Electrochemical Biosensors Driving Model Transformation for Food Testing
Abstract
1. Introduction
2. Electrochemical Sensing Principles
2.1. Mechanisms of Signal Output
2.2. Signal Acquisition Modalities
2.3. Signal Amplification Strategies
3. Nanostructure-Sensitized Electrochemical Assay
3.1. Metallic Nanomaterials for Sensitivity Enhancement

3.2. Carbon-Based Materials for Sensitivity Enhancement
3.3. Porous Frameworks for Sensitivity Enhancement
4. Recognition Elements in Electrochemical Sensing Toward Food Testing
4.1. Aptamer-Enhanced Selectivity in Electrochemical Sensing
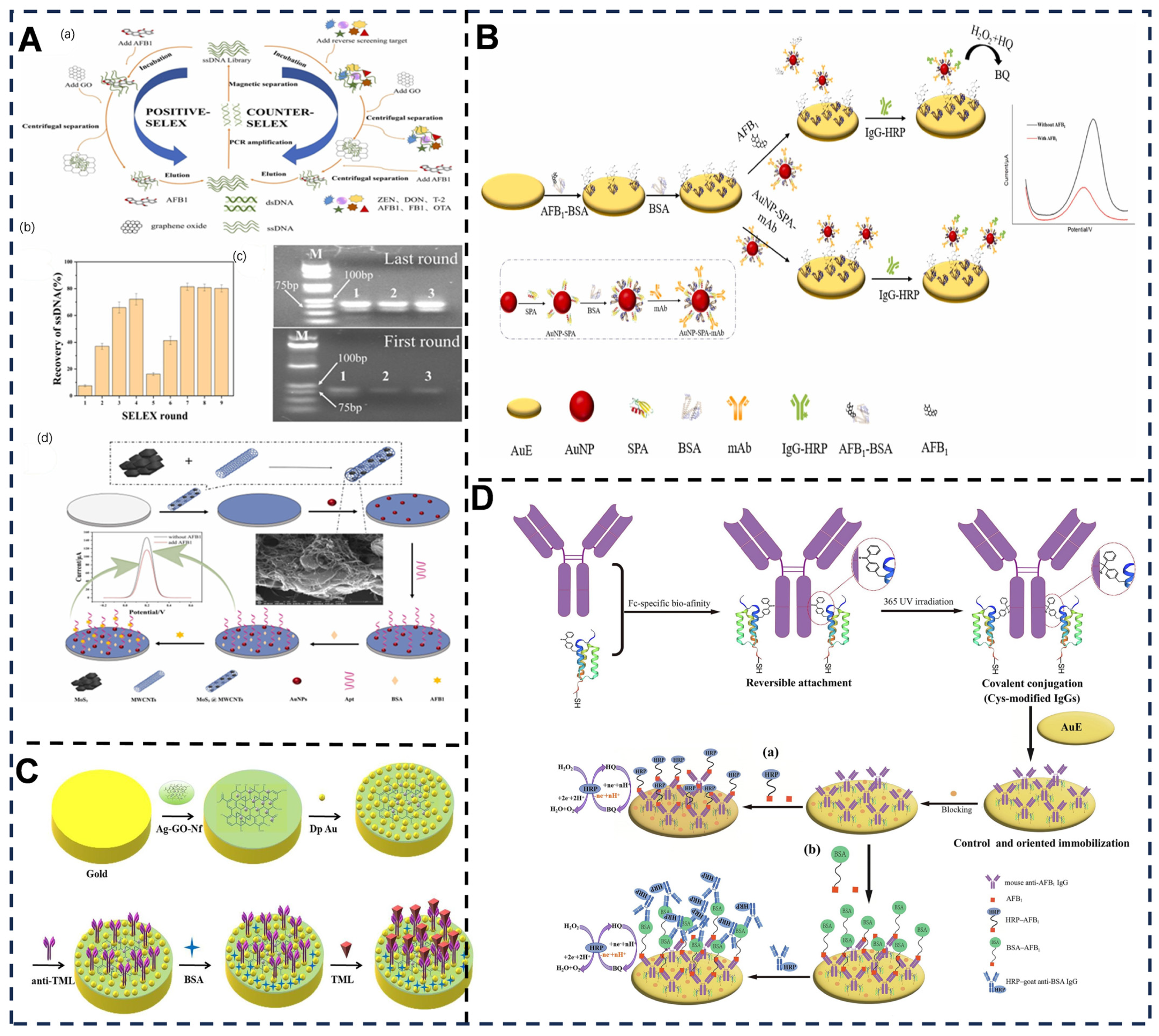
4.2. Antibody-Enhanced Selectivity in Electrochemical Sensing
4.3. Cell-Enhanced Selectivity in Electrochemical Sensing
4.4. Protein Scaffold-Enhanced Selectivity in Electrochemical Sensing
4.5. MIP-Enhanced Selectivity in Electrochemical Sensing
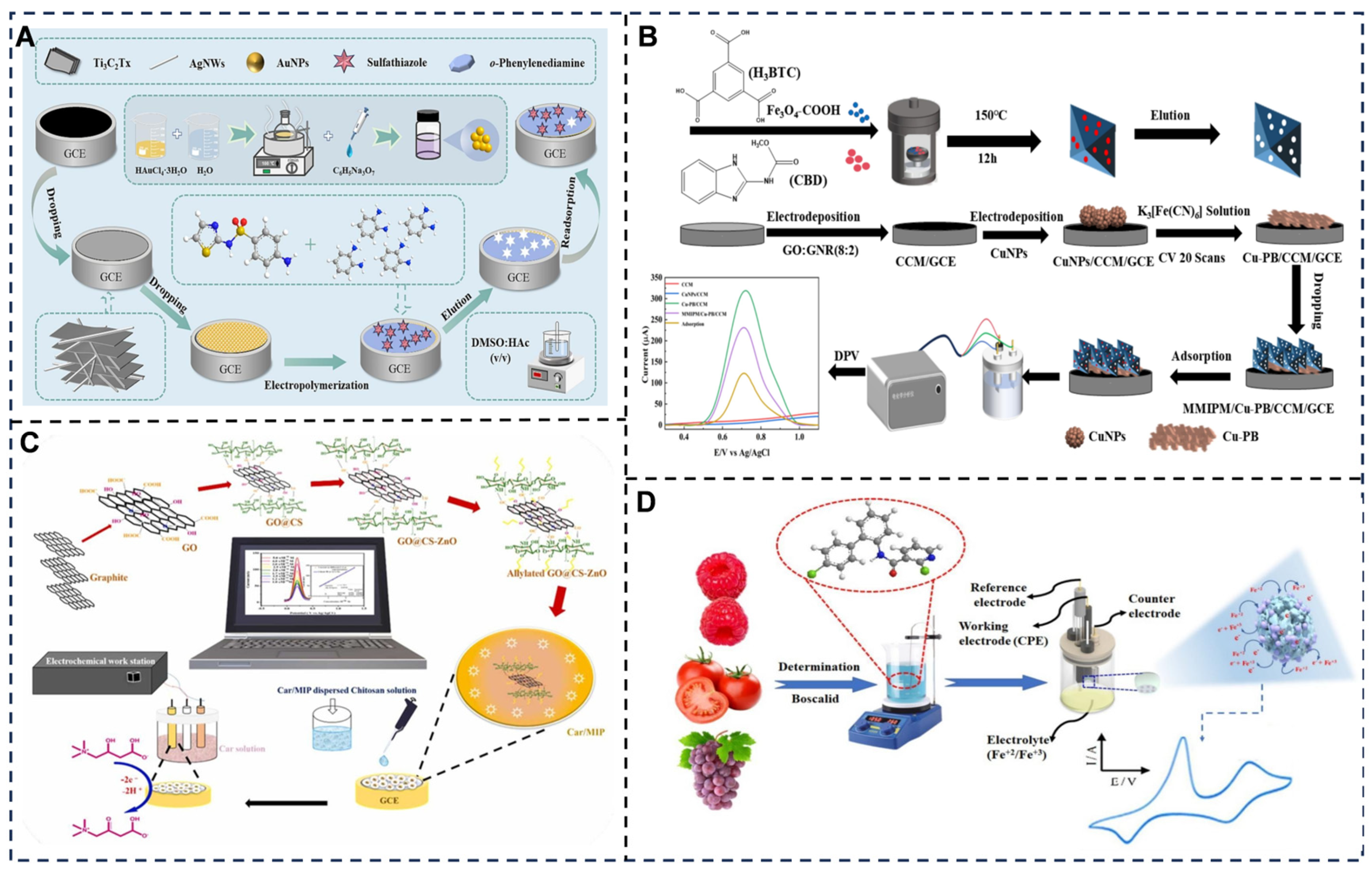
5. Electrochemical Sensing Toward Food Testing
5.1. Electrochemical Sensing Toward Food Contaminant

5.2. Electrochemical Sensing Toward Additive and Nutritional Analysis
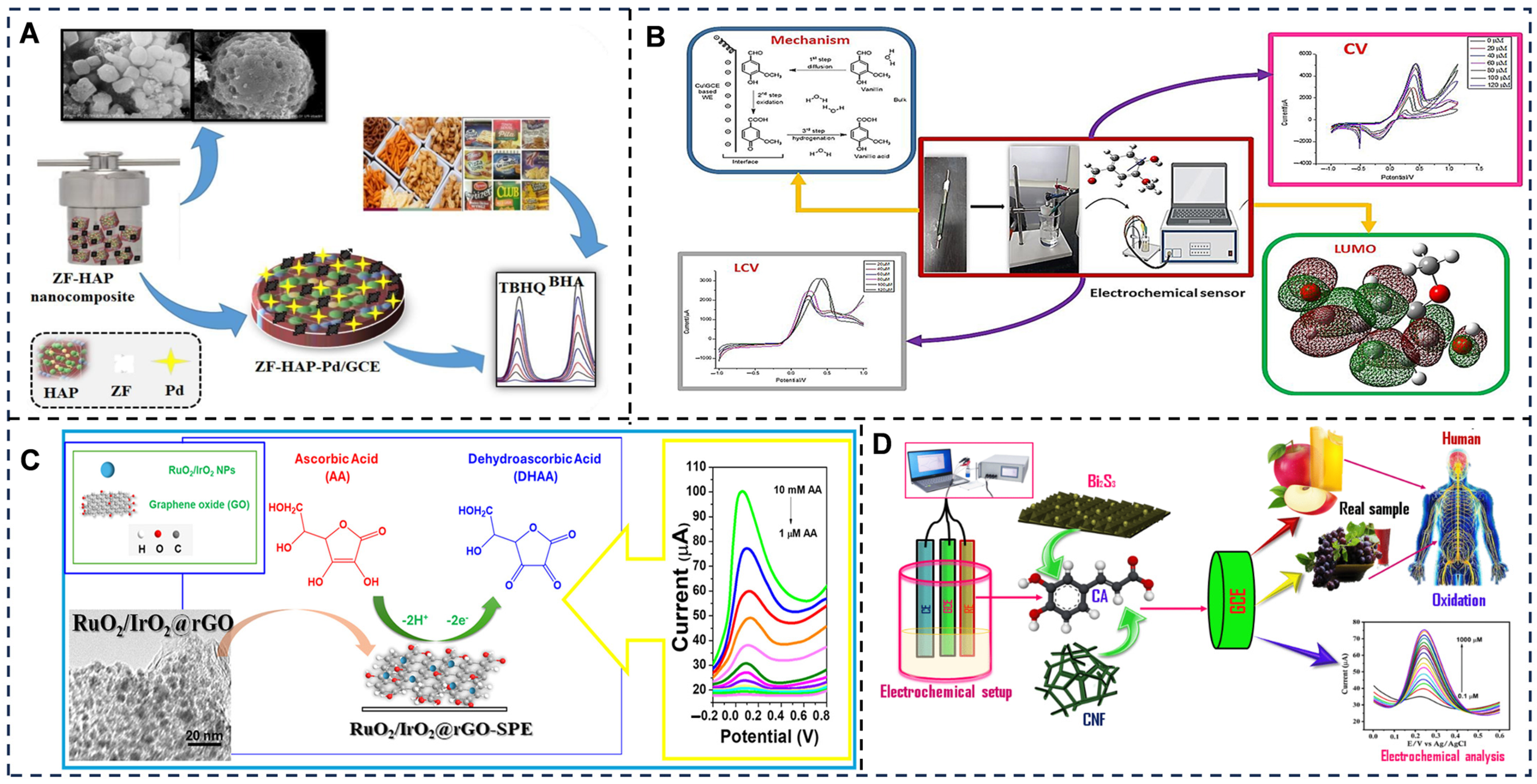
5.3. Electrochemical Sensing Toward Food Authenticity
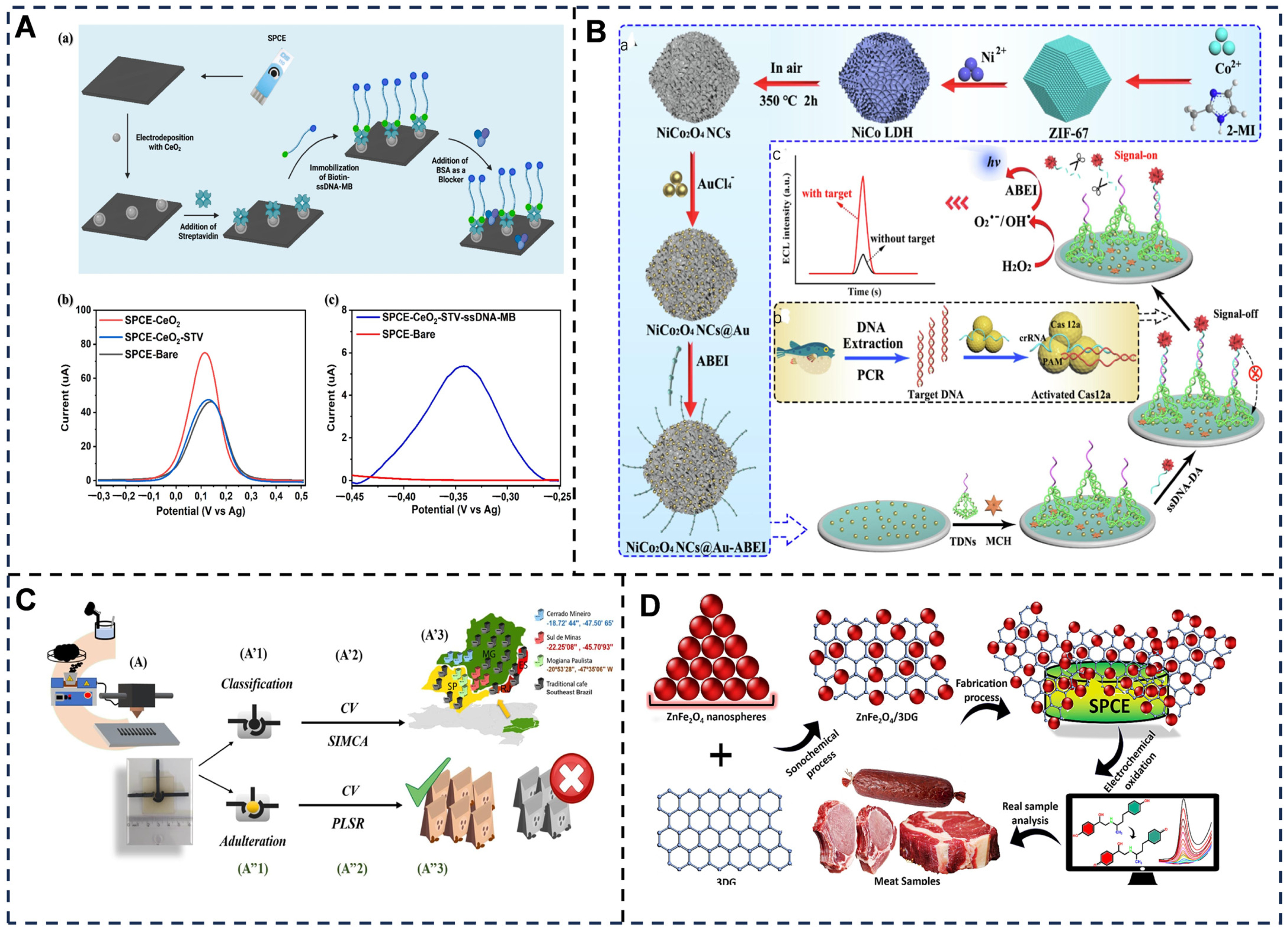
5.4. Multiplexing Capability of Electrochemical Biosensors
6. Current Achievements and Future Horizons
6.1. State of the Art in Electrochemical Biosensors
6.2. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiao, H.; Shi, Y.; Sun, J.; Lu, X.; Zhang, H.; Li, Y.; Fu, Y.; Guo, J.; Wang, Q.; Liu, H.; et al. Sawdust-derived cellulose nanofibrils with high biosafety for potential bioprinting. Ind. Crops Prod. 2024, 209, 118025. [Google Scholar] [CrossRef]
- Gao, S.P.; Zhou, R.Y.; Zhang, D.; Zheng, X.Y.; El-Seedi, H.R.; Chen, S.Q.; Niu, L.D.; Li, X.; Guo, Z.M.; Zou, X.B. Magnetic nanoparticle-based immunosensors and aptasensors for mycotoxin detection in foodstuffs: An update. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13266. [Google Scholar] [CrossRef]
- Xue, S.; Yin, L.; Gao, S.; Zhou, R.; Zhang, Y.; Jayan, H.; El-Seedi, H.R.; Zou, X.; Guo, Z. A film-like SERS aptasensor for sensitive detection of patulin based on GO@Au nanosheets. Food Chem. 2024, 441, 138364. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, R.; Chen, H.; Zhang, X. Recent Advances in Food Safety: Nanostructure-Sensitized Surface-Enhanced Raman Sensing. Foods 2025, 14, 1115. [Google Scholar] [CrossRef]
- Wu, X.; Yin, L.; Gao, S.; Zhou, R.; Zhang, Y.; Xue, S.; Jayan, H.; El-Seedi, H.R.; Zou, X.; Guo, Z. Core-satellite nanoassembly system with aptamer-conjugated Au@Ag nanoparticles for SERS detection of patulin in apples. Food Control 2024, 159, 110293. [Google Scholar] [CrossRef]
- Jayan, H.; Yin, L.; Xue, S.; Zou, X.; Guo, Z. Raman spectroscopy-based microfluidic platforms: A promising tool for detection of foodborne pathogens in food products. Food Res. Int. 2024, 180, 114052. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; De Filippis, A.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Beneficial Effects of Plant Extracts and Bioactive Food Components in Childhood Supplementation. Nutrients 2021, 13, 3157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.H.; Sheng, R.; Chen, Q.S. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Guo, Z.M.; Chen, P.; Yosri, N.; Chen, Q.S.; Elseedi, H.R.; Zou, X.B.; Yang, H.S. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Sharma, A.S.; Li, H.H.; Chen, Q.S. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 486–504. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.Y.; Wang, C.Q.; Tian, X.Y.; Chang, X.H.; Ren, Y.; Yu, S.S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef]
- Zhang, X.N.; Huang, X.Y.; Wang, Z.L.; Zhang, Y.; Huang, X.W.; Li, Z.H.; Daglia, M.; Xiao, J.B.; Shi, J.Y.; Zou, X.B. Bioinspired nanozyme enabling glucometer readout for portable monitoring of pesticide under resource-scarce environments. Chem. Eng. J. 2022, 429, 132243. [Google Scholar] [CrossRef]
- Kang, W.C.; Lin, H.; Jiang, H.; Adade, S.; Xue, Z.L.; Chen, Q.S. Advanced applications of chemo-responsive dyes based odor imaging technology for fast sensing food quality and safety: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5145–5172. [Google Scholar] [CrossRef]
- Wu, X.H.; Liang, X.Y.; Wang, Y.X.; Wu, B.; Sun, J. Non-Destructive Techniques for the Analysis and Evaluation of Meat Quality and Safety: A Review. Foods 2022, 11, 3713. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.B.; Zou, X.B.; Meng, G.W.; Wu, N.Q. Raman spectroscopy for food quality assurance and safety monitoring: A review. Curr. Opin. Food Sci. 2022, 47, 100910. [Google Scholar] [CrossRef]
- Lin, H.; Jiang, H.; Adade, S.; Kang, W.C.; Xue, Z.L.; Zareef, M.; Chen, Q.S. Overview of advanced technologies for volatile organic compounds measurement in food quality and safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 8226–8248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Li, C.Z.; Aliakbarlu, J.; Cui, H.Y.; Lin, L. Typical application of electrostatic layer-by-layer self-assembly technology in food safety assurance. Trends Food Sci. Technol. 2022, 129, 88–97. [Google Scholar] [CrossRef]
- Li, H.H.; Sheng, W.; Haruna, S.A.; Hassan, M.M.; Chen, Q.S. Recent advances in rare earth ion-doped upconversion nanomaterials: From design to their applications in food safety analysis. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3732–3764. [Google Scholar] [CrossRef]
- Fakhlaei, R.; Babadi, A.A.; Sun, C.J.; Ariffin, N.M.; Khatib, A.; Selamat, J.; Xiaobo, Z. Application, challenges and future prospects of recent nondestructive techniques based on the electromagnetic spectrum in food quality and safety. Food Chem. 2024, 441, 138402. [Google Scholar] [CrossRef]
- Yang, N.; Xie, L.L.; Pan, C.; Yuan, M.F.; Tao, Z.H.; Mao, H.P. A novel on-chip solution enabling rapid analysis of melamine and chloramphenicol in milk by smartphones. J. Food Process Eng. 2019, 42, e12976. [Google Scholar] [CrossRef]
- Shoaib, M.; Li, H.H.; Khan, I.M.; Zareef, M.; Hassan, M.M.; Niazi, S.; Chen, Q.S. Emerging MXenes-based aptasensors: A paradigm shift in food safety detection. Trends Food Sci. Technol. 2024, 151, 104635. [Google Scholar] [CrossRef]
- Long, L.L.; Cao, S.Y.; Jin, B.; Yuan, X.Q.; Han, Y.Y.; Wang, K. Construction of a Novel Fluorescent Probe for On-site Measuring Hydrogen Sulfide Levels in Food Samples. Food Anal. Methods 2019, 12, 852–858. [Google Scholar] [CrossRef]
- Li, Y.Q.; Luo, S.L.; Sun, L.; Kong, D.Z.; Sheng, J.G.; Wang, K.; Dong, C.W. A Green, Simple, and Rapid Detection for Amaranth in Candy Samples Based on the Fluorescence Quenching of Nitrogen-Doped Graphene Quantum Dots. Food Anal. Methods 2019, 12, 1658–1665. [Google Scholar] [CrossRef]
- Zhang, J.N.; Zhang, J.J.; Zhang, X.A.; Huang, X.W.; Shi, J.Y.; Sobhy, R.; Khalifa, I.; Zou, X.B. Ammonia-Responsive Colorimetric Film of Phytochemical Formulation (Alizarin) Grafted onto ZIF-8 Carrier with Poly(vinyl alcohol) and Sodium Alginate for Beef Freshness Monitoring. J. Agric. Food Chem. 2024, 72, 11706–11715. [Google Scholar] [CrossRef]
- Yuan, Z.; Huang, X.; Zhang, X.; Gao, S.; Chen, H.; Li, Z.; El-Mesery, H.S.; Shi, J.; Zou, X. Unveiling rheological behavior of hydrogels toward Magic 3D printing patterns. Food Hydrocoll. 2025, 168, 111505. [Google Scholar] [CrossRef]
- Gao, S.; Huang, X.; Zhang, X.; Yuan, Z.; Chen, H.; Li, Z.; El-Mesery, H.S.; Shi, J.; Zou, X. Empowering protein single-molecule sequencing: Nanopore technology toward sensing gene sequences. Anal. Methods 2025, 17, 3902–3924. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.T.; Liu, Z.J.; Fu, X.L.; Du, D.L. High-performance liquid chromatography for the sensitive zearalenone determination by the automated immunomagnetic beads purifier for one-step sample pre-treatment. Eur. Food Res. Technol. 2022, 248, 109–117. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, B.; Yu, M.; Han, J.; Wang, Y.; Tan, Z.J.; Yan, Y.S. Simultaneous separation/enrichment and detection of trace ciprofloxacin and lomefloxacin in food samples using thermosensitive smart polymers aqueous two-phase flotation system combined with HPLC. Food Chem. 2016, 210, 1–8. [Google Scholar] [CrossRef]
- Shao, Y.X.; Chen, G.H.; Fang, R.; Zhang, L.; Yi, L.X.; Meng, H.L. Analysis of Six β-Lactam Residues in Milk and Egg by Micellar Electrokinetic Chromatography with Large-Volume Sample Stacking and Polarity Switching. J. Agric. Food Chem. 2016, 64, 3456–3461. [Google Scholar] [CrossRef]
- Lv, R.Q.; Huang, X.Y.; Aheto, J.H.; Mu, L.J.; Tian, X.Y. Analysis of fish spoilage by gas chromatography-mass spectrometry and electronic olfaction bionic system. J. Food Safety 2018, 38, e12557. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zou, X.B.; Huang, X.W.; Shi, J.Y.; Mariod, A.A. Discrimination of honeys using colorimetric sensor arrays, sensory analysis and gas chromatography techniques. Food Chem. 2016, 206, 37–43. [Google Scholar] [CrossRef]
- Li, H.H.; Geng, W.H.; Haruna, S.A.; Zhou, C.G.; Wang, Y.; Ouyang, Q.; Chen, Q.S. Identification of characteristic volatiles and metabolomic pathway during pork storage using HS-SPME-GC/MS coupled with multivariate analysis. Food Chem. 2022, 373, 131431. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.Y.; Aheto, J.H.; Osae, R. Application of electronic nose as a non-invasive technique for odor fingerprinting and detection of bacterial foodborne pathogens: A review. J. Food Sci. Technol.-Mysore 2020, 57, 1977–1990. [Google Scholar] [CrossRef]
- Kutsanedzie, F.Y.H.; Guo, Z.M.; Chen, Q.S. Advances in Nondestructive Methods for Meat Quality and Safety Monitoring. Food Rev. Int. 2019, 35, 536–562. [Google Scholar] [CrossRef]
- Zareef, M.; Chen, Q.S.; Hassan, M.M.; Arslan, M.; Hashim, M.M.; Ahmad, W.; Kutsanedzie, F.Y.H.; Agyekum, A.A. An Overview on the Applications of Typical Non-linear Algorithms Coupled with NIR Spectroscopy in Food Analysis. Food Eng. Rev. 2020, 12, 173–190. [Google Scholar] [CrossRef]
- Ouyang, Q.; Rong, Y.N.; Wu, J.Q.; Wang, Z.; Lin, H.; Chen, Q.S. Application of colorimetric sensor array combined with visible near-infrared spectroscopy for the matcha classification. Food Chem. 2023, 420, 136078. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jiang, H.; Lin, J.J.; Chen, Q.S.; Ali, S.; Teng, S.W.; Zuo, M. Rice Freshness Identification Based on Visible Near-Infrared Spectroscopy and Colorimetric Sensor Array. Food Anal. Methods 2021, 14, 1305–1314. [Google Scholar] [CrossRef]
- Sun, J.; Nirere, A.; Dusabe, K.D.; Zhong, Y.H.; Adrien, G. Rapid and nondestructive watermelon (Citrullus lanatus) seed viability detection based on visible near-infrared hyperspectral imaging technology and machine learning algorithms. J. Food Sci. 2024, 89, 4403–4418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.A.; Wang, Z.L.; Huang, X.W.; Huang, Q.L.; Wen, Y.B.; Li, B.; Holmes, M.; Shi, J.Y.; Zou, X.B. Uniform stain pattern of robust MOF-mediated probe for flexible paper-based colorimetric sensing toward environmental pesticide exposure. Chem. Eng. J. 2023, 451, 138928. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Z.Y.; Adade, S.; Yang, W.J.; Chen, Q.S. Detection of Maize Mold Based on a Nanocomposite Colorimetric Sensor Array under Different Substrates. J. Agric. Food Chem. 2024, 72, 11164–11173. [Google Scholar] [CrossRef]
- Chen, X.D.; Zhao, C.L.; Zhao, Q.W.; Yang, Y.F.; Yang, S.X.; Zhang, R.M.; Wang, Y.Q.; Wang, K.; Qian, J.; Long, L.L. Construction of a Colorimetric and Near-Infrared Ratiometric Fluorescent Sensor and Portable Sensing System for On-Site Quantitative Measurement of Sulfite in Food. Foods 2024, 13, 1758. [Google Scholar] [CrossRef]
- Xu, F.Y.; Huang, X.Y.; Tian, X.Y.; Yu, S.S.; Zhang, X.R.; Zareef, M. Application of hyperspectral imaging and colorimetric sensor array coupled with multivariate analysis for quality detection during salted duck eggs processing. J. Food Process Eng. 2024, 47, e14589. [Google Scholar] [CrossRef]
- Rong, Y.W.; Hassan, M.M.; Ouyang, Q.; Chen, Q.S. Lanthanide ion (Ln3+)-based upconversion sensor for quantification of food contaminants: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3531–3578. [Google Scholar] [CrossRef]
- Sharma, A.S.; Ali, S.; Sabarinathan, D.; Murugavelu, M.; Li, H.H.; Chen, Q.S. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar] [CrossRef]
- Ding, X.D.; Ahmad, W.; Rong, Y.W.; Wu, J.Z.; Ouyang, Q.; Chen, Q.S. A dual-mode fluorescence and colorimetric sensing platform for efficient detection of ofloxacin in aquatic products using iron alkoxide nanozyme. Food Chem. 2024, 442, 138417. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, X.D.; Xu, Y.W.; Liu, C.; Zou, X.B.; Li, Z.H.; Shi, J.Y.; Huang, X.W. Facile fabrication of three-dimensional gold nanodendrites decorated by silver nanoparticles as hybrid SERS-active substrate for the detection of food contaminants. Food Control 2021, 122, 107772. [Google Scholar] [CrossRef]
- Hassan, M.M.; Ahmad, W.; Zareef, M.; Rong, Y.W.; Xu, Y.; Jiao, T.H.; He, P.H.; Li, H.H.; Chen, Q.S. Rapid detection of mercury in food via rhodamine 6G signal using surface-enhanced Raman scattering coupled multivariate calibration. Food Chem. 2021, 358, 129844. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Chen, Q.S.; Belwal, T.; Lin, X.Y.; Luo, Z.S. Insights into chemometric algorithms for quality attributes and hazards detection in foodstuffs using Raman/surface enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2476–2507. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; He, P.H.; Zareef, M.; Li, H.H.; Chen, Q.S. Rapid detection and prediction of chloramphenicol in food employing label-free HAu/Ag NFs-SERS sensor coupled multivariate calibration. Food Chem. 2022, 374, 131765. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Xu, Y.; Zareef, M.; Li, H.H.; Rong, Y.W.; Chen, Q.S. Recent advances of nanomaterial-based optical sensor for the detection of benzimidazole fungicides in food: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 2851–2872. [Google Scholar] [CrossRef]
- Arslan, M.; Zareef, M.; Tahir, H.E.; Guo, Z.; Rakha, A.; Hu, X.T.; Shi, J.Y.; Li, Z.H.; Zou, X.B.; Khan, M.R. Discrimination of rice varieties using smartphone-based colorimetric sensor arrays and gas chromatography techniques. Food Chem. 2022, 368, 130783. [Google Scholar] [CrossRef]
- Tahir, H.E.; Mariod, A.A.; Hashim, S.B.H.; Arslan, M.; Mahunu, G.K.; Huang, X.W.; Li, Z.H.; Abdalla, I.I.H.; Zou, X.B. Classification of Black Mahlab seeds (Monechma ciliatum) using GC-MS and FT-NIR and simultaneous prediction of their major volatile compounds using chemometrics. Food Chem. 2023, 408, 134948. [Google Scholar] [CrossRef]
- Pang, J.; Xiao, Q.; Yan, H.; Cao, Y.; Miao, J.; Wang, S.; Li, X.; Li, H.; Cheng, Z. Bovine Lactoferrin Quantification in Dairy Products by a Simple Immunoaffinity Magnetic Purification Method Coupled with High-Performance Liquid Chromatography with Fluorescence Detection. J. Agric. Food Chem. 2020, 68, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.S.; Marimuthu, M.; Varghese, A.W.; Wu, J.Z.; Xu, J.; Luo, X.F.; Devaraj, S.; Lan, Y.; Li, H.H.; Chen, Q.S. A review of biomolecules conjugated lanthanide up-conversion nanoparticles-based fluorescence probes in food safety and quality monitoring applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 6129–6159. [Google Scholar] [CrossRef]
- Hassan, M.M.; Xu, Y.; He, P.H.; Zareef, M.; Li, H.H.; Chen, Q.S. Simultaneous determination of benzimidazole fungicides in food using signal optimized label-free HAu/Ag NS-SERS sensor. Food Chem. 2022, 397, 133755. [Google Scholar] [CrossRef]
- Chen, P.; Yin, L.M.; El-Seedi, H.R.; Zou, X.B.; Guo, Z.M. Green reduction of silver nanoparticles for cadmium detection in food using surface-enhanced Raman spectroscopy coupled multivariate calibration. Food Chem. 2022, 394, 133481. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Ezeorba, T.P.C.; Okoye, C.O.; Chen, Y.; Mao, G.H.; Feng, W.W.; Wu, X.Y. Analytical detection methods for azo dyes: A focus on comparative limitations and prospects of bio-sensing and electrochemical nano-detection. J. Food Compos. Anal. 2022, 114, 104778. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Shi, Y.; Li, Y.; Tan, W.; Zhang, X.; Zou, Y.; Wang, T.; Shi, J.; Zou, X. Electrochemical Sensing toward Noninvasive Evaluation of High-Starch Food Digestion via Point-of-Use Monitoring Glucose Level in Saliva. J. Agric. Food Chem. 2025, 73, 11422–11434. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhou, Y.; Wang, J.L.; Huang, X.W.; El-Mesery, H.S.; Shi, Y.Q.; Zou, Y.C.; Li, Z.H.; Li, Y.H.; Shi, J.Y.; et al. Simple-easy electrochemical sensing mode assisted with integrative carbon-based gel electrolyte for in-situ monitoring of plant hormone indole acetic acid. Food Chem. 2025, 467, 142342. [Google Scholar] [CrossRef]
- Chen, H.L.; Wang, J.L.; Zhang, W.W.; Li, Y.H.; Zhang, X.N.; Huang, X.W.; Shi, Y.Q.; Zou, Y.C.; Li, Z.H.; Shi, J.Y.; et al. Highly catalytic Ce-based MOF for powering electrochemical aptasensing toward evaluating dissolution rate of microelement copper from tea-leaves. J. Food Compos. Anal. 2025, 140, 107266. [Google Scholar] [CrossRef]
- Lu, W.J.; Dai, X.L.; Yang, R.Q.; Liu, Z.Y.; Chen, H.L.; Zhang, Y.F.; Zhang, X.N. Fenton-like catalytic MOFs driving electrochemical aptasensing toward tracking lead pollution in pomegranate fruit. Food Control 2025, 169, 111006. [Google Scholar] [CrossRef]
- Zhang, X.N.; Huang, X.Y.; Xu, Y.W.; Wang, X.; Guo, Z.M.; Huang, X.W.; Li, Z.H.; Shi, J.Y.; Zou, X.B. Single-step electrochemical sensing of ppt-level lead in leaf vegetables based on peroxidase-mimicking metal-organic framework. Biosens. Bioelectron. 2020, 168, 112544. [Google Scholar] [CrossRef]
- Wu, H.; Xie, R.; Hao, Y.; Pang, J.; Gao, H.; Qu, F.; Tian, M.; Guo, C.; Mao, B.; Chai, F. Portable smartphone-integrated AuAg nanoclusters electrospun membranes for multivariate fluorescent sensing of Hg2+, Cu2+ and L-histidine in water and food samples. Food Chem. 2023, 418, 135961. [Google Scholar] [CrossRef]
- Su, X.; Yang, J.; Li, Q.; Yang, X.; Fang, R.-Y.; Zhang, Y.; Chen, L.; Chen, F.; Tian, Y.; Shen, Y.-D.; et al. Development of Highly Sensitive Immunochromatography Using Time-Resolved Fluorescence Microspheres for Amantadine Detection. J. Agric. Food Chem. 2024, 72, 21794–21803. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, S.; Yuan, Z.; Yang, R.; Zhang, X.; El-Mesery, H.S.; Dai, X.; Lu, W.; Xu, R. Exploring Formation and Control of Hazards in Thermal Processing for Food Safety. Foods 2025, 14, 2168. [Google Scholar] [CrossRef]
- Yan, H.; He, B.; Zhao, R.; Ren, W.; Suo, Z.; Xu, Y.; Xie, D.; Zhao, W.; Wei, M.; Jin, H. Electrochemical aptasensor based on CRISPR/Cas12a-mediated and DNAzyme-assisted cascade dual-enzyme transformation strategy for zearalenone detection. Chem. Eng. J. 2024, 493, 152431. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zhou, Y.; Wang, H.; Huang, X.W.; Shi, Y.Q.; Zou, Y.C.; Hu, X.T.; Li, Z.H.; Shi, J.Y.; Zou, X.B. Energy difference-driven ROS reduction for electrochemical tracking crop growth sensitized with electron-migration nanostructures. Anal. Chim. Acta 2024, 1304, 342515. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Zou, X.B.; Xiao, J.B.; Mahunu, G.K.; Shi, J.Y.; Xu, J.L.; Sun, D.W. Recent Progress in Rapid Analyses of Vitamins, Phenolic, and Volatile Compounds in Foods Using Vibrational Spectroscopy Combined with Chemometrics: A Review. Food Anal. Methods 2019, 12, 2361–2382. [Google Scholar] [CrossRef]
- Fan, X.B.; Peng, L.L.; Wang, X.H.; Han, S.Q.; Yang, L.Z.; Wang, H.L.; Hao, C. Efficient capture of lead ion and methylene blue by functionalized biomass carbon-based adsorbent for wastewater treatment. Ind. Crops Prod. 2022, 183, 114966. [Google Scholar] [CrossRef]
- Tong, Y.Q.; Wang, S.Y.; Han, K.G.; Song, X.X.; Zhang, W.; Ye, Y.X.; Ren, X.D. Development of a Novel Metal Grating and Its Applications of Terahertz Spectroscopic Detection of CuSO4 in Fruit. Food Anal. Methods 2021, 14, 1590–1599. [Google Scholar] [CrossRef]
- Mu, W.Y.; Huang, P.Z.; Chen, Q.Y.; Wang, W. Determination of melamine and melamine-Cu(II) complexes in milk using a DNA-Ag hydrocolloid as the sensor. Food Chem. 2020, 311, 125889. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, P.; Sudalaimani, S.; Giribabu, K.; Suresh, C. Nanomolar Detection of Vitamin C in Artificial Urine using a Glassy Carbon Electrode Modified with Molybdenum Disulfide. J. Electrochem. Soc. 2021, 168, 087507. [Google Scholar] [CrossRef]
- Zeng, K.; Chen, B.; Li, Y.X.; Meng, H.; Wu, Q.Y.; Yang, J.; Liang, H.F. Gold nanoparticle-carbon nanotube nanohybrids with peroxidase-like activity for the highly-sensitive immunoassay of kanamycin in milk. Int. J. Food Sci. Technol. 2022, 57, 6028–6037. [Google Scholar] [CrossRef]
- Tang, C.Y.; He, Y.; Yuan, B.Z.; Li, L.B.; Luo, L.J.; You, T.Y. Simultaneous detection of multiple mycotoxins in agricultural products: Recent advances in optical and electrochemical sensing methods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70062. [Google Scholar] [CrossRef]
- Liu, G.X.; Liu, Z.Y.; Sun, Y.M.; Sun, M.N.; Duan, J.S.; Tian, Y.; Du, D.L.; Li, M. Cascade Amplifying Electrochemical Bioanalysis for Zearalenone Detection in Agricultural Products: Utilizing a Glucose-Fenton-HQ System on Bimetallic-ZIF@CNP Nanocomposites. Foods 2024, 13, 3192. [Google Scholar] [CrossRef]
- Shouket, S.; Khurshid, S.; Khan, J.; Batool, R.; Sarwar, A.; Aziz, T.; Alhomrani, M.; Alamri, A.S.; Sameeh, M.Y.; Filimban, F.Z. Enhancement of shelf-life of food items via immobilized enzyme nanoparticles on varied supports. A sustainable approach towards food safety and sustainability. Food Res. Int. 2023, 169, 112940. [Google Scholar] [CrossRef]
- Zhang, X.N.; Wang, Z.L.; Huang, X.W.; Hu, X.T.; Li, Y.X.; Zhou, Y.; Wang, X.; Zhang, R.J.; Wei, X.O.; Zhai, X.D.; et al. H-Bond Modulation Mechanism for Moisture-driven Bacteriostat Evolved from Phytochemical Formulation. Adv. Funct. Mater. 2024, 34, 2312053. [Google Scholar] [CrossRef]
- Huang, X.W.; Huang, C.Y.; Zhou, L.L.; Hou, G.Y.; Sun, J.Y.; Zhang, X.A.; Zou, X.B. Allosteric switch for electrochemical aptasensor toward heavy metals pollution of Lentinus edodes sensitized with porphyrinic metal-organic frameworks. Anal. Chim. Acta 2023, 1278, 341752. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhou, Y.; Huang, X.Y.; Hu, X.T.; Huang, X.W.; Yin, L.M.; Huang, Q.L.; Wen, Y.B.; Li, B.; Shi, J.Y.; et al. Switchable aptamer-fueled colorimetric sensing toward agricultural fipronil exposure sensitized with affiliative metal-organic framework. Food Chem. 2023, 407, 135115. [Google Scholar] [CrossRef]
- Rong, J.H.; Zhou, Z.J.; Wang, Y.; Han, J.; Li, C.M.; Zhang, W.L.; Ni, L. Immobilization of Horseradish Peroxidase on Multi-Armed Magnetic Graphene Oxide Composite: Improvement of Loading Amount and Catalytic Activity. Food Technol. Biotechnol. 2019, 57, 260–271. [Google Scholar] [CrossRef]
- Xu, Y.W.; Zhang, W.; Shi, J.Y.; Zou, X.B.; Li, Y.X.; Tahir, H.E.; Huang, X.W.; Li, Z.H.; Zhai, X.D.; Hu, X.T. Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in fish. Food Chem. 2017, 237, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.X.; Liu, D.; Li, Y.Y.; Chen, T.; You, T.Y. Label-free ratiometric homogeneous electrochemical aptasensor based on hybridization chain reaction for facile and rapid detection of aflatoxin B1 in cereal crops. Food Chem. 2022, 373, 131443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Jiang, Y.J.; Zhu, M.C.; Xu, Y.W.; Guo, Z.M.; Shi, J.Y.; Han, E.; Zou, X.B.; Wang, D. Electrochemical DNA sensor for inorganic mercury(II) ion at attomolar level in dairy product using Cu(II)-anchored metal-organic framework as mimetic catalyst. Chem. Eng. J. 2020, 383, 123182. [Google Scholar] [CrossRef]
- Luo, L.J.; Liu, X.H.; Ma, S.; Li, L.B.; You, T.Y. Quantification of zearalenone in mildewing cereal crops using an innovative photoelectrochemical aptamer sensing strategy based on ZnO-NGQDs composites. Food Chem. 2020, 322, 126778. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.W.; Li, Y.H.; Li, Y.X.; Li, Z.H.; Zhang, W.; Zou, X.B.; Shi, J.Y.; Huang, X.W.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Qin, C.C.; Guo, W.L.; Liu, Y.; Liu, Z.C.; Qiu, J.; Peng, J.B. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles-Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Zhang, X.A.; Huang, C.Y.; Jiang, Y.J.; Jiang, Y.X.; Shen, J.Z.; Han, E. Structure-Switching Electrochemical Aptasensor for Single-Step and Specific Detection of Trace Mercury in Dairy Products. J. Agric. Food Chem. 2018, 66, 10106–10112. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Liu, F.H.; Zou, X.B.; Xu, Y.W.; Xu, X.C. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef]
- Sun, X.X.; Zhang, D.; Zhao, L.; Shi, B.L.; Xiao, J.B.; Shi, J.Y.; Zou, X.B. Development of differential pulse voltammetric method for rapid quantification of total hydroxyl-sanshools in Sichuan Pepper. Lwt-Food Sci. Technol. 2020, 130, 109640. [Google Scholar] [CrossRef]
- Liu, J.; Lang, Q.; Liang, B.; Zheng, Z.; Zhang, Y.; Liu, A. Sensitive electrochemical sequential enzyme biosensor for glucose and starch based on glucoamylase- and glucose oxidase-controllably co-displayed yeast recombinant. Anal. Chim. Acta 2022, 1221, 340173. [Google Scholar] [CrossRef]
- Torre, R.; Cerrato-Alvarez, M.; Nouws, H.P.A.; Delerue-Matos, C.; Fernández-Abedul, M.T.; Costa-Rama, E. A hand-drawn graphite electrode for affordable analysis: Application to the enzymatic determination of tyramine in fish. Sens. Actuators B Chem. 2025, 423, 136705. [Google Scholar] [CrossRef]
- Søpstad, S.; Imenes, K.; Johannessen, E.A. Hybrid electrochemical sensor platform for capsaicin determination using coarsely stepped cyclic squarewave voltammetry. Biosens. Bioelectron. 2019, 130, 374–381. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, W.; Hao, T.; Xie, J.; Wu, Y.; Jiang, X.; Hu, Y.; Wang, S.; Guo, Z. Electrochemical sensor for single-cell determination of bacteria based on target-triggered click chemistry and fast scan voltammetry. Food Chem. 2023, 417, 135906. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, C.; Chau, Y.; Lee, Y.-K. The synergy of chemical immobilization and electrical orientation of T4 bacteriophage on a micro electrochemical sensor for low-level viable bacteria detection via Differential Pulse Voltammetry. Biosens. Bioelectron. 2020, 151, 111914. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ü.T.; Calik, E.; Akdulum, B.; Yilmaz, H. Determination of carnosic acid in Rosmarinus officinalis L. using square wave voltammetry and electrochemical behavior. J. Food Drug Anal. 2018, 26, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zou, J.; Yu, Q.; Gao, Y.; Qu, F.; Liu, S.; Zhou, H.; Lu, L. Ultrasensitive indirect electrochemical sensing of thiabendazole in fruit and water by the anodic stripping voltammetry of Cu2+ with hierarchical Ti3C2Tx-TiO2 for signal amplification. Food Chem. 2023, 402, 134379. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, A.D.; Broadhurst, D.H.; Symes, M.D. Electrochemical reduction of nitrobenzene via redox-mediated chronoamperometry. STAR Protoc. 2022, 3, 101817. [Google Scholar] [CrossRef]
- Cheng, J.H.; Yu, P.P.; Huang, Y.R.; Zhang, G.; Lu, C.L.; Jiang, X.P. Application Status and Prospect of Impedance Spectroscopy in Agricultural Product Quality Detection. Agriculture 2022, 12, 1525. [Google Scholar] [CrossRef]
- Li, M.Q.; Li, J.Y.; Wei, X.H.; Zhu, W.J. Early diagnosis and monitoring of nitrogen nutrition stress in tomato leaves using electrical impedance spectroscopy. Int. J. Agric. Biol. Eng. 2017, 10, 194–205. [Google Scholar]
- Sun, J.; Liu, Y.H.; Wu, G.S.; Zhang, Y.C.; Zhang, R.B.; Li, X.J. A Fusion Parameter Method for Classifying Freshness of Fish Based on Electrochemical Impedance Spectroscopy. J. Food Qual. 2021, 2021, 6664291. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, R.B.; Zhang, Y.C.; Liang, Q.F.; Zhang, F.; Xu, P.F.; Li, G.X. Evaluation of fish freshness using impedance spectroscopy based on the characteristic parameter of orthogonal direction difference. J. Sci. Food Agric. 2020, 100, 4124–4131. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, R.B.; Zhang, Y.C.; Li, G.X.; Liang, Q.F. Estimating freshness of carp based on EIS morphological characteristic. J. Food Eng. 2017, 193, 58–67. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, R.B.; Zhang, Y.C.; Liang, Q.F.; Li, G.X.; Yang, N.; Xu, P.F.; Guo, J.J. Classifying fish freshness according to the relationship between EIS parameters and spoilage stages. J. Food Eng. 2018, 219, 101–110. [Google Scholar] [CrossRef]
- Sun, Z.B.; Liang, L.M.; Li, J.K.; Liu, X.Y.; Sun, J.; Zou, X.B.; Zuo, M.; Guo, Z.M. Rapid detection of Atlantic salmon multi-quality based on impedance properties. Food Sci. Nutr. 2020, 8, 862–869. [Google Scholar] [CrossRef]
- Zhang, Z.; Hedhili, M.N.; Zhu, H.; Wang, P. Electrochemical reduction induced self-doping of Ti3+ for efficient water splitting performance on TiO2 based photoelectrodes. Phys. Chem. Chem. Phys. 2013, 15, 15637–15644. [Google Scholar] [CrossRef] [PubMed]
- Cotta, F.C.; Amaral, R.; Bacellar, F.L.; Correia, D.; Asadi, K.; Rocha, P.R.F. A 3D porous electrode for real-time monitoring of microalgal growth and exopolysaccharides yields using Electrochemical Impedance Spectroscopy. Biosens. Bioelectron. 2025, 277, 117260. [Google Scholar] [CrossRef] [PubMed]
- Filippas, A.; Onua, L.; Manavbasi, A.; Liska, J.; Liu, N. A platform for continuous monitoring of the degradation of aluminum beverage can lids in realistic conditions through electrochemical impedance spectroscopy. Food Packag. Shelf Life 2024, 44, 101321. [Google Scholar] [CrossRef]
- Zhang, N.; Lim, S.J.; Toh, J.M.; Wei, Y.F.; Rusli; Ke, L. Investigation of spoilage in salmon by electrochemical impedance spectroscopy and time-domain terahertz spectroscopy. ChemPhysMater 2022, 1, 148–154. [Google Scholar] [CrossRef]
- Sui, X.; Zou, J.; Geng, Z.; Yang, H.; Hou, J.; Feng, L. Electrochemical impedance spectroscopy for pear ripeness detection and integration with robotic manipulators. Food Control 2025, 177, 111425. [Google Scholar] [CrossRef]
- Du, X.J.; Du, W.H.; Sun, J.; Jiang, D. Self-powered photoelectrochemical sensor for chlorpyrifos detection in fruit and vegetables based on metal-ligand charge transfer effect by Ti3C2 based Schottky junction. Food Chem. 2022, 385, 132731. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, L.; Liu, D.; Yuan, W.; Chen, S.; Li, H.; Bian, Z.; Wang, J.; Wang, Z.L. Improved Degradation Efficiency of Levofloxacin by a Self-Powered Electrochemical System with Pulsed Direct-Current. ACS Nano 2021, 15, 5478–5485. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.Y.; Li, L.B.; Luo, L.J.; Liu, X.H.; Li, J.M.; You, T.Y. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Jiang, D.; Du, X.; Wang, Y.; Shan, X.; Wang, W.; Shiigi, H.; Chen, Z. Portable self-powered electrochemical aptasensor for ultrasensitive and real-time detection of microcystin-RR based on hydrovoltaic-photothermal coupling effect. Biosens. Bioelectron. 2025, 267, 116834. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Meng, S.; Dong, N.; Liu, C.; Wei, Y.; You, T. Signal-enhanced strategy for ratiometric aptasensing of aflatoxin B1: Plasmon-modulated competition between photoelectrochemistry-driven and electrochemistry-driven redox of methylene blue. Biosens. Bioelectron. 2022, 218, 114759. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Guan, H.; Xu, W.; Zhao, J.; Liu, Y.; Cui, L.; Zhou, J. Recent advance for enantiorecognition of chiral drugs sensing: Electrochemical, electrochemiluminescent and photoelectrochemical application. Biosens. Bioelectron. 2025, 273, 117141. [Google Scholar] [CrossRef]
- Ning, K.; Wang, Z.; Qin, H.; Ahmed, M.; Azat, S.; Hu, X.; Xu, Q.; Yang, D. Novel self-powered sandwich type Aptamer sensor for estrogen detection assisted with peroxidase mimic Cu-MOF. Food Chem. 2024, 460, 140780. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, L.; Cai, Z.; Zhu, F.; Huang, B.; Lu, Q. Ultra-flexible anti-freezing cellulose conductive hydrogel for energy storage and self-powered sensors. Ind. Crops Prod. 2025, 232, 121222. [Google Scholar] [CrossRef]
- Lai, J.J.; Ding, L.J.; Liu, Y.; Fan, C.H.; You, F.H.; Wei, J.; Qian, J.; Wang, K. A miniaturized organic photoelectrochemical transistor aptasensor based on nanorod arrays toward high-sensitive T-2 toxin detection in milk samples. Food Chem. 2023, 423, 136285. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.Z.; Zhou, R.Y.; He, Y.; Wu, Y.T.; Yi, Y.H.; Zhu, G.B. Highly sensitive electrochemical detection of paraoxon ethyl in water and fruit samples based on defect-engineered graphene nanoribbons modified electrode. J. Food Meas. Charact. 2022, 16, 2596–2603. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, X.; Huang, X.W.; Zou, X.B.; Shi, J.Y.; Xu, Y.W.; Hu, X.T.; Sun, Y.; Zhai, X.D. Hypha-templated synthesis of carbon/ZnO microfiber for dopamine sensing in pork. Food Chem. 2021, 335, 127646. [Google Scholar] [CrossRef]
- Meng, S.Y.; Liu, D.; Li, Y.Y.; Dong, N.; Chen, T.; You, T.Y. Engineering the Signal Transduction between CdTe and CdSe Quantum Dots for in Situ Ratiometric Photoelectrochemical Immunoassay of Cry1Ab Protein. J. Agric. Food Chem. 2022, 70, 13583–13591. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.Y.; Chen, T.; Meng, S.Y.; Liu, D.; Dong, D.M.; You, T.Y. Electric Field-Induced Specific Preconcentration to Enhance DNA-Based Electrochemical Sensing of Hg2+ via the Synergy of Enrichment and Self-Cleaning. J. Agric. Food Chem. 2022, 70, 7412–7419. [Google Scholar] [CrossRef]
- Wu, S.J.; Duan, N.; Qiu, Y.T.; Li, J.H.; Wang, Z.P. Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- He, B.S.; Dong, X.Z. Nb.BbvCI powered DNA walking machine-based Zr-MOFs-labeled electrochemical aptasensor using Pt@AuNRs/Fe-MOFs/PEI-rGO as electrode modification material for patulin detection. Chem. Eng. J. 2021, 405, 126642. [Google Scholar] [CrossRef]
- He, B.S.; Yan, X.H. Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@Co-MOF networks for the detection of zearalenone. Sens. Actuators B-Chem. 2020, 306, 127558. [Google Scholar] [CrossRef]
- Guo, W.; Guo, Y.; Xu, H.; Li, C.; Zhang, X.; Zou, X.; Sun, Z. Ultrasensitive “On–Off” Ratiometric Fluorescence Biosensor Based on RPA-CRISPR/Cas12a for Detection of Staphylococcus aureus. J. Agric. Food Chem. 2025, 73, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.W.; Yu, L.J.; Song, J.F.; Wu, C.E.; Li, Y.; Zhang, C.C. Hybridization of glucosyl stevioside and hydroxypropyl methylcellulose to improve the solubility of lutein. Food Chem. 2022, 394, 133490. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Gao, Z.F.; Guo, X.; Chen, G.H. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Liu, S.D.; Meng, S.Y.; Wang, M.; Li, W.J.; Dong, N.; Liu, D.; Li, Y.Y.; You, T.Y. In-depth interpretation of aptamer-based sensing on electrode: Dual-mode electrochemical-photoelectrochemical sensor for the ratiometric detection of patulin. Food Chem. 2023, 410, 135450. [Google Scholar] [CrossRef]
- Qiao, W.; He, B.; Yang, J.; Ren, W.; Zhao, R.; Zhang, Y.; Bai, C.; Suo, Z.; Xu, Y.; Wei, M.; et al. Pt@AuNF nanozyme and horseradish peroxidase-based lateral flow immunoassay dual enzymes signal amplification strategy for sensitive detection of zearalenone. Int. J. Biol. Macromol. 2024, 254, 127746. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Q.; Meng, X.; Yan, C.; Yao, B.; Chen, Z.; Wang, Z.; Chen, W. CRISPR/Cas12a-mediated cyclic signal amplification and electrochemical reporting strategy for rapid and accurate sensing of Vibrio parahaemolyticus in aquatic foods. Biosens. Bioelectron. 2025, 277, 117284. [Google Scholar] [CrossRef]
- Wang, R.; Kong, L.; Xia, M.; Chai, Y.; Yuan, R. An efficient signal amplification strategy with low background based on a G-quadruplex-enriched DNA nanonetwork for ultrasensitive electrochemical detection of mucin 1. Biosens. Bioelectron. 2025, 275, 117204. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, X.; Gong, X.; Deng, L.; Tan, G.; Zhang, Q.-e.; Xiao, Z.; Yao, Q.; Liu, S.; Gao, Y.; et al. Highly sensitive turn-on electrochemical sensing of organophosphorus pesticides by integration of homogeneous reaction and heterogeneous catalytic signal amplification. Food Chem. 2024, 458, 140275. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; He, B.; Xie, L.; Cao, X.; Wei, M.; Jin, H.; Ren, W.; Suo, Z.; Xu, Y. Simultaneous Hg2+ and Pb2+ detection in water samples using an electrochemical aptasensor with dual signal amplification by exonuclease III and metal-organic frameworks. Anal. Chim. Acta 2024, 1316, 342800. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.W.; Zhang, W.; Shi, J.Y.; Li, Z.H.; Huang, X.W.; Zou, X.B.; Tan, W.L.; Zhang, X.N.; Hu, X.T.; Wang, X.; et al. Impedimetric aptasensor based on highly porous gold for sensitive detection of acetamiprid in fruits and vegetables. Food Chem. 2020, 322, 126762. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wei, D.L.; Zeng, K.; Shao, J.; Zhu, F.; Du, D.L. An Enhanced Direct Competitive Immunoassay for the Detection of Kanamycin and Tobramycin in Milk Using Multienzyme-Particle Amplification. Food Anal. Methods 2018, 11, 2066–2075. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Wang, X.; Hong, L.; Yin, X.; Zhang, Y.; Hu, B.; Zheng, Q.; Cao, J. CRISPR/Cas12a integrated electrochemiluminescence biosensor for pufferfish authenticity detection based on NiCo2O4 NCs@Au as a coreaction accelerator. Food Chem. 2024, 445, 138781. [Google Scholar] [CrossRef]
- Li, Y.Y.; Meng, S.Y.; Dong, N.; Wei, Y.; Wang, Y.; Li, X.; Liu, D.; You, T.Y. Space-Confined Electrochemical Aptasensing with Conductive Hydrogels for Enhanced Applicability to Aflatoxin B1 Detection. J. Agric. Food Chem. 2023, 71, 14806–14813. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.C.; Lin, X.H.; Zhu, X.T.; Ma, W.; Chen, J. The Electrochemical Detection of Bisphenol A and Catechol in Red Wine. Foods 2025, 14, 133. [Google Scholar] [CrossRef]
- Xia, J.J.; Zou, B.; Liu, F.; Wang, P.Y.; Yan, Y. Sensitive glucose biosensor based on cyclodextrin modified carbon nanotubes for detecting glucose in honey. J. Food Compos. Anal. 2022, 105, 104221. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, S.J.; Xue, J.Y.; Liu, Z.H.; Xu, S.Q.; He, Z.X.; Yang, H.X. Signal-enhanced electrochemical sensor employing MWCNTs/CMK-3/AuNPs and Au@Pd core-shell structure for sensitive determination of AFB1 in complex matrix. Microchim. Acta 2024, 191, 594. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.W.; Nallathambi, V.; Liu, Y.W.; Kresse, J.; Huebner, R.; Reichenberger, S.; Gault, B.; Zhan, J.H.; Eychmueller, A.; et al. Structural Regulation of Au-Pt Bimetallic Aerogels for Catalyzing the Glucose Cascade Reaction. Adv. Mater. 2024, 36, 2405200. [Google Scholar] [CrossRef]
- Ma, S.; Pan, L.G.; You, T.Y.; Wang, K. g-C3N4/Fe3O4 Nanocomposites as Adsorbents Analyzed by UPLC-MS/MS for Highly Sensitive Simultaneous Determination of 27 Mycotoxins in Maize: Aiming at Increasing Purification Efficiency and Reducing Time. J. Agric. Food Chem. 2021, 69, 4874–4882. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.M.; Yu, F.F.; Quan, Y. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef]
- Khademi, F.; Motamedzadegan, A.; Farahmandfar, R.; Hamzeh, S.; Shahidi, S.A. Development of an Ionic Liquid based-Fe3O4/Gr Nanocomposite for Sensitive Electrochemical Sensing and Monitoring of Vanillin in Food Products. Top. Catal. 2025, 68, 718–725. [Google Scholar] [CrossRef]
- Yang, Z.W.; Zhu, A.F.; Adade, S.; Ali, S.; Chen, Q.M.; Wei, J.; Chen, X.M.; Jiao, T.H.; Chen, Q.S. Ag@Au core-shell nanoparticle-based surface-enhanced Raman scattering coupled with chemometrics for rapid determination of chloramphenicol residue in fish. Food Chem. 2024, 438, 138026. [Google Scholar] [CrossRef]
- Liu, Y.X.; Chen, P.S.; Lin, K.Y.; Zhou, Q.; Zhou, L.; Zhai, H.Y. An ultrasensitive electrochemical aptasensor based on different oxidation nanoprobes for single or simultaneous detection of aflatoxin B1 and ochratoxin A. Sens. Actuators B-Chem. 2025, 432, 137471. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar Sinha, S. Development of a highly sensitive non-enzymatic electrochemical cholesterol biosensor based on Ag with Cu2O nanomaterial deposited on TiO2 nanotubes. Measurement 2024, 232, 114707. [Google Scholar] [CrossRef]
- Venkateswara Raju, C.; Hwan Cho, C.; Mohana Rani, G.; Manju, V.; Umapathi, R.; Suk Huh, Y.; Pil Park, J. Emerging insights into the use of carbon-based nanomaterials for the electrochemical detection of heavy metal ions. Coord. Chem. Rev. 2023, 476, 214920. [Google Scholar] [CrossRef]
- Chen, T.-W.; Chen, S.-M.; Anushya, G.; Kannan, R.; Veerakumar, P.; Al-Sehemi, A.G.; Mariyappan, V.; Alargarsamy, S.; Alam, M.M.; Mahesh, T.C.; et al. Electrochemical energy storage applications of functionalized carbon-based nanomaterials: An overview. Int. J. Electrochem. Sci. 2024, 19, 100548. [Google Scholar] [CrossRef]
- Selvam, A.; Gopika, M.G.; Kuo, C.-Y.; Yusuf, K.; Saraswathyamma, B.; Govindasamy, M. Design of an ultrasensitive electrochemical sensor using a carbon black/zinc-organic framework nanocomposite for quantifying quercetin in fruits. Food Chem. 2025, 491, 145222. [Google Scholar] [CrossRef]
- Dey, B.; Kushwaha, K.S.; Ullah, I.; Kamal, T.; Khan, S.; Wasi Ahmad, M.; Hossain, S.K.S.; Choudhury, A. Highly sensitive and selective electrochemical sensor for carbendazim detection in fruit juice using novel bi-metallic metal organic framework anchored graphite rod electrode. Inorg. Chem. Commun. 2025, 178, 114643. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.W.; Zou, X.B. Rapid determination of cadmium in rice using an all-solid RGO-enhanced light addressable potentiometric sensor. Food Chem. 2018, 261, 1–7. [Google Scholar] [CrossRef]
- Han, E.; Li, L.; Gao, T.; Pan, Y.Y.; Cai, J.R. Nitrite determination in food using electrochemical sensor based on self-assembled MWCNTs/AuNPs/poly-melamine nanocomposite. Food Chem. 2024, 437, 137773. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, M.; You, T.Y.; Wang, K. Using Magnetic Multiwalled Carbon Nanotubes as Modified QuEChERS Adsorbent for Simultaneous Determination of Multiple Mycotoxins in Grains by UPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wei, W.; Jiang, L.; Zhu, F.; Du, D.L. Use of Carbon Nanotubes as a Solid Support To Establish Quantitative (Centrifugation) and Qualitative (Filtration) Immunoassays To Detect Gentamicin Contamination in Commercial Milk. J. Agric. Food Chem. 2016, 64, 7874–7881. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, Y.X.; Zhang, D.; Tan, W.L.; Shi, J.Y.; Li, Z.H.; Liu, H.Y.; Yu, Y.Y.; Yang, L.; Wang, X.; et al. A fluorescence resonance energy transfer probe based on functionalized graphene oxide and upconversion nanoparticles for sensitive and rapid detection of zearalenone. Lwt-Food Sci. Technol. 2021, 147, 111541. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhou, X.C.; Wang, K.; Zou, X.B.; Shi, J.Y.; Huang, X.W.; Holmes, M. A novel sensor for determination of dopamine in meat based on ZnO-decorated reduced graphene oxide composites. Innov. Food Sci. Emerg. Technol. 2015, 31, 196–203. [Google Scholar] [CrossRef]
- Chao, Y.H.; Pang, J.Y.; Bai, Y.; Wu, P.W.; Luo, J.; He, J.; Jin, Y.; Li, X.W.; Xiong, J.; Li, H.M.; et al. Graphene-like BN@SiO2 nanocomposites as efficient sorbents for solid-phase extraction of Rhodamine B and Rhodamine 6G from food samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef]
- Gomez, E.; Mehmood, A.; Bian, Z.Y.; Lee, S.A.; Tauzin, L.J.; Adhikari, S.; Gruebele, M.; Levine, B.G.; Link, S. Single-Particle Correlated Imaging Reveals Multiple Chromophores in Carbon Dot Fluorescence. J. Am. Chem. Soc. 2025, 147, 17784–17794. [Google Scholar] [CrossRef]
- Du, J.; Liu, Y.C.; Sun, M.; Guan, J.; Chen, A.B.; Han, B.X. Highly Selective Oxygen Electroreduction to Hydrogen Peroxide on Sulfur-Doped Mesoporous Carbon. Angew. Chem.-Int. Ed. 2025, 64, e202503385. [Google Scholar] [CrossRef]
- Gu, C.M.; Wang, Q.; Zhang, L.; Yang, P.P.; Xie, Y.X.; Fei, J.J. Ultrasensitive non-enzymatic pesticide electrochemical sensor based on HKUST-1-derived copper oxide @ mesoporous carbon composite. Sens. Actuators B-Chem. 2020, 305, 127478. [Google Scholar] [CrossRef]
- Sharma, R.; Bhat, G.P.; Gandhi, S. MXene-rGO nanocomposite based electrochemical immunosensor for detection of endosulfan—An organochlorine pesticide. Chemosphere 2025, 370, 143997. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lv, S.; Gan, L.; Wang, C.; Wang, Z.; Zhang, Z. Single-Fe-Atom Catalyst for Sensitive Electrochemical Detection of Caffeic Acid. Acs Appl. Mater. Interfaces 2023, 15, 53189–53197. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.N.; Hu, X.T.; Zhang, X.A.; Li, W.T.; Guo, Z.; Huang, X.W.; Li, Z.H.; Zhang, R.J.; Shen, T.T.; Zou, X.B.; et al. Ratiometric Sensing for Ultratrace Tetracycline Using Electrochemically Active Metal-Organic Frameworks as Response Signals. J. Agric. Food Chem. 2023, 71, 7584–7592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Zhang, Y.; Jayan, H.; Gao, S.P.; Zhou, R.Y.; Yosri, N.; Zou, X.B.; Guo, Z.M. Recent and emerging trends of metal-organic frameworks (MOFs)-based sensors for detecting food contaminants: A critical and comprehensive review. Food Chem. 2024, 448, 139051. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.T.; Zhai, X.D.; Huang, X.W.; Li, Z.H.; Zou, X.B.; Shi, J.Y. A simple and sensitive electrochemical sensing based on amine-functionalized metal-organic framework and polypyrrole composite for detection of lead ions in meat samples. J. Food Meas. Charact. 2024, 18, 5813–5825. [Google Scholar] [CrossRef]
- Wang, S.L.; Liang, N.N.; Hu, X.T.; Li, W.T.; Guo, Z.; Zhang, X.A.; Huang, X.W.; Li, Z.H.; Zou, X.B.; Shi, J.Y. Carbon dots and covalent organic frameworks based FRET immunosensor for sensitive detection of Escherichia coli O157:H7. Food Chem. 2024, 447, 138663. [Google Scholar] [CrossRef]
- Ma, G.X.; Shi, Q.; Hou, X.L.; Peng, Y.X.; Liu, Q. An electrochemical sensor for simultaneous voltammetric detection of ascorbic acid and dopamine enabled by higher electrocatalytic activity of co-modified MCM-41 mesoporous molecular sieve. Front. Sustain. Food Syst. 2024, 8, 1448421. [Google Scholar] [CrossRef]
- Shi, Y.H.; Rong, X.S.; Chen, C.; Wu, M.; Takai, Y.; Qiu, X.C.; Wang, C.C.; Shimasaki, Y.; Oshima, Y. Effects of ZIF-8 Nanoparticles on the Survival, Development, and Locomotor Activity of Early-life-stages of Zebrafish (Danio rerio). J. Fac. Agric. Kyushu Univ. 2021, 66, 211–216. [Google Scholar] [CrossRef]
- Ou, X.X.; He, M.; Chen, B.B.; Hu, B. Covalent organic frameworks based hierarchical porous hybrid monolithic capillary: Synthesis, characterization, and applications in trace metals analysis. J. Hazard. Mater. 2024, 462, 132680. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.Y.; Yu, S.S.; Xiong, F.; Wang, Y.; Zhang, X.R.; Ren, Y. Characterization of the volatile flavor profiles of Zhenjiang aromatic vinegar combining a novel nanocomposite colorimetric sensor array with HS-SPME-GC/MS. Food Res. Int. 2022, 159, 111585. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.N.; Shi, B.Q.; Hu, X.T.; Li, W.T.; Huang, X.W.; Li, Z.H.; Zhang, X.N.; Zou, X.B.; Shi, J.Y. A ternary heterostructure aptasensor based on metal-organic framework and polydopamine nanoparticles for fluorescent detection of sulfamethazine. Food Chem. 2024, 460, 140570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhou, R.Y.; Ke, L.J.; Li, J.B.; Jayan, H.; El-Seedi, H.R.; Zou, X.B.; Guo, Z.M. Development of multifunctional metal-organic frameworks (MOFs)-based nanofiller materials in food packaging: A comprehensive review. Trends Food Sci. Technol. 2024, 154, 104771. [Google Scholar] [CrossRef]
- Shen, X.Q.; Yan, B. Regulating the Azo-Functionalization of Covalent Organic Frameworks for Photo-Modulated Adsorption and Sensing of Aniline. Adv. Funct. Mater. 2025, 202424688. [Google Scholar] [CrossRef]
- Elashery, S.E.A.; Attia, N.F.; El Badry Mohamed, M. Exploitation of 2D Mn-MOF nanosheets for developing rapid, sensitive, and selective sensor for determination of Mn(II) ions in food and biological samples. Talanta 2025, 294, 128217. [Google Scholar] [CrossRef]
- Liu, R.; Ali, S.; Huang, D.; Zhang, Y.L.; Lü, P.; Chen, Q.S. A Sensitive Nucleic Acid Detection Platform for Foodborne Pathogens Based on CRISPR-Cas13a System Combined with Polymerase Chain Reaction. Food Anal. Methods 2023, 16, 356–366. [Google Scholar] [CrossRef]
- Wu, S.J.; Liu, L.H.; Duan, N.; Li, Q.; Zhou, Y.; Wang, Z.P. Aptamer-Based Lateral Flow Test Strip for Rapid Detection of Zearalenone in Corn Samples. J. Agric. Food Chem. 2018, 66, 1949–1954. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, H.Y.; Han, Y.F.; Yu, F.F.; Shi, X.M. Development of a biomimetic enzyme-linked immunosorbent assay based on molecularly imprinted polymers on paper for the detection of carbaryl. Food Chem. 2018, 240, 893–897. [Google Scholar] [CrossRef]
- Dong, Z.Q.; Lu, J.; Wu, Y.L.; Meng, M.J.; Yu, C.; Sun, C.; Chen, M.N.; Da, Z.L.; Yan, Y.S. Antifouling molecularly imprinted membranes for pretreatment of milk samples: Selective separation and detection of lincomycin. Food Chem. 2020, 333, 127477. [Google Scholar] [CrossRef]
- You, F.H.; Wen, Z.R.; Yuan, R.S.; Qian, J.; Long, L.L.; Wang, K. Sensitive and stable detection of deoxynivalenol based on electrochemiluminescence aptasensor enhanced by 0D/2D homojunction effect in food analysis. Food Chem. 2023, 403, 134397. [Google Scholar] [CrossRef]
- Wang, C.Q.; Zhao, X.; Gu, C.D.; Xu, F.Y.; Zhang, W.H.; Huang, X.Y.; Qian, J. Fabrication of a Versatile Aptasensing Chip for Aflatoxin B1 in Photothermal and Electrochemical Dual Modes. Food Anal. Methods 2022, 15, 3390–3399. [Google Scholar] [CrossRef]
- Duan, N.; Gong, W.H.; Wu, S.J.; Wang, Z.P. Selection and Application of ssDNA Aptamers against Clenbuterol Hydrochloride Based on ssDNA Library Immobilized SELEX. J. Agric. Food Chem. 2017, 65, 1771–1777. [Google Scholar] [CrossRef]
- Luo, L.J.; Ma, S.; Li, L.B.; Liu, X.H.; Zhang, J.Y.; Li, X.; Liu, D.; You, T.Y. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@SiO2 luminophore. Food Chem. 2019, 292, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Sun, X.X.; Liu, Y.X.; Zhou, Y.J.; Shin, H.C.; Wang, Y.; Shen, L.Q.; Wang, C.T.; Wang, S.Y.; et al. Voltammetric, spectroscopic, and cellular characterization of redox functionality of eckol and phlorofucofuroeckol-A: A comparative study. J. Food Biochem. 2019, 43, e12845. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.L.; Ali, S.; Haruna, S.A.; He, P.H.; Li, H.H.; Ouyang, Q.; Chen, Q.S. Development of a fluorescence aptasensor for rapid and sensitive detection of Listeria monocytogenes in food. Food Control 2021, 122, 107808. [Google Scholar] [CrossRef]
- Liu, R.; Ali, S.; Haruna, S.A.; Ouyang, Q.; Li, H.H.; Chen, Q.S. Development of a fluorescence sensing platform for specific and sensitive detection of pathogenic bacteria in food samples. Food Control 2022, 131, 108419. [Google Scholar] [CrossRef]
- Guo, Z.M.; Chen, P.; Yin, L.M.; Zuo, M.; Chen, Q.S.; El-Seedi, H.R.; Zou, X.B. Determination of lead in food by surface-enhanced Raman spectroscopy with aptamer regulating gold nanoparticles reduction. Food Control 2022, 132, 108498. [Google Scholar] [CrossRef]
- Wu, S.W.; Hsieh, C.Y.; Liu, B.H.; Lin, X.J.; Yu, F.Y. Novel antibody- and aptamer-based approaches for sensitive detection of mycotoxin fusaric acid in cereal. Food Chem. 2025, 463, 141245. [Google Scholar] [CrossRef]
- Tang, S.; He, B.; Liu, Y.; Wang, L.; Liang, Y.; Wang, J.; Jin, H.; Wei, M.; Ren, W.; Suo, Z.; et al. A dual-signal mode electrochemical aptasensor based on tetrahedral DNA nanostructures for sensitive detection of citrinin in food using PtPdCo mesoporous nanozymes. Food Chem. 2024, 460, 140739. [Google Scholar] [CrossRef]
- Jin, Y.N.; Zhang, Y.Z.; Xu, H.; Lu, X.; Yuan, Y.W.; Zhang, W. A ratiometric electrochemical aptasensor for sensitive detection of kanamycin in food based on entropy-driven strand displacement reaction. Food Control 2024, 161, 110390. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, Y.; Zhang, H.; Xia, Z.; Chen, D.; Yuan, Z.; Zhao, W. Screening and optimization of AFB1 aptamer for target-capture electrochemical aptasensor development. Sens. Actuators B Chem. 2025, 442, 138111. [Google Scholar] [CrossRef]
- Xu, L.; Hao, X.; Chen, L.; Qu, W.; Duan, S.; Wang, Q.; Yang, Q.; Wu, J.; Gong, Z.; Dai, H. Electrochemical immunosensor based on antibody-oriented probe for the detection of AFB1 in rice bran oil. J. Food Compos. Anal. 2024, 130, 106198. [Google Scholar] [CrossRef]
- Wang, A.; You, X.; Liu, H.; Zhou, J.; Chen, Y.; Zhang, C.; Ma, K.; Liu, Y.; Ding, P.; Qi, Y.; et al. Development of a label free electrochemical sensor based on a sensitive monoclonal antibody for the detection of tiamulin. Food Chem. 2022, 366, 130573. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Yang, H.-M.; Xu, C.-M.; Ju, J.-Y.; Xue, T.-S.; Jiang, Y.; Jia, Z.-Y.; Gong, X.-M.; Zeng, X.-Z.; Tang, J.-B. A facile and effective electrochemical immunosensor based on controlled and oriented immobilization of natural antibodies for sensitive detection of aflatoxin B1. Food Chem. 2025, 475, 143324. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.F.; Ling, L.; Deng, N.N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xu, J.L.; Qiu, Y.L.; Li, P.; Liu, B.B.; Yang, L.F.; Barnych, B.; Hammock, B.D.; Zhang, C.Z. Highly Specific Monoclonal Antibody and Sensitive Quantum Dot Beads-Based Fluorescence Immunochromatographic Test Strip for Tebuconazole Assay in Agricultural Products. J. Agric. Food Chem. 2019, 67, 9096–9103. [Google Scholar] [CrossRef]
- Su, X.Y.; Chen, Z.Y.; Wang, H.; Yuan, L.; Zheng, K.Y.; Zhang, W.; Zou, X.B. Ratiometric immunosensor with DNA tetrahedron nanostructure as high-performance carrier of reference signal and its applications in selective phoxim determination for vegetables. Food Chem. 2022, 383, 132445. [Google Scholar] [CrossRef]
- Wan, J.W.; Tian, Y.Y.; Wu, D.; Ye, Z.J.; Chen, S.M.; Hu, Q.; Wang, M.G.; Lv, J.P.; Xu, W.H.; Zhang, X.Y.; et al. Site-Directed Electrochemical Grafting for Amplified Detection of Antibody Pharmaceuticals. Anal. Chem. 2024, 96, 9278–9284. [Google Scholar] [CrossRef]
- Kim, G.; Yang, H. Electrochemical biosensor using direct electron transfer and an antibody-aptamer hybrid sandwich for target detection in complex biological samples. Biosens. Bioelectron. 2024, 253, 116184. [Google Scholar] [CrossRef]
- Cho, Y.K.; Choi, Y.; Kim, S.; Kim, H.; Chow, K.F.; Shin, I.S.; Park, J.H.; Lee, H. Scalable electrochemical system for rapid on-site detection of food allergens. Biosens. Bioelectron. 2025, 273, 117142. [Google Scholar] [CrossRef]
- Torati, S.R.; Slaughter, G. Advanced laser-induced graphene-based electrochemical immunosensor for the detection of C-reactive protein. Bioelectrochemistry 2025, 161, 108842. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, H.; Wu, Y.; Luo, S.; Hong, Q.; Zhang, X.; Zou, X.; Sun, Z. Conductive MoS2-Au nanocomposite-based electrochemical biosensor for CRISPR/Cas12a-driven Staphylococcus aureus detection. Sens. Actuators B Chem. 2025, 442, 138078. [Google Scholar] [CrossRef]
- Niu, M.; Ge, K.; Yi, L.; Duan, F.; Fan, X.; Gu, Y.; Wang, S. A zwitterionic phosphorylcholine-based antifouling electrochemical aptasensor for aflatoxin B1 detection in food. J. Food Compos. Anal. 2025, 139, 107178. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, X.; Wang, W.; Liu, C.; Yang, W.; Wang, D. Nanobody@Biomimetic mineralized MOF as a sensing immunoprobe in detection of aflatoxin B1. Biosens. Bioelectron. 2023, 220, 114906. [Google Scholar] [CrossRef] [PubMed]
- Cong, R.; Hu, K.; Hu, D.; Sun, J.; Yao, J.; Guo, Z.; Wang, S.; Hu, Y. Ag-enhanced MOF-based electrochemical biosensor for sensitive detection of uracil-DNA glycosylase activity in cellular systems. Microchem. J. 2025, 212, 113236. [Google Scholar] [CrossRef]
- Sun, D.; Wang, H.; Li, W.; Wang, R.; Zhang, Y.; Zhang, H.; Cai, C. Electrochemical detection of cancer cell-derived small extracellular vesicles based on three aptamer recognition and dual signal amplification. Sens. Actuators B Chem. 2025, 442, 138096. [Google Scholar] [CrossRef]
- Aye, N.N.S.; Maraming, P.; Tippayawat, P.; Daduang, S.; Techasen, A.; Jamnongkan, W.; Sithithaworn, P.; Daduang, J. Functionalized graphene oxide–antibody conjugate-based electrochemical immunosensors to detect Opisthorchis viverrini antigen in urine. Mater. Adv. 2024, 5, 4491–4503. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, J.; Zhou, S.; Zhao, Y.; Li, J.; Wu, D. A dual-functional module cellular electrochemical sensing platform for simultaneous detection guanine and xanthine. Biosens. Bioelectron. 2023, 226, 115104. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, Z.; Yu, W.; Ma, Y.; Kim, K.; Rivera, N.; Mohammed, J.; Lantelme, E.; Hsu-Kim, H.; Chilkoti, A.; et al. Biomolecular condensates regulate cellular electrochemical equilibria. Cell 2024, 187, 5951–5966.e5918. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Kumar, L.; Das, S.; Gupta, D.; Anand, R. Ultrasensitive detection of aromatic water pollutants through protein immobilization driven organic electrochemical transistors. Chem. Sci. 2024, 15, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, C.; Xie, H.; Lin, Y.; Yang, J.; Yin, T. Integrating aptamer-functionalized covalent organic frameworks with electrochemical sensing platform: A novel approach for exosome analysis. Microchem. J. 2025, 215, 114210. [Google Scholar] [CrossRef]
- Gao, G.; Fang, D.; Yu, Y.; Wu, L.; Wang, Y.; Zhi, J. A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta 2017, 167, 208–216. [Google Scholar] [CrossRef]
- Jain, S.; Panwar, R.; Mathur, J. Design and development of colorimetric, whole-cell based, electrochemical biosensors for arsenic detection. Inorg. Chem. Commun. 2023, 153, 110730. [Google Scholar] [CrossRef]
- Hatamie, A.; He, X.; Zhang, X.-W.; Oomen, P.E.; Ewing, A.G. Advances in nano/microscale electrochemical sensors and biosensors for analysis of single vesicles, a key nanoscale organelle in cellular communication. Biosens. Bioelectron. 2023, 220, 114899. [Google Scholar] [CrossRef]
- Ozbakir, Y.; Min, H.J.; Zheng, Q.; Kim, Y.J.; Carraro, C.; Kim, J.H.; Maboudian, R. Atomically dispersed palladium supported on graphene oxide for advanced electrochemical biosensing of dopamine. Electrochim. Acta 2025, 532, 146414. [Google Scholar] [CrossRef]
- Stasyuk, N.; Zakalskiy, A.; Nogala, W.; Holdynski, M.; Gawinkowski, S.; Zakalska, O.; Demkiv, O.; Salyha, Y.; Gonchar, M. Genetically engineered yeast cells enriched with nanocomposites containing a natural enzyme and nanozyme for the construction of microbial sensors. Electrochim. Acta 2024, 497, 144605. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Chen, W.-T.; An, X.; Liu, H.; Chang, F.; Gao, S.; Hu, G. Recent advances and perspectives in functionalized nanocomposites for electrochemical sensing of toxic environmental heavy metal ions. Coord. Chem. Rev. 2025, 542, 216859. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, J.; Zhang, Y.; He, S.; Esakkimuthu, S.; Zhu, K.; Kumar, S.; Lv, G.; Hu, X. Biochar assisted cultivation of Chlorella protothecoides for adsorption of tetracycline and electrochemical study on self-cultured Chlorella protothecoides. Bioresour. Technol. 2023, 389, 129810. [Google Scholar] [CrossRef]
- Tang, X.; Tang, J.; Zhang, Q.; Suonanmu, D.; Zhang, Y.; Ren, Q.; Tao, F.; Li, C.; Wang, F. An integrated microfluidic cytosensor coupled with self-calibration electrochemical/colorimetric dual-signal output for sensitive and accurate detection of hepatoma cells. Sens. Actuators B Chem. 2024, 403, 135176. [Google Scholar] [CrossRef]
- Surendhiran, S.; Savitha, S.; Karthik, A.; Sruthi, N.; Balu, K.S.; Jagan, K.S.G.; Naren Vidaarth, T.M. Bio electrochemical and in-vitro bioactivity evaluation of Araucaria columnaris interceded α-MoO3 nano rods fused chitosan-sodium alginate scaffold for tissue engineering applications. J. Mol. Struct. 2025, 1328, 141384. [Google Scholar] [CrossRef]
- Cui, M.; Gong, Y.; Du, M.; Wang, K.; Li, T.; Zhu, X.; Wang, S.; Luo, X. An antifouling electrochemical biosensor based on a protein imprinted hydrogel for human immunoglobulin G recognition in complex biological media. Sens. Actuators B Chem. 2021, 337, 129820. [Google Scholar] [CrossRef]
- Ziółkowski, R.; Kaczmarek, A.; Kośnik, I.; Malinowska, E. Reduced nonspecific protein adsorption by application of diethyldithiocarbamate in receptor layer of diphtheria toxoid electrochemical immunosensor. Bioelectrochemistry 2020, 132, 107415. [Google Scholar] [CrossRef]
- Kong, L.; Li, H.; Xu, S.; Xia, M.; Han, Z.; Zhuo, Y.; Chai, Y.; Tan, X.; Yuan, R. A novel cascade catalytic electrochemical biosensor based on bifunctional Mn3O4@AuNPs with self-supplied O2 property and glucose oxidase-like activity for ultrasensitive detection of microRNA. Chem. Eng. J. 2024, 479, 147627. [Google Scholar] [CrossRef]
- Kim, J.-W.; Choi, S.H.; Lillehei, P.T.; Chu, S.-H.; King, G.C.; Watt, G.D. Electrochemically controlled reconstitution of immobilized ferritins for bioelectronic applications. J. Electroanal. Chem. 2007, 601, 8–16. [Google Scholar] [CrossRef]
- Shin, K.M.; Lee, J.W.; Wallace, G.G.; Kim, S.J. Electrochemical properties of SWNT/ferritin composite for bioapplications. Sens. Actuators B Chem. 2008, 133, 393–397. [Google Scholar] [CrossRef]
- Nicul, M.; Liendo, F.; Paz de la vega, A.; Pichún, B.; Penagos, J.; Pizarro, J.; Godoy, F.; Arancibia, V.; Segura, R. Electrochemical determination of Se(IV) in food and water samples using reduced graphene oxide and gold nanoparticle-modified glassy carbon electrodes. Electrochim. Acta 2025, 528, 146270. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, A.; Sajid, A.; Akyürekli, S.; Ahmed, A.Y.; Bayach, I.; Buzid, A.; Alharthi, A.F. NiO@CNTs wrapped graphene oxide nanohybrid towards simultaneous electrochemical detection of ascorbic acid and uric acid. Inorg. Chem. Commun. 2025, 179, 114774. [Google Scholar] [CrossRef]
- Tian, R.; Ma, W.; Wang, L.; Xie, W.; Wang, Y.; Yin, Y.; Weng, T.; He, S.; Fang, S.; Liang, L.; et al. The combination of DNA nanostructures and materials for highly sensitive electrochemical detection. Bioelectrochemistry 2024, 157, 108651. [Google Scholar] [CrossRef]
- Ukirade, N.; Choudhari, U.; Jadhav, U.; Jagtap, S.; Rane, S. Horseradish peroxidase/graphene electrochemical biosensor for glutathione detection. Nanomater. Energy 2024, 13, 102–109. [Google Scholar] [CrossRef]
- Shao, K.; You, J.; Ye, S.; Gu, D.; Wang, T.; Teng, Y.; Shen, Z.; Pan, Z. Gold nanoclusters-poly(9,9-dioctylfluorenyl-2,7-diyl) dots@zeolitic imidazolate framework-8 (ZIF-8) nanohybrid based probe for ratiometric analysis of dopamine. Anal. Chim. Acta 2020, 1098, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.P.G.; Oliveira-Brett, A.M. Calcium-induced calmodulin conformational change. Electrochemical evaluation. Bioelectrochemistry 2017, 113, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, T. Electrochemical detection of cortisol using a molecularly imprinted Au-decorated SiC/β-cyclodextrin nanocomposite sensor in human sweat. Int. J. Electrochem. Sci. 2025, 20, 101093. [Google Scholar] [CrossRef]
- Shaikh, T.; Tan, E.M.M.; Cingil, H.E. L-cysteine gold nanoparticles for colorimetric and electrochemical detection of spermine and histamine. Microchem. J. 2024, 207, 112196. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, M.; Guo, H.; Wang, Y.; Zhang, Y.; He, M.; Cai, Z. Stimuli-responsive hydrogels based on protein/peptide and their sensing applications. Prog. Mater. Sci. 2025, 148, 101355. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Y.; Zhang, K.; Jiao, J.; Zhao, Y. A reusable electrochemical biosensor for NF-κB p50: Precise detection of a critical biological macromolecule via CRISPR/Cas12a and pH-responsive signal regeneration. Microchem. J. 2025, 214, 114131. [Google Scholar] [CrossRef]
- Ding, S.; Chen, X.; Yu, B.; Liu, Z. Electrochemical biosensors for clinical detection of bacterial pathogens: Advances, applications, and challenges. Chem. Commun. 2024, 60, 9513–9525. [Google Scholar] [CrossRef]
- Flores-Ramírez, A.Y.; González-Estrada, R.R.; Chacón-López, M.A.; García-Magaña, M.d.L.; Montalvo-González, E.; Álvarez-López, A.; Rodríguez-López, A.; López-García, U.M. Detection of foodborne pathogens in contaminated food using nanomaterial-based electrochemical biosensors. Anal. Biochem. 2024, 693, 115600. [Google Scholar] [CrossRef]
- Subak, H.; Baş, H.; Talay Pınar, P. Nanomolar detection and electrochemical mechanism of nintedanib via square-wave voltammetry using a MWCNT-modified glassy carbon electrode. Diam. Relat. Mater. 2025, 153, 112008. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.Y.; Li, L.; Cai, J.R. Bisphenol A detection based on nano gold-doped molecular imprinting electrochemical sensor with enhanced sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef]
- Jiang, T.; Lin, B.; Yan, Y.; Lv, D.; Xu, Y.; Ren, X.; Wang, T.; Qiao, S.; Jiang, X. Fabrication of an electrochemical sensor based on molecular imprinting technology for detecting elemene. Int. J. Electrochem. Sci. 2023, 18, 100361. [Google Scholar] [CrossRef]
- Parihar, A.; Sharma, P.; Choudhary, N.K.; Khan, R.; Mostafavi, E. Internet-of-things-integrated molecularly imprinted polymer-based electrochemical nano-sensors for pesticide detection in the environment and food products. Environ. Pollut. 2024, 351, 124029. [Google Scholar] [CrossRef]
- Li, X.; Yu, P.; Feng, Y.; Yang, Q.; Li, Y.; Ye, B.-C. Specific adsorption and highly sensitive detection of methyl red in wastewater using an iron paste electrode modified with a molecularly imprinted polymer. Electrochem. Commun. 2021, 132, 107144. [Google Scholar] [CrossRef]
- Khan, N.; Ullah, R.; Khan, M. Almas. Enhanced electrochemical performances of ternary polypyrrole/MXene/Gum arabic composites as supercapacitors electrode materials. Electrochim. Acta 2024, 505, 144988. [Google Scholar] [CrossRef]
- Haotian, R.; Zhu, Z.; Zhang, H.; Lv, T.; Tang, S.; Zhang, J.; Luo, A.; Liang, A. Engineering conductive covalent-organic frameworks enable highly sensitive and anti-interference molecularly imprinted electrochemical biosensor. Biosens. Bioelectron. 2025, 273, 117195. [Google Scholar] [CrossRef]
- Mao, H.; Liu, M.; Cao, Z.; Ji, C.; Sun, Y.; Liu, D.; Wu, S.; Zhang, Y.; Song, X.-M. Poly(4-vinylphenylboronic acid) functionalized polypyrrole/graphene oxide nanosheets for simultaneous electrochemical determination of catechol and hydroquinone. Appl. Surf. Sci. 2017, 420, 594–605. [Google Scholar] [CrossRef]
- Siler, T.; Stanley, L.; Saleem, M.; Badalyan, A. A non-covalently bound redox indicator for electrochemical CRISPR-Cas12a and DNase I biosensors. Anal. Chim. Acta 2025, 1336, 343480. [Google Scholar] [CrossRef]
- Zhang, S.; Xi, J.; Yu, X.; He, H.; Yang, X.; Zhao, Y.; Wang, X.; Xu, M.; Du, S.; Li, C.-P.; et al. Fabricating a sensitive electrochemical aptasensor based on HOF/MXene nanocomposites for monitoring trace ampicillin. Microchem. J. 2025, 213, 113625. [Google Scholar] [CrossRef]
- Mei, X.; Yang, J.; Yu, X.; Peng, Z.; Zhang, G.; Li, Y. Wearable molecularly imprinted electrochemical sensor with integrated nanofiber-based microfluidic chip for in situ monitoring of cortisol in sweat. Sens. Actuators B Chem. 2023, 381, 133451. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Ma, Z.; Gu, H.; Luo, X.; Yin, X.; Yi, H.; Chen, Y. Hybrid recognition-enabled ratiometric electrochemical sensing of Staphylococcus aureus via in-situ growth of MOF/Ti3C2Tx-MXene and a self-reporting bacterial imprinted polymer. Food Chem. 2025, 463, 141496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Zhang, X.B.; Yuan, J.L.; Zheng, Q.Y.; Hu, B.; Liu, R.G.; Deng, R.J.; Cao, J.J. Metal-organic frameworks-based photoelectrochemical Biosensors: From material design to intelligent detection. Trends Food Sci. Technol. 2025, 162, 105103. [Google Scholar] [CrossRef]
- Aschemacher, N.A.; Gegenschatz, S.A.; Teglia, C.M.; Siano, Á.S.; Gutierrez, F.A.; Goicoechea, H.C. Highly sensitive and selective electrochemical sensor for simultaneous determination of gallic acid, theophylline and caffeine using poly(l-proline) decorated carbon nanotubes in biological and food samples. Talanta 2024, 267, 125246. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Zhang, M.; Wang, Q.; Wang, Y.; Yu, S.; Dang, R.; Wang, X.; Yang, Z.; Fan, S.; et al. N/O co-doping porous biomass carbon constructed electrochemical sensor for universal and sensitive detection to mycotoxins. Food Chem. 2025, 475, 143397. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, B.; Li, G.; Shen, Q.; Zou, L. NiCoFeS/rGO nanozyme-mediated multifunctional homogeneous sensing system for ultrasensitive electrochemical assay of pesticides residues in fruits and vegetables. Sens. Actuators B Chem. 2025, 422, 136664. [Google Scholar] [CrossRef]
- Aljabri, M.D.; Mahmud Alam, M.; Fazle Rabbee, M.; Ahmed, J.; Al-Humaidi, J.Y.; Abdel-Fadeel, M.A.; Almahri, A.; Rahman, M.M. Electrochemical detection of salicylic acid in fruit products with ternary ZnO/CuO/Y2O3 nanocomposites embedded PEDOT:PSS by differential pulse voltammetry. Measurement 2025, 240, 115583. [Google Scholar] [CrossRef]
- Don Disouza, F.P.; Vincent John, A.J.; Chen, T.-W.; Sengodan, S.; Yu, J.; Chen, S.-M. Bismuth molybdate incorporates functionalized carbon nanofiber for the sensitive electrochemical determination of sulfonamide-based antibiotics in foods and environmental samples. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]
- Gao, P.; Hussain, M.Z.; Gryc, D.; Mukherjee, S.; Zhou, Z.; Li, W.; Jentys, A.; Elsner, M.; Fischer, R.A. Enhanced electrochemical activity by MOF superstructure derived Ni2P@C for ultrasensitive sensing of Bisphenol A. Biosens. Bioelectron. 2025, 286, 117598. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, W.; Guo, M.; Cai, L.; Zhang, B.; Fang, G.; Wang, S. Fabrication of a new molecularly imprinted electrochemical sensor by AgNWs/Ti3C2Tx synergistic AuNPs for ultrasensitive and targeted detection of sulfathiazole in food. Microchem. J. 2025, 215, 114217. [Google Scholar] [CrossRef]
- Zhou, B.; Li, X.; Zheng, X.; Liang, M.; Yang, Z.; Liu, A.; Chen, L. A self-reporting electrochemical sensor for carbendazim in food based on magnetic molecularly imprinted MOFs. Food Chem. 2025, 487, 144789. [Google Scholar] [CrossRef]
- Athira, M.; Lekshmi, G.S.; Rajeev, M.R.; Anirudhan, T.S. Zinc oxide and chitosan incorporated graphene oxide based molecular imprinted electrochemical sensor for L-Carnitine. J. Environ. Chem. Eng. 2025, 13, 116239. [Google Scholar] [CrossRef]
- Tarek, M.; Molouk, A.F.S.; El-Shinawi, H.; El-Dafrawy, S.M.; Abdallah, A.B. Rational design of a novel, ultrasensitive molecularly imprinted polymer-based electrochemical sensor for boscalid detection in grape, tomato, and berry samples. Food Chem. 2025, 490, 145186. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, D.C.; Dhamu, V.N.; Samson, M.; Muthukumar, S.; Prasad, S. Pesticide analytical screening system (PASS): A novel electrochemical system for multiplex screening of glyphosate and chlorpyrifos in high-fat and low-fat food matrices. Food Chem. 2023, 400, 134075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Zhu, M.C.; Jiang, Y.J.; Wang, X.; Guo, Z.M.; Shi, J.Y.; Zou, X.B.; Han, E. Simple electrochemical sensing for mercury ions in dairy product using optimal Cu2+-based metal-organic frameworks as signal reporting. J. Hazard. Mater. 2020, 400, 123222. [Google Scholar] [CrossRef] [PubMed]
- Kerouad, S.; Forsal, I.; Talfana, A.; Kasbaji, M.; Edaala, M.-A. Simultaneous electrochemical detection of Pb(II) and Hg(II) in drinking water using a carbon paste electrode modified with bio-magnesium ferrite nanoparticles: A comparative study. Inorg. Chem. Commun. 2025, 177, 114232. [Google Scholar] [CrossRef]
- Chi, H.; Wen, X.; Li, H.; Tang, J.; Zhang, X.; Chen, H. An electrochemical immunosensing of electrochemically modified carbon cloth electrode based on functionalized gold nanoparticle-Prussian blue for the detection of aflatoxin B1 in vegetable oil industry. Food Chem. 2025, 471, 142765. [Google Scholar] [CrossRef]
- Cheng, F.; Huang, K.L.; Liu, S.Q.; Liu, J.L.; Deng, R.J. Surfactant carbonization to synthesize pseudocubic α-Fe2O3/C nanocomposite and its electrochemical performance in lithium-ion batteries. Electrochim. Acta 2011, 56, 5593–5598. [Google Scholar] [CrossRef]
- Aishwarya, K.G.; Nayaka, Y.A.; Pradeepa, E.; Sahana, H.R. Electrochemical determination of ascorbic acid using sensitive and disposable methylene blue modified pencil graphite electrode. Anal. Biochem. 2025, 698, 115733. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Dhanabalan, S.P.; Shanmugavel, B.; Jaffer, S.B.; Marimuthu, S.; Radhika, K.; Nagappan, E.; Johnson, I.; Periyannan, S. Aflatoxin evaluation and integrated management strategies to minimize toxin contamination in maize and peanuts. J. Agric. Food Res. 2025, 21, 101809. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, R. Electrochemical Determination of Monocrotophos in Water and Fruits using acetylcholinesterase immobilized on CNTs modified Carbon paste electrode. Int. J. Electrochem. Sci. 2021, 16, 211221. [Google Scholar] [CrossRef]
- Wei, J.; Wang, L.; Hu, J.; Wei, W.; Yang, Y.; Song, Y.; Li, Y.; Gao, G. Development of an origami paper-based electrochemical sensor using N-doped graphene for simultaneous detection of Cd(II), Pb(II), and Hg(II) in water. Microchem. J. 2025, 212, 113223. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Liu, M.; Jiang, P.; Guo, X.; Gan, T.; Wu, C.; Wu, K. Rapid room-temperature synthesis of disposable Co nanoparticles modified 3D N-doped porous carbon electrode device for efficient electrochemical detection of veterinary drug and pesticide residue. Carbon 2024, 219, 118806. [Google Scholar] [CrossRef]
- Bacchu, M.S.; Ali, M.R.; Das, S.; Akter, S.; Sakamoto, H.; Suye, S.I.; Rahman, M.M.; Campbell, K.; Khan, M.Z.H. A DNA functionalized advanced electrochemical biosensor for identification of the foodborne pathogen Salmonella enterica serovar Typhi in real samples. Anal. Chim. Acta 2022, 1192, 339332. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.A.M.; Moharam, M.M.; Jabir, M.S.; Ahmad, I.; Roopashree, R.; Kashyap, A.; Krithiga, T.; Ray, S.; Mohammed, I.A.; Jasim, H.A. Recent advances in photo/electrochemical biosensing of chemical food contaminants based on the porphyrin-MOFs nanohybrids. Microchem. J. 2025, 213, 113671. [Google Scholar] [CrossRef]
- Mohan, B.; Priyanka; Singh, G.; Chauhan, A.; Pombeiro, A.J.L.; Ren, P. Metal-organic frameworks (MOFs) based luminescent and electrochemical sensors for food contaminant detection. J. Hazard. Mater. 2023, 453, 131324. [Google Scholar] [CrossRef]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly imprinted polymer-based electrochemical sensors for food contaminants determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Wang, H.; Gao, X.; Niu, B.; Li, W.; Wang, H. Electrochemical biosensor based on copper sulfide/reduced graphene oxide/glucose oxidase construct for glucose detection. Anal. Biochem. 2025, 696, 115696. [Google Scholar] [CrossRef]
- Jana, J.; Sharma, T.S.K.; Chowdhury, S.; Ghanem, M.A.; Babu, B.M.; Kang, S.G.; Chung, J.S.; Choi, W.M.; Hur, S.H. Au-decorated α-Fe2O3-based electrochemical sensor for the detection of propyl gallate for monitoring preservative levels in packaged foods. Food Chem. 2025, 486, 144597. [Google Scholar] [CrossRef]
- Yemmi, R.; Swamy, B.E.K.; Manjunath, K.M.; Yusuf, K.; Aljuwayid, A.M.; Govindasamy, M. Advanced ZnFe2O4@f-CNF electrode: A robust electrochemical sensor for Tert-butyl hydroquinone detection in food and edible oil. Food Chem. 2025, 486, 144648. [Google Scholar] [CrossRef]
- Kotteeswaran, S.; Kondee, S.; Pon-On, W.; Al-Qahtani, W.H.; Wongchoosuk, C.; Govindasamy, M. CuZnS microflowers anchored on MXene nanosheets based electrochemical sensor for pico-molar determination of chloramphenicol in biological samples and dairy products. Microchem. J. 2025, 212, 113291. [Google Scholar] [CrossRef]
- Zhuang, L.H.; Gao, Y.M.; Wei, H.R.; Pei, L.Z.; Zhang, Y. Synthesis of bismuth antimony nanomaterials and electrochemical detection of benzoic acid. J. Appl. Electrochem. 2024, 54, 1075–1083. [Google Scholar] [CrossRef]
- Manuel, M.; Kanchi, S. Construction of a waste-derived graphite electrode integrated IL/Ni-MOF flowers/Co3O4 NDs for specific enrichment and signal amplification to detect aspartame. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135648. [Google Scholar] [CrossRef]
- Bindu, K.; Anusha, T.; Brahman, P.K. A novel electrochemical sensor for vitamin D3 detection in real samples using NiNPs-ZIF-8-modified electrode. Chem. Phys. Lett. 2025, 865, 141936. [Google Scholar] [CrossRef]
- Qin, Z.-N.; Chen, J.-M.; Li, J.-W.; Zhen, Z.-P.; Chen, Q.-Z.; Zhang, Z.-Q.; Wang, G.-H.; Gao, Y.-F. Rapid absolute quantification of glucose and fructose isomers in honey using a boronic acid-based reactive matrix by MALDI-TOF/TOF tandem mass spectrometry. Food Chem. 2025, 477, 143623. [Google Scholar] [CrossRef]
- Jyoti; Deepeka; Kaur, P.; Rana, S.; Singhal, S. Palladium-zinc ferrite varnished hydroxyapatite spherocuboids for electrochemical detection of carcinogenic food preservatives. Food Chem. 2025, 464, 141626. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, H.; Yadav, A.; Sharma, R.; Kumari, A.; Kumar, A. Metal nanocomposites-based electrochemical sensor for the detection of Vanillin (food additives): Experimental and theoretical approach. Food Biosci. 2023, 52, 102464. [Google Scholar] [CrossRef]
- Cirillo, C.; Iuliano, M.; Astorga, E.N.; Sarno, M. RuO2/IrO2 nanoparticles on graphene modified screen-printed electrode for enhanced electrochemical detection of ascorbic acid. Surf. Interfaces 2025, 72, 106943. [Google Scholar] [CrossRef]
- Parasuraman, B.; Chinnapayan, S.; Rangaraju, H.; Paramasivam, S.; Sangaraju, S.; Thangavelu, P.; Huang, C.-H. Rapid detection of caffeic acid in food beverages using a non-enzymatic electrochemical sensor based on a Bi2S3/CNF nanocomposite. Sustain. Food Technol. 2024, 2, 717–728. [Google Scholar] [CrossRef]
- Wang, K.; Pei, L.; Liu, H. A point-of-care electrochemical DNA sensor for rapid and sensitive detection of human papillomavirus type 18. Int. J. Electrochem. Sci. 2025, 20, 101074. [Google Scholar] [CrossRef]
- Silveri, F.; Scroccarello, A.; Della Pelle, F.; Del Carlo, M.; Compagnone, D. Rapid pretreatment-free evaluation of antioxidant capacity in extra virgin olive oil using a laser-nanodecorated electrochemical lab-on-strip. Food Chem. 2023, 420, 136112. [Google Scholar] [CrossRef]
- Brazys, E.; Ratautaite, V.; Brasiunas, B.; Ramanaviciene, A.; Rodríguez, L.; Pinto, A.; Milea, D.; Prentice, U.; Ramanavicius, A. Molecularly imprinted polypyrrole-based electrochemical melamine sensors. Microchem. J. 2024, 199, 109890. [Google Scholar] [CrossRef]
- Ting, W.-T.; Ali, M.Y.; Mitea, V.; Wang, M.-J.; Howlader, M.M.R. Polyaniline-based bovine serum albumin imprinted electrochemical sensor for ultra-trace-level detection in clinical and food safety applications. Int. J. Biol. Macromol. 2024, 277, 134137. [Google Scholar] [CrossRef]
- Wu, Y.; Al-Huqail, A.; Farhan, Z.A.; Alkhalifah, T.; Alturise, F.; Ali, H.E. Enhanced artificial intelligence for electrochemical sensors in monitoring and removing of azo dyes and food colorant substances. Food Chem. Toxicol. 2022, 169, 113398. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.; Liu, N.; Liu, C.; Jiang, A.; Chen, L. Integrating AI with detection methods, IoT, and blockchain to achieve food authenticity and traceability from farm-to-table. Trends Food Sci. Technol. 2025, 158, 104925. [Google Scholar] [CrossRef]
- Seleka, W.M.; Makhado, E.; Kganyakgo, L.K.; Mofokeng, L.E.; Makwakwa, D.; Botlhoko, O.J. Development of a rapid responsive conductive electrochemical sensor for sensitive hydrogen detection: Chitosan-based GO/Fe3O4/PANi hydrogel nanocomposite. Int. J. Hydrog. Energy 2025, 142, 498–510. [Google Scholar] [CrossRef]
- Lu, C.; Wang, G.; Zhang, X.; Fang, Z.; Wang, X.; Tang, F.; Ning, K.; Xu, M.; Wang, J.; Jiang, H.; et al. Cost-effective, label-free electrochemical aptasensors for rapid detection of concanavalin A with screen printed electrodes. Food Chem. 2025, 476, 143338. [Google Scholar] [CrossRef]
- Sedighi, V.; Eshlaghi, S.N.; Faridbod, F. Polyelectrolyte Dot Approached and Evaluation of Functionalized Graphene Modified Electrode Electrochemical Behavior for Detection of the dye, as an Additive color Food Abused. Food Chem. Adv. 2025, 100971. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, W.; Wu, D.; Yang, Y.; Feng, X.; Gao, C.; Meng, L.; Shen, X.; Zhang, Y.; Tang, X. An electrochemical method for determination of amaranth in drinks using functionalized graphene oxide/chitosan/ionic liquid nanocomposite supported nanoporous gold. Food Chem. 2022, 367, 130727. [Google Scholar] [CrossRef]
- Zein, M.I.H.L.; Kharismasari, C.Y.; Hardianto, A.; Zakiyyah, S.N.; Amalia, R.; Ozsoz, M.; Mirasoli, M.; Irkham; Hartati, Y.W. A CRISPR/Cas12a electrochemical biosensing to detect pig mtDNA D-loop for ensuring food authenticity. Sens. Bio-Sens. Res. 2025, 47, 100755. [Google Scholar] [CrossRef]
- Mutz, Y.S.; do Rosario, D.; Silva, L.R.G.; Galvan, D.; Stefano, J.S.; Janegitz, B.C.; Weitz, D.A.; Bernardes, P.C.; Conte-Junior, C.A. Lab-made 3D printed electrochemical sensors coupled with chemometrics for Brazilian coffee authentication. Food Chem. 2023, 403, 134411. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Alharthi, S.S.; Al-Saidi, H.M.; Kumar, D. Nanomolar electrochemical detection of feed additive ractopamine in meat samples using spinel zinc ferrite decorated 3-dimensional graphene nanosheets to combat food fraud in livestock industries. Food Chem. 2024, 437, 137868. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qi, X.; Wu, J.; Wan, X.; Wang, T.; Liu, Y.; Chen, Y.; Xia, Y. Highly stable electrochemical sensing platform for the selective determination of pefloxacin in food samples based on a molecularly imprinted-polymer-coated gold nanoparticle/black phosphorus nanocomposite. Food Chem. 2024, 436, 137753. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, J.; Liu, H.; Yi, J.; Xia, F.; Tian, D.; Zhou, C. Multiplexed electrochemical aptasensor based on mixed valence Ce(III, IV)-MOF for simultaneous determination of malathion and chlorpyrifos. Anal. Chim. Acta 2022, 1230, 340364. [Google Scholar] [CrossRef]
- Liang, Q.; Xiao, W.; Zhang, C.; Zhu, D.; Wang, S.-L.; Tian, S.-Y.U.; Long, T.; Yue, E.-L.; Wang, J.-J.; Hou, X.Y. MOFs-based Fe@YAU-101/GCE electrochemical sensor platform for highly selective detecting trace multiplex heavy metal ions. Talanta 2023, 259, 124491. [Google Scholar] [CrossRef]
- Yin, H.; He, H.; Li, T.; Hu, M.; Huang, W.; Wang, Z.; Yang, X.; Yao, W.; Xiao, F.; Wu, Y.; et al. Ultra-sensitive detection of multiplexed heavy metal ions by MOF-derived carbon film encapsulating BiCu alloy nanoparticles in potable electrochemical sensing system. Anal. Chim. Acta 2023, 1239, 340730. [Google Scholar] [CrossRef]
- Jiang, B.; Jiang, N.; Cui, Y.; Wang, H.; Zhang, G.; Li, J.; Zhang, Y. Rapid Synthesis and Microenvironment Optimization of Hierarchical Porous Fe―N―C Catalysts for Enhanced ORR in Microbial Fuel Cells. Adv. Sci. 2024, 11, 2402610. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, D.; Wen, W.; Tian, Z.; Zhang, X.; Wang, S. Self-powered electrochemical sensor based on photoelectrode: An up-to-date review. Coord. Chem. Rev. 2024, 518, 216095. [Google Scholar] [CrossRef]
- Chen, H.; Yao, Y.; Zhang, C.; Ping, J. Determination of Heavy Metal Ions in Infant Milk Powder Using a Nanoporous Carbon Modified Disposable Sensor. Foods 2023, 12, 730. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghorai, S.; Dhara, M.; Naskar, H.; Ali, S.B.; Das, N.; Saha, P.; Tudu, B.; Chatterjee, A.; Bandyopadhyay, R.; et al. Accurate prediction of piperine content in black pepper using combined CNN and regression modelling with PDMAM@G electrode and cyclic voltammetry. J. Food Compos. Anal. 2025, 141, 107355. [Google Scholar] [CrossRef]
- Sun, X.; Yin, Y.; Chen, H.; Zhao, L.; Wang, C.; Wang, J. Effect of iron-based oxide-modified electrodes on the selectivity of carbon chain elongation metabolites in electro-fermentation system. Bioresour. Technol. 2025, 431, 132588. [Google Scholar] [CrossRef]
- Jia, X.; Guo, H.; Hao, Y.; Shi, J.; Chai, R.; Wang, S.; Wu, H.; Feng, Y.; Ji, W.; Wu, S. Dual recognition electrochemical sensor for detection of gallic acid in teas, apples and grapes and derived products. Food Chem. 2025, 490, 145063. [Google Scholar] [CrossRef]
- Tao, Q.; Liu, T.; Si, M.; Wang, X. High-reliability simultaneous detection of trace heavy metal ions in complex aquatic products: Performance evaluation and optimization of electrochemical strategies. Food Chem. 2025, 490, 145094. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Rafi, J.; Reza, S.; Thapa, R.; Mathur, S.; Bernaurdshaw, N. Enhancing host-guest interactions through interfacial modulation of IRMOF-MXene hybrids: A detailed study on the significance of accessible functional groups in electrochemical detection. Mater. Res. Bull. 2025, 192, 113611. [Google Scholar] [CrossRef]
- Shahtouri, M.G.; Fooladi, E.; Marrazza, G.; Jahani, M.; Feizy, J. Development of laccase electrochemical biosensor for detection of Dephostatin using ZrO2/β-cyclodextrin/Polyaniline nanocomposite. Microchem. J. 2025, 210, 112930. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Evaluation of Olive Oil Quality with Electrochemical Sensors and Biosensors: A Review. Int. J. Mol. Sci. 2021, 22, 12708. [Google Scholar] [CrossRef]
- Salazar-Avalos, S.; Soliz, A.; Caceres, L.; Conejeros, S.; Brito, I.; Galvez, E.; Galleguillos Madrid, F.M. Metal Recovery from Natural Saline Brines with an Electrochemical Ion Pumping Method Using Hexacyanoferrate Materials as Electrodes. Nanomaterials 2023, 13, 2557. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Wang, Z.; Wu, F.; He, Y.; Liu, Y.; Deng, Y.; Bing, T.; Qiu, L.; Tan, W. DNA aptamer-based sensitive electrochemical biosensor for NAD (H) detection. Biosens. Bioelectron. 2025, 271, 116996. [Google Scholar] [CrossRef]
- Song, D.; Zhang, S.; Ning, H.; Fei, X.; Wang, M.; Wang, X.; Wu, W.; Zhao, Q.; Li, Y.; Wu, M. Self-supporting BiCu/carbon hybrid nanofiber membrane promotes efficient CO2 electroreduction to formate. Sci. China-Mater. 2024, 67, 788–795. [Google Scholar] [CrossRef]
- Liu, H.; Yao, K.; Hu, M.; Li, S.; Yang, S.; Zhao, A. On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells. Nanomaterials 2025, 15, 417. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Qin, C.; Nie, Q.; Luo, Y.; Qin, Q.-P.; Wang, R.; Liu, B.; Luo, D. An Electrochemical Sensor Based on Electropolymerization of β-Cyclodextrin on Glassy Carbon Electrode for the Determination of Fenitrothion. Sensors 2023, 23, 435. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghorai, S.; Naskar, H.; Manna, S.; Ahmed, A.H.M.T.; Biswas, D.; Dasgupta, S.; Bhattacharya, I.; Tudu, B.; Chatterjee, A.; et al. A novel TMS@G-MIP electrochemical sensor for selective detection of p-coumaric acid in fruits using voltammetry signal and CNN. J. Food Compos. Anal. 2025, 146, 107925. [Google Scholar] [CrossRef]
- Li, C.-Y.; Tian, Z.-Q. Sixty years of electrochemical optical spectroscopy: A retrospective. Chem. Soc. Rev. 2024, 53, 3579–3605. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.; Wang, Y.; Chen, X.; Niu, H.; Gu, S.; Kang, H.; Liu, J.; Luo, L.; Liu, L.; et al. Research progress on construction and application of aminoglycosides optical and electrochemical sensors. Food Ferment. Ind. 2024, 50, 351–360. [Google Scholar]
- Al Borhani, W.; Rhouati, A.; Cialla-May, D.; Popp, J.; Zourob, M. Multiplex electrochemical aptasensor for the simultaneous detection of linomycin and neomycin antibiotics. Talanta 2025, 282, 126922. [Google Scholar] [CrossRef]
- Lin, H.; Yan, T.; Yang, Q.; Lin, L.; Liu, L.; Xi, J. Electrochemical In Situ Characterization Techniques in the Field of Energy Conversion. Small Methods 2025, 9, 2401701. [Google Scholar] [CrossRef]
- McGowan, M.P.; Trowbridge, A.J.; Reitemeier, J.; Jordan, K.M.; Fu, K.X. Surface charge effects of monovalent and zwitterionic monolayers to differentiate structurally similar aminoglycosides with electrochemical aptamer biosensors. Biosens. Bioelectron. 2025, 276, 117229. [Google Scholar] [CrossRef]
- Wu, S.; Wu, S.; Zhang, X.; Feng, T.; Wu, L. Chitosan-Based Hydrogels for Bioelectronic Sensing: Recent Advances and Applications in Biomedicine and Food Safety. Biosensors 2023, 13, 93. [Google Scholar] [CrossRef]
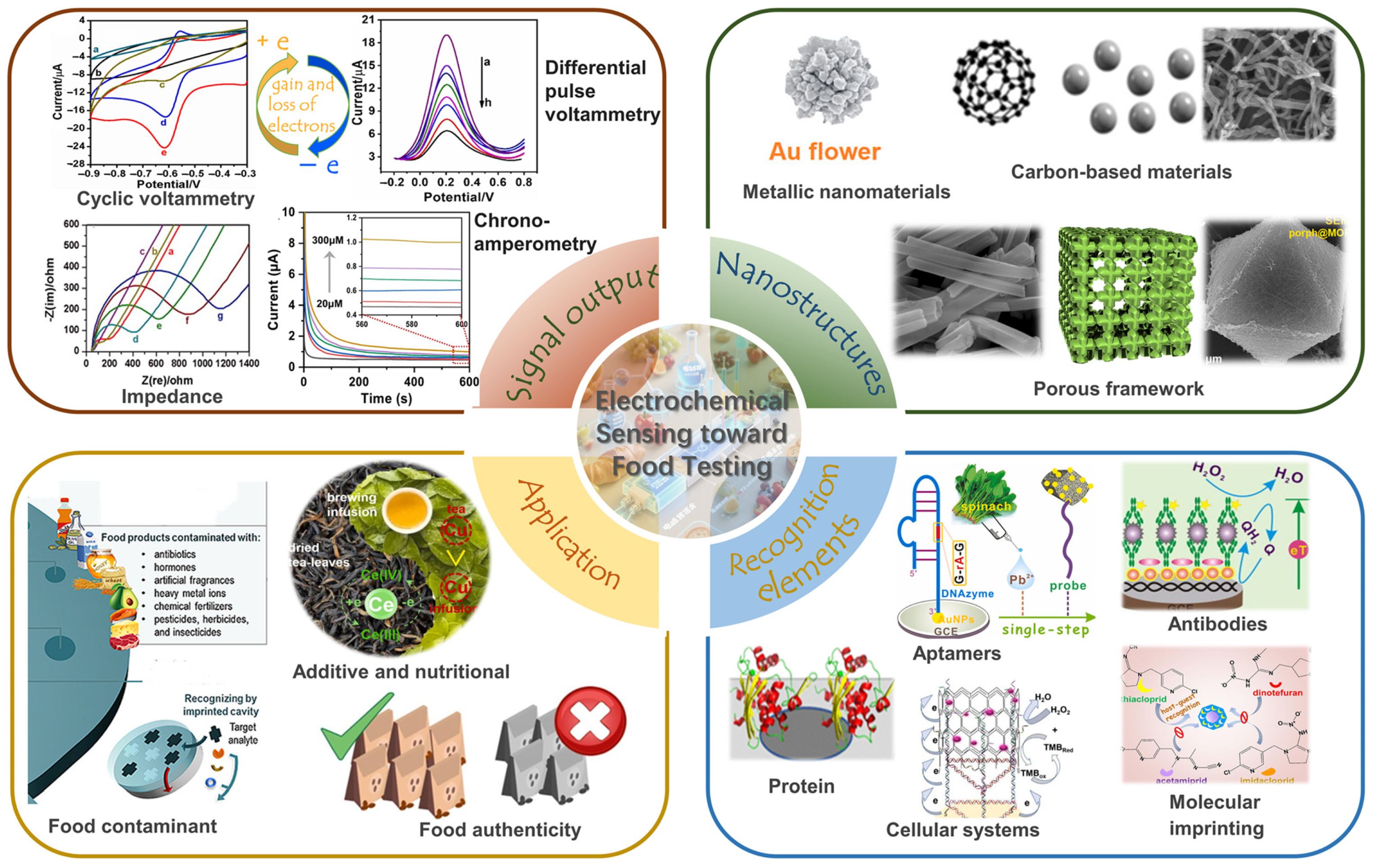

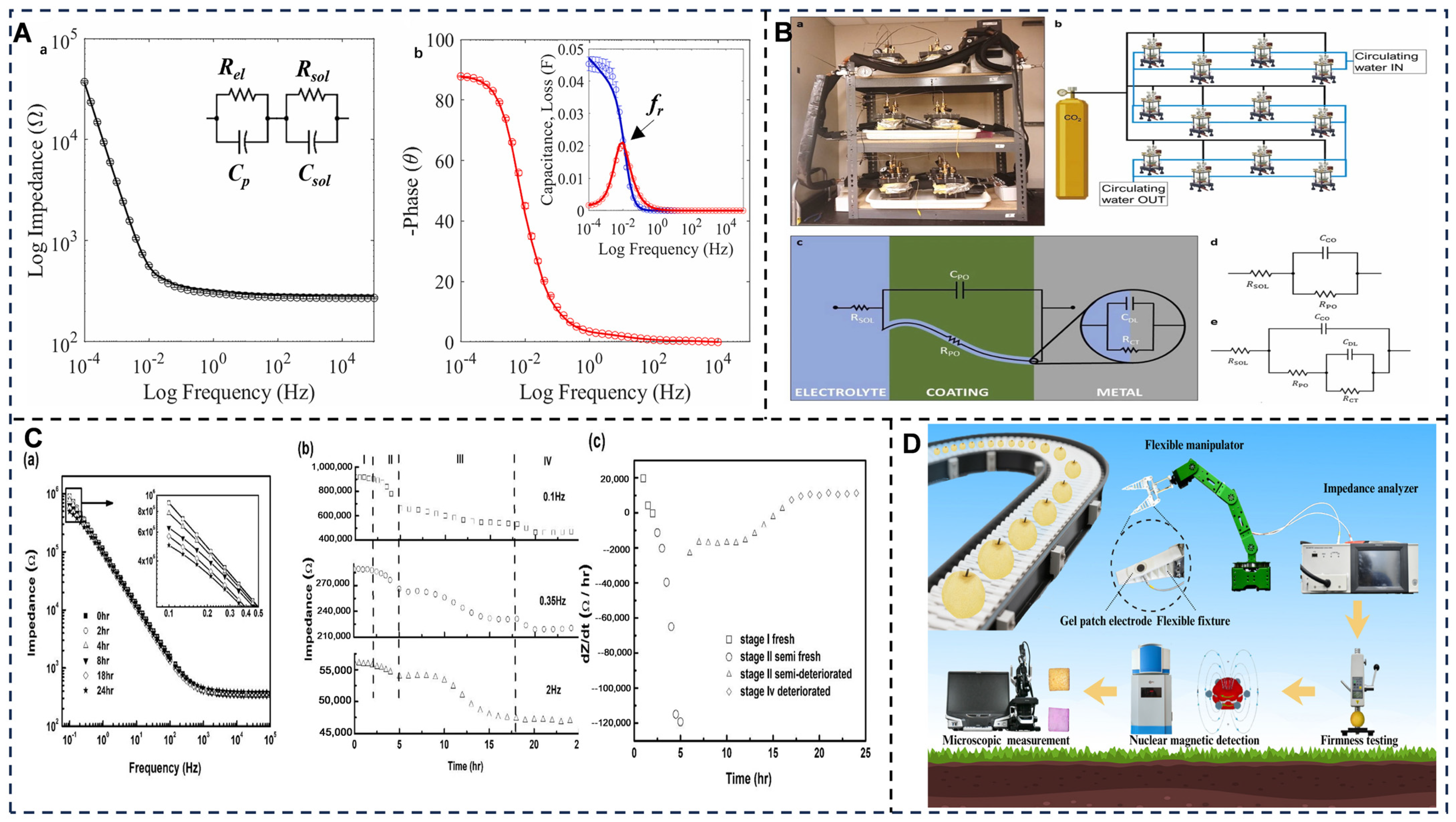
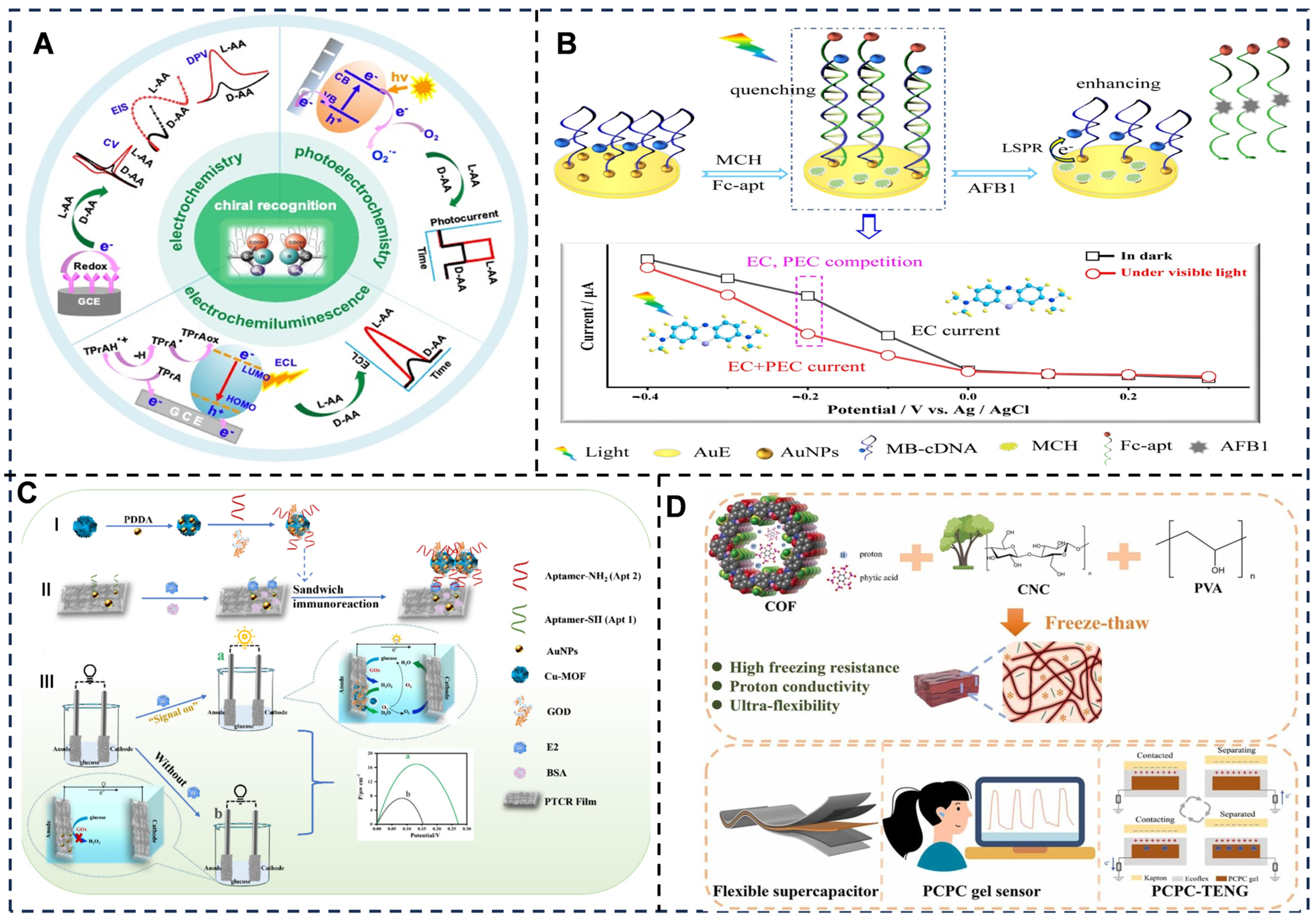

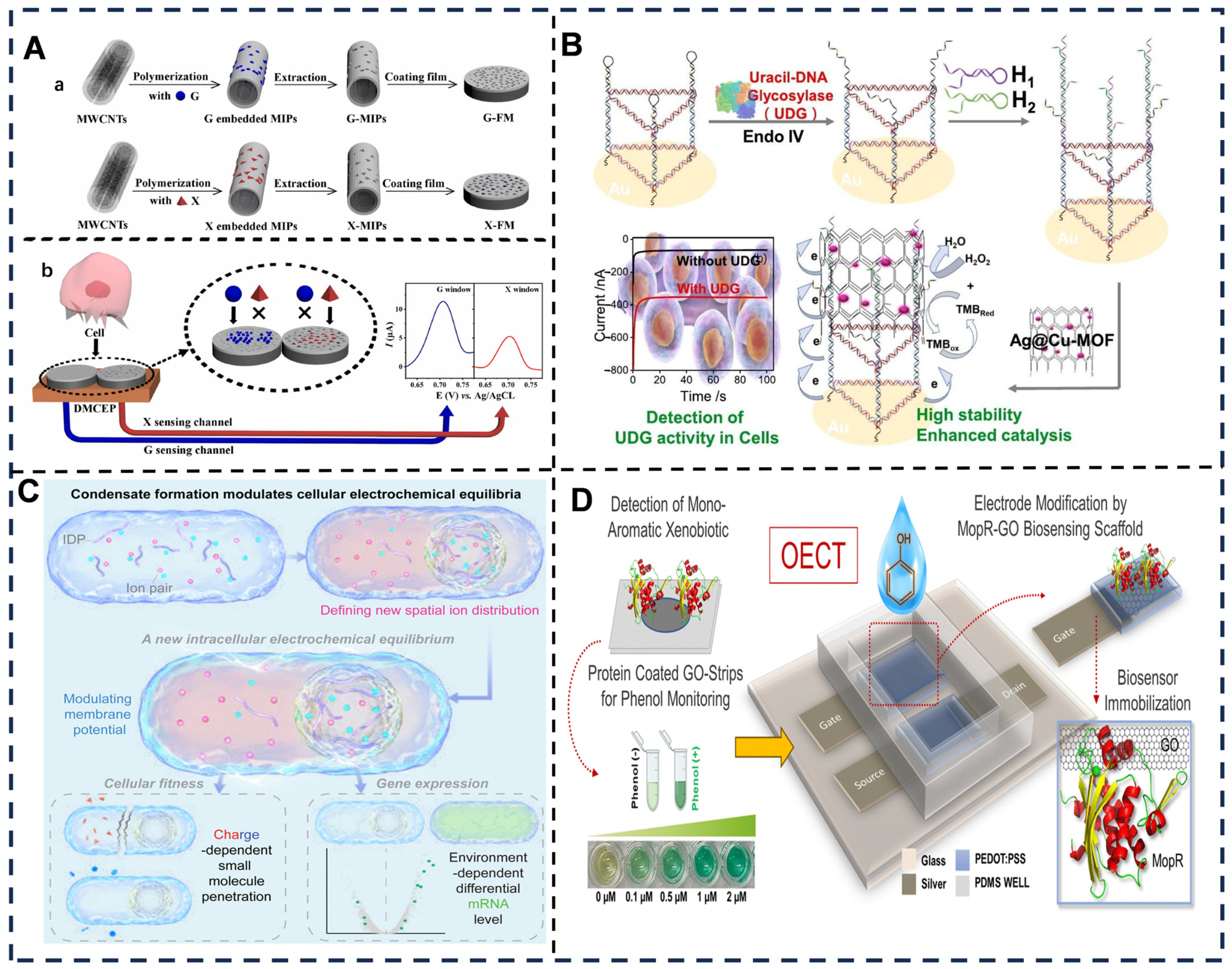
| Category | Key Content | Technical Highlights and Performance Parameters |
|---|---|---|
| Detection Targets | ||
| Small Molecules | Toxins (aflatoxin B1), additives (benzoic acid/aspartame), and pesticides (organophosphates) |
|
| Microorganisms | Pathogens (Salmonella and E. coli) and viruses |
|
| Ions | Heavy metals (Pb2+ and Hg2+) and trace elements |
|
| Macromolecules | Allergenic proteins, DNA adulteration markers |
|
| Recognition Elements | ||
| Aptamers | SELEX-selected DNA/RNA and conformational switching for small molecules |
|
| Antibodies | Sandwich/competitive assays for pathogens/toxins |
|
| Molecularly Imprinted Polymers (MIPs) | Synthetic cavities for pesticides/additives |
|
| Cells/Enzymes | Whole-cell metabolic monitoring (histamine) and enzymatic catalysis (glucose oxidase) |
|
| Detection Principles | ||
| Direct Current (DC) | Voltammetry (DPV/SWV): heavy metals/additives; Amperometry: steady-state catalytic current |
|
| Alternating Current (AC) | EIS for interface changes (e.g., bacterial adhesion); Conductometric sensors |
|
| Emerging Modes | Photoelectrochemistry (PEC): light-controlled e− transfer; Self-powered systems: microcystin-RR detection |
|
| Signal Amplification | ||
| Nanomaterials | Au/Ag NPs, graphene, and MOFs for enhanced surface area |
|
| Catalytic Cascades | Enzyme-linked reactions (HRP), nanozymes (Fe3O4@MIL-101), and CRISPR-Cas amplification |
|
| Redox Cycling | [Ru(NH3)6]3+/mediator pairs |
|
| Application Scenarios | ||
| Contaminant Screening | Mycotoxins (AFB1), heavy metals, and pathogens |
|
| Nutrients and Additives | Antioxidants (ascorbic acid), preservatives, and sweeteners |
|
| Authenticity Verification | DNA sensors for meat adulteration; MIPs for olive oil markers |
|
| Future Directions | ||
| Intelligent Systems | AI-assisted EIS analysis (neural networks); Blockchain data traceability |
|
| Sustainability | Biodegradable electrodes (chitosan/cellulose); Microbial fuel cells |
|
| System Integration | Microfluidic chip coupling; Flexible wearable sensors (patch-type detection) |
|
| Standardization Challenges | Lack of unified performance criteria; Matrix interference in real samples |
|
| Parameter | Electrochemical Biosensors | Optical Biosensors | Technical Impact | SERS |
|---|---|---|---|---|
| Detection Limit | 0.01–0.1 ppb (Nanomaterial amplification) | 0.1–10 ppb (SERS/Fluorescence) | Electrochemical biosensors superior for trace contaminants | [322] |
| Response Time | <10 min (Direct electron transfer) | ≥30 min (SPR equilibrium required) | Electrochemical biosensors ideal for on-site decisions | [322] |
| Equipment Cost | USD 50–USD 500 (Handheld reader) | USD 5000–USD 50,000 (SPR/Raman systems) | Electrochemical cost: 1–10% of optical | [323] |
| Multiplexing Capability | Excellent (16-channel microarray) | Moderate (3-channel fluorescence) | Electrochemical biosensors enable high-throughput | [305,324] |
| Matrix Interference Resistance | Poor >30% signal attenuation (e.g., olive oil) | Excellent (SPR resists nonspecific adsorption) | Optical biosensors are better for dark/turbid samples (e.g., juice) | [322] |
| Long-term Stability | <30 days (Enzyme activity decay) | >6 months (Fiber-optic sensors) | Optical biosensors suitable for long-term monitoring | [323] |
| Spatial Resolution | None | Micrometer-level (Fluorescence imaging) | Optical biosensors uniquely visualize contaminant distribution | [325] |
| Sample Pretreatment Demand | High (Enzymatic digestion for meat) | Low (SERS direct detection of turbid liquids) | Optical biosnesors reduce processing by 50% | [322] |
| Typical Applications | -Pesticide screenin -Cold chain monitoring | -Food fraud lab verification -Non-destructive packaged food testing | Complementarity > Competition | [323] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yuan, Z.; Gao, S.; Zhang, X.; El-Mesery, H.S.; Lu, W.; Dai, X.; Xu, R. Electrochemical Biosensors Driving Model Transformation for Food Testing. Foods 2025, 14, 2669. https://doi.org/10.3390/foods14152669
Wu X, Yuan Z, Gao S, Zhang X, El-Mesery HS, Lu W, Dai X, Xu R. Electrochemical Biosensors Driving Model Transformation for Food Testing. Foods. 2025; 14(15):2669. https://doi.org/10.3390/foods14152669
Chicago/Turabian StyleWu, Xinxin, Zhecong Yuan, Shujie Gao, Xinai Zhang, Hany S. El-Mesery, Wenjie Lu, Xiaoli Dai, and Rongjin Xu. 2025. "Electrochemical Biosensors Driving Model Transformation for Food Testing" Foods 14, no. 15: 2669. https://doi.org/10.3390/foods14152669
APA StyleWu, X., Yuan, Z., Gao, S., Zhang, X., El-Mesery, H. S., Lu, W., Dai, X., & Xu, R. (2025). Electrochemical Biosensors Driving Model Transformation for Food Testing. Foods, 14(15), 2669. https://doi.org/10.3390/foods14152669





