Development of a Rapid and Sensitive Visual Pesticide Detection Card Using Crosslinked and Surface-Decorated Electrospun Nanofiber Mat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Thermal Crosslinking of PCNM
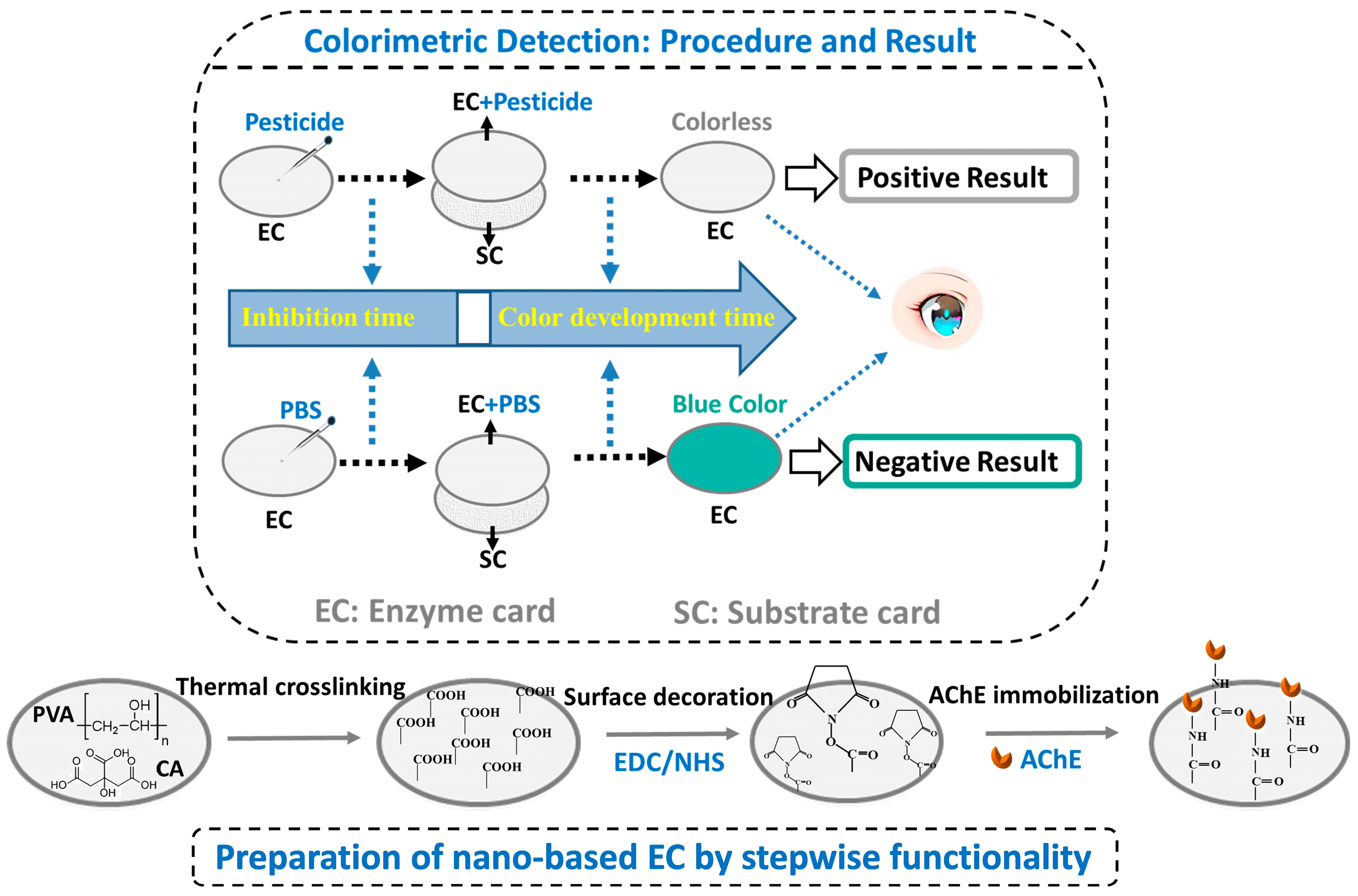
2.3. Construction of Detection Card Using Surface-Decorated C-PCNM
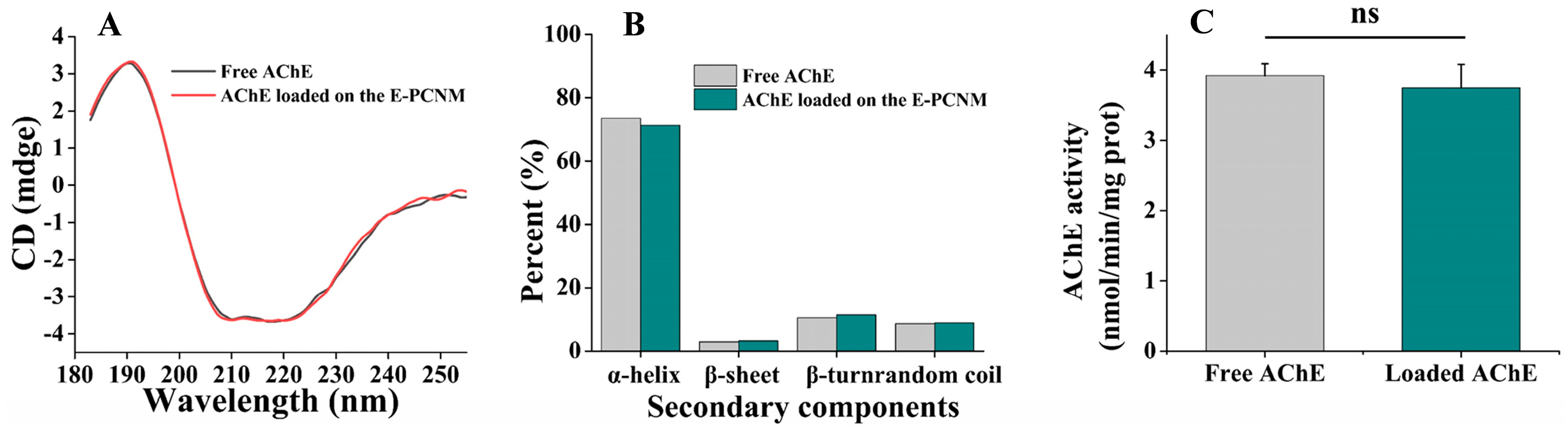
2.4. Stability of AChE After Immobilization
2.5. Characterization of Electrospun Nanofiber Mat
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5.3. Thermogravimetric Analysis (TGA)
2.5.4. Mechanical Analysis
2.6. Water Resistance Performance
2.6.1. Swelling Property
2.6.2. Contact Angle
2.7. Detection Conditions of Pesticides
2.8. Sensitivity of the Nano-Based Card
2.9. Storage Stability of Detection Card
2.10. Detection in Real Food
2.11. Statistical Analysis
3. Results and Discussion
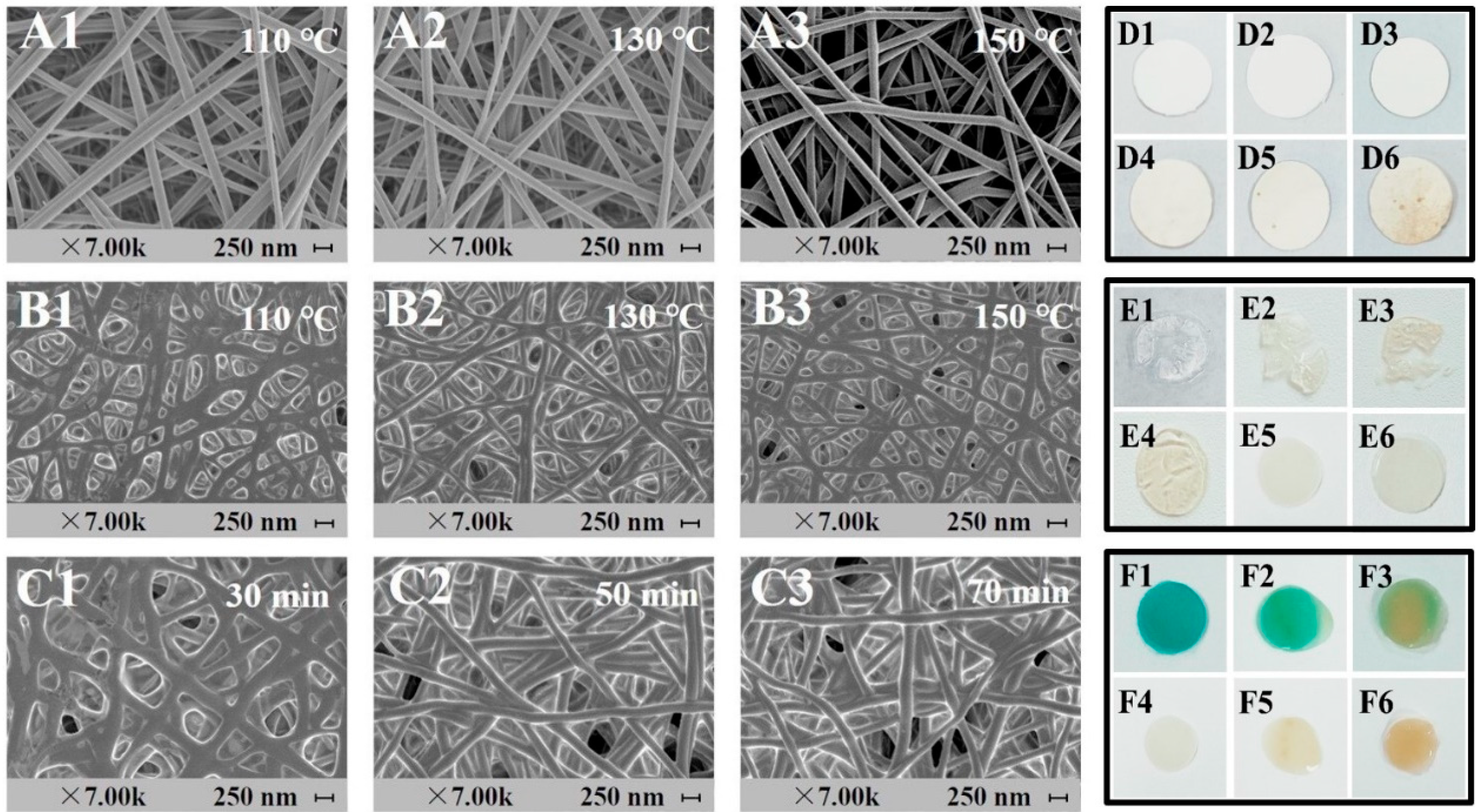
3.1. The Effect of Thermal Crosslinking on the Properties of PCNM
3.1.1. Effect of Crosslinking Temperature on the Properties of PC Nanofiber Mat
3.1.2. Effect of Crosslinking Time on the Properties of PCNM
3.2. Property of the Enzyme Card (EC) Prepared by Surface Decoration
3.2.1. Effect of Surface Decoration on AChE Immobilization
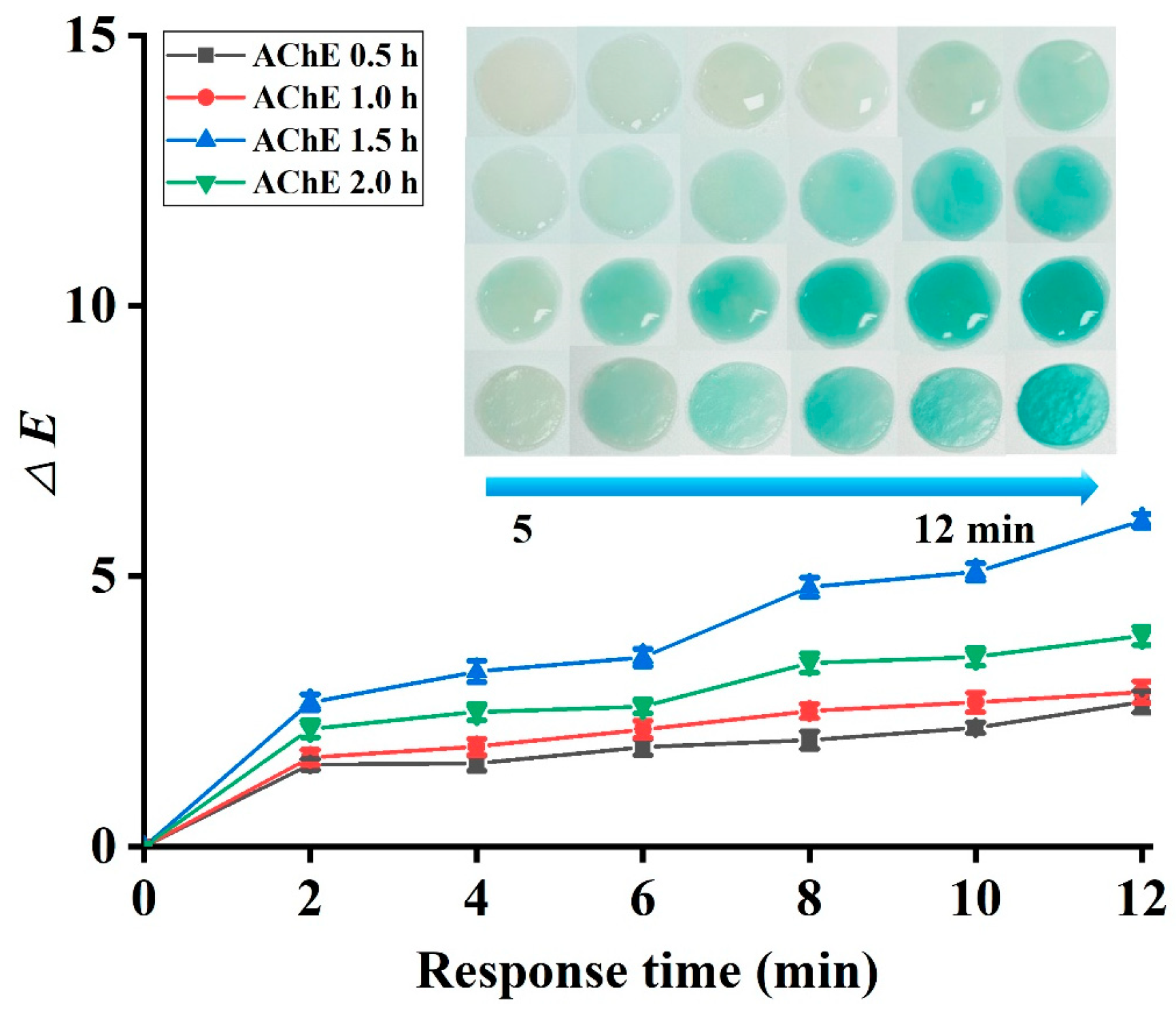
3.2.2. Effect of Decoration Time and Absorption Time on the Detection Efficacy of Pesticides
3.3. Characterization of Different Nanofiber Mats
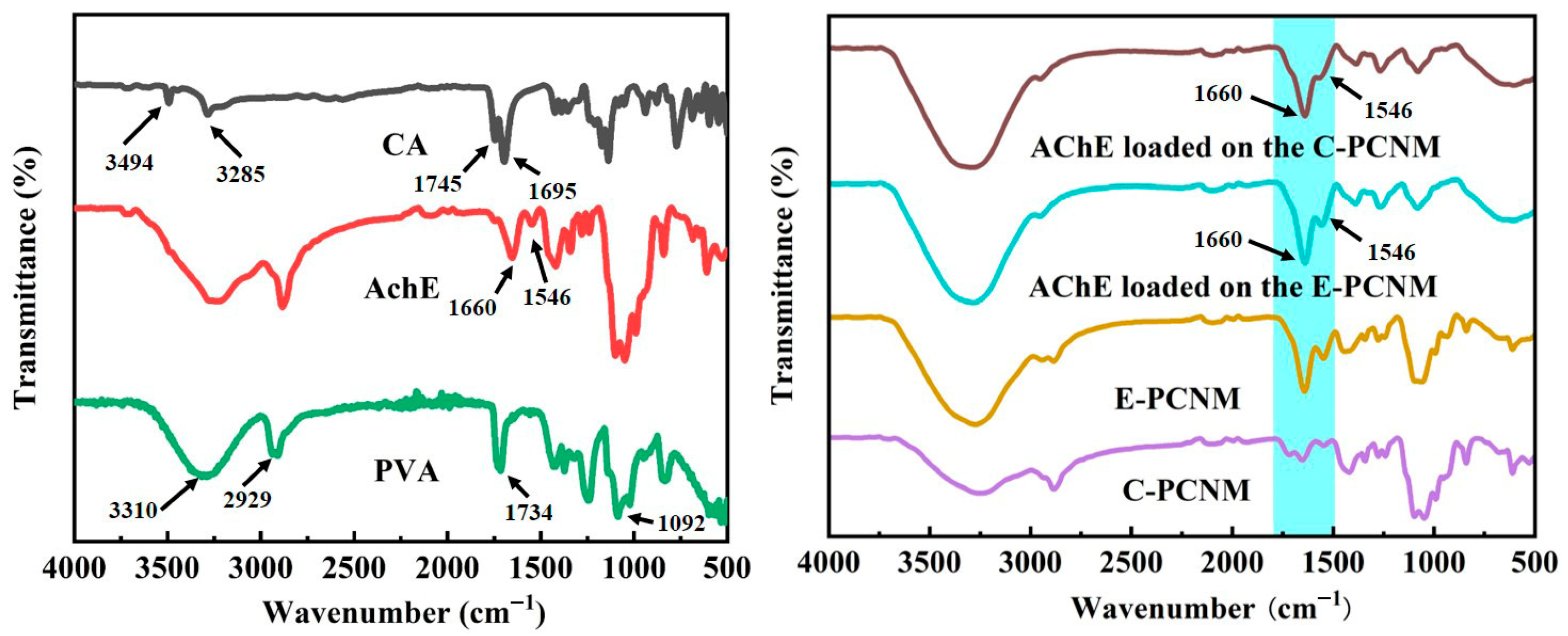
3.3.1. FTIR Analysis
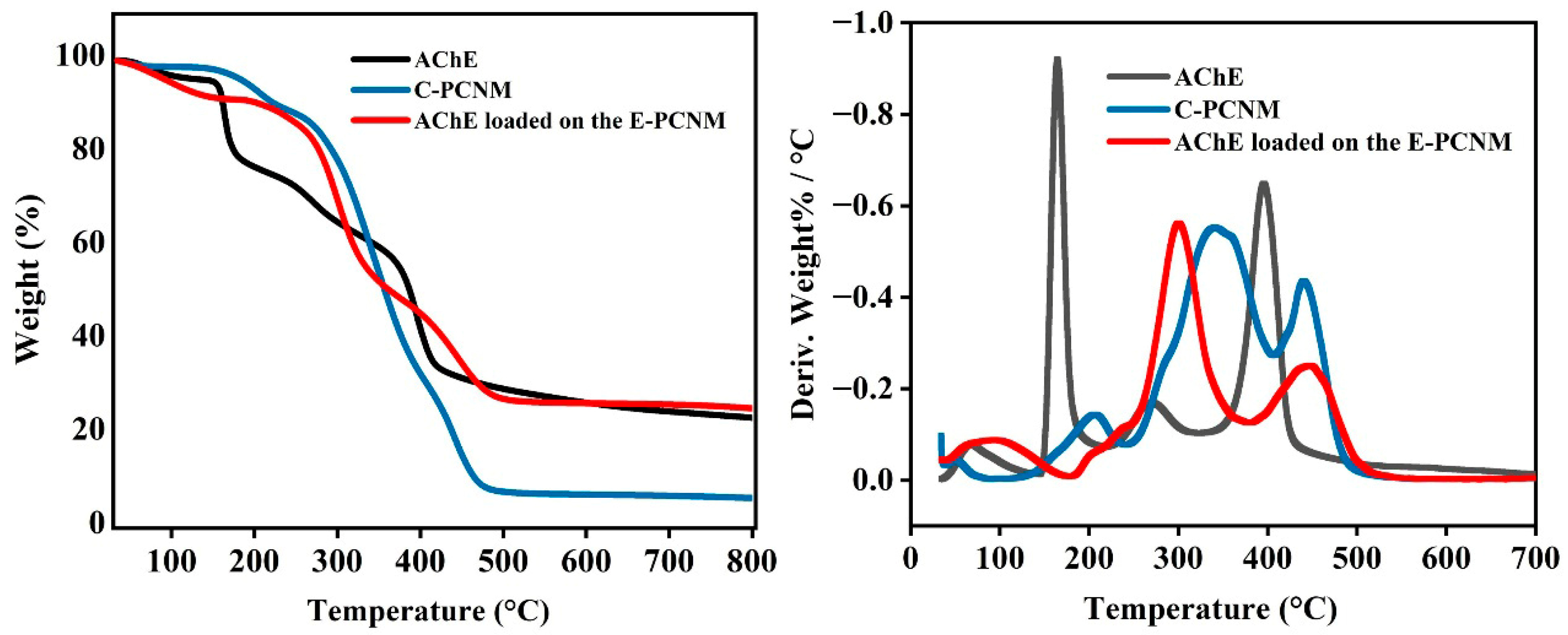
3.3.2. Thermal Properties
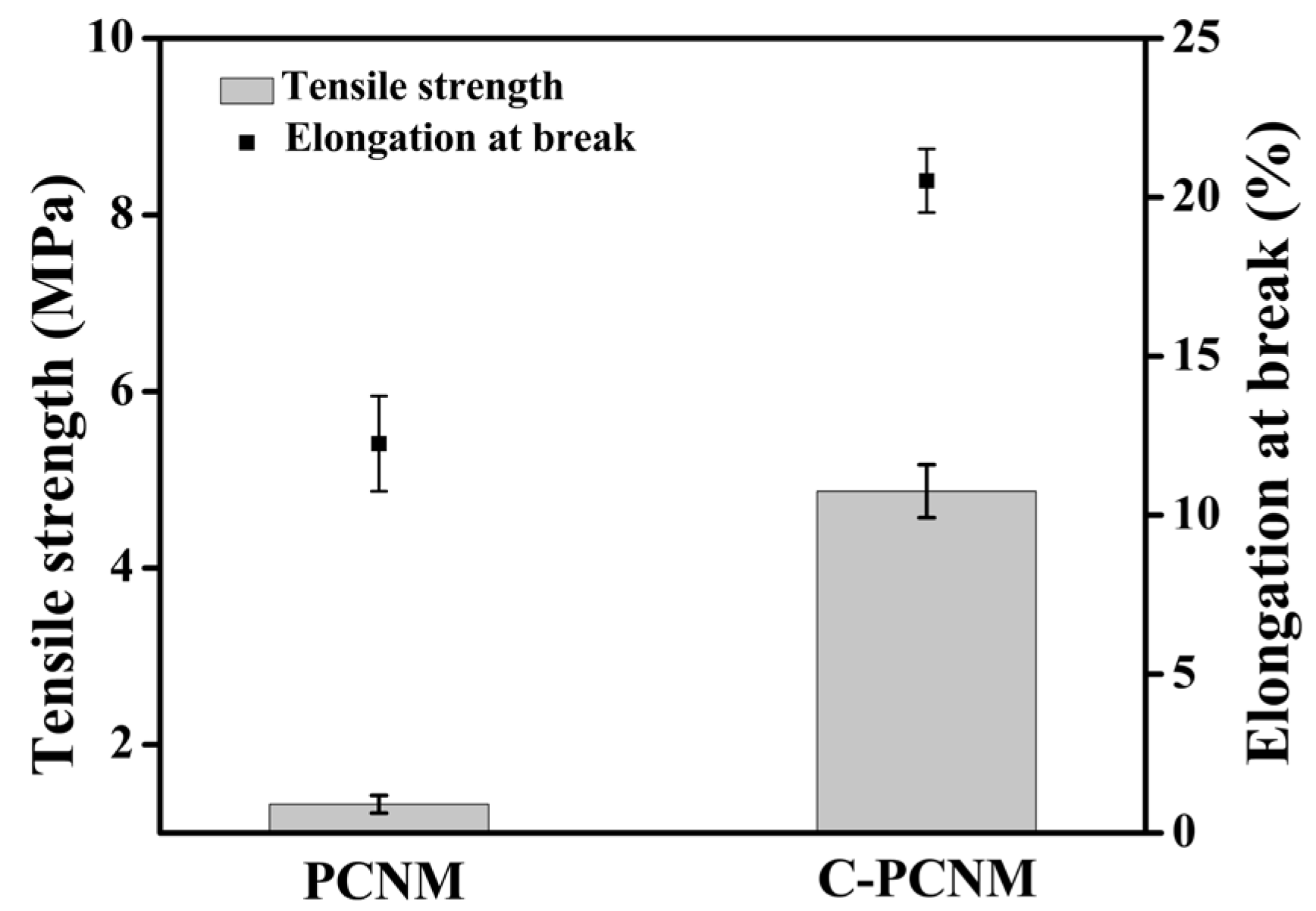
3.3.3. Mechanical Properties
3.4. Optimization of the Detection System
3.5. Sensitivity of the Detection Card
3.6. Storage Stability
3.7. Applicability of Detection Card in Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| EC | enzyme card |
| SC | substrate card |
| PVA | polyvinyl alcohol |
| CA | citric acid |
| PNM | PVA nanofiber mat |
| PCNM | PVA/CA nanofiber mat |
| EDC | 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride |
| NHS | N-hydroxysuccinimide |
| EN | EDC/NHS |
| C-PCNM | thermally crosslinked PCNM |
| E-PCNM | EN-treated C-PCNM |
| IA | indolyl acetate |
| CD | circular dichroism |
| SEM | scanning electron microscopy |
| ATR-FTIR | attenuated total reflection–Fourier transform infrared |
| ATR | attenuated total reflection |
| TGA | thermogravimetric analysis |
| DTG | derivative thermogravimetry |
| PBS | phosphate-buffered saline |
| PLA | polylactic acid |
References
- Liu, M.; Khan, A.; Wang, Z.F.; Liu, Y.; Yang, G.J.; Deng, Y.; He, N.Y. Aptasensors for pesticide detection. Biosens. Bioelectron. 2019, 123, 1–10. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Kaur, N.; Khunger, A.; Wallen, S.L.; Kaushik, A.; Chaudhary, G.R.; Varma, R.S. Advanced green analytical chemistry for environmental pesticide detection. Curr. Opin. Green Sustain. Chem. 2021, 30, 100488. [Google Scholar] [CrossRef]
- Xu, L.Y.; El-Aty, A.M.A.; Eun, J.B.; Shim, J.H.; Zhao, J.; Lei, X.M.; Gao, S.; She, Y.X.; Jin, F.; Wang, J.; et al. Recent advances in rapid detection techniques for pesticide residue: A review. J. Agric. Food Chem. 2022, 70, 1301–1315. [Google Scholar] [CrossRef]
- Gumber, K. Naked eye sensors for on-site pesticide detection: A review. J. Plant Prot. Res. 2023, 63, 173–184. [Google Scholar] [CrossRef]
- Umapathi, R.; Sonwal, S.; Lee, M.J.; Rani, G.M.; Lee, E.S.; Jeon, T.J.; Kang, S.M.; Oh, M.H.; Huh, Y.S. Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: New horizons, perspectives, and challenges. Coord. Chem. Rev. 2021, 446, 214061. [Google Scholar] [CrossRef]
- Anboo, S.; Lau, S.Y.; Kansedo, J.; Yap, P.S.; Hadibarata, T.; Jeevanandam, J.; Kamaruddin, A.H. Recent advancements in enzyme-incorporated nanomaterials: Synthesis, mechanistic formation, and applications. Biotechnol. Bioeng. 2022, 119, 1234–1250. [Google Scholar] [CrossRef]
- Xue, J.J.; Wu, T.; Dai, Y.Q.; Xia, Y.N. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Matinfar, G.; Ye, H.; Bashiry, M.; Hashami, Z.; Yang, T. Electrospinning-based sensing technologies: Opportunities for food applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13415. [Google Scholar] [CrossRef]
- Pandey, V.K.; Singh, G.; Choudhary, P.; Pathak, A.; Saini, A. Chapter 4—Nanosensors in food shelf-life extension and quality monitoring. In Advancements in Nanotechnology for Food and Packaging; Ghosh, T., Roy, S., Łopusiewicz, Ł., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2025; pp. 79–102. [Google Scholar]
- Wong, D.E.; Senecal, K.J.; Goddard, J.M. Immobilization of chymotrypsin on hierarchical nylon 6,6 nanofiber improves enzyme performance. Colloids Surf. B: Biointerfaces 2017, 154, 270–278. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; de Farias, B.S.; Sant’Anna Cadaval, T.R., Jr.; de Almeida Pinto, L.A.; Diaz, P.S. Chitosan-based nanofibers for enzyme immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef]
- Zhai, M.Y.; Feng, K.; Hu, T.G.; Zong, M.H.; Wu, H. Development of a novel nano-based detection card by electrospinning for rapid and sensitive analysis of pesticide residues. J. Sci. Food Agric. 2020, 100, 4400–4408. [Google Scholar] [CrossRef]
- Feng, K.; Zhai, M.Y.; Wei, Y.S.; Zong, M.H.; Wu, H.; Han, S.Y. Fabrication of nano/micro-structured electrospun detection card for the detection of pesticide residues. Foods 2021, 10, 889. [Google Scholar] [CrossRef]
- Zhang, W.L.; Roy, S.; Assadpour, E.; Cong, X.L.; Jafari, S.M. Cross-linked biopolymeric films by citric acid for food packaging and preservation. Adv. Colloid Interface Sci. 2023, 314, 102886. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloids Surf. B Biointerfaces 2020, 188, 110757. [Google Scholar] [CrossRef]
- Yu, D.; Feng, Y.; Xu, J.; Kong, B.; Liu, Q.; Wang, H. Fabrication, characterization, and antibacterial properties of citric acid crosslinked PVA electrospun microfibre mats for active food packaging. Packag. Technol. Sci. 2021, 34, 361–370. [Google Scholar] [CrossRef]
- Sun, Z.W.; Tian, L.T.; Guo, M.; Xu, X.T.; Li, Q.; Weng, H.B. A double-film screening card for rapid detection of organophosphate and carbamate pesticide residues by one step in vegetables and fruits. Food Control 2017, 81, 23–29. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Yang, W.J.; He, X.Y.; Luzi, F.; Dong, W.F.; Zheng, T.; Kenny, J.M.; Puglia, D.; Ma, P. Thermomechanical, antioxidant and moisture behaviour of PVA films in presence of citric acid esterified cellulose nanocrystals. Int. J. Biol. Macromol. 2020, 161, 617–626. [Google Scholar] [CrossRef]
- Li, J.; Meng, L.; Xu, Y.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Hybrid nanoparticles of quaternary ammonium cellulose derivatives and citric acid for enhancing the antibacterial activity of polyvinyl alcohol composites. Cellulose 2023, 30, 3625–3638. [Google Scholar] [CrossRef]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Beigh, M.A.; Sheikh, F.A. Overview on immobilization of enzymes on synthetic polymeric nanofibers fabricated by electrospinning. Biotechnol. Bioeng. 2022, 119, 9–33. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Lai, Q.M.; Jiang, B.; Gong, R.M. Covalent immobilization of lipase onto citric acid-esterified loofah sponge. Bioresources 2013, 8, 3289–3298. [Google Scholar] [CrossRef]
- Jakubow, K.; Kowalewska, B. The impact of immobilization process on the electrochemical performance, bioactivity and conformation of glucose oxidase enzyme. Sens. Actuators B Chem. 2017, 244, 852–861. [Google Scholar]
- Danesh, N.; Navaee Sedighi, Z.; Beigoli, S.; Beigoli, A.; Sharifi-Rad, A.; Saberi, M.R.; Chamani, J. Determining the binding site and binding affinity of estradiol to human serum albumin and holo-transferrin: Fluorescence spectroscopic, isothermal titration calorimetry and molecular modeling approaches. J. Biomol. Struct. Dyn. 2018, 36, 1747–1763. [Google Scholar] [CrossRef]
- Liao, Y.J.; Hu, X.; Pan, J.H.; Zhang, G.W. Inhibitory mechanism of baicalein on acetylcholinesterase: Inhibitory interaction, conformational change, and computational simulation. Foods 2022, 11, 168. [Google Scholar] [CrossRef]
- Deng, G.Q.; Nagy, C.; Yu, P.Q. Combined molecular spectroscopic techniques (SR-FTIR, XRF, ATR-FTIR) to study physiochemical and nutrient profiles of Avena sativa grain and nutrition and structure interactive association properties. Crit. Rev. Food Sci. 2023, 63, 7225–7237. [Google Scholar] [CrossRef]
- Canizo, B.V.; Quintas, P.Y.; Wuilloud, R.; Silva, M.F.; Gomez, F. Fluorescent behavior of melatonin and related indoleamines in natural deep eutectic solvents. J. Mol. Liq. 2022, 363, 119902. [Google Scholar] [CrossRef]
- Olvera Bernal, R.A.; Olekhnovich, R.O.; Uspenskaya, M.V. Chitosan/PVA nanofibers as potential material for the development of soft actuators. Polymers 2023, 15, 2037. [Google Scholar] [CrossRef]
- Doostan, M.; Doostan, M.; Mohammadi, P.; Khoshnevisan, K.; Maleki, H. Wound healing promotion by flaxseed extract-loaded polyvinyl alcohol/chitosan nanofibrous scaffolds. Int. J. Biol. Macromol. 2023, 228, 506–516. [Google Scholar] [CrossRef]
- Yin, W.J.; Zhang, J.X.; Wang, H.; Wang, Y.; Zeng, X.; Xu, Z.L.; Yang, J.Y.; Xiao, Z.L.; Hammock, B.D.; Wen, P. A highly sensitive electrochemical immunosensor based on electrospun nanocomposite for the detection of parathion. Food Chem. 2023, 404, 134371. [Google Scholar] [CrossRef]
- Rodríguez-deLuna, S.E.; Moreno-Cortez, I.E.; Garza-Navarro, M.A.; Lucio-Porto, R.; Luis, L.P.; González-González, V.A. Thermal stability of the immobilization process of horseradish peroxidase in electrospun polymeric nanofibers. J. Appl. Polym. Sci. 2017, 134, 45318. [Google Scholar] [CrossRef]
- Chang, X.L.; Zhang, X.R.; Qiang, Y.; Cao, Y.H.; Shang, X.Y.; Wang, W.F.; Yang, J.L. In situ biomineralization and citric acid etching strategy for enhancing activity of immobilized acetylcholinesterase. Langmuir 2024, 40, 22794–22802. [Google Scholar] [CrossRef]
- Atiroğlu, V.; Atiroğlu, A.; Atiroğlu, A.; Al-Hajri, A.S.; Özacar, M. Green immobilization: Enhancing enzyme stability and reusability on eco-friendly support. Food Chem. 2024, 448, 138978. [Google Scholar] [CrossRef]
- Khaldi, K.; Sam, S.; Gouget-Laemmel, A.C.; Henry de Villeneuve, C.; Moraillon, A.; Ozanam, F.; Yang, J.; Kermad, A.; Gabouze, N. Active acetylcholinesterase immobilization on a functionalized silicon surface. Langmuir 2015, 31, 8421–8428. [Google Scholar] [CrossRef]
- Wiecinska, P. Thermal degradation of organic additives used in colloidal shaping of ceramics investigated by the coupled DTA/TG/MS analysis. J. Therm. Anal. Calorim. 2016, 123, 1419–1430. [Google Scholar] [CrossRef]
- Siddaiah, T.; Ojha, P.; Kumar, N.O.; Ramu, C. Structural, optical and thermal characterizations of PVA/MAA: EA polyblend films. Mater. Res. 2018, 21, e20170987. [Google Scholar] [CrossRef]
- Altun, S.; Akrolu, B.; Münteha, Z.; Zacar, M. A facile and effective immobilization of glucose oxidase on tannic acid modified CoFe2O4 magnetic nanoparticles. Colloids Surf. B Biointerfaces 2015, 136, 963–970. [Google Scholar] [CrossRef]
- Liu, J.; Ma, R.; Ha, W.; Zhang, H.X.; Shi, Y.P. An MnO2-ZIF-67 immobilized acetylcholinesterase method for acetylcholinesterase activity assay and inhibitor screening from Inula macrophylla based on capillary electrophoresis. Talanta 2023, 253, 124025. [Google Scholar] [CrossRef]
- Poppe, J.K.; Costa, A.P.O.; Brasil, M.C.; Rodrigues, R.C.; Ayub, M.A.Z. Multipoint covalent immobilization of lipases on aldehyde-activated support: Characterization and application in transesterification reaction. J. Mol. Catal. B-Enzym. 2013, 94, 57–62. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Thai, L.A.T. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocoll. 2017, 71, 141–148. [Google Scholar] [CrossRef]
- Malkappa, K.; Jana, T. Simultaneous improvement of tensile strength and elongation: An unprecedented observation in the case of hydroxyl terminated polybutadiene polyurethanes. Ind. Eng. Chem. Res. 2013, 52, 12887–12896. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.L.; Zhang, Z.Z.; Zhu, A.C.; Chen, D.F.; Zhou, X.H.; Zhang, L.Q.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Csiszár, E.; Herceg, I.; Fekete, E. Effect of heating and citric acid on the performance of cellulose nanocrystal thin films. Polymers 2023, 15, 1698. [Google Scholar] [CrossRef]
- Guo, X.S.; Zhang, X.Y.; Cai, Q.; Shen, T.; Zhu, S.M. Developing a novel sensitive visual screening card for rapid detection of pesticide residues in food. Food Control 2013, 30, 15–23. [Google Scholar] [CrossRef]
- Apilux, A.; Isarankura-Na-Ayudhya, C.; Tantimongcolwat, T.; Prachayasittikul, V. Paper-based acetylcholinesterase inhibition assay combining a wet system for organophosphate and carbamate pesticides detection. EXCLI J. 2015, 14, 307–319. [Google Scholar]
- GB/T 5009.199−2003; Rapid Determination for Organophosphate and Carbamate Pesticide Residues in Vegetables. Standardization Administration of China: Beijing, China, 2003.



| Card Material | Response Time (min) | Storage Stability (d) | Detection Limits | References |
|---|---|---|---|---|
| Polyester fiber, glass fiber, and absorbent paper | 15 | 60 | 0.05, 0.1, 0.5, 0.5, 0.04, and 0.09 mg/mL for phoxim, acephate, malathion, omethoate, carbofuran, and aldicarb | [45] |
| PVDF | 25 | 60 | 1.0, 0.1, 2.0, 0.05, 1.5, and 0.8 mg/mL for omethoate, dichlorvos, methamidophos, chlorpyrifos, carbaryl, and pirimicarb | [18] |
| PVA | 15 | 120 | 0.5, 1.5, 0.1, and 0.02 mg/mL for omethoate, malathion, carbaryl, and carbofuran | [13] |
| PLA | 20 | 60 | 0.2, 1.0, 2.5, and 0.1 mg/mL for Trichlorfon, malathion, carbaryl, and carbofuran | [14] |
| Filter paper | 30 | 2 | 0.5, 0.005, 0.1, 0.5, and 0.5 ppm for carbaryl, carbofuran, dichlovos, paraoxon, and pirimicarb | [46] |
| Pesticide | Detection Limit of Rapid Determination (mg/L) | Detection Limit of Rapid Detection Card (mg/L) | Result | |||
|---|---|---|---|---|---|---|
| Phoxim | 0.01 | 0.007 |  |  |  |  |
| 0 | 0.01 | 0.007 | 0.005 | |||
| Methomyl | 0.15 | 0.1 |  |  |  |  |
| 0 | 0.15 | 0.1 | 0.007 | |||
| Sample | Temperature | Storage Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | ||
| Control | 4 °C |  |  |  |  |
| RT |  |  |  |  | |
| Phoxim | 4 °C |  |  |  |  |
| RT |  |  |  |  | |
| Methomyl | 4 °C |  |  |  |  |
| RT |  | 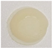 |  |  | |
| Sample | Detection Card | Methomyl Concentration (mg/L) | |||
|---|---|---|---|---|---|
| 0 | 0.1 | 0.15 | 0.2 | ||
| Chinese cabbage | Commercial |  |  |  |  |
| Self-made |  |  |  |  | |
| Orange | Commercial |  |  |  |  |
| Self-made |  |  |  |  | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhou, H.; Kang, J.; Wu, Y.; Feng, K. Development of a Rapid and Sensitive Visual Pesticide Detection Card Using Crosslinked and Surface-Decorated Electrospun Nanofiber Mat. Foods 2025, 14, 2628. https://doi.org/10.3390/foods14152628
Wei Y, Zhou H, Kang J, Wu Y, Feng K. Development of a Rapid and Sensitive Visual Pesticide Detection Card Using Crosslinked and Surface-Decorated Electrospun Nanofiber Mat. Foods. 2025; 14(15):2628. https://doi.org/10.3390/foods14152628
Chicago/Turabian StyleWei, Yunshan, Huange Zhou, Jingxuan Kang, Yongmei Wu, and Kun Feng. 2025. "Development of a Rapid and Sensitive Visual Pesticide Detection Card Using Crosslinked and Surface-Decorated Electrospun Nanofiber Mat" Foods 14, no. 15: 2628. https://doi.org/10.3390/foods14152628
APA StyleWei, Y., Zhou, H., Kang, J., Wu, Y., & Feng, K. (2025). Development of a Rapid and Sensitive Visual Pesticide Detection Card Using Crosslinked and Surface-Decorated Electrospun Nanofiber Mat. Foods, 14(15), 2628. https://doi.org/10.3390/foods14152628






