Potential and Challenges of a Targeted Membrane Pre-Fouling: Process Performance of Milk Protein Fractionation After the Application of a Transglutaminase Treatment of Casein Micelles
Abstract
1. Introduction
2. Materials and Methods
2.1. Retentate Material
2.2. Compositional Analysis of Filtration Media
2.3. Enzymatic Pretreatment with Transglutaminase (Tgase)
2.4. Dead-End Filtrations on a Laboratory Scale
2.5. Cross-Flow Filtration Tests on a Pilot Scale
2.6. Data Analysis
3. Results
3.1. Composition of Filtration Media and Analyses of TGase-Induced Modifications in SM
3.2. Testing of Different Tgase Treatments in Laboratory-Scale Dead-End Filtration
3.3. Cross-Flow Microfiltration of Untreated and Tgase-Pretreated Skim Milk
3.4. Cross-Flow Microfiltration of Untreated and Tgase-Pretreated Micellar Casein
3.5. Tests to Implement the Setup of an Optimized Deposit Layer of MCC-2U Prior to MF of SM-0U
- Although pressure conditions were kept stable, the higher viscosity of SM-0U, which is mainly related to the presence of lactose, induces a possibly relevant abrupt change in the flow profile at the membrane surface to less turbulent conditions [26].
- The increased amount of relatively small whey proteins in SM-0U could lead to the abrupt pore blocking of the less compacted, more porous pre-fouling layer.
- The structural state of casein micelles in the retentate changes from a slightly swollen state in MCC-2U to a nearly natural and more compact state in SM-0U [54], which might also lead to a pore-blocking effect.
- The ionic environment changes from the water-like state in MCC induced by DF to the serum phase of SM-0U. The higher calcium level of the latter medium has been described to increase interaction within deposits built from natural casein micelles, resulting in their compaction [25]. Despite the Tgase treatment, comparatively strong inter- or intramicellar interaction and compaction might be induced in a deposit built from less regularly structured CM in MCC when increasing the calcium level and ionic strength.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MF | Microfiltration |
| SM | Skim milk |
| MCC | Micellar casein concentrate |
| MPI | Milk protein isolate |
| RD | Deposit resistance |
| ΔpTM | Transmembrane pressure difference |
| J | Flux |
| Tgase | Transglutaminase |
| DF | Diafiltration |
References
- De Boer, R. From Milk By-Products to Milk Ingredients: Upgrading the Cycle; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-0-470-67222-8. [Google Scholar]
- Pierre, A.; Fauquant, J.; Le Graet, Y.; Piot, M.; Maubois, J.L. Préparation de phosphocaséinate natif par microfiltration sur membrane. Lait 1992, 72, 461–474. [Google Scholar] [CrossRef]
- Sisay, E.J.; Al-Tayawi, A.N.; László, Z.; Kertész, S. Recent Advances in Organic Fouling Control and Mitigation Strategies in Membrane Separation Processes: A Review. Sustainability 2023, 15, 13389. [Google Scholar] [CrossRef]
- Vetier, C.; Bennasar, M.; de la Fuente, B.T. Study of the fouling of a mineral microfiltration membrane using scanning electron microscopy and physicochemical analyses in the processing of milk. J. Dairy Res. 1988, 55, 381–400. [Google Scholar] [CrossRef]
- Qu, P.; Gésan-Guiziou, G.; Bouchoux, A. Dead-end filtration of sponge-like colloids: The case of casein micelle. J. Membr. Sci. 2012, 417–418, 10–19. [Google Scholar] [CrossRef]
- Huppertz, T.; Gazi, I.; Luyten, H.; Nieuwenhuijse, H.; Alting, A.; Schokker, E. Hydration of casein micelles and caseinates: Implications for casein micelle structure. Int. Dairy J. 2017, 74, 1–11. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef]
- Trejo, R.; Dokland, T.; Jurat-Fuentes, J.; Harte, F. Cryo-transmission electron tomography of native casein micelles from bovine milk. J. Dairy Sci. 2011, 94, 5770–5775. [Google Scholar] [CrossRef]
- Reitmaier, M.; Bachmann, I.; Heidebrecht, H.-J.; Kulozik, U. Effect of changes in ionic composition induced by different diafiltration media on deposited layer properties and separation efficiency in milk protein fractionation by microfiltration. Int. Dairy J. 2021, 120, 105089. [Google Scholar] [CrossRef]
- Adams, M.C.; Barbano, D.M. Serum protein removal from skim milk with a 3-stage, 3× ceramic Isoflux membrane process at 50 °C. J. Dairy Sci. 2013, 96, 2020–2034. [Google Scholar] [CrossRef]
- Adams, M.C.; Hurt, E.E.; Barbano, D.M. Effect of soluble calcium and lactose on limiting flux and serum protein removal during skim milk microfiltration. J. Dairy Sci. 2015, 98, 7483–7497. [Google Scholar] [CrossRef]
- Schorsch, C.; Carrie, H.; Clark, A.; Norton, I. Cross-linking casein micelles by a microbial transglutaminase conditions for formation of transglutaminase-induced gels. Int. Dairy J. 2000, 10, 519–528. [Google Scholar] [CrossRef]
- Huppertz, T.; de Kruif, C.G. Structure and stability of nanogel particles prepared by internal cross-linking of casein micelles. Int. Dairy J. 2008, 18, 556–565. [Google Scholar] [CrossRef]
- Akal, H.C.; Koçak, C.; Özer, H.B. Transglutaminase Applications in Dairy Technology. In Microbial Cultures and Enzymes in Dairy Technology; Budak, S.O., Akal, H.C., Eds.; IGI Global Medical Information Science Reference (An Imprint of IGI Global): Hershey, PA, USA, 2018; ISBN 1522553630. [Google Scholar]
- Smiddy, M.A.; Martin, J.-E.; Kelly, A.L.; de Kruif, C.G.; Huppertz, T. Stability of Casein Micelles Cross-Linked by Transglutaminase. J. Dairy Sci. 2006, 89, 1906–1914. [Google Scholar] [CrossRef]
- Mounsey, J.S.; O’Kennedy, B.T.; Kelly, P.M. Influence of transglutaminase treatment on properties of micellar casein and products made therefrom. Lait 2005, 85, 405–418. [Google Scholar] [CrossRef]
- Puri, R.; Bot, F.; Singh, U.; O’Mahony, J.A. Influence of Transglutaminase Crosslinking on Casein Protein Fractionation during Low Temperature Microfiltration. Foods 2021, 10, 3146. [Google Scholar] [CrossRef]
- Vasbinder, A.J.; Rollema, H.S.; Bot, A.; de Kruif, C.G. Gelation Mechanism of Milk as Influenced by Temperature and pH.; Studied by the Use of Transglutaminase Cross-Linked Casein Micelles. J. Dairy Sci. 2003, 86, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R.; Steinhauer, T.; Meyer, P.; Sterr, J.; Perlich, J.; Kulozik, U. Structural changes of deposited casein micelles induced by membrane filtration. Faraday Discuss. 2012, 158, 77–88; discussion 105–124. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, S.; Hartinger, M.; Matyssek, A.; Kulozik, U. On the reversibility of deposit formation in low temperature milk microfiltration with ceramic membranes depending on mode of adjustment of transmembrane pressure and wall shear stress. Sep. Purif. Technol. 2020, 247, 116962. [Google Scholar] [CrossRef]
- De Kort, E. Stabilized Micellar Casein and Compositions. 2015 Patent: WO2015156662A1, 15 October 2015. [Google Scholar]
- Hinz, K.; Huppertz, T.; Kulozik, U.; Kelly, A.L. Influence of enzymatic cross-linking on milk fat globules and emulsifying properties of milk proteins. Int. Dairy J. 2007, 17, 289–293. [Google Scholar] [CrossRef]
- Gauche, C.; Vieira, J.T.; Ogliari, P.J.; Bordignon-Luiz, M.T. Crosslinking of milk whey proteins by transglutaminase. Process Biochem. 2008, 43, 788–794. [Google Scholar] [CrossRef]
- Salunke, P.; Metzger, L.E. Impact of transglutaminase treatment given to the skim milk before or after microfiltration on the functionality of micellar casein concentrate used in process cheese product and comparison with rennet casein. Int. Dairy J. 2022, 128, 105317. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Han, J.; Yuan, D.; Lu, Z.; Zhang, L. Influence of transglutaminase-induced modification of milk protein concentrate (MPC) on yoghurt texture. Int. Dairy J. 2018, 78, 65–72. [Google Scholar] [CrossRef]
- Anantharaman, A.; Chun, Y.; Hua, T.; Chew, J.W.; Wang, R. Pre-deposited dynamic membrane filtration—A review. Water Res. 2020, 173, 115558. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Hess, H.; Höflinger, W. Influence of operating parameters on precoat layers built up under crossflow condition. Sep. Purif. Technol. 2003, 31, 269–280. [Google Scholar] [CrossRef]
- Obermeyer, H.D.; Kulozik, U.; Kessler, H.G. Controlled deposit formation to influence the retention of solutes in reverse osmosis and ultrafiltration. Desalination 1993, 90, 161–172. [Google Scholar] [CrossRef]
- Dumpler, J.; Kieferle, I.; Wohlschläger, H.; Kulozik, U. Milk ultrafiltrate analysis by ion chromatography and calcium activity for SMUF preparation for different scientific purposes and prediction of its supersaturation. Int. Dairy J. 2017, 68, 60–69. [Google Scholar] [CrossRef]
- Warncke, M.; Kulozik, U. Impact of temperature and high pressure homogenization on the solubility and rheological behavior of reconstituted dairy powders of different composition. Powder Technol. 2020, 376, 285–295. [Google Scholar] [CrossRef]
- Dumpler, J.; Wohlschläger, H.; Kulozik, U. Dissociation and coagulation of caseins and whey proteins in concentrated skim milk heated by direct steam injection. Dairy Sci. Technol. 2016, 96, 807–826. [Google Scholar] [CrossRef]
- Reiter, M.; Reitmaier, M.; Haslbeck, A.; Kulozik, U. Acid gelation functionality of casein micelles in concentrated state: Influence of calcium supplementation or chelation combined with enzymatic stabilization. Food Hydrocoll. 2023, 143, 108927. [Google Scholar] [CrossRef]
- Li, Y.; Joyner, H.S.; Carter, B.G.; Drake, M.A. Effects of fat content, pasteurization method, homogenization pressure, and storage time on the mechanical and sensory properties of bovine milk. J. Dairy Sci. 2018, 101, 2941–2955. [Google Scholar] [CrossRef]
- Manji, B.; Kakuda, Y. Determination of Whey Protein Denaturation in Heat-Processed Milks: Comparison of Three Methods. J. Dairy Sci. 1987, 70, 1355–1361. [Google Scholar] [CrossRef]
- Svanborg, S.; Johansen, A.-G.; Abrahamsen, R.K.; Skeie, S.B. Initial pasteurisation effects on the protein fractionation of skimmed milk by microfiltration. Int. Dairy J. 2014, 37, 26–30. [Google Scholar] [CrossRef]
- Granger-Delacroix, M.; Leconte, N.; Garnier-Lambrouin, F.; Le Goff, F.; van Audenhaege, M.; Gésan-Guiziou, G. Transmembrane Pressure and Recovery of Serum Proteins During Microfiltration of Skimmed Milk Subjected to Different Storage and Treatment Conditions. Foods 2020, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, T.; Lonfat, J.; Hager, I.; Gebhardt, R.; Kulozik, U. Effect of pH, transmembrane pressure and whey proteins on the properties of casein micelle deposit layers. J. Membr. Sci. 2015, 493, 452–459. [Google Scholar] [CrossRef]
- Lipnizki, F.; Boelsmand, J.; Madsen, R.F. Concepts of industrial-scale diafiltration systems. Desalination 2002, 144, 179–184. [Google Scholar] [CrossRef]
- Nyambura, H.L.; Janssen, A.E.; van der Padt, A.; Boom, R.M. A geometric model to predict protein retentions during skim milk microfiltration. J. Membr. Sci. 2025, 722, 123865. [Google Scholar] [CrossRef]
- Doudiès, F.; Loginov, M.; Hengl, N.; Karrouch, M.; Leconte, N.; Garnier-Lambrouin, F.; Pérez, J.; Pignon, F.; Gésan-Guiziou, G. Build-up and relaxation of membrane fouling deposits produced during crossflow ultrafiltration of casein micelle dispersions at 12 °C and 42 °C probed by in situ SAXS. J. Membr. Sci. 2021, 618, 118700. [Google Scholar] [CrossRef]
- Ribadeau-Dumas, B.; Grappin, R. Milk protein analysis: Review. Lait 1989, 69, 357–416. [Google Scholar] [CrossRef]
- ISO 11814/IDF 162; Dried Milk—Assessment of Heat Treatment Intensity—Method Using High Performance Liquid Chromatography. American National Standards Institute (ANSI): Washington, D.C., USA, 2002.
- Mazuknaite, I.; Guyot, C.; Leskauskaite, D.; Kulozik, U. Influence of transglutaminase on the physical and chemical properties of acid milk gel and cottage type cheese. J. Food Agric. Environ. 2013, 11, 119–124. [Google Scholar] [CrossRef]
- Guyot, C.; Kulozik, U. Effect of transglutaminase-treated milk powders on the properties of skim milk yoghurt. Int. Dairy J. 2011, 21, 628–635. [Google Scholar] [CrossRef]
- Lauber, S.; Henle, T.; Klostermeyer, H. Relationship between the crosslinking of caseins by transglutaminase and the gel strength of yoghurt. Eur. Food Res. Technol. 2000, 210, 305–309. [Google Scholar] [CrossRef]
- Creamer, L.K.; Berry, G.P.; Mills, O.E. A study of the dissociation of beta -casein from the bovine casein micelle at low temperature. New Zealand J. Dairy Sci. Technol. 1977, 1, 58–66. [Google Scholar]
- Velazquez-Dominguez, A.; Hiolle, M.; Abdallah, M.; Delaplace, G.; Peixoto, P.P. Transglutaminase cross-linking on dairy proteins: Functionalities, patents, and commercial uses. Int. Dairy J. 2023, 143, 105688. [Google Scholar] [CrossRef]
- Salunke, P.; Metzger, L.E. Functional properties of milk protein concentrate and micellar casein concentrate as affected by transglutaminase treatment. Food Hydrocoll. 2023, 137, 108367. [Google Scholar] [CrossRef]
- Heidebrecht, H.-J.; Kulozik, U. Fractionation of casein micelles and minor proteins by microfiltration in diafiltration mode. Study of the transmission and yield of the immunoglobulins IgG, IgA and IgM. Int. Dairy J. 2019, 93, 1–10. [Google Scholar] [CrossRef]
- Raak, N.; Corredig, M. Kinetic aspects of casein micelle cross-linking by transglutaminase at different volume fractions. Food Hydrocoll. 2022, 128, 107603. [Google Scholar] [CrossRef]
- Heidebrecht, H.-J.; Kainz, B.; Schopf, R.; Godl, K.; Karcier, Z.; Kulozik, U.; Förster, B. Isolation of biofunctional bovine immunoglobulin G from milk- and colostral whey with mixed-mode chromatography at lab and pilot scale. J. Chromatogr. A 2018, 1562, 59–68. [Google Scholar] [CrossRef]
- Weinberger, M.E.; Andlinger, D.J.; Kulozik, U. A novel approach for characterisation of stabilising bonds in milk protein deposit layers on microfiltration membranes. Int. Dairy J. 2021, 118, 105044. [Google Scholar] [CrossRef]
- Hiller, B.; Lorenzen, P.C. Surface hydrophobicity of physicochemically and enzymatically treated milk proteins in relation to techno-functional properties. J. Agric. Food Chem. 2008, 56, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, M.; Heidebrecht, H.-J.; Schiffer, S.; Dumpler, J.; Kulozik, U. Milk Protein Fractionation by Means of Spiral-Wound Microfiltration Membranes: Effect of the Pressure Adjustment Mode and Temperature on Flux and Protein Permeation. Foods 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.S.; Haribabu, M.; Harvie, D.J.; Dunstan, D.E.; Martin, G.J. Mechanisms of flux decline in skim milk ultrafiltration: A review. J. Membr. Sci. 2017, 523, 144–162. [Google Scholar] [CrossRef]
- Doudiès, F.; Arsène, A.-S.; Garnier-Lambrouin, F.; Famelart, M.-H.; Bouchoux, A.; Pignon, F.; Gésan-Guiziou, G. Major Role of Voluminosity in the Compressibility and Sol-Gel Transition of Casein Micelle Dispersions Concentrated at 7 °C and 20 °C. Foods 2019, 8, 652. [Google Scholar] [CrossRef]
- Reitmaier, M.; Barbosa, B.; Sigler, S.; Heidebrecht, H.-J.; Kulozik, U. Impact of different aqueous phases on casein micelles: Kinetics of physicochemical changes under variation of water hardness and diafiltration conditions. Int. Dairy J. 2020, 109, 104776. [Google Scholar] [CrossRef]
- Lu, Y.; McMahon, D.J.; Vollmer, A.H. Investigating cold gelation properties of recombined highly concentrated micellar casein concentrate and cream for use in cheese making. J. Dairy Sci. 2016, 99, 5132–5143. [Google Scholar] [CrossRef] [PubMed]
- Subhir, S.; McSweeney, P.L.; Murphy, E.G.; Tobin, J.T. The impact of temperature on flux, protein transmission and energy usage during microfiltration of skim milk using polymeric membranes. Int. Dairy J. 2023, 147, 105729. [Google Scholar] [CrossRef]
- Gésan-Guiziou, G.; Boyaval, E.; Daufin, G. Critical stability conditions in cross-flow microfiltration of skimmed milk: Transition to irreversible deposition. J. Membr. Sci. 1999, 158, 211–222. [Google Scholar] [CrossRef]
- Thomar, P.; Nicolai, T. Heat-induced gelation of casein micelles in aqueous suspensions at different pH. Colloids Surf. B Biointerfaces 2016, 146, 801–807. [Google Scholar] [CrossRef]
- Dunn, M.; Barbano, D.M.; Drake, M. Viscosity changes and gel formation during storage of liquid micellar casein concentrates. J. Dairy Sci. 2021, 104, 12263–12273. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Silva, J.V.C.; Nicolai, T.; Chassenieux, C.; Bovay, C.; Buczkowski, J.; Schmitt, C. Specific effect of calcium ions on thermal gelation of aqueous micellar casein suspensions. Colloids Surf. B Biointerfaces 2018, 163, 218–224. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D.J. Influence of NaCl and CaCl2 on Cold-Set Gelation of Heat-denatured Whey Protein. J. Food Sci. 2000, 65, 801–804. [Google Scholar] [CrossRef]
- Kharlamova, A.; Nicolai, T.; Chassenieux, C. Mixtures of sodium caseinate and whey protein aggregates: Viscosity and acid- or salt-induced gelation. Int. Dairy J. 2018, 86, 110–119. [Google Scholar] [CrossRef]
- Alting, A.C.; Hamer, R.J.; de Kruif, C.G.; Paques, M.; Visschers, R.W. Number of thiol groups rather than the size of the aggregates determines the hardness of cold set whey protein gels. Food Hydrocoll. 2003, 17, 469–479. [Google Scholar] [CrossRef]
- Jimenez-Lopez, A.; Leconte, N.; Garnier-Lambrouin, F.; Bouchoux, A.; Rousseau, F.; Gésan-Guiziou, G. Ionic strength dependence of skimmed milk microfiltration: Relations between filtration performance, deposit layer characteristics and colloidal properties of casein micelles. J. Membr. Sci. 2011, 369, 404–413. [Google Scholar] [CrossRef]
- Fu, L.F.; Dempsey, B.A. Modeling the effect of particle size and charge on the structure of the filter cake in ultrafiltration. J. Membr. Sci. 1998, 149, 221–240. [Google Scholar] [CrossRef]

 ) at 10 °C (A,C) and 50 °C (B,D), respectively, under sequential step-wise adjustment of ΔpTM (-).
) at 10 °C (A,C) and 50 °C (B,D), respectively, under sequential step-wise adjustment of ΔpTM (-).
 ) at 10 °C (A,C) and 50 °C (B,D), respectively, under sequential step-wise adjustment of ΔpTM (-).
) at 10 °C (A,C) and 50 °C (B,D), respectively, under sequential step-wise adjustment of ΔpTM (-).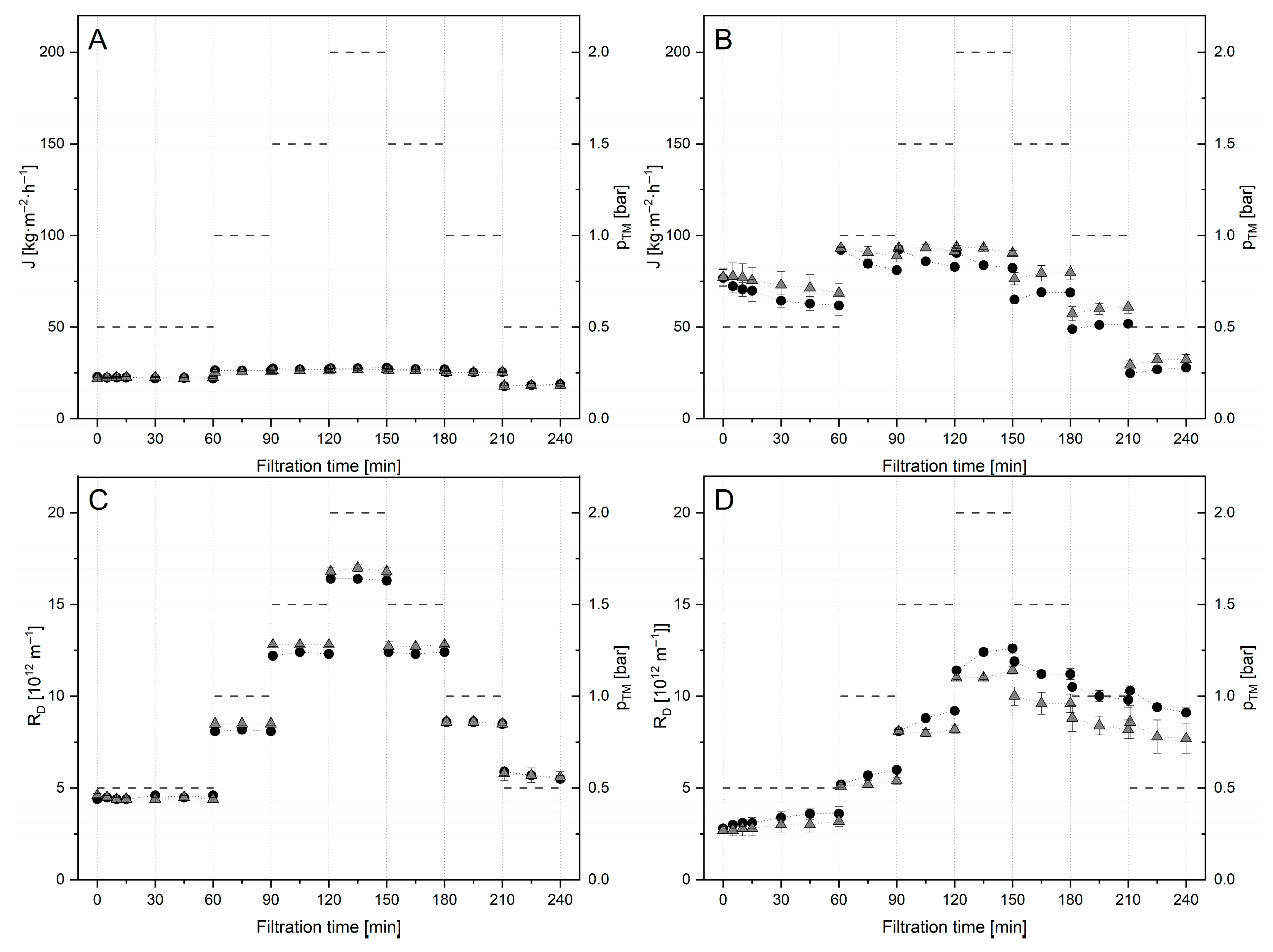
 ) at 10 °C (A) and 50 °C (B) applying sequential step-wise adjustment of ΔpTM.
) at 10 °C (A) and 50 °C (B) applying sequential step-wise adjustment of ΔpTM.
 ) at 10 °C (A) and 50 °C (B) applying sequential step-wise adjustment of ΔpTM.
) at 10 °C (A) and 50 °C (B) applying sequential step-wise adjustment of ΔpTM.
 ) at 10 °C (A,C) and 50 °C (B,D) under sequential step-wise adjustment of ΔpTM (-).
) at 10 °C (A,C) and 50 °C (B,D) under sequential step-wise adjustment of ΔpTM (-).
 ) at 10 °C (A,C) and 50 °C (B,D) under sequential step-wise adjustment of ΔpTM (-).
) at 10 °C (A,C) and 50 °C (B,D) under sequential step-wise adjustment of ΔpTM (-).
 ).
).
 ).
).
 ) and 0.5 bar ΔpTM.
) and 0.5 bar ΔpTM.
 ) and 0.5 bar ΔpTM.
) and 0.5 bar ΔpTM.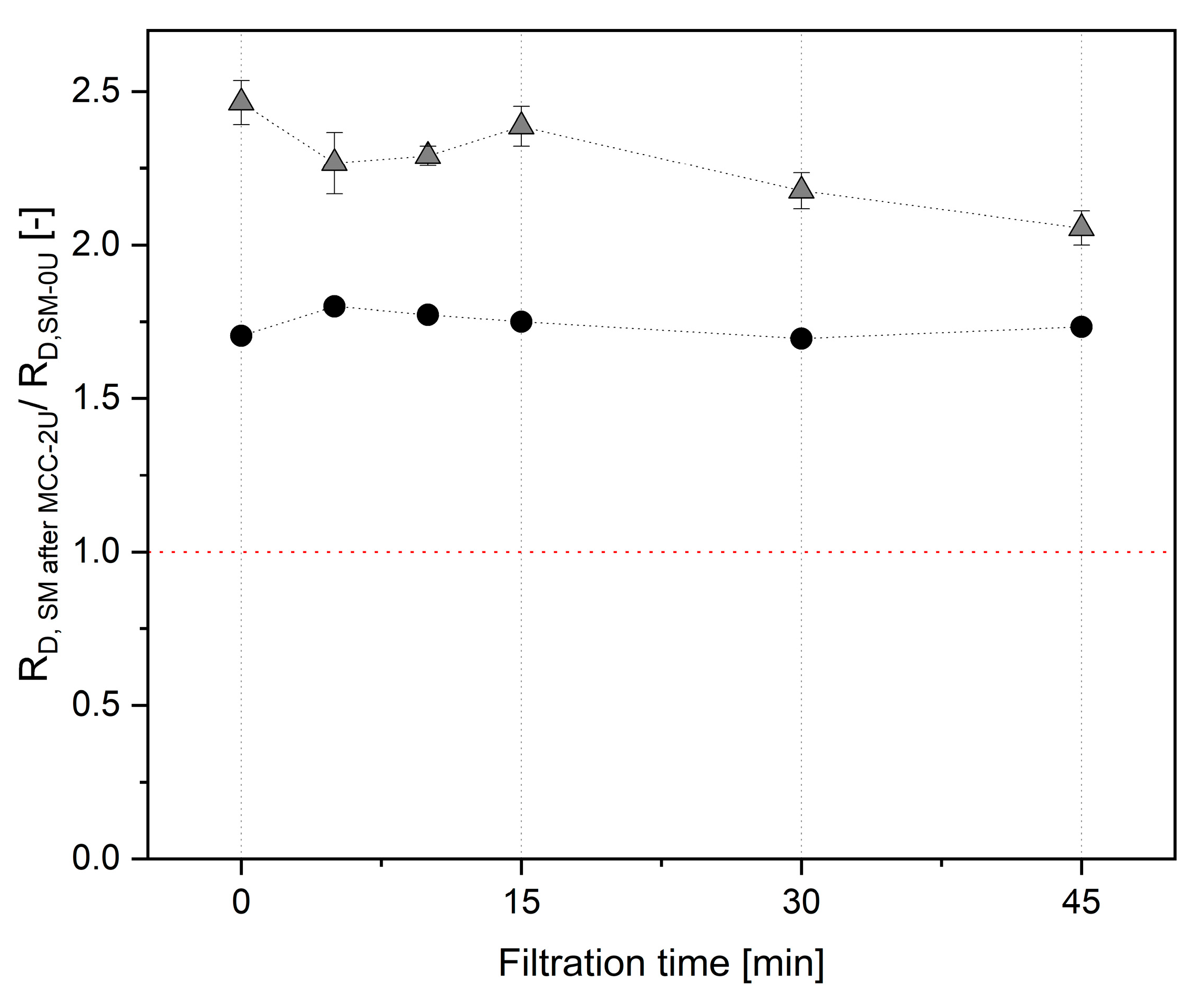
 ) and at 0.5 bar ΔpTM.
) and at 0.5 bar ΔpTM.
 ) and at 0.5 bar ΔpTM.
) and at 0.5 bar ΔpTM.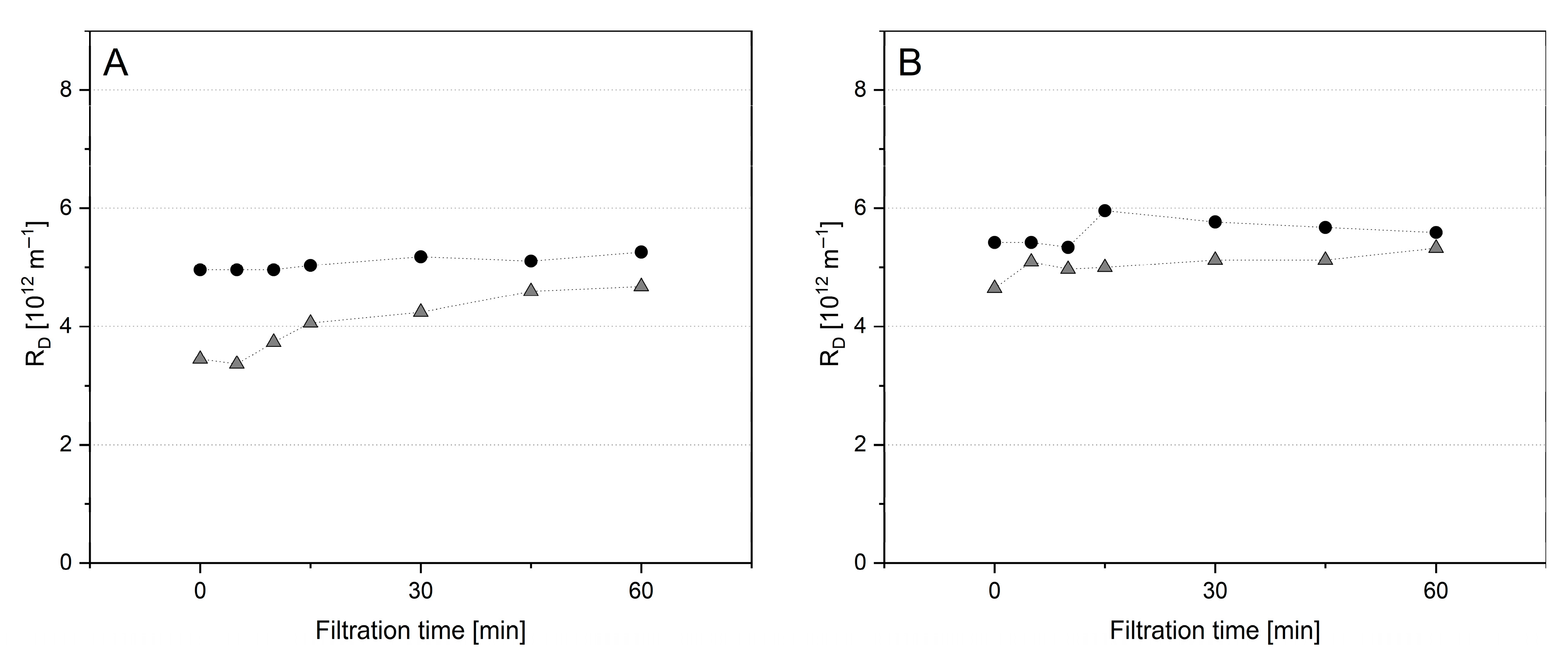
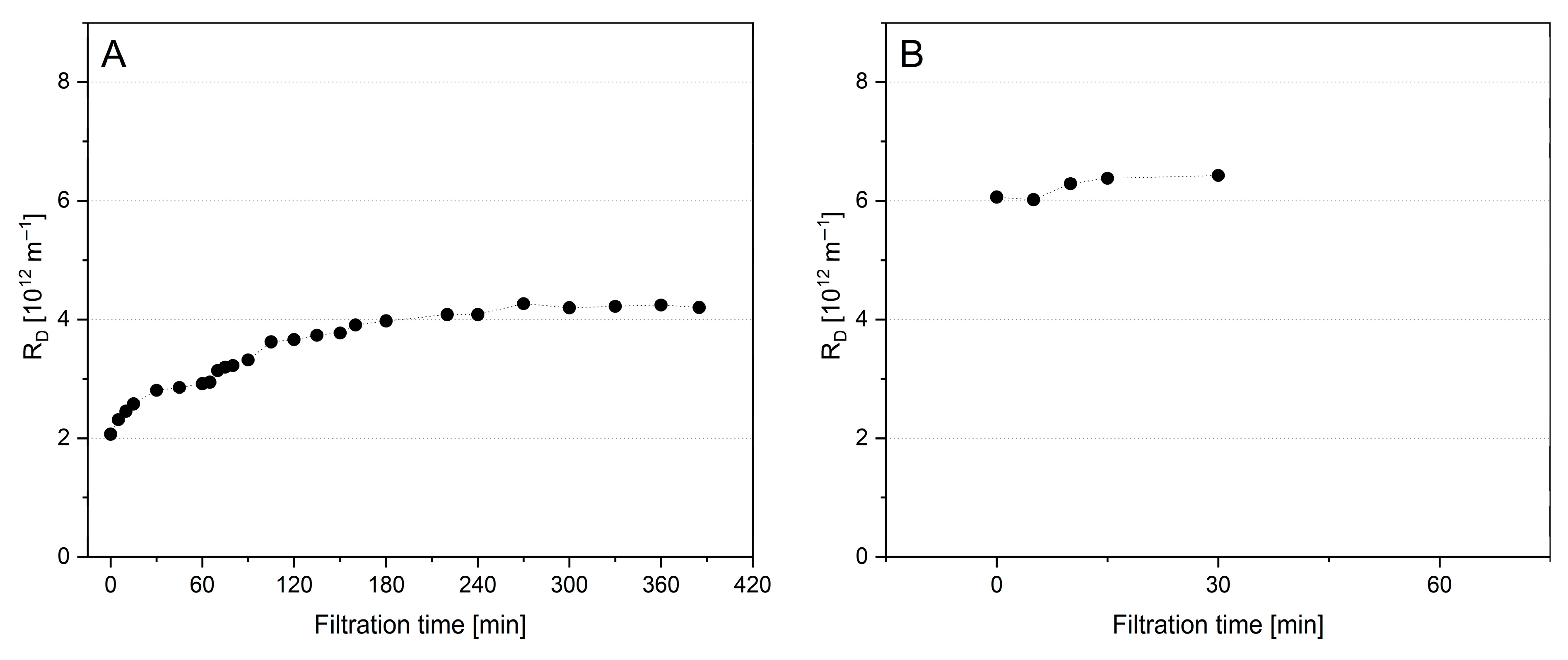
 ) at 10 °C, and Tgase-pretreated micellar casein (MCC-2U,
) at 10 °C, and Tgase-pretreated micellar casein (MCC-2U,  ) at 50 °C and related subsequent filtrations of untreated skim milk (SM-0U) (B) at 0.5 bar ΔpTM.
) at 50 °C and related subsequent filtrations of untreated skim milk (SM-0U) (B) at 0.5 bar ΔpTM.
 ) at 10 °C, and Tgase-pretreated micellar casein (MCC-2U,
) at 10 °C, and Tgase-pretreated micellar casein (MCC-2U,  ) at 50 °C and related subsequent filtrations of untreated skim milk (SM-0U) (B) at 0.5 bar ΔpTM.
) at 50 °C and related subsequent filtrations of untreated skim milk (SM-0U) (B) at 0.5 bar ΔpTM.
| Feed Material | κ−Casein (mg mL−1) | αs2-Casein (mg mL−1) | αs1-Casein (mg mL−1) | β-Casein (mg mL−1) | α-Lac * (mg mL−1) | β-Lg ** B (mg mL−1) | β-Lg ** A (mg mL−1) |
|---|---|---|---|---|---|---|---|
| SM-0U | 3.90 ± 0.15 | 4.90 ± 0.41 | 11.13 ± 0.38 | 11.92 ± 0.39 | 1.50 ± 0.16 | 1.36 ± 0.03 | 3.11 ± 0.10 |
| MCC-0U | 3.54 ± 0.09 | 3.64 ± 0.40 | 9.89 ± 0.35 | 10.97 ± 0.33 | 0.73 ± 0.11 | 0.58 ± 15 | 0.56 ± 0.02 |
| MPI-0U | 3.80 ± 0.04 | 3.71 ± 0.15 | 10.89 ± 0.17 | 11.86 ± 0.20 | 1.68 ± 0.02 | 1.99 ± 0.03 | 2.47 ± 0.03 |
| Tgase | κ-Casein | αs2-Casein | αs1-Casein | β-Casein | ||||
|---|---|---|---|---|---|---|---|---|
| Addition (U/gprot) | c (mg/mL) | Nativity (cxU/c0U) | c (mg/mL) | Nativity (cxU/c0U) | c (mg/mL) | Nativity (cxU/c0U) | c (mg/mL) | Nativity (cxU/c0U) |
| 0 | 3.94 | 1.00 | 5.36 | 1.00 | 11.4 | 1.00 | 11.91 | 1.00 |
| 1 | 2.72 | 0.69 | 4.84 | 0.90 | 10.27 | 0.90 | 9.48 | 0.80 |
| 2 | 1.61 | 0.41 | 4 | 0.75 | 8.57 | 0.75 | 7.49 | 0.63 |
| 3 | 1.24 | 0.31 | 3.85 | 0.72 | 8.66 | 0.76 | 7.12 | 0.60 |
| Tgase Addition | R D, 0.5 bar | R D, 3.0 bar | m D, 0.5 bar | m D, 3.0 bar | DM D, 0.5 bar | DM D, 3.0 bar |
|---|---|---|---|---|---|---|
| (U/gprot) | (1018 m−2) | (1018 m−2) | (g) | (g) | (g) | (g) |
| 0 | 6.95 ± 0.23 | 29.15 ± 1,54 | 0.219 ± 0.040 | 0.242 ± 0.019 | 0.083 ± 0.010 | 0.090 ± 0.005 |
| 1 | 7.39 ± 0.02 | 25.05 ± 0.25 | 0.243 ± 0.010 | 0.244 ± 0.001 | 0.094 ± 0.002 | 0.086 ± 0.14 |
| 2 | 6.15 ± 0.13 | 23.06 ± 1.42 | 0.228 ± 0.060 | 0.234 ± 0.018 | 0.081 ± 0.015 | 0.087 ± 0.02 |
| 3 | 6.02 ± 0.29 | 22.26 ± 2.41 | 0.256 ± 0.037 | 0.254 ± 0.026 | 0.094 ± 0.002 | 0.094 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitmaier, M.; Kulozik, U.; Först, P. Potential and Challenges of a Targeted Membrane Pre-Fouling: Process Performance of Milk Protein Fractionation After the Application of a Transglutaminase Treatment of Casein Micelles. Foods 2025, 14, 2682. https://doi.org/10.3390/foods14152682
Reitmaier M, Kulozik U, Först P. Potential and Challenges of a Targeted Membrane Pre-Fouling: Process Performance of Milk Protein Fractionation After the Application of a Transglutaminase Treatment of Casein Micelles. Foods. 2025; 14(15):2682. https://doi.org/10.3390/foods14152682
Chicago/Turabian StyleReitmaier, Michael, Ulrich Kulozik, and Petra Först. 2025. "Potential and Challenges of a Targeted Membrane Pre-Fouling: Process Performance of Milk Protein Fractionation After the Application of a Transglutaminase Treatment of Casein Micelles" Foods 14, no. 15: 2682. https://doi.org/10.3390/foods14152682
APA StyleReitmaier, M., Kulozik, U., & Först, P. (2025). Potential and Challenges of a Targeted Membrane Pre-Fouling: Process Performance of Milk Protein Fractionation After the Application of a Transglutaminase Treatment of Casein Micelles. Foods, 14(15), 2682. https://doi.org/10.3390/foods14152682








