Evaluating the Effect of Thermal Treatment on Phenolic Compounds in Functional Flours Using Vis–NIR–SWIR Spectroscopy: A Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Flour Samples and Chemicals
2.2. Granulometric Analysis
2.3. Chemical Characterization of Flours
2.3.1. Heat Treatment of Flours
2.3.2. Determination of Total Phenolic Content (TPC)
2.4. Spectroscopic Characterization of Flours
2.4.1. Vis–NIR–SWIR Analysis
2.4.2. Spectral Processing Techniques
Spectral Pre-Treatments
Machine Learning Modeling
Explainability Analysis
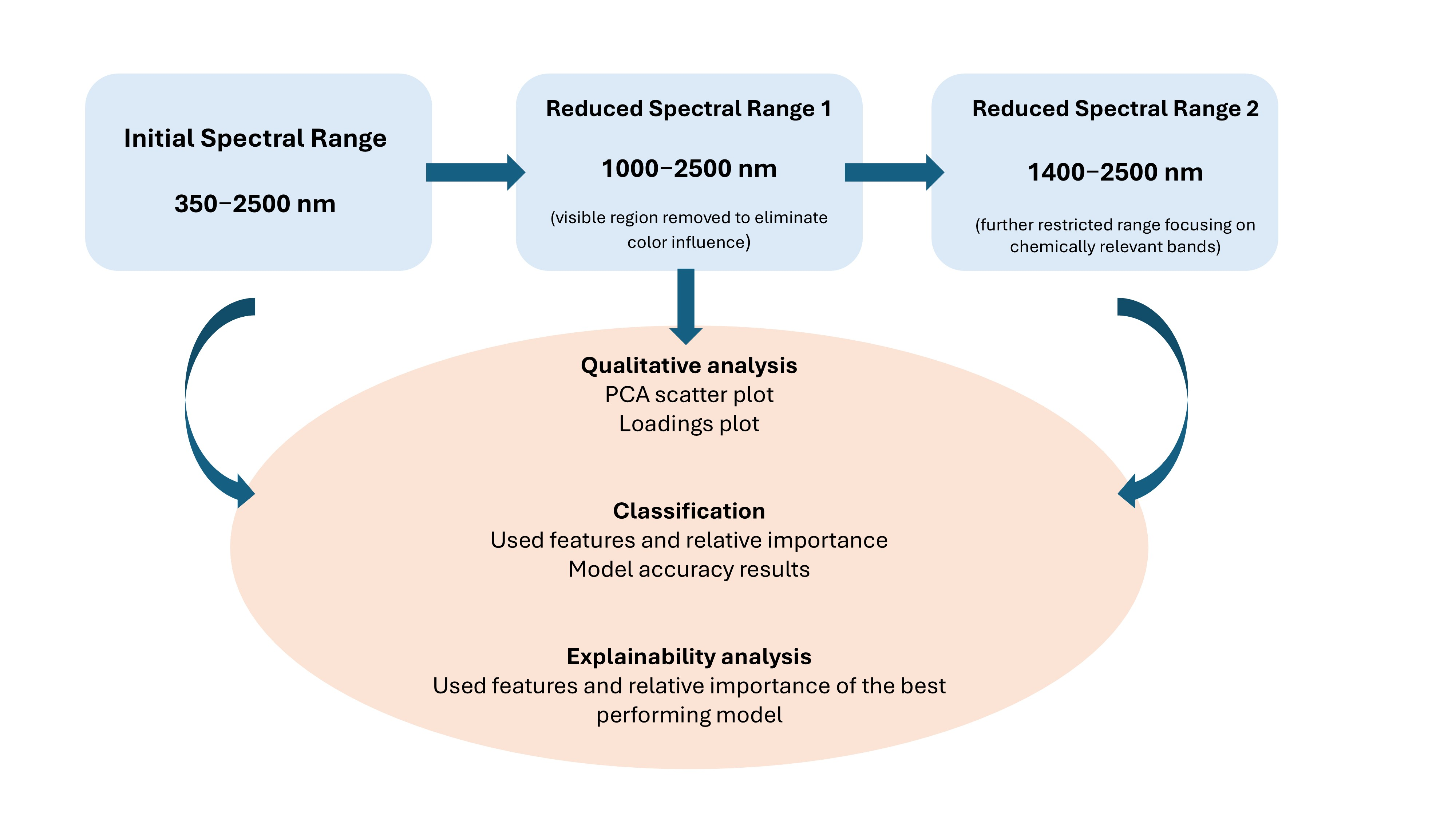
Spectral Range Selection and Processing Workflow
2.5. Statistical Analysis
3. Results and Discussion
3.1. Color Assesment of Functional Flours
3.2. Chemical Characterization of Functional Flours
3.3. Initial Evaluation Using the Complete Vis–NIR–SWIR Spectral Range (350–2500 nm)
3.4. Spectral Interpretation and Performance
3.4.1. Qualitative Analysis
3.4.2. Classification of Functional Flours from Vis–NIR–SWIR Spectra
3.4.3. Explainability Analysis from Vis–NIR–SWIR Spectra
3.5. Study Strengths, Limitations and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannoutsos, K.; Zalidis, A.P.; Koukoumaki, D.I.; Menexes, G.; Mourtzinos, I.; Sarris, D.; Gkatzionis, K. Production of functional crackers based on non-conventional flours. Study of the physicochemical and sensory properties. Food Chem. Adv. 2023, 2, 100194. [Google Scholar] [CrossRef]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgis, A.Z.; Beyene, F. Nutritional quality and health benefits of okra (Abelmoschus esculentus): A review. J. Food Process Technol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Seal, C.J.; Courtin, C.M.; Venema, K.; de Vries, J. Health benefits of whole grain: Effects on dietary carbohydrate quality, the gut microbiome, and consequences of processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Dietary phenolic compounds, health benefits and bioaccessibility. Arch. Latinoam. De Nutr. 2016, 66, 87–100. [Google Scholar]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals derived from agricultural residues and their valuable properties and applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef] [PubMed]
- Leváková, Ľ.; Lacko-Bartošová, M. Phenolic acids and antioxidant activity of wheat species: A review. Agriculture 2017, 63, 92. [Google Scholar] [CrossRef]
- Vollmannova, A.; Lidikova, J.; Musilova, J.; Snirc, M.; Bojnanska, T.; Urminska, D.; Zetochova, E. White Lupin as a promising source of antioxidant phenolics for functional food production. J. Food Qual. 2021, 2021, 5512236. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Health risks and benefits of chickpea (Cicer arietinum) consumption. J. Agric. Food Chem. 2017, 65, 6–22. [Google Scholar] [CrossRef]
- Zalidis, A.P.; Kalogiouri, N.P.; Mourtzinos, I.; Sarris, D.; Gkatzionis, K. A Novel Liquid Chromatographic Time-of-Flight Tandem Mass Spectrometric Method for the Determination of Secondary Metabolites in Functional Flours Produced from Grape Seed and Olive Stone Waste. Molecules 2025, 30, 1527. [Google Scholar] [CrossRef]
- El-Safy, F.S.; Salem, R.H.; Abd El-Ghany, M.E. Chemical and nutritional evaluation of different seed flours as novel sources of protein. World J. Dairy Food Sci. 2012, 7, 59–65. [Google Scholar]
- Tadini, C.C.; Gut, J.A. The importance of heating unit operations in the food industry to obtain safe and high-quality products. Front. Nutr. 2022, 9, 853638. [Google Scholar] [CrossRef] [PubMed]
- Dias-Martins, A.M.; Pessanha, K.L.F.; Pacheco, S.; Rodrigues, J.A.S.; Carvalho, C.W.P. Potential use of pearl millet (Pennisetum glaucum (L.) R. Br.) in Brazil: Food security, processing, health benefits and nutritional products. Food Res. Int. 2018, 109, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Forghani, F.; den Bakker, M.; Liao, J.Y.; Payton, A.S.; Futral, A.N.; Diez-Gonzalez, F. Salmonella and enterohemorrhagic Escherichia coli serogroups O45, O121, O145 in wheat flour: Effects of long-term storage and thermal treatments. Front. Microbiol. 2019, 10, 323. [Google Scholar] [CrossRef]
- Ferreyra, S.; Bottini, R.; Fontana, A. Temperature and light conditions affect stability of phenolic compounds of stored grape cane extracts. Food Chem. 2023, 405, 134718. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Jolayemi, O.S.; Giovenzana, V.; Tugnolo, A.; Squeo, G.; Conte, P.; Alamprese, C. Near infrared spectroscopy as a green technology for the quality prediction of intact olives. Foods 2021, 10, 1042. [Google Scholar] [CrossRef]
- Chaukhande, P.; Luthra, S.K.; Patel, R.N.; Padhi, S.R.; Mankar, P.; Mangal, M.; Riar, A.S. Development and validation of near-infrared reflectance spectroscopy prediction modeling for the rapid estimation of biochemical traits in potato. Foods 2024, 13, 1655. [Google Scholar] [CrossRef]
- Ozaki, Y.; Huck, C.; Tsuchikawa, S.; Engelsen, S.B. Near-Infrared Spectroscopy: Theory, Spectral Analysis, Instrumentation, and Applications; Springer: Singapore, 2021; p. 978-9811586477. [Google Scholar]
- Falcioni, R.; Moriwaki, T.; Antunes, W.C.; Nanni, M.R. Rapid quantification method for yield, calorimetric energy and chlorophyll a fluorescence parameters in nicotiana tabacum l. using vis-nir-swir hyperspectroscopy. Plants 2022, 11, 2406. [Google Scholar] [CrossRef]
- Freitag, S.; Sulyok, M.; Logan, N.; Elliott, C.T.; Krska, R. The potential and applicability of infrared spectroscopic methods for the rapid screening and routine analysis of mycotoxins in food crops. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5199–5224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xing, Y.; Lu, R.; Huang, M.; Ng, P.K. Visible/shortwave near infrared spectroscopy and hyperspectral scattering for determining bulk density and particle size of wheat flour. J. Near Infrared Spectrosc. 2017, 25, 116–126. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Zhang, G.; Wang, D.; Tilley, M.; Li, Y. Rapid determination of total phenolic content of whole wheat flour using near-infrared spectroscopy and chemometrics. Food Chem. 2021, 344, 128633. [Google Scholar] [CrossRef] [PubMed]
- Albanell, E.; Martínez, M.; De Marchi, M.; Manuelian, C.L. Prediction of bioactive compounds in barley by near-infrared reflectance spectroscopy (NIRS). J. Food Compos. Anal. 2021, 97, 103763. [Google Scholar] [CrossRef]
- Bolea, C.; Turturică, M.; Stănciuc, N.; Vizireanu, C. Thermal degradation kinetics of bioactive compounds from black rice flour (Oryza sativa L.) extracts. J. Cereal Sci. 2016, 71, 160–166. [Google Scholar] [CrossRef]
- Tsakanikas, P.; Karnavas, A.; Panagou, E.Z.; Nychas, G.J. A machine learning workflow for raw food spectroscopic classification in a future industry. Sci. Rep. 2020, 10, 11212. [Google Scholar] [CrossRef]
- Kusumiyati, K.; Asikin, Y. Machine learning-based prediction of total phenolic and flavonoid in horticultural products. Open Agric. 2023, 8, 20220163. [Google Scholar] [CrossRef]
- Tziolas, N.; Ordoudi, S.A.; Tavlaridis, A.; Karyotis, K.; Zalidis, G.; Mourtzinos, I. Rapid Assessment of Anthocyanins Content of Onion Waste through Visible-Near-Short-Wave and Mid-Infrared Spectroscopy Combined with Machine Learning Techniques. Sustainability 2021, 13, 6588. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, N.; Sharma, P.; Ananthan, R.; Singh, M.; Singh, A.K.; Bhardwaj, R. Optimizing protein content prediction in rice bean (Vigna umbellata L.) by integrating near-infrared reflectance spectroscopy, MPLS, deep learning, and key wavelengths selection algorithms. J. Food Compos. Anal. 2024, 135, 106655. [Google Scholar] [CrossRef]
- Jahreis, G.; Brese, M.; Leiterer, M.; Schaefer, U.; Boehm, V. Legume flours: Nutritionally important sources of protein and dietary fiber. Ernahr. Umsch. 2016, 63, 36–42. [Google Scholar]
- AACC. AACC Method 66-20.01. Determination of granularity of semolina and farina: Sieving method. In AACC Approved Methods of Analysis, 11th ed.; American Association of Cereal Chemists International: St. Paul, MN, USA, 1999. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Zhang, Z. Introduction to machine learning: K-nearest neighbors. Ann. Transl. Med. 2016, 4, 218. [Google Scholar] [CrossRef]
- Lowe, M.; Qin, R.; Mao, X. A review on machine learning, artificial intelligence, and smart technology in water treatment and monitoring. Water 2022, 14, 1384. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Arrieta, A.B.; Díaz-Rodríguez, N.; Del Ser, J.; Bennetot, A.; Tabik, S.; Barbado, A.; Herrera, F. Explainable Artificial Intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion 2020, 58, 82–115. [Google Scholar] [CrossRef]
- Linardatos, P.; Papastefanopoulos, V.; Kotsiantis, S. Explainable ai: A review of machine learning interpretability methods. Entropy 2020, 23, 18. [Google Scholar] [CrossRef]
- Antwarg, L.; Miller, R.M.; Shapira, B.; Rokach, L. Explaining anomalies detected by autoencoders using Shapley Additive Explanations. Expert Syst. Appl. 2021, 186, 115736. [Google Scholar] [CrossRef]
- Ross, B.C. Mutual information between discrete and continuous data sets. PLoS ONE 2014, 9, e87357. [Google Scholar] [CrossRef]
- Maray, A.R.M. Physicochemical and Functional Properties, Nutritional Value and Bioactive Compounds of Some Composite Flours. Assiut J. Agric. Sci. 2023, 54, 116–131. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Thermal processing for the release of phenolic compounds from wheat and oat bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between phenolic acids, proteins, and carbohydrates—Influence on dough and bread properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef]
- Mazumder, K.; Nabila, A.; Aktar, A.; Farahnaky, A. Bioactive variability and in vitro and in vivo antioxidant activity of unprocessed and processed flour of nine cultivars of Australian lupin species: A comprehensive substantiation. Antioxidants 2020, 9, 282. [Google Scholar] [CrossRef]
- Madurapperumage, A.; Tang, L.; Thavarajah, P.; Bridges, W.; Shipe, E.; Vandemark, G.; Thavarajah, D. Chickpea (Cicer arietinum L.) as a source of essential fatty acids–a biofortification approach. Front. Plant Sci. 2021, 12, 734980. [Google Scholar] [CrossRef]
- Rumiyati, R.; James, A.P.; Jayasena, V. Effects of lupin incorporation on the physical properties and stability of bioactive constituents in muffins. Int. J. Food Sci. Technol. 2015, 50, 103–110. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Zhao, Y. Phenolic compounds in whole grain sorghum and their health benefits. Foods 2021, 10, 1921. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef]
- Mark, H.; Workman, J. Derivatives in spectroscopy Part I-The behavior of the derivative. Spectroscopy 2003, 18, 32–37. [Google Scholar]
- Francisco García Martín, J. A Guide to Near-Infrared Spectroscopy; Nova Science Publishers: Hauppauge, NY, USA, 2023. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhang, Y.; Wang, D.; Wang, X.; Yu, L.; Li, P. Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils. Trends Food Sci. Technol. 2020, 101, 172–181. [Google Scholar] [CrossRef]
- Qi, Y.; Hu, D.; Jiang, Y.; Wu, Z.; Zheng, M.; Chen, E.X.; Chen, Y.P. Recent progresses in machine learning assisted Raman spectroscopy. Adv. Opt. Mater. 2023, 11, 2203104. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, Y.; Liu, C.; Li, J.; Sun, X.; Wu, J. Discrimination of wheat flour grade based on PSO-SVM of hyperspectral technique. Spectrochim. Acta: Part A: Mol. Biomol. Spectrosc. 2023, 302, 123050. [Google Scholar] [CrossRef]
- Ding, X.; Ni, Y.; Kokot, S. NIR spectroscopy and chemometrics for the discrimination of pure, powdered, purple sweet potatoes and their samples adulterated with the white sweet potato flour. Chemom. Intell. Lab. Syst. 2015, 144, 17–23. [Google Scholar] [CrossRef]
- Sampaio, P.S.; Castanho, A.; Almeida, A.S.; Oliveira, J.; Brites, C. Identification of rice flour types with near-infrared spectroscopy associated with PLS-DA and SVM methods. Eur. Food Res. Technol. 2020, 246, 527–537. [Google Scholar] [CrossRef]
- Badaró, A.T.; e Tavares, J.P.H.; Blasco, J.; Aleixos-Borrás, N.; Barbin, D.F. Near infrared techniques applied to analysis of wheat-based products: Recent advances and future trends. Food Control. 2022, 140, 109115. [Google Scholar] [CrossRef]
- Verdú, S.; Vásquez, F.; Ivorra, E.; Sánchez, A.J.; Barat, J.M.; Grau, R. Hyperspectral image control of the heat-treatment process of oat flour to model composite bread properties. J. Food Eng. 2017, 192, 45–52. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Research. 2018, 25, 266. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Fasoli, M.; Dell’Anna, R.; Dal Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Zenoni, S. Pectins, hemicelluloses and celluloses show specific dynamics in the internal and external surfaces of grape berry skin during ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef]
- Mahesar, S.A.; Lucarini, M.; Durazzo, A.; Santini, A.; Lampe, A.I.; Kiefer, J. Application of infrared spectroscopy for functional compounds evaluation in olive oil: A current snapshot. J. Spectrosc. 2019, 2019, 5319024. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins–A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Golea, C.M.; Codină, G.G.; Oroian, M. Prediction of wheat flours composition using fourier transform infrared spectrometry (FT-IR). Food Control. 2023, 143, 109318. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Cecchini, F. Grape seeds: Chromatographic profile of fatty acids and phenolic compounds and qualitative analysis by FTIR-ATR spectroscopy. Foods 2019, 9, 10. [Google Scholar] [CrossRef]
- Singh, S.; Majer, M.; Czarnecki, M.A.; Morisawa, Y.; Ozaki, Y. Solvent effect on assembling and interactions in solutions of phenol: Infrared spectroscopic and density functional theory study. Appl. Spectrosc. 2022, 76, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Ayub, H.; Sehrish, A.; Ambreen, S.; Khan, F.A.; Itrat, N.; Rocha, J.M. Essential components from plant source oils: A review on extraction, detection, identification, and quantification. Molecules 2023, 28, 6881. [Google Scholar] [CrossRef]






| Model | Hyperparameter | Values Tested |
|---|---|---|
| k-NN | Number of neighbors (k) | [1,20] |
| Distance metric | Euclidean and Cosine | |
| Decision Tree | Max features | |
| Random Forest | Max features | |
| Number of estimators | {10, 50, 100, 200} |
| °C (Mean ± SD) | WF | CF | LF | GSF | OSF |
|---|---|---|---|---|---|
| 25 °C | 0.662 ± 0.065 a | 0.721 ± 0.005 a | 5.547 ± 0.348 a | 88.121 ± 2.393 c | 18.774 ± 1.177 a |
| 74 °C | 0.852 ± 0.072 a | 0.787 ± 0.004 a | 7.320 ± 0.699 a | 91.807 ± 3.436 c | 19.885 ± 1.011 a |
| 110 °C | 0.541 ± 0.145 a | 0.528 ± 0.027 a | 5.276 ± 0.004 a | 91.590 ± 0.948 c | 18.684 ± 1.382 a |
| 145 °C | 0.543 ± 0.038 a | 0.565 ± 0.039 a | 4.562 ± 0.012 a | 79.677 ± 0.355 c | 17.886 ± 0.742 a |
| 180 °C | 0.745 ± 0.023 a | 0.669 ± 0.014 a | 5.621 ± 0.515 a | 60.912 ± 2.630 b | 15.713 ± 1.036 a |
| Class | 350 to 2500 nm | 1000 to 2500 nm | 1400 to 2500 nm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Prec. | Recall | F1-Score | Prec. | Recall | F1-Score | Prec. | Recall | F1-Score | |
| Flour type | |||||||||
| Wheat | 1.00 | 0.99 | 0.99 | 1.00 | 0.82 | 0.90 | 0.91 | 0.91 | 0.91 |
| Lupin | 0.98 | 0.98 | 0.98 | 0.97 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 |
| Chickpea | 0.97 | 0.98 | 0.98 | 0.97 | 0.97 | 0.97 | 0.92 | 0.92 | 0.92 |
| Grape seed | 0.98 | 0.98 | 0.98 | 0.98 | 0.96 | 0.97 | 0.98 | 0.98 | 0.98 |
| Olive stone | 0.98 | 0.98 | 0.98 | 0.96 | 0.98 | 0.97 | 0.98 | 0.98 | 0.98 |
| accuracy | 0.98 | 0.96 | 0.95 | ||||||
| Temperature | |||||||||
| 25 | 0.98 | 0.98 | 0.98 | 0.92 | 0.92 | 0.92 | 1.00 | 0.92 | 0.96 |
| 74 | 0.98 | 0.98 | 0.98 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| 110 | 0.98 | 0.97 | 0.97 | 0.79 | 0.85 | 0.81 | 0.77 | 0.77 | 0.77 |
| 145 | 0.97 | 0.98 | 0.98 | 0.60 | 0.75 | 0.67 | 0.58 | 0.88 | 0.70 |
| 180 | 0.98 | 0.98 | 0.98 | 0.90 | 0.69 | 0.78 | 0.80 | 0.62 | 0.70 |
| accuracy | 0.98 | 0.82 | 0.80 | ||||||
| Phenolics | |||||||||
| Low | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Medium | 0.97 | 0.98 | 0.98 | 0.94 | 0.97 | 0.95 | 0.94 | 0.98 | 0.95 |
| High | 1.00 | 0.99 | 0.99 | 0.99 | 0.98 | 0.98 | 0.99 | 0.98 | 0.98 |
| accuracy | 0.99 | 0.98 | 0.98 | ||||||
| Best model and optimal hyperparameters | |||||||||
| Random Forest with Ref. Max. feat.= , est. = 100 | Random Forest with Ref. + SG2 Max. feat.= , est. = 50 | Random Forest with Ref. + SG2 Max. feat.= , est. = 100 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalidis, A.P.; Tsakiridis, N.; Zalidis, G.; Mourtzinos, I.; Gkatzionis, K. Evaluating the Effect of Thermal Treatment on Phenolic Compounds in Functional Flours Using Vis–NIR–SWIR Spectroscopy: A Machine Learning Approach. Foods 2025, 14, 2663. https://doi.org/10.3390/foods14152663
Zalidis AP, Tsakiridis N, Zalidis G, Mourtzinos I, Gkatzionis K. Evaluating the Effect of Thermal Treatment on Phenolic Compounds in Functional Flours Using Vis–NIR–SWIR Spectroscopy: A Machine Learning Approach. Foods. 2025; 14(15):2663. https://doi.org/10.3390/foods14152663
Chicago/Turabian StyleZalidis, Achilleas Panagiotis, Nikolaos Tsakiridis, George Zalidis, Ioannis Mourtzinos, and Konstantinos Gkatzionis. 2025. "Evaluating the Effect of Thermal Treatment on Phenolic Compounds in Functional Flours Using Vis–NIR–SWIR Spectroscopy: A Machine Learning Approach" Foods 14, no. 15: 2663. https://doi.org/10.3390/foods14152663
APA StyleZalidis, A. P., Tsakiridis, N., Zalidis, G., Mourtzinos, I., & Gkatzionis, K. (2025). Evaluating the Effect of Thermal Treatment on Phenolic Compounds in Functional Flours Using Vis–NIR–SWIR Spectroscopy: A Machine Learning Approach. Foods, 14(15), 2663. https://doi.org/10.3390/foods14152663








