed oil during storage. Antioxidants 2023, 12, 1231. [Google Scholar] [CrossRef]
- Monteiro, G.C.; Minatel, I.O.; Junior, A.P.; Gomez-Gomez, H.A.; de Camargo, J.P.; Diamante, M.S.; Basílio, L.S.; Tecchio, M.A.; Lima, G.P. Bioactive compounds and antioxidant capacity of grape pomace flours. LWT 2021, 135, 110053. [Google Scholar] [CrossRef]
- Lo Turco, V.; Nava, V.; Potortì, A.G.; Sgrò, B.; Arrigo, M.A.; Di Bella, G. Total polyphenol contents and mineral profiles in commercial wellness herbal infusions: Evaluation of the differences between two preparation methods. Foods 2024, 13, 2145. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union. 2006, 404, 9–25. [Google Scholar]
- Vidrih, R.; Hribar, J.; Prgomet, Ž.; Poklar Ulrih, N. The physico-chemical properties of strawberry tree (Arbutus unedo L.) fruits. Croat. J. Food Sci. Technol. 2013, 5, 29–33. [Google Scholar]
- Trad, M.; Boge, M.; Ben Hamda, H.; Renard, C.M.; Harbi, M. The glucose-fructose ratio of wild Tunisian grapes. Cogent Food Agric. 2017, 3, 1374156. [Google Scholar] [CrossRef]
- Akšić, M.F.; Tosti, T.; Sredojević, M.; Milivojević, J.; Meland, M.; Natić, M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants 2019, 8, 205. [Google Scholar] [CrossRef]
- Topcu, H.; Degirmenci, I.; Sonmez, D.A.; Paizila, A.; Karci, H.; Kafkas, S.; Kafkas, E.; Ercisli, S.; Alatawi, A. Sugar, invertase enzyme activities and invertase gene expression in different developmental stages of strawberry Fruits. Plants 2022, 11, 509. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enteral Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Oliveira, I.; Baptista, P.; Malheiro, R.; Casal, S.; Bento, A.; Pereira, J.A. Influence of strawberry tree (Arbutus unedo L.) fruit ripening stage on chemical composition and antioxidant activity. Food Res. Int. 2011, 44, 1401–1407. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.; Carvalho, A.M.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Morales, R.; Tardío, J. Wild edible fruits as a potential source of phytochemicals with capacity to inhibit lipid peroxidation. Eur. J. Lipid Sci. Technol. 2013, 115, 176–185. [Google Scholar] [CrossRef]
- Zwyrzykowska-Wodzińska, A.; Jarosz, B.; Okińczyc, P.; Szperlik, J.; Bąbelewski, P.; Zadák, Z.; Jankowska-Mąkosa, A.; Knecht, D. GC-MS and PCA Analysis of Fatty Acid Profile in Various Ilex Species. Molecules 2024, 29, 4833. [Google Scholar] [CrossRef]
- Bastürk, F.N.; Özcan, T.; Gercek, Y.C. Populational segregation of Echium plantagineum L. based on seed oil fatty acid ratios as chemotaxonomical marker sets. J. Food Compos. Anal. 2024, 136, 106752. [Google Scholar] [CrossRef]
- Izadi-Darbandi, A.; Akbari, A.; Bahmani, K.; Warner, R.; Ebrahimi, M.; Ramshini, H. Fatty acid profiling and oil content variation among Iranian fennel (Foeniculum vulgare Mill. var. vulgare) landraces. Int. J. Hortic. Sci. Technol. 2023, 10, 193–202. [Google Scholar]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Comp. Anal. 2006, 19, 196–204. [Google Scholar] [CrossRef]
- Charoensiri, R.; Kongkachuichai, R.; Suknicom, S.; Sungpuag, P. Beta-carotene, lycopene, and alpha-tocopherol contents of selected Thai fruits. Food Chem. 2009, 113, 202–207. [Google Scholar] [CrossRef]
- Gündeşli, M.A.; Korkmaz, N.; Okatan, V. Polyphenol content and antioxidant capacity of berries: A review. Int. J. Agric. For. Life Sci. 2019, 3, 350–361. [Google Scholar]
- Mrázová, M.; Rampáčková, E.; Šnurkovič, P.; Ondrášek, I.; Nečas, T.; Ercisli, S. Determination of selected beneficial substances in peach fruits. Sustainability 2021, 13, 14028. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, Y.; Zhou, D.; Pan, L.; Tu, K. Differences in commercial quality and carotenoids profile of yellow-and white-fleshed nectarine fruit during low temperature storage and the regulation of carotenoids by sugar. Postharvest Biol. Technol. 2023, 197, 112206. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Milošević, S.; Teslić, N.; Pavlić, B.; Putnik, P.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Bursać Kovačević, D. development of a pressurized green liquid extraction procedure to recover antioxidant bioactive compounds from strawberry tree fruit (Arbutus unedo L.). Plants 2023, 12, 2006. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Un. 2023, 119, 103–157. [Google Scholar]
- Mihai, R.A.; Rodríguez Valencia, K.E.; Sivizaca Flores, N.G.; Ramiro Fernando, V.G.; Nelson Santiago, C.I.; Catana, R.D. Consequences of volcanic ash on antioxidants, nutrient composition, heavy metal accumulation, and secondary metabolites in key crops of Cotopaxi province, Ecuador. Toxics 2025, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Wang, Y.; Sun, H.; Fu, L.; Zhu, L.; Liu, J.; Zhi, Z.; He, J.; Wang, W.; Wu, C. Effects of soil conditioner (volcanic ash) on yield quality and rhizosphere soil characteristics of melon. Plants 2024, 13, 1787. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.; Messina, E.M.; Cammilleri, G.; Galluzzo, F.G.; Pantano, L.; Buscemi, M.D.; Macaluso, A.; Bertuglia, T.; Pulvirenti, A.; Lo Dico, G.M.; et al. Detection of polyphenols in carob pods (Ceratonia siliqua) from Southern Italy by a LC-HRMS method. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
| Sample Code | Geographical Origin | Geographical Coordinates | Sampling Date | No. of Replicates |
|---|---|---|---|---|
| S1 | Castanea delle Furie (Messina, Italy) | 38°15′46.2″ N 15°31′22.4″ E | November 2024 | 3 |
| S2 | Castelbuono (Palermo, Italy) | 37°57′28.4″ N 14°05′20.3″ E | 3 | |
| S3 | Caltagirone (Catania, Italy) | 37°14′14.2″ N 14°30′43.7″ E | 3 | |
| S4 | Pedara (Catania, Italy) | 37°37′06.2″ N 15°03′42.4″ E | 3 |
| Parameter | Setting Value |
|---|---|
| Radio-frequency power | 1500 W |
| Plasma gas flow rate (Ar) | 14 L/min |
| Auxiliary gas flow rate (Ar) | 0.8 L/min |
| Carrier gas flow rate (Ar) | 1.1 L/min |
| Collision gas flow rate (He) | 4.7 mL/min |
| Spray chamber temperature | 2.7 °C |
| Injection volume | 200 μL |
| Sample introduction flow rate | 0.93 mL/min |
| Acquisition mode | Full scan mode |
| Integration time | 0.5 or 0.1 s/point based on the analyte |
| Sample | Moisture | Carbohydrates | Protein | Lipids | Crude Fiber | Ash |
| S1 | 56.27 ± 1.06 a | 18.00 ± 1.46 a | 1.63 ± 0.09 a | 1.65 ± 0.07 a | 9.88 ± 1.55 a | 1.13 ± 0.12 a |
| S2 | 56.31 ± 0.59 a | 19.87 ± 0.63 a | 1.68 ± 0.04 a | 1.75 ± 0.12 a | 9.81 ± 2.40 a | 1.28 ± 0.10 a |
| S3 | 46.82 ± 0.57 b | 22.53 ± 2.78 b | 2.50 ± 0.06 b | 2.43 ± 0.15 b | 12.84 ± 1.50 a | 2.00 ± 0.07 b |
| S4 | 47.03 ± 0.90 b | 24.62 ± 2.62 b | 2.48 ± 0.07 b | 2.34 ± 0.12 b | 13.00 ± 2.37 a | 2.58 ± 0.11 c |
| Sample | Fructose | Glucose | Sucrose | Xylose | Arabinose | Maltose |
|---|---|---|---|---|---|---|
| S1 | 6.37 ± 0.56 a | 2.24 ± 0.30 a | 0.96 ± 0.08 a | 0.25 ± 0.09 a | 0.09 ± 0.02 a | <LOD |
| S2 | 6.93 ± 0.44 a | 1.91 ± 0.24 a | 0.95 ± 0.11 a | 0.41 ± 0.10 a | 0.07 ± 0.02 a | <LOD |
| S3 | 8.60 ± 0.39 b | 4.55 ± 0.72 b | 1.17 ± 0.17 a | 0.85 ± 0.10 b | 0.14 ± 0.04 b | <LOD |
| S4 | 9.37 ± 0.42 b | 5.40 ± 0.81 b | 1.16 ± 0.06 a | 0.77 ± 0.08 b | 0.22 ± 0.03 c | <LOD |
| FA | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| C16:0 | 16.54 ± 0.30 a | 15.43 ± 0.20 b | 21.66 ± 0.12 c | 19.92 ± 0.18 d |
| C16:1 n-7 | 0.32 ± 0.03 a | 0.23 ± 0.02 b | 0.64 ± 0.05 c | 0.52 ± 0.04 d |
| C17:0 | 0.62 ± 0.04 a | 0.79 ± 0.04 a | 0.92 ± 0.18 b | 0.84 ± 0.06 a,b |
| C17:1 | 1.37 ± 0.05 a | 1.14 ± 0.03 b | 1.56 ± 0.12 a | 1.78 ± 0.10 c |
| C18:0 | 6.52 ± 0.18 a | 5.48 ± 0.37 b | 5.01 ± 0.10 b | 9.34 ± 0.24 c |
| C18:1 n-9 | 18.27 ± 0.17 a | 19.20 ± 0.20 b | 7.37 ± 0.36 c | 15.86 ± 0.18 d |
| C18:1 n-7 | 1.02 ± 0.07 a | 1.08 ± 0.03 a | 0.72 ± 0.05 b | 1.09 ± 0.04 a |
| C18:2 n-6 | 33.30 ± 0.55 a | 34.32 ± 0.16 a | 37.35 ± 0.32 b | 30.58 ± 0.44 c |
| C18:3 n-3 | 20.76 ± 0.25 a | 21.11 ± 0.03 a | 23.22 ± 0.72 b | 18.75 ± 0.45 c |
| C20:0 | 1.04 ± 0.13 a | 0.84 ± 0.04 a | 0.97 ± 0.12 a | 0.84 ± 0.04 a |
| C20:1 n-9 | 0.33 ± 0.07 a | 0.15 ± 0.02 b | 0.35 ± 0.04 a | 0.25 ± 0.04 a |
| SFA | 24.71 ± 0.15 a | 22.54 ± 0.27 b | 28.56 ± 0.10 c | 30.93 ± 0.45 d |
| MUFA | 21.32 ± 0.10 a | 21.80 ± 0.26 a | 10.65 ± 0.51 b | 19.50 ± 0.26 c |
| PUFA | 54.06 ± 0.80 a | 55.43 ± 0.16 a | 60.57 ± 0.70 b | 49.33 ± 0.20 c |
| UFA/SFA | 3.05 ± 0.05 a | 3.18 ± 0.24 b | 3.28 ± 0.24 c | 2.23 ± 0.05 d |
| n-6/n-3 | 1.60 ± 0.01 a | 1.63 ± 0.01 a | 1.61 ± 0.05 a | 1.63 ± 0.06 a |
| Sample | α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total |
|---|---|---|---|---|---|
| S1 | 6.42 ± 0.32 a | 0.08 ± 0.01 a | 2.05 ± 0.18 a | <LOD | 8.55 ± 0.50 a |
| S2 | 6.23 ± 0.34 a | 0.12 ± 0.02 a | 2.29 ± 0.17 a | <LOD | 8.65 ± 0.36 a |
| S3 | 7.64 ± 0.33 b | 0.35 ± 0.06 b | 3.20 ± 0.16 b | <LOD | 11.19 ± 0.32 b |
| S4 | 8.58 ± 0.39 c | 0.28 ± 0.08 a | 2.87 ± 0.13 b | <LOD | 11.73 ± 0.33 b |
| Sample | Total Carotenoids | Total Polyphenols |
|---|---|---|
| S1 | 11.73 ± 0.23 a | 3324.27 ± 119.85 a,b |
| S2 | 9.60 ± 0.50 b | 3027.61 ± 138.90 a |
| S3 | 15.45 ± 0.17 c | 3100.17 ± 140.26 a |
| S4 | 12.11 ± 0.17 a | 3666.17 ± 231.04 b |
| Element | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Na | 224.01 ± 5.84 a | 234.48 ± 15.49 a | 194.92 ± 4.37 b | 192.97 ± 5.28 b |
| Ca | 948.61 ± 20.99 a | 938.87 ± 25.65 a | 1286.03 ± 45.90 b | 1196.50 ± 74.36 b |
| Mg | 113.11 ± 7.54 a | 114.31 ± 6.15 a | 148.68 ± 5.77 b | 182.03 ± 7.73 c |
| K | 2517.74 ± 45.24 a | 2435.99 ± 42.48 a | 3425.87 ± 60.28 b | 3940.53 ± 184.06 c |
| P | 736.96 ± 6.71 a | 733.84 ± 9.81 a | 900.33 ± 56.85 b | 951.98 ± 51.20 b |
| Zn | 7.03 ± 0.14 a | 7.15 ± 0.15 a | 5.30 ± 0.18 b | 5.16 ± 0.09 b |
| Fe | 6.11 ± 0.13 a | 5.48 ± 0.34 a | 7.74 ± 0.13 b | 9.27 ± 1.43 b |
| Cu | 0.83 ± 0.06 a | 0.88 ± 0.05 a | 1.47 ± 0.10 b | 2.64 ± 0.10 c |
| Mn | 0.60 ± 0.06 a | 0.61 ± 0.09 a | 1.03 ± 0.12 b | 2.00 ± 0.08 c |
| Mo | 0.031 ± 0.010 a | 0.036 ± 0.008 a | 0.017 ± 0.009 a | 0.016 ± 0.006 a |
| Cr | 1.71 ± 0.07 a | 1.72 ± 0.14 a | 0.76 ± 0.06 b | 0.75 ± 0.03 b |
| Ni | 0.033 ± 0.011 a | 0.032 ± 0.013 a | 0.010 ± 0.009 a | 0.011 ± 0.010 a |
| Al | 4.14 ± 0.08 a | 4.16 ± 0.11 a | 3.35 ± 0.09 b | 3.77 ± 0.15 c |
| Cd | 0.022 ± 0.006 a | 0.020 ± 0.005 a | 0.015 ± 0.003 a | 0.012 ± 0.010 a |
| Pb | 0.012 ± 0.004 a | 0.014 ± 0.007 a | <LOD | <LOD |
| As | 0.013 ± 0.002 a | 0.015 ± 0.005 a | 0.013 ± 0.003 a | 0.011 ± 0.002 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litrenta, F.; Nava, V.; Albergamo, A.; Potortì, A.G.; Sturniolo, R.; Lo Turco, V.; Di Bella, G. Unveiling the Nutritional Quality of the Sicilian Strawberry Tree (Arbutus unedo L.), a Neglected Fruit Species. Foods 2025, 14, 2734. https://doi.org/10.3390/foods14152734
Litrenta F, Nava V, Albergamo A, Potortì AG, Sturniolo R, Lo Turco V, Di Bella G. Unveiling the Nutritional Quality of the Sicilian Strawberry Tree (Arbutus unedo L.), a Neglected Fruit Species. Foods. 2025; 14(15):2734. https://doi.org/10.3390/foods14152734
Chicago/Turabian StyleLitrenta, Federica, Vincenzo Nava, Ambrogina Albergamo, Angela Giorgia Potortì, Roberto Sturniolo, Vincenzo Lo Turco, and Giuseppa Di Bella. 2025. "Unveiling the Nutritional Quality of the Sicilian Strawberry Tree (Arbutus unedo L.), a Neglected Fruit Species" Foods 14, no. 15: 2734. https://doi.org/10.3390/foods14152734
APA StyleLitrenta, F., Nava, V., Albergamo, A., Potortì, A. G., Sturniolo, R., Lo Turco, V., & Di Bella, G. (2025). Unveiling the Nutritional Quality of the Sicilian Strawberry Tree (Arbutus unedo L.), a Neglected Fruit Species. Foods, 14(15), 2734. https://doi.org/10.3390/foods14152734








