Study on the Microbial Inactivation and Quality Assurance of Ultrasonic-Assisted Slightly Acidic Electrolyzed Water for Mirror Carp (Cyprinus carpio L.) Fillets During Refrigerated Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Material Preparation
2.2. US-Assisted SAEW Treatment
2.3. Microbiological Characterization
2.4. pH
2.5. Total Volatile Basic Nitrogen (TVB-N)
2.6. Protein and Lipid Oxidation
2.7. Water Distribution
2.8. Color
2.9. Texture
2.10. Statistical Analysis
3. Results and Discussion
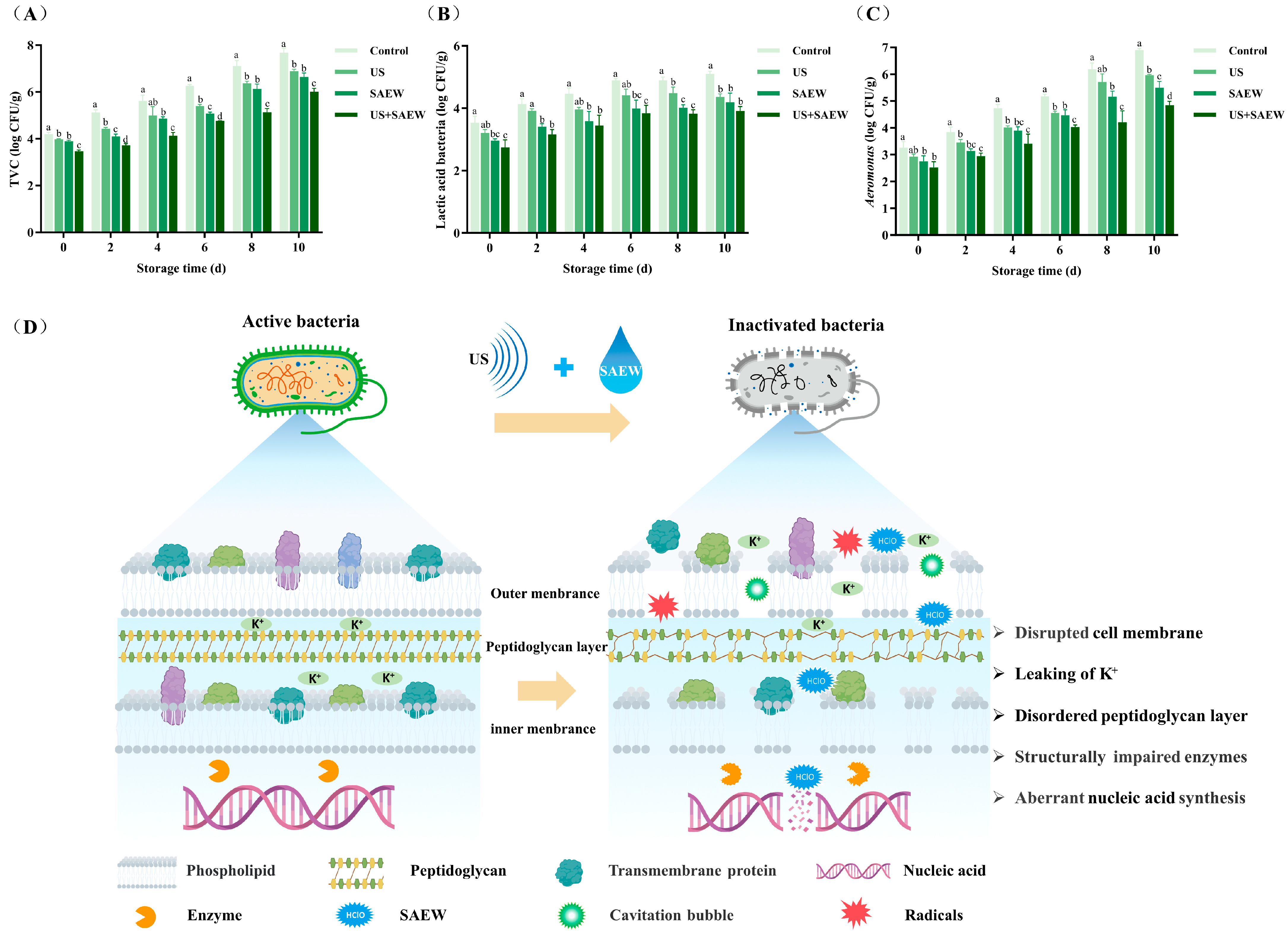
3.1. Bactericidal Effect Analysis
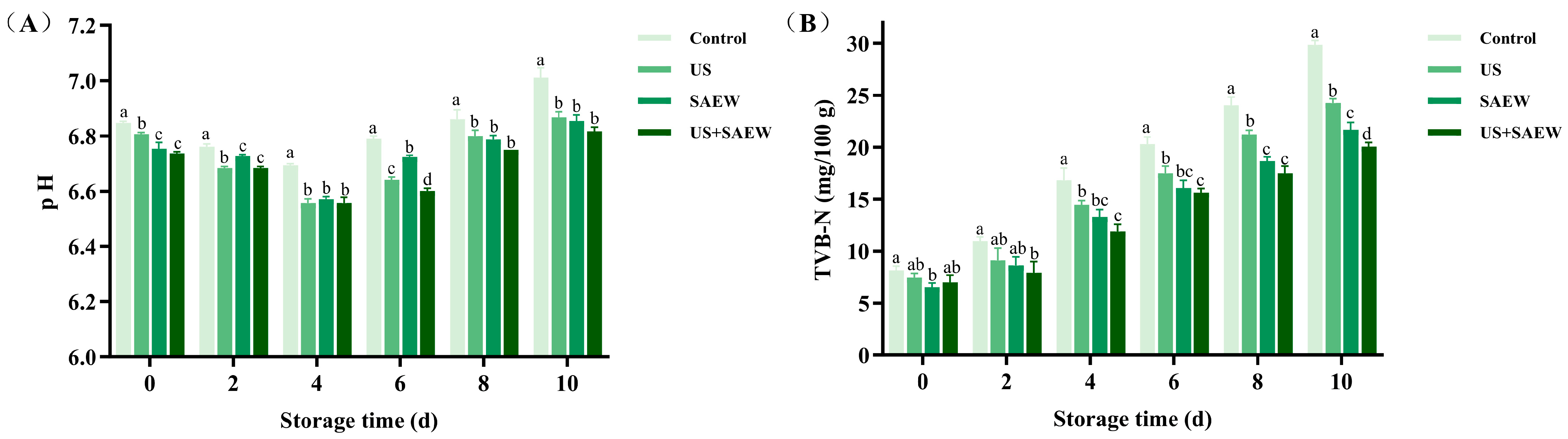
3.2. pH Analysis
3.3. Total Volatile Basic Nitrogen (TVB-N) Analysis
3.4. Protein and Lipid Oxidation Analysis
3.5. Water Distribution Analysis
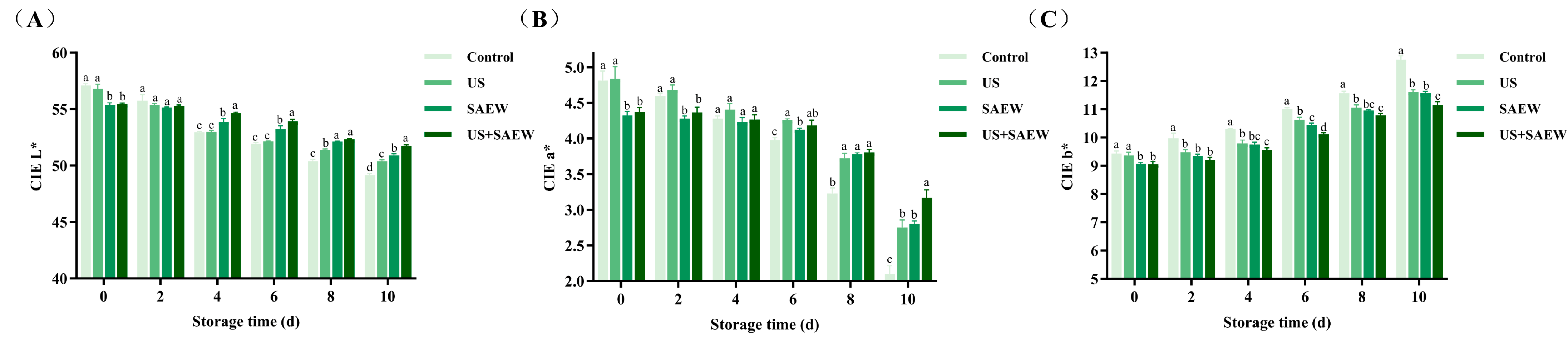
3.6. Color Analysis
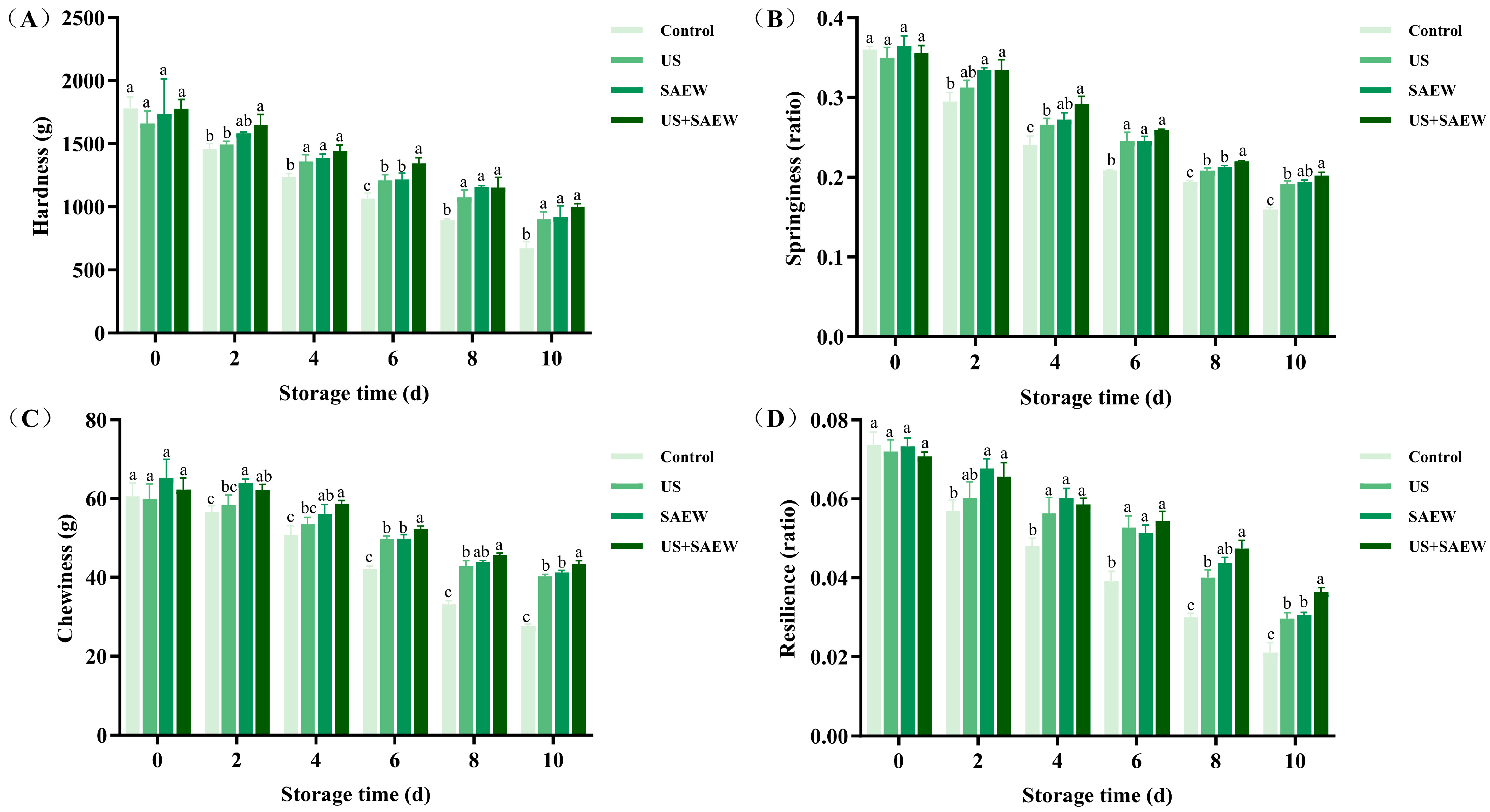
3.7. Texture Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, X.; Zhao, M.N.; Pan, N.; Wang, S.P.; Xia, X.F.; Zhang, D.J. Tracking aggregation behaviour and gel properties induced by structural alterations in myofibrillar protein in mirror carp (Cyprinus carpio) under the synergistic effects of pH and heating. Food Chem. 2021, 362, 130222. [Google Scholar] [CrossRef] [PubMed]
- Rostini, I.; Junianto; Warsiki, E. Myofibrillar Protein–Based edible film with sappan wood extract for color sensor: Application distance determination and correlation analysis between its response and fish fillet qualities during storage. Food Control 2025, 171, 111106. [Google Scholar] [CrossRef]
- Shen, Q.J.; Sun, J.; Pan, J.N.; Zheng, X.D.; Zhong, J.J.; Zhou, W.W. Ultrasound-synergized gas in ensuring the sterilization and physicochemical quality of fruit and vegetables: A review. Postharvest Biol. Technol. 2024, 209, 112705. [Google Scholar] [CrossRef]
- Chai, T.T.; Huang, Y.N.; Ren, S.T.; Jin, D.L.; Fu, J.J.; Guo, J.Y.; Chen, Y.W. Inhibitory effects of ultrasonic and rosmarinic acid on lipid oxidation and lipoxygenase in large yellow croaker during cold storage. Ultrason. Sonochem. 2023, 92, 106229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, L.K.; Deng, S.G.; Xie, C.; Qiu, X.H. Shelf-life of pacific white shrimp (Litopenaeus vannamei) as affected by weakly acidic electrolyzed water ice-glazing and modified atmosphere packaging. Food Control 2015, 51, 114–121. [Google Scholar] [CrossRef]
- Zhou, S.C.; Chen, W.J.; Fan, K. Recent advances in combined ultrasound and microwave treatment for improving food processing efficiency and quality: A review. Food Biosci. 2024, 58, 103683. [Google Scholar] [CrossRef]
- Carneiro, G.R.; Pimentel, T.C. Unraveling the potential of ultrasound processing from the consumer perspective: A review on sensory characteristics and perception. Food Biosci. 2025, 68, 106789. [Google Scholar] [CrossRef]
- Bariya, A.R.; Rathod, N.B.; Patel, A.S.; Nayak, J.K.B.; Ranveer, R.C.; Hashem, A.; Abd_Allah, E.F.; Ozogul, F.; Jambrak, A.R.; Rocha, J.M. Recent developments in ultrasound approach for preservation of animal origin foods. Ultrason. Sonochem. 2023, 101, 106676. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, C.C.; Wang, J.F.; Xie, J. Effects of orthogonal dual-frequency ultrasound-assisted treatment combined with bioactive coating containing Melissa officinalis L. essential oil on changes in quality, lipid, and protein of large yellow croaker (Pseudosciaena crocea) during cold storage. Food Chem. X 2024, 24, 101861. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.J.; Li, X.; Li, X.F.; Wu, Y.M.; An, F.P.; Zhang, L.; Geng, F.; Huang, Q.; Liu, Z.D.; Tian, Y.T. The improvement mechanism of volatile for cooked Tibetan pork assisted with ultrasound at low-temperature: Based on the differences in oxidation of lipid and protein. Ultrason. Sonochem. 2024, 110, 107060. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Ge, Z.; Shi, J.; Xu, Y.T.; Jones, C.L.; Liu, D.H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasonic on microbial loads and quality of fresh fruits. LWT-Food Sci. Technol. 2015, 60, 1195–1199. [Google Scholar] [CrossRef]
- Jia, Z.X.; Zhou, J.W.; Han, J.Z.; Liu, D.H.; Lv, R.L. Proteomics-based analysis of the stress response of Bacillus cereus spores under ultrasonic and electrolyzed water treatment. Ultrason. Sonochem. 2023, 98, 106523. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.R.; Ai, C.M.; Liao, X.Y.; Liu, D.H.; Ding, T. Effect of slightly acidic electrolyzed water (SAEW) and ultraviolet light illumination pretreatment on microflora inactivation of coriander. LWT 2020, 132, 109898. [Google Scholar] [CrossRef]
- Li, F.F.; Zhong, Q.; Kong, B.; Pan, N.; Xia, X.F.; Bao, Y.H. Synergistic effect and disinfection mechanism of combined treatment with ultrasonic and slightly acidic electrolyzed water and associated preservation of mirror carp (Cyprinus carpio L.) during refrigeration storage. Food Chem. 2022, 386, 132858. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.L.; Tian, Q.; Li, G.J.; Yi, J.J.; Hu, X.S.; Jiang, Y.L. Advanced application of slightly acidic electrolyzed water for fresh-cut fruits and vegetables preservation. Food Res. Int. 2024, 195, 114996. [Google Scholar] [CrossRef] [PubMed]
- Bing, S.; Zang, Y.T.; Li, Y.J.; Shu, D.Q. The synergistic effects of slightly acidic electrolyzed water and UV-C light on the inactivation of Salmonella enteritidis on contaminated eggshells. Poult. Sci. 2019, 98, 6914–6920. [Google Scholar] [CrossRef] [PubMed]
- Cichoski, A.J.; Flores, D.R.M.; De Menezes, C.R.; Jacob-Lopes, E.; Zepka, L.Q.; Wagner, R.; Barin, J.S.B.; de Moraes Flores, É.M.; da Cruz Fernandes, M.; Campagnol, P.C.B. Ultrasonic and slightly acid electrolyzed water application: An efficient combination to reduce the bacterial counts of chicken breast during pre-chilling. Int. J. Food Microbiol. 2019, 301, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Sun, H.N.; Zhang, M.; Mu, T.H. Effects of ultrasound with slightly acid electrolytic water on storage of sweet potato: Physiological, nutritional, sensory and microstructural characteristics. Food Control 2025, 167, 110830. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.Y.; Wang, Y.H.; Xu, W.P.; Zhang, Z.Y.; Zhang, C.L.; Shan, Z.G.; Wang, X.; Shi, C. Dual functionality of ultrasound-CaCl2-slightly acidic electrolyzed water: Efficient Salmonella thompson reduction and onion freshness retention. Food Control. 2025, 175, 111312. [Google Scholar] [CrossRef]
- Lan, W.Q.; Lang, A.; Zhou, D.P.; Xie, J. Combined effects of ultrasonic and slightly acidic electrolyzed water on quality of sea bass (Lateolabrax japonicus) fillets during refrigerated storage. Ultrason. Sonochem. 2021, 81, 105854. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhao, X.; Chen, H.; Zhang, C.; Kong, B. Impact of spice extracts on the formation of biogenic amines and the physicochemical, microbiological and sensory quality of dry sausage. Food Control. 2018, 92, 190–200. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.X.; Xia, X.F.; Sun, Q.X.; Sun, F.D.; Kong, B.H. Changes in protein oxidation, structure, and thermal stability of chicken breast subjected to ultrasound-assisted immersion freezing during frozen storage. Food Chem. 2023, 398, 133874. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Zhong, Q.; Kong, B.H.; Wang, B.; Pan, N.; Xia, X.F. Deterioration in quality of quick-frozen pork patties induced by changes in protein structure and lipid and protein oxidation during frozen storage. Food Res. Int. 2020, 133, 109142. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Li, H.J.; Deng, S.Y.; Ren, Y.M.; Kong, B.H.; Xia, X.F. Tannic acid-induced changes in water distribution and protein structural properties of bacon during the curing process. LWT 2021, 137, 110381. [Google Scholar] [CrossRef]
- Xu, G.C.; Tang, X.; Tang, S.H.; You, H.B.; Shi, H.W.; Gu, R.B. Combined effect of electrolyzed oxidizing water and chitosan on the microbiological, physicochemical, and sensory attributes of American shad (Alosa sapidissima) during refrigerated storage. Food Control 2014, 46, 397–402. [Google Scholar] [CrossRef]
- Abd El-Fatah, R.A.; Rozan, M.A.; Ziena, H.M.; Imre, K.; Morar, A.; Herman, V.; Abdel-Naeem, H.H.S. Improvement of microbial quality, physicochemical properties, fatty acids profile, and shelf life of Basa (Pangasius bocourti) fillets during chilling storage using pepsin, rosemary oil, and citric acid. Foods 2023, 12, 4170. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Sun, X.J.; Chen, X.Q.; Zheng, K.L.; Li, J.R.; Li, X.X. Effect of slightly acidic electrolyzed water (SAEW) combined with ultrasound sterilization on quality of Bigeye tuna (Thunnus obesus) during cryogenic storage. J. Food Compos. Anal. 2023, 115, 104999. [Google Scholar] [CrossRef]
- Suo, K.; Zhang, Y.; Feng, Y.B.; Yang, Z.F.; Zhou, C.S.; Chen, W.; Wang, J.C. Ultrasonic synergistic slightly acidic electrolyzed water processing to improve postharvest storage quality of Chinese bayberry. Ultrason. Sonochem. 2023, 101, 106668. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Tan, Y.Q.; Hong, H.; Li, D.P.; Zhang, L.T.; Luo, Y.K. Exploration of the roles of spoilage bacteria in degrading grass carp proteins during chilled storage: A combined metagenomic and metabolomic approach. Food Res. Int. 2022, 152, 110926. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, P.; Dalzini, E.; Cosciani-Cunico, E.; Abdul, M.E.; Monastero, P.; Merigo, D.; Ducoli, S.; Norton, A.; Losio, M.N.; Pavoni, E. Growth rate determination of Listeria monocytogenes in ready-to-eat fish products under different storage conditions for possible shelf-life extension. Foods 2025, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.J.; Wu, X.Y.; Xiong, Z.Y.; Shi, T.; Sun, Q.C.; Yuan, L.; Gao, R.C. Study on antibacterial properties of mucus extract of snakehead (Channa argus) against Escherichia coli and its application in chilled fish fillets preservation. LWT 2022, 167, 113840. [Google Scholar] [CrossRef]
- Shao, L.T.; Dong, Y.; Chen, X.J.; Xu, X.L.; Wang, H.H. Modeling the elimination of mature biofilms formed by Staphylococcus aureus and Salmonella spp. Using combined ultrasound and disinfectants. Ultrason. Sonochem. 2020, 69, 105269. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.R.; Quan, Y.; Liu, S.K.; Hao, J.X. Effectiveness of ultrasound (US) and slightly acidic electrolyzed water (SAEW) treatments for removing Listeria monocytogenes biofilms. Ultrason. Sonochem. 2025, 112, 107190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Xie, J. Ultrasonic-assisted slightly acidic electrolyzed water in aquatic product sterilization: A Review. Foods 2022, 11, 3863. [Google Scholar] [CrossRef] [PubMed]
- Nyamende, N.E.; Belay, Z.A.; Caleb, O.J. Recent advances in electrolyzed water treatments: Mechanisms of action and its effect on browning, bioactive compounds, and disinfection of fresh-cut fruit and vegetables—A review. Food Chem. Adv. 2023, 3, 100569. [Google Scholar] [CrossRef]
- Zhao, L.; Poh, C.N.; Wu, J.; Zhao, X.; He, Y.; Yang, H.S. Effects of electrolysed water combined with ultrasonic on inactivation kinetics and metabolite profiles of Escherichia coli biofilms on food contact surface. Innov. Food Sci. Emerg. Technol. 2022, 76, 102917. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Yang, R.; Zhao, W. Combined effect of slightly acidic electrolyzed water and ascorbic acid to improve quality of whole chilled freshwater prawn (Macrobrachium rosenbergii). Food Control 2020, 108, 135341. [Google Scholar] [CrossRef]
- Xuan, X.T.; Fan, Y.F.; Ling, J.G.; Hu, Y.Q.; Liu, D.H.; Chen, S.G.; Ye, X.Q.; Ding, T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control 2017, 73, 1483–1489. [Google Scholar] [CrossRef]
- He, Y.; Xie, Z.Y.; Xu, Y.R.; Zhao, X.; Zhao, L.; Yang, H.S. Preservative effect of slightly acid electrolysed water ice generated by the developed sanitising unit on shrimp (Penaeus vannamei). Food Control 2022, 136, 108876. [Google Scholar] [CrossRef]
- Li, Y.H.; Mei, J.; Xie, J. Effect of air-conditioned packaging combined with temperature fluctuations on the preservation of mandarin fish (Siniperca chuatsi). Food Chem. 2025, 480, 143893. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.Q.; Shao, Z.; Lang, A.; Xie, J. Effects of slightly acidic electrolyzed water combined with ɛ-polylysine-chitooligosaccharide Maillard reaction products treatment on the quality of vacuum packaged sea bass (Lateolabrax japonicas). Int. J. Biol. Macromol. 2024, 260, 129554. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.P.; Chen, C.W.; Xie, J. Development of antimicrobial active films based on poly (vinyl alcohol) containing nano-TiO2 and its application in macrobrachium rosenbergii packaging. J. Food Process. Preserv. 2018, 42, e13702. [Google Scholar] [CrossRef]
- Du, J.T.; Lan, W.Q.; Xie, J. Quality characteristics and moisture migration of refrigerated bullfrog (Lithobates catesbeiana) under slightly acidic electrolyzed water combined with composite preservative treatment. Food Biosci. 2023, 55, 102947. [Google Scholar] [CrossRef]
- Shi, P.; Mei, J.; Xie, J. Impact of pretreatment sterilization techniques and ginger (Zingiber officinale roscoe) essential oil-based active packaging on the quality of crucian carp (Carassius auratus) during cold storage. J. Stored Prod. Res. 2025, 112, 102598. [Google Scholar] [CrossRef]
- Günal-Köroglu, D.; Yılmaz, H.; Subasi, B.G.; Capanoglu, E. Protein oxidation: The effect of different preservation methods or phenolic additives during chilled and frozen storage of meat/meat products. Food Res. Int. 2025, 200, 115378. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.H.; Liu, Y.; Luo, Z.H.; Ni, K.; Zhang, P.F.; Zhou, T.; Bai, L.; Zhang, C.L.; Wang, X. Response surface methodology to optimize the sterilization process of slightly acidic electrolyzed water for Chinese shrimp (Fenneropenaeus chinensis) and to investigate its effect on shrimp quality. Food Chem. X 2024, 21, 101180. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.X.; Sun, R.X.; Jiang, N.; Om, A.S. Effects of ultrasonication coupled with plasma-activated water cleaning on the sterilization and preservation of fresh crucian carp fillets. LWT 2025, 215, 117246. [Google Scholar] [CrossRef]
- Zhao, M.M.; You, X.P.; Wu, Y.W.; Wang, L.; Wu, W.J.; Shi, L.; Sun, W.Q.; Xiong, G.Q. Acute heat stress during transportation deteriorated the qualities of rainbow trout (Oncorhynchus mykiss) fillets during chilling storage and its relief attempt by ascorbic acid. LWT 2022, 156, 112844. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, J.; Zhou, L.; Wang, W.; Liu, D.Y.; Zhang, Y.; Bai, T.; Pan, D.D.; Zhang, L.; Pan, S.F.; et al. Investigation of physicochemical properties, volatilome and microbial dynamics in goat meat under modified atmosphere and vacuum packaging. LWT 2025, 223, 117735. [Google Scholar] [CrossRef]
- Yang, C.; Wu, G.Y.; Yue, J.P.; Li, Y.B.; Li, Q.Q.; Liu, J.Q.; Zhang, C.H.; Liu, C.J.; Li, X. Effect of low-voltage electrostatic field-assisted subfreezing preservation on the quality of long-term frozen lamb meat: A new strategy alternative to conventional frozen storage. LWT 2025, 220, 117527. [Google Scholar] [CrossRef]
- Izadi, H.; Zandi, M.; Rafeiee, G.; Bimakr, M. Tomato seed mucilage-whey protein isolate coating enriched with shallot essential oil: Effect on quality changes of the trout fish fillet during cold storage. Biocatal. Agric. Biotechnol. 2024, 58, 103149. [Google Scholar] [CrossRef]
- Zhu, W.H.; Tan, G.Z.; Han, M.L.; Bu, Y.; Li, X.P.; Li, J.R. Evaluating the effects of plasma-activated slightly acidic electrolyzed water on bacterial inactivation and quality attributes of Atlantic salmon fillets. Innov. Food Sci. Emerg. Technol. 2023, 84, 103286. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Q.; Xia, X.; Li, F. Study on the Microbial Inactivation and Quality Assurance of Ultrasonic-Assisted Slightly Acidic Electrolyzed Water for Mirror Carp (Cyprinus carpio L.) Fillets During Refrigerated Storage. Foods 2025, 14, 2652. https://doi.org/10.3390/foods14152652
Zhong Q, Xia X, Li F. Study on the Microbial Inactivation and Quality Assurance of Ultrasonic-Assisted Slightly Acidic Electrolyzed Water for Mirror Carp (Cyprinus carpio L.) Fillets During Refrigerated Storage. Foods. 2025; 14(15):2652. https://doi.org/10.3390/foods14152652
Chicago/Turabian StyleZhong, Qiang, Xiufang Xia, and Fangfei Li. 2025. "Study on the Microbial Inactivation and Quality Assurance of Ultrasonic-Assisted Slightly Acidic Electrolyzed Water for Mirror Carp (Cyprinus carpio L.) Fillets During Refrigerated Storage" Foods 14, no. 15: 2652. https://doi.org/10.3390/foods14152652
APA StyleZhong, Q., Xia, X., & Li, F. (2025). Study on the Microbial Inactivation and Quality Assurance of Ultrasonic-Assisted Slightly Acidic Electrolyzed Water for Mirror Carp (Cyprinus carpio L.) Fillets During Refrigerated Storage. Foods, 14(15), 2652. https://doi.org/10.3390/foods14152652





