Effect of Homogenization and Pectin on Chemical, Textural, Antioxidant and Sensory Characteristics of L. bulgaricus-Fermented Oat-Based Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Oat Base Preparation
2.2. Starter Culture
2.3. Samples
2.4. Fermentation Processing
2.5. Chemical Assays
2.6. Analysis of Viscosity and Structural–Mechanical and Textural Parameters
2.7. Antioxidant Assays
2.8. Enzyme Inhibition Assays
2.9. Method of Sensory Evaluation
2.10. Statistical Analysis
3. Results
3.1. Preparation Oat-Fermented Base for Plant Beverages
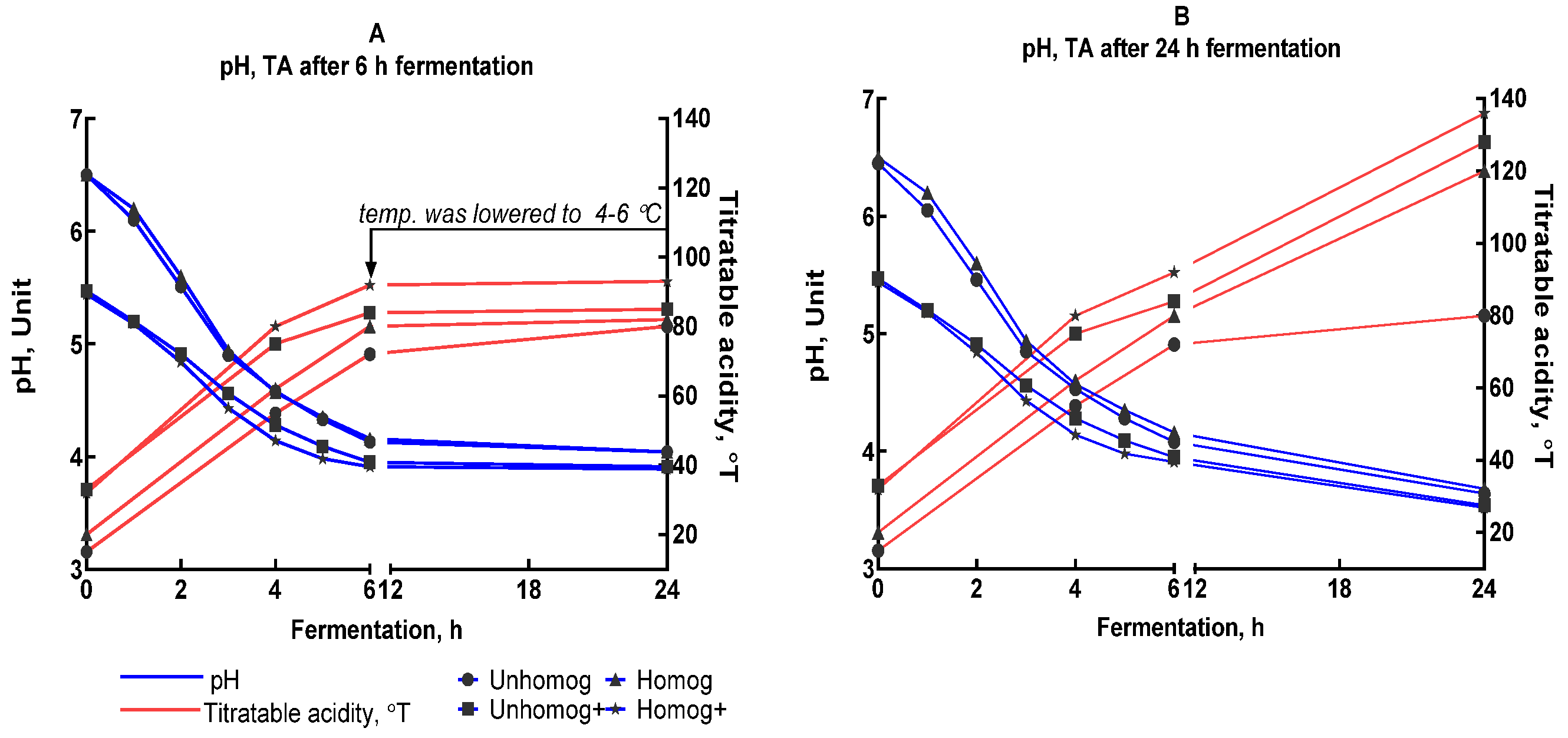
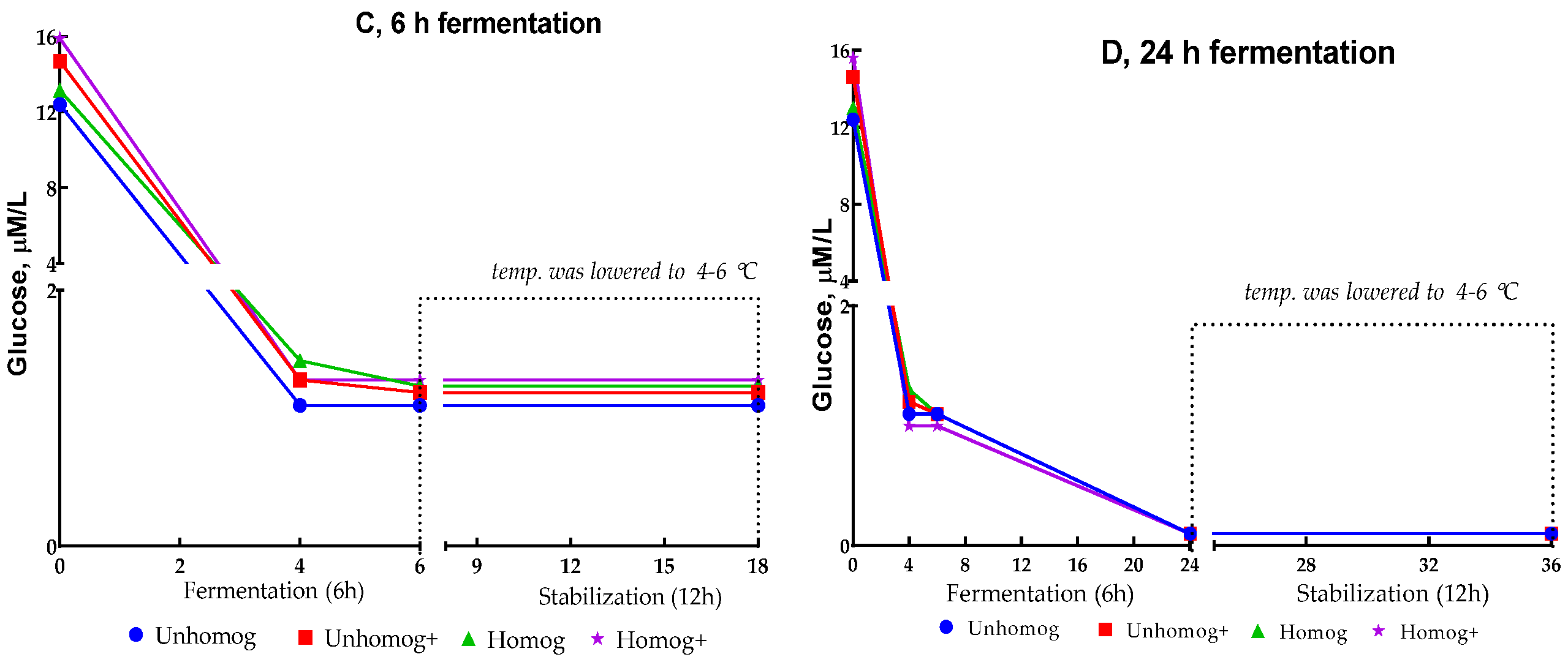
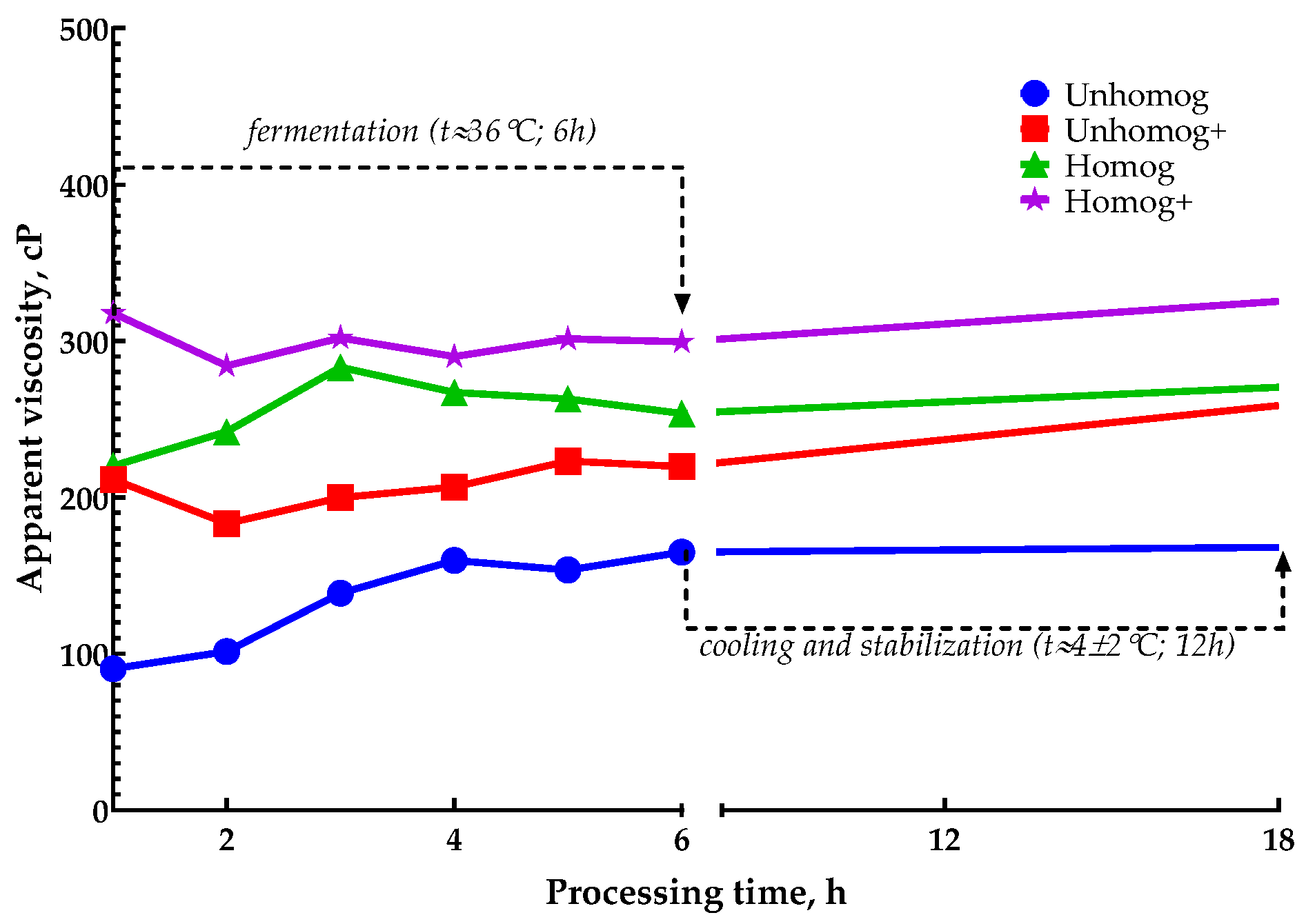
3.2. The Oat Base Fermentation Processing
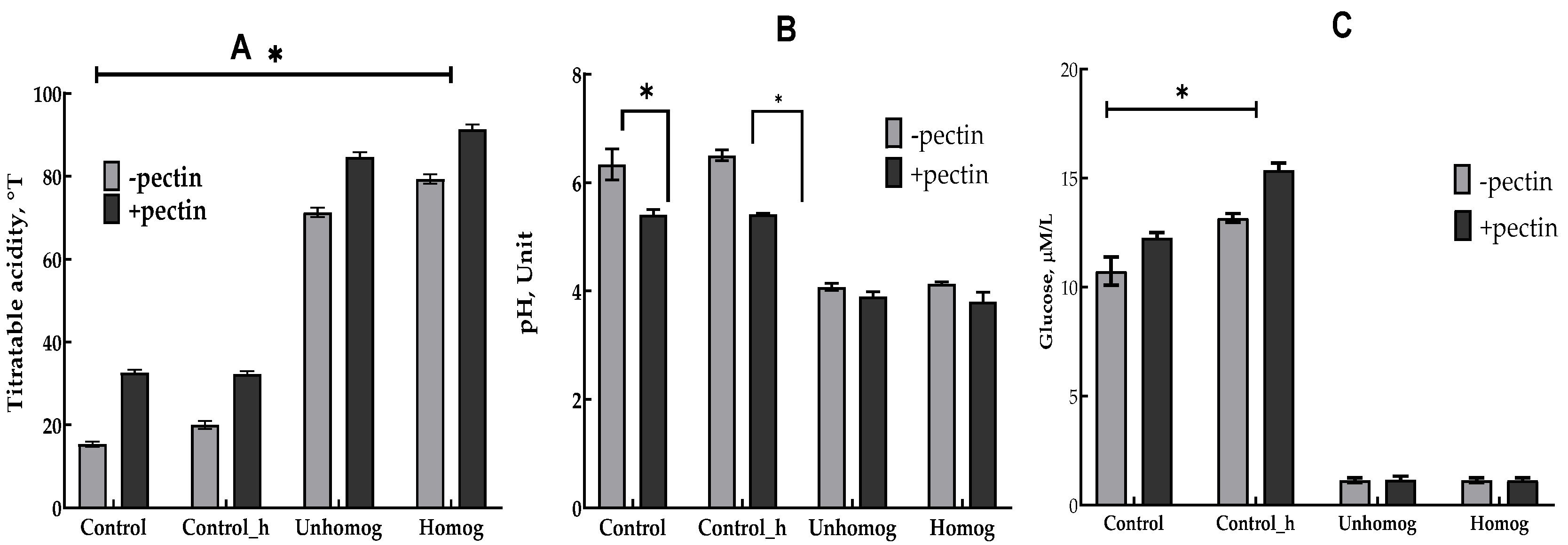
3.3. Properties of Oat-Fermented Base After 6 h of Fermentation
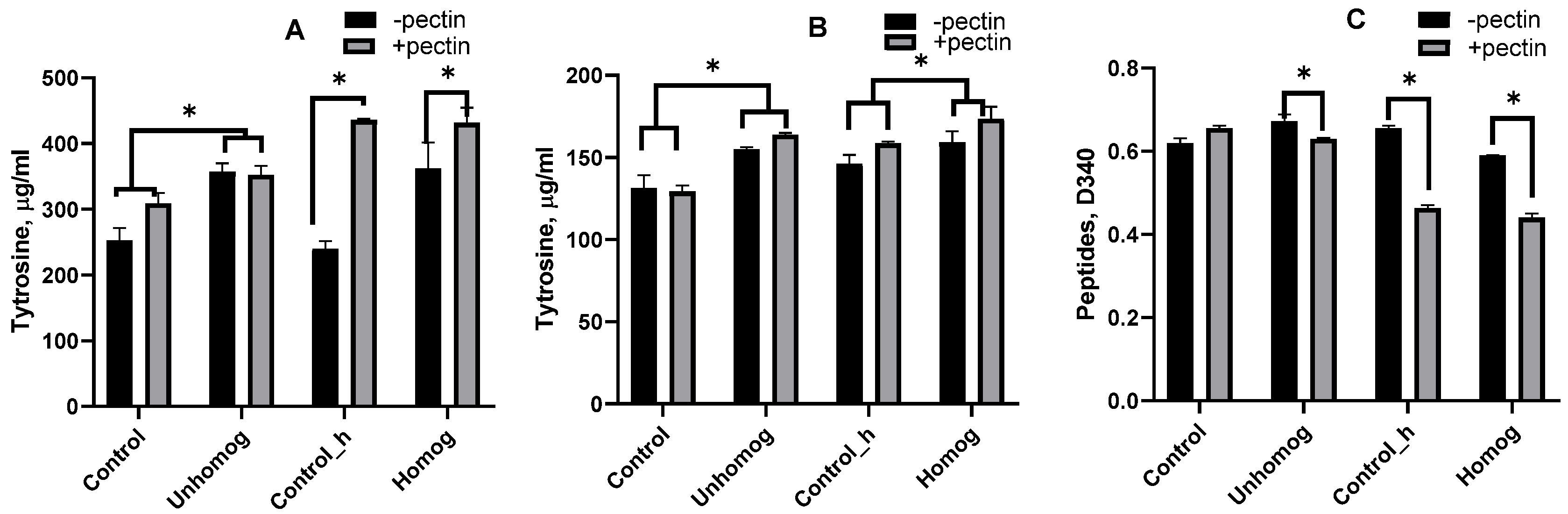
3.3.1. Chemical Composition Changes
3.3.2. Structural and Textural Properties
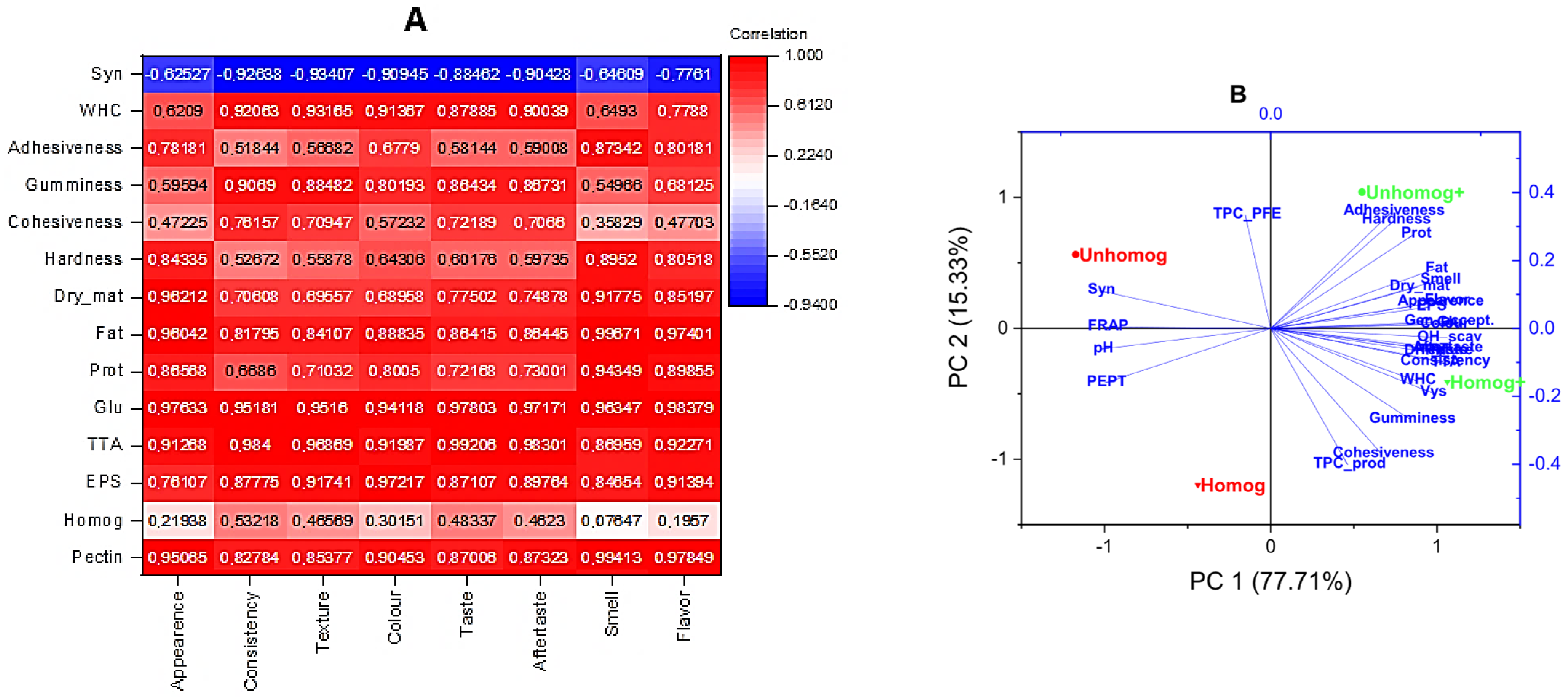
3.3.3. Sensory Evaluation
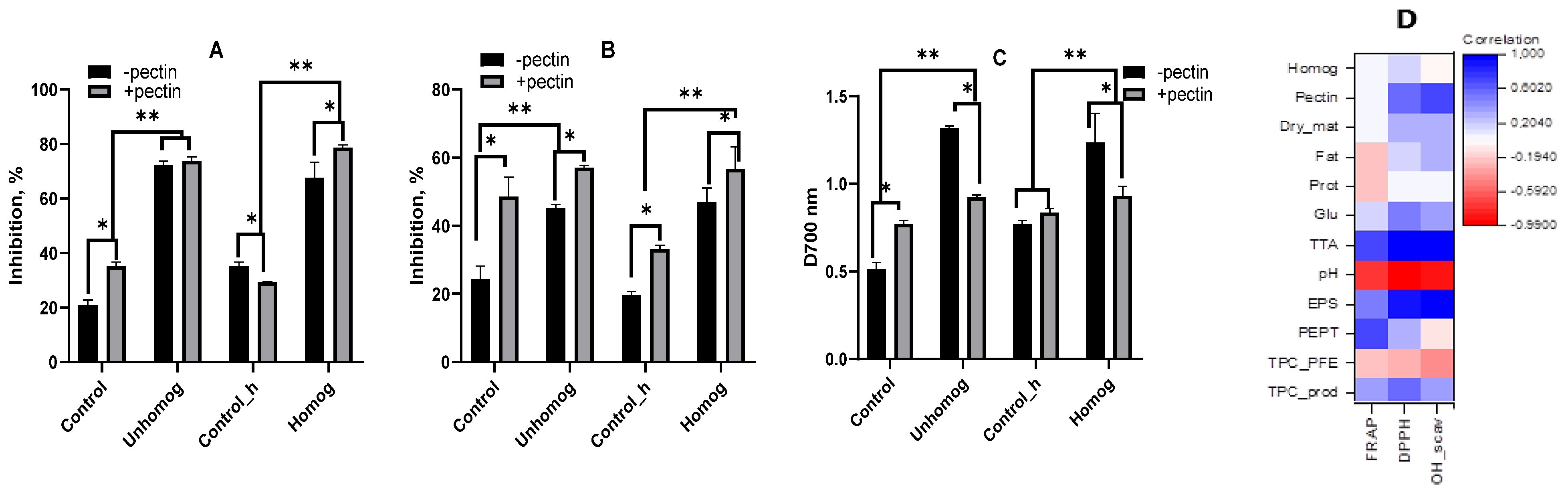
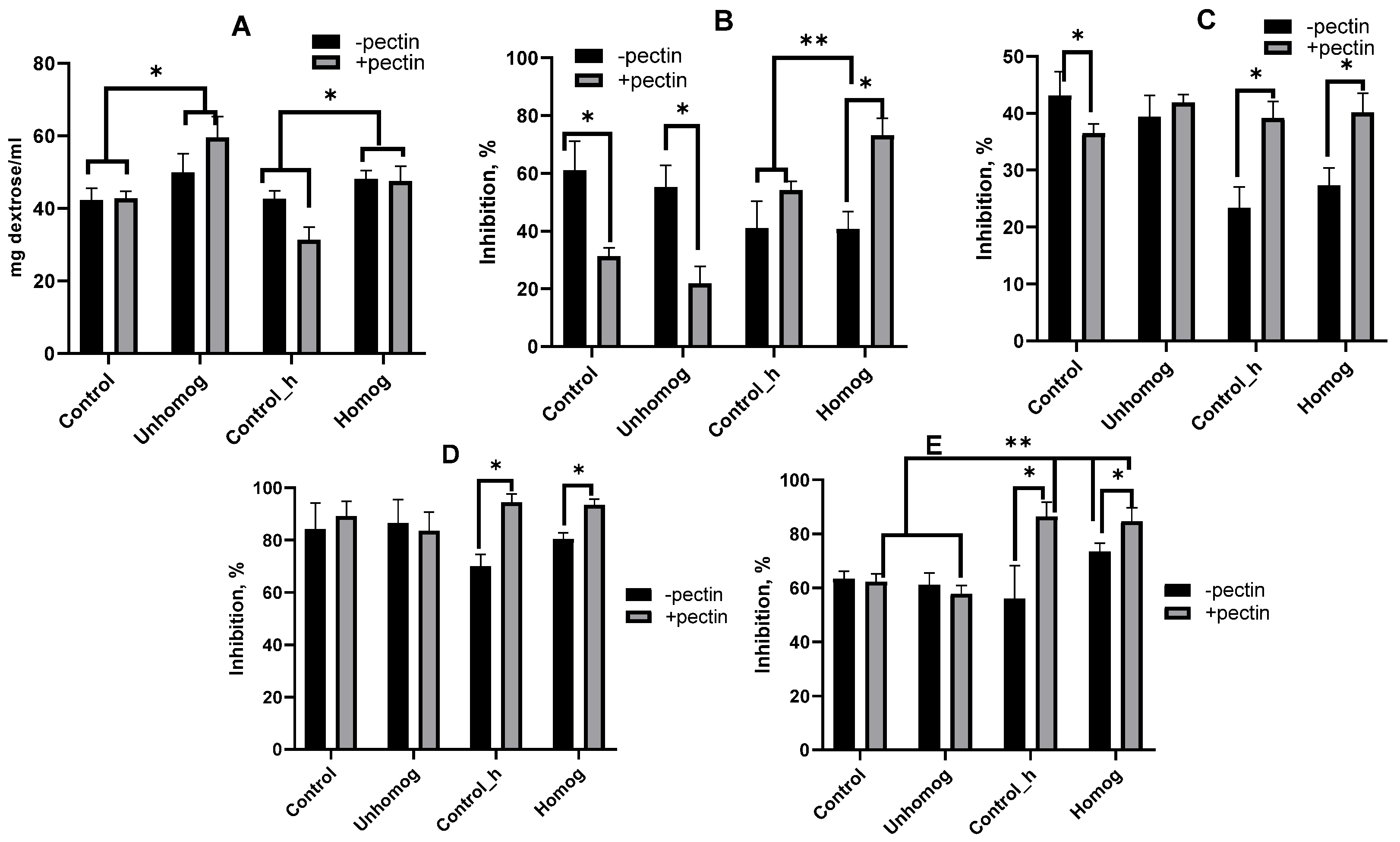
3.4. Total Phenol-Containing Compounds and Antioxidant Properties
3.5. Extractable Polysaccharides (PSs) and Their Antioxidant Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, S.-S.; Wicklund, R.; Bridges, J.; Whaley, J. Starch Swelling Behavior and Texture Development in Stirred Yogurt. Food Hydrocoll. 2020, 98, 105274. [Google Scholar] [CrossRef]
- Weerathilake, W.A.D.V.; Rasika, D.M.D.; Ruwanmali, J.K.U.; Munasinghe, M.A.D.D. The Evolution, Processing, Varieties and Health Benefits of Yogurt. Int. J. Sci. Res. Publ. 2014, 4. Available online: https://www.ijsrp.org/research-paper-0414/ijsrp-p2855.pdf (accessed on 22 July 2025).
- Kesika, P.; Thangaleela, S.; Sivamaruthi, B.S.; Bharathi, M.; Chaiyasut, C. Fermented Foods and Their Role in Respiratory Health: A Mini-Review. Fermentation 2022, 8, 162. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Yeboah, P.J.; Ayivi, R.D.; Eddin, A.S.; Wijemanna, N.D.; Paidari, S.; Bakhshayesh, R.V. A Review and Comparative Perspective on Health Benefits of Probiotic and Fermented Foods. Int. J. Food Sci. Technol. 2023, 58, 4948–4964. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Process Optimization for the Development of a Functional Beverage Based on Lactic Acid Fermentation of Oats. Biochem. Eng. J. 2010, 52, 199–204. [Google Scholar] [CrossRef]
- Andressa, I.; do Nascimento, G.K.S.; do Santos, T.M.; da Silva Rodrigues, R.; de Oliveira Teotônio, D.; Paucar-Menacho, L.M.; Benassi, V.M.; Schmiele, M. Technological and Health Properties and Main Challenges in the Production of Vegetable Beverages and Dairy Analogs. Food Funct. 2024, 15, 460–480. [Google Scholar] [CrossRef]
- Lopez, S.H.; Dias, J.V.; Mol, H.G.J.; de Kok, A. Selective Multiresidue Determination of Highly Polar Anionic Pesticides in Plant-Based Milk, Wine and Beer Using Hydrophilic Interaction Liquid Chromatography Combined with Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1625, 461226. [Google Scholar] [CrossRef]
- Haas, R.; Schnepps, A.; Pichler, A.; Meixner, O. Cow Milk versus Plant-Based Milk Substitutes: A Comparison of Product Image and Motivational Structure of Consumption. Sustainability 2019, 11, 5046. [Google Scholar] [CrossRef]
- Meena, K.K.; Taneja, N.K.; Jain, D.; Ojha, A.; Saravanan, C.; Bunkar, D.S. Spontaneously Fermented Cereal Based Products: An Ancient Health Promoting Formulae for Consumption of Probiotic Lactic Acid Bacteria. Biointerface Res. Appl. Chem. 2023, 13, 465. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Mustafa, M.A.; Hassan, M.A.; Al-Asmari, F. Probiotic-Fermentation of Oat: Safety, Strategies for Improving Quality, Potential Food Applications and Biological Activities. Trends Food Sci. Technol. 2024, 5, 104640. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Varga, L.; Babett, G. Nutritional and Functional Aspects of European Cereal-Based Fermented Foods and Beverages. Food Res. Int. 2025, 209, 116221. [Google Scholar] [CrossRef]
- Vahini, R.V.; Many, N.J. An Overview on Recent Technological Aspects and Health Concerns of Plant Based Milk Alternatives. Int. J. Multidiscip. Res. Arts Sci. Commer. 2022, 2, 46–57. [Google Scholar]
- Clark, B.E.; Pope, L.; Belarmino, E.H. Perspectives from Healthcare Professionals on the Nutritional Adequacy of Plant-Based Dairy Alternatives: Results of a Mixed Methods Inquiry. BMC Nutr. 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; de J. Cabrera-Hidalgo, A.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toalá, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef]
- Aydar, A.Y. (Ed.) Plant-Based Foods: Ingredients, Technology and Health Aspects; Springer: Cham, Switzerland, 2023; ISBN 978-3-031-27443-5. [Google Scholar]
- Vashisht, P.; Sharma, A.; Awasti, N.; Wason, S.; Singh, L.; Sharma, S.; Charles, A.P.R.; Sharma, S.; Gill, A.; Khattra, A.K. Comparative Review of Nutri-Functional and Sensorial Properties, Health Benefits and Environmental Impact of Dairy (Bovine Milk) and Plant-Based Milk (Soy, Almond, and Oat Milk). Food Humanit. 2024, 2, 100301. [Google Scholar] [CrossRef]
- Giugliano, R.; Musolino, N.; Ciccotelli, V.; Ferraris, C.; Savio, V.; Vivaldi, B.; Ercolini, C.; Bianchi, D.M.; Decastelli, L. Soy, Rice and Oat Drinks: Investigating Chemical and Biological Safety in Plant-Based Milk Alternatives. Nutrients 2023, 15, 2258. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, A.R.A.; Ribeiro, B.D. Health Issues and Technological Aspects of Plant-Based Alternative Milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Kamal, N.; Renhuldt, N.T.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhász, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The Mosaic Oat Genome Gives Insights into a Uniquely Healthy Cereal Crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef]
- Varma, P.; Bhankharia, H.; Bhatia, S. Oats A Multi-Functional Grain. J. Clin. Prev. Cardiol. 2016, 5, 9–17. [Google Scholar] [CrossRef]
- Luana, N.; Rossana, C.; Curiel, J.A.; Poutanen, K.; Gobbetti, M.; Rizzello, C.G. Manufacture and Characterization of a Yogurt-like Beverage Made with Oat Flakes Fermented by Selected Lactic Acid Bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef]
- Kumar, L.; Sehrawat, R.; Kong, Y. Oat Proteins: A Perspective on Functional Properties. LWT 2021, 152, 112307. [Google Scholar] [CrossRef]
- Sang, S.; Chu, Y. Whole Grain Oats, More than Just a Fiber: Role of Unique Phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef]
- Yu, Q.; Qian, J.; Guo, Y.; Qian, H.; Yao, W.; Cheng, Y. Applicable Strains, Processing Techniques and Health Benefits of Fermented Oat Beverages: A Review. Foods 2023, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Sontag-Strohm, T.; Lehtinen, P.; Kaukovirta-Norja, A. Oat Products and Their Current Status in the Celiac Diet. In Gluten-Free Cereal Products and Beverages; Arendt, E., Dal Bello, F., Eds.; Food Science and Technology International Series; Elsevier; Academic Press: San Diego, CA, USA, 2008; pp. 191–202. [Google Scholar]
- Walsh, H.; Ross, J.; Hendricks, G.; Guo, M. Physico-Chemical Properties, Probiotic Survivability, Microstructure, and Acceptability of a Yogurt-Like Symbiotic Oats-Based Product Using Pre-Polymerized Whey Protein as a Gelation Agent. J. Food Sci. 2010, 75, M327–M337. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bao, Y.; Guo, M.; Hendricks, G.M. Consistency, Microstructure and Probiotic Survivability of Goats’ Milk Yoghurt Using Polymerized Whey Protein as a Co-Thickening Agent. Int. Dairy J. 2012, 24, 113–119. [Google Scholar] [CrossRef]
- McCann, T.H.; Fabre, F.; Day, L. Microstructure, Rheology and Storage Stability of Low-Fat Yoghurt Structured by Carrot Cell Wall Particles. Food Res. Int. 2011, 44, 884–892. [Google Scholar] [CrossRef]
- Ghahfaroki, M.G.; Yousefvand, A.; Gavlighi, H.A.; Zarei, M.; Peyman, F. Developing Novel Synbiotic Low-fat Yogurt with Fucoxylogalacturonan from Tragacanth Gum: Investigation of Quality Parameters and Lactobacillus Casei Survival. Food Sci. Nutr. 2020, 8, 4491–4504. [Google Scholar] [CrossRef]
- Sun, R.; Li, M.; Yuan, Y.; Liu, Y.; Wang, K.; Yue, T.Y.; Gao, Z. Emerging Trends in Pectin Functional Processing and Its Fortification for Synbiotics: A Review. Trends Food Sci. Technol. 2023, 134, 80–97. [Google Scholar] [CrossRef]
- Hua, Y.; Wei, Z.; Xue, C.; Si, J. Stability and Programmed Sequential Release of Lactobacillus Plantarum and Curcumin Encapsulated in Bilayer-Stabilized W1/O/W2 Double Emulsion: Effect of Pectin as Protective Shell. Int. J. Biol. Macromol. 2024, 265 Pt 1, 130805. [Google Scholar] [CrossRef]
- Fan, Z.; Jia, W.; Du, A.; Shi, L. Complex Pectin Metabolism by Lactobacillus and Streptococcus Suggests an Effective Control Approach for Maillard Harmful Products in Brown Fermented Milk. Fundam. Res. 2022, 4, 1171–1184. [Google Scholar] [CrossRef]
- Haijuan, H.; Zhang, P.; Liu, F.; Pan, S. Regulations of Citrus Pectin Oligosaccharide on Cholesterol Metabolism: Insights from Integrative Analysis of Gut Microbiota and Metabolites. Nutrients 2024, 16, 2002. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sehrawat, N.; Sharma, A.K.; Kumar, S.; Singh, R.; Kumar, A.; Kumar, A. Synbiotics as Potent Functional Food: Recent Updates on Therapeutic Potential and Mechanistic Insight. J. Food Sci. Technol. 2022, 61, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khrundin, D.V.; Nikitina, E.V. Chemical, Textural and Antioxidant Properties of Oat-Fermented Beverages with Different Starter Lactic Acid Bacteria and Pectin. BioTech 2024, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Mikshina, P.V.; Kharina, M.; Sungatullina, A.; Petrova, T.; Sibgatullin, T.A.; Nikitina, E. Influence of Flaxseed Mucilage on the Formation, Composition, and Properties of Exopolysaccharides Produced by Different Strains of Lactic Acid Bacteria. Int. J. Biol. Macromol. 2024, 281, 136092. [Google Scholar] [CrossRef]
- Nikitina, E.; Petrova, T.; Sungatullina, A.; Bondar, O.; Kharina, M.; Mikshina, P.V.; Gavrilova, E.; Kayumov, A.R. The Profile of Exopolysaccharides Produced by Various Lactobacillus Species from Silage during Not-Fat Milk Fermentation. Fermentation 2023, 9, 197. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Nikitina, E.; Petrova, T.; Vafina, A.; Ezhkova, A.; Yahia, M.N.; Kayumov, A.R. Textural and Functional Properties of Skimmed and Whole Milk Fermented by Novel Lactiplantibacillus Plantarum AG10 Strain Isolated from Silage. Fermentation 2022, 8, 290. [Google Scholar] [CrossRef]
- Sungatullina, A.; Petrova, T.; Kharina, M.; Mikshina, P.V.; Nikitina, E. Effect of Flaxseed Mucilage on the Probiotic, Antioxidant, and Structural-Mechanical Properties of the Different Lactobacillus Cells. Fermentation. Fermentation 2023, 9, 486. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Rosenthal, A.J. Human Oral Processing and Texture Profile Analysis Parameters—Bridging the Gap between the Sensory Evaluation and the Instrumental Measurements. J. Texture Stud. 2019, 50, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.S.Q.; Dias, L.G.; Teixeira, A. Emerging Methods for the Evaluation of Sensory Quality of Food: Technology at Service. Curr. Food Sci. Technol. Rep. 2024, 2, 77–90. [Google Scholar] [CrossRef]
- Grujić, S.; Odžaković, B.; Ciganović, M. Sensory Analysis as a Tool in the New Food Product Development. In Proceedings of the II International Congress Food Technology Quality and Safety, Novi Sad, Serbia, 28–30 October 2014; pp. 325–330. [Google Scholar]
- Lopatin, S.A.; Sharonov, A.N.; Tyts, V.V.; Zharikov, M.V. Taste Sensations: The Role of Sensory Analysis in Controlling the Quality of Food Products. Pharm. Formulas 2022, 4, 48–55. (In Russian) [Google Scholar] [CrossRef]
- Tang, W.; Dong, M.; Wang, W.; Shuo, H.; Rui, X.; Chen, X.; Jiang, M.; Zhang, Q.; Wu, J.; Li, W. Structural Characterization and Antioxidant Property of Released Exopolysaccharides from Lactobacillus delbrueckii ssp. Bulgaricus SRFM-1. Carbohydr. Polym. 2017, 173, 654–664. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Li, L. The Relationship between Charge Intensity and Bioactivities/Processing Characteristics of Exopolysaccharides from Lactic Acid Bacteria. LWT 2021, 153, 112345. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Ma, H.; Ding, Z.-C.; Yang, Y.; Wang, W.-H.; Zhang, H.-N.; Yan, J.-K. Three-Phase Partitioning for the Direct Extraction and Separation of Bioactive Exopolysaccharides from the Cultured Broth of Phellinus baumii. Int. J. Biol. Macromol. 2019, 123, 201–209. [Google Scholar] [CrossRef]
- Németh, R.; Turóczi, F.; Csernus, D.; Solymos, F.; Jaksics, E.; Tömösközi, S. Characterization of Chemical Composition and Techno-functional Properties of Oat Cultivars. Cereal Chem. 2021, 98, 1183–1192. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional Advantages of Oats and Opportunities for Its Processing as Value Added Foods—A Review. J. Food Sci. Technol. 2013, 52, 662–675. [Google Scholar] [CrossRef]
- Decker, E.A.; Rose, D.; Stewart, D. Processing of Oats and the Impact of Processing Operations on Nutrition and Health Benefits. Br. J. Nutr. 2014, 112, S58–S64. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. Oats. In Cereal Grains for the Food and Beverage Industries; Woodhead Publishing: Cambridge, UK, 2013; pp. 243–282. [Google Scholar]
- Cui, L.; Jia, Q.; Zhao, J.; Zhou, S.; Hou, D. A Comprehensive Review on Oat Milk: From Oat Nutrients and Phytochemicals to Its Processing Technologies, Product Features, and Potential Applications. Food Funct. 2023, 14, 5858–5869. [Google Scholar] [CrossRef]
- Smith, N.; Dave, A.C.; Hill, J.P.; Mcnabb, W. Nutritional Assessment of Plant-Based Beverages in Comparison to Bovine Milk. Front. Nutr. 2022, 9, 957486. [Google Scholar] [CrossRef] [PubMed]
- Ahola, H.G.; Sontag-Strohm, T.S.; Tanhuanpää, P.; Schulman, A.H.; Viitala, S.; Huang, X. Immunochemical Analysis of Oat Avenins in an Oat Cultivar and Landrace Collection. J. Cereal Sci. 2020, 95, 103053. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, A.; Orsat, V. An Overview of Different Homogenizers, Their Working Mechanisms and Impact on Processing of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2023, 63, 2004–2017. [Google Scholar] [CrossRef]
- Salehi, F. Physico-Chemical and Rheological Properties of Fruit and Vegetable Juices as Affected by High Pressure Homogenization: A Review. Int. J. Food Prop. 2020, 23, 1136–1149. [Google Scholar] [CrossRef]
- Shao, Y.; Yuan, Y.; Xi, Y.; Zhao, T.; Ai, N. Effects of Homogenization on Organoleptic Quality and Stability of Pasteurized Milk Samples. Agriculture 2023, 13, 205. [Google Scholar] [CrossRef]
- Merenkova, S.P.; Tesalova, D.G. Analysis of the Extraction Methods Effectiveness for Obtaining Plant-Based Beverages with Optimal Properties. Bull. S. Ural. State Univ. Ser. Food Biotechnol. 2021, 9, 48–56. (In Russian) [Google Scholar] [CrossRef]
- Egorova, E.Y.; Khmelev, V.N.; Morozhenko, Y.V.; Reznichenko, I.Y. Production of Vegetable “Milk” from Oilcakes Using Ultrasonic Cavitation. Foods Raw Mater. 2017, 5, 24–35. [Google Scholar] [CrossRef]
- Briviba, K.; Gräf, V.; Walz, E.; Guamis, B.; Butz, P. Ultra-High-Pressure Homogenization of Almond Milk: Physico-Chemical and Physiological Effects. Food Chem. 2016, 192, 82–89. [Google Scholar] [CrossRef]
- Khrundin, D.; Ponomarev, V.Y.; Yunusov, E.S. Fermented Oat Milk as a Base for Lactose-Free Sauce. Foods Raw Mater. 2022, 10, 155–162. [Google Scholar] [CrossRef]
- Alshevskaya, M.; Anistratova, O.; Kochina, A.; Ustich, V.; Alshevskiy, D. Rheological Structure Assessment of the Plant Alternative to Yoghurt. BIO Web Conf. 2023, 64, 01020. [Google Scholar] [CrossRef]
- Alinovi, M.; Bancalari, E.; Monica, S.; Del Vecchio, L.; Cirlini, M.; Chiavaro, E.; Bot, F. Tailoring the Physico-Chemical Properties and VOCs of Pea-Based Fermented Beverages through Lactobacillus Delbrueckii Subsp. Bulgaricus and Streptococcus Thermophilus Fermentation. Food Res. Int. 2025, 209, 116250. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Vázquez-Araújo, L.; Estrada, O.; Puente, T.; Regefalk, J. Exploring the Role of Lactic Acid Bacteria Blends in Shaping the Volatile Composition of Fermented Dairy and Rice-Based Beverages: A Step towards Innovative Plant-Based Alternatives. Foods 2024, 13, 664. [Google Scholar] [CrossRef]
- McMullen, R.L.; Gorcea, M.; Chen, S.S. Emulsions and Their Characterization by Texture Profile Analysis. In Apply Topically: A Practical Guide to Formulating Topical Application; Dayan, N., Ed.; Allured Publishing: Carol Stream, IL, USA, 2016; Chapter 6; pp. 131–154. [Google Scholar]
- Nasseri, A.T.; Thibault, J.-F.; Ralet, M.-C. Citrus Pectin: Structure and Application in Acid Dairy Drinks. In Tree and Forestry Science and Biotechnology; Global Science Books: Ikenobe, Japan, 2008. [Google Scholar]
- Khule, G.D.; Ranvare, A.R.; Singh, A.; Babu, C.S. Texture Profile Analysis: A Comprehensive Insight Ito Food Texture Evaluation. J. Dyn. Control. 2024, 8, 30–45. [Google Scholar] [CrossRef]
- Peleg, M. The Instrumental Texture Profile Analysis (TPA) Revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Krishnan, P.G.; Araña, M.; Oneca, M.; Díaz, J.V.; Barajas, M.; Rovai, M. Development, Analysis, and Sensory Evaluation of Improved Bread Fortified with a Plant-Based Fermented Food Product. Foods 2023, 12, 2817. [Google Scholar] [CrossRef] [PubMed]
- Huang, K. The Development of Sensory Analysis Techniques in the Food Industry and the Research Progress. Theor. Nat. Sci. 2024, 71, 164–169. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, V.; Singh, L. Pectin from Fruit Peels and Its Uses as Pharmaceutical and Food Grade: A Descriptive Review. Eur. J. Biomed. Pharm. Sci. 2018, 5, 185–199. [Google Scholar]
- Srivastava, P.; Malviya, R. Sources of Pectin, Extraction and Its Applications in Pharmaceutical Industry—An Overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Castro, W.F.; Cruz, A.G.; Bisinotto, M.S.; Guerreiro, L.M.R.; Faria, J.A.F.; Bolini, H.M.A.; Cunha, R.L.; Deliza, R. Development of Probiotic Dairy Beverages: Rheological Properties and Application of Mathematical Models in Sensory Evaluation. J. Dairy Sci. 2012, 96, 16–25. [Google Scholar] [CrossRef]
- Rahman, M.S.; Singh, V.; Guizani, N.; Shah, H. Correlation between Sensory and Instrumental Textural Attributes of Date Palm (Phoenix dactylifera, L.) Fruits: Technical Note. J. Agric. Mar. Sci. 2021, 26, 57–61. [Google Scholar] [CrossRef]
- He, A.; Xu, B. High-Pressure Homogenisation Improves Food Quality of Plant-Based Milk Alternatives. Int. J. Food Sci. Technol. 2024, 59, 399–407. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. B-Glucan: A Valuable Functional Ingredient in Foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, E. Effect of Carbon Source on the Synthesis and Antioxidant Properties of Exopolysaccharides of Lactic Acid Bacteria. BIO Web Conf. 2025, 181, 02021. [Google Scholar] [CrossRef]
- Ryan, P.M.; Ross, R.P.; Fitzgerald, G.F.; Caplice, N.; Stanton, C. Sugar Coated: Exopolysaccharide Producing Lactic Acid Bacteria for Food and Human Health Applications. Food Funct. 2014, 6, 679–693. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascón, A.; Dueñas, M.T.; López, P.; Spano, G. Lactobacillus Plantarum Strains for Multifunctional Oat-Based Foods. LWT 2015, 68, 288–294. [Google Scholar] [CrossRef]
- Kütt, M.-L.; Orgusaar, K.; Stulova, I.; Priidik, R.; Pismennõi, D.; Vaikma, H.; Kallastu, A.; Zhogoleva, A.; Morell, I.; Kriščiunaite, T. Starter Culture Growth Dynamics and Sensory Properties of Fermented Oat Drink. Heliyon 2023, 9, e15627. [Google Scholar] [CrossRef]
- Dhakal, D.; Kumar, G.; Devkota, L.; Subedi, D.; Dhital, S. The Choice of Probiotics Affects the Rheological, Structural, and Sensory Attributes of Lupin-Oat-Based Yoghurt. Food Hydrocoll. 2024, 156, 110353. [Google Scholar] [CrossRef]
- Farida, B.; Belleili, I. An Innovative Approach: Formulation and Evaluation of Fermented Oat Milk Using Native Yoghurt Cultures. Meas. Food 2023, 12, 100113. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Exopolysaccharides Produced by Lactic Acid Bacteria and Bifidobacteria as Fermentable Substrates by the Intestinal Microbiota. Crit. Rev. Food Sci. Nutr. 2015, 56, 1440–1453. [Google Scholar] [CrossRef]
- Ramos, P.; Cerqueira, M.; Teixeira, J.A.; Vicente, A. Physiological Protection of Probiotic Microcapsules by Coatings. Crit. Rev. Food Sci. Nutr. 2017, 58, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P. Application of Pectin in Oral Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Ventura, I.; Jammal, J.; Bianco-Peled, H. Insights into the Nanostructure of Low-Methoxyl Pectin-Calcium Gels. Carbohydr. Polym. 2013, 97, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, E. Potato Starch as a Component Increasing the Antioxidant Potential of Yogurt. Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012072. [Google Scholar] [CrossRef]
- Wei, X.; Yao, J.; Wang, F.; Wu, D.; Zhang, R. Extraction, Isolation, Structural Characterization, and Antioxidant Activity of Polysaccharides from Elderberry Fruit. Front. Nutr. 2022, 9, 947706. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Sheng, L.; Zhang, D.; Zheng, X.; Pan, Y.; Yu, X.; Liang, X.; Wang, Q.; Wang, B.; et al. Effect of Ultrasonic Degradation on the Structural Feature, Physicochemical Property and Bioactivity of Plant and Microbial Polysaccharides: A Review. Int. J. Biol. Macromol. 2023, 236, 123924. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, J.; Wang, K.; Cheng, Y.; Sheng, Q.; Kurtovic, I.; Yuan, Y.; Yue, T. Tibetan Kefir Grains Fermentation Alters Physicochemical Properties and Improves Antioxidant Activities of Lycium barbarum Pulp Polysaccharides. Food Chem. 2024, 453, 139659. [Google Scholar] [CrossRef]
- Aparicio-García, N.; Martinez-Villaluenga, C.; Frias, J.; Peñas, E. Sprouted Oat as a Potential Gluten-Free Ingredient with Enhanced Nutritional and Bioactive Properties. Food Chem. 2021, 338, 127972. [Google Scholar] [CrossRef]
- Wronkowska, M.; Rostek, D.; Lenkiewicz, M.; Kurantowicz, E.; Yaneva, T.G.; Starowicz, M. Oat Flour Fermented by Lactobacillus Strains—Kinetics of Volatile Compound Formation and Antioxidant Capacity. J. Cereal Sci. 2021, 103, 103392. [Google Scholar] [CrossRef]
- Wen, J.; Ma, L.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; Tang, D.; Zou, B.; Li, L. Effects of Probiotic Litchi Juice on Immunomodulatory Function and Gut Microbiota in Mice. Food Res. Int. 2020, 137, 109433. [Google Scholar] [CrossRef]
- Guo, C.; Cui, Q.; Cheng, J.; Chen, J.; Zhao, Z.; Guo, R.; Dai, X.; Wei, Z.; Li, W. Probiotic-Fermented Chinese Dwarf Cherry [Cerasus humilis (Bge.) Sok.] Juice Modulates the Intestinal Mucosal Barrier and Increases the Abundance of Akkermansia in the Gut in Association with Polyphenols. J. Funct. Foods 2021, 80, 104424. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; El-Naggar, E.A.; Kenawi, M.N. Morenga Leaves for Promotion the Healthy Benefits of Oat Fermented by Probiotic Bacteria: The First Investigation. Appl. Food Res. 2022, 2, 100166. [Google Scholar] [CrossRef]
- Fabiano, G.; Oliveira, R.P.S.; Rodrigues, S.; Santos, B.N.; Venema, K.; Antunes, A. Evidence of Synbiotic Potential of Oat Beverage Enriched with Inulin and Fermented by L. rhamnosus LR B in a Dynamic in Vitro Model of Human Colon. Food Res. Int. 2025, 211, 116489. [Google Scholar] [CrossRef]
- Lin, H.; Fei, T.; Liu, X.; Lin, X.; Wang, L. Oat (Avena sativa, L.) Fermented by GRAS-Grade Microorganisms: From Improvement of the Quality Properity and Health Benefits, Safety Assessment to Potential Industrial Applications. Trends Food Sci. Technol. 2025, 160, 105020. [Google Scholar] [CrossRef]
- Gungor, G.; Akpinar, A.; Yerlikaya, O. Production of Plant-Based Fermented Beverages Using Probiotic Starter Cultures and Propionibacterium spp. Food Biosci. 2024, 59, 103840. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Yuthaworawit, N.; Whanmek, K.; Suttisansanee, U.; Santivarangkna, C. Health Beneficial Properties of a Novel Plant-Based Probiotic Drink Produced by Fermentation of Brown Rice Milk with GABA-Producing Lactobacillus pentosus Isolated from Thai Pickled Weed. J. Funct. Foods 2021, 86, 104710. [Google Scholar] [CrossRef]

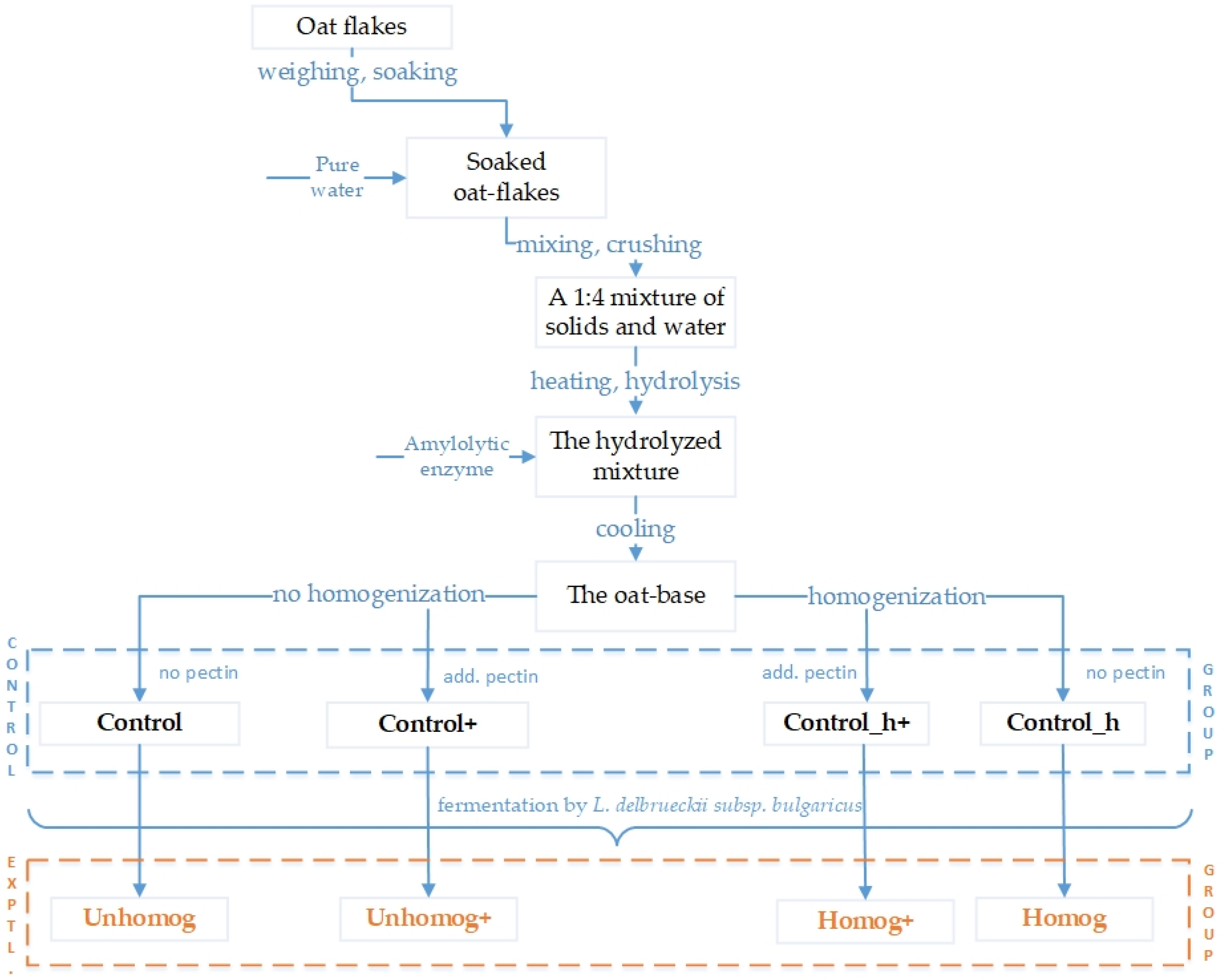
| Sample | Starter Culture: L. delbrueckii subsp. bulgaricus | Pectin Content | Short Description |
|---|---|---|---|
| Control group | |||
| Control | No | No | The crushed 1:4 mixture oat flakes and water treated by amylolytic enzymes. Not fermented by L. bulgaricus. |
| Control+ | No | Yes | The crushed 1:4 mixture oat flakes and water treated by amylolytic enzymes. Add. pectin (1%). Not fermented by L. bulgaricus. |
| Control_h | No | No | The “control” additionally milled by the homogenizer. Not fermented by L. bulgaricus. |
| Control_h+ | No | Yes | The “Control_h” additionally milled by the homogenizer. Add. pectin (1%). Not fermented by L. bulgaricus. |
| Experimental group | |||
| Unhomog | Yes | No | The “Control” fermented by L. bulgaricus. |
| Unhomog+ | Yes | Yes | The “Control+” fermented by L. bulgaricus. |
| Homog | Yes | No | The “Control_h” fermented by L. bulgaricus. |
| Homog+ | Yes | Yes | The “Control_h+” fermented by L. bulgaricus. |
| Indicator | Short Description | Significance Coefficient, k |
|---|---|---|
| Appearance | A characteristic of a product formed by sight in transmitted and reflected light during mixing and pouring (if necessary). It is necessary to exclude the other senses as much as possible. | 0.10 |
| Color | A characteristic of the color or color scheme of a product formed by a visual evaluation of the product. | 0.10 |
| Consistency | A set of rheological characteristics of products perceived by mechanical and tactile receptors. It is reasonable to understand consistency as a characteristic of the mobility (density) of viscous liquids. | 0.15 |
| Texture | A set of mechanical, geometric and surface characteristics of a product that are perceived by mechanical, tactile and, where possible, visual and auditory receptors. Texture is perceived tactilely in the oral cavity when consuming the product with the involvement of elements of the mechanical impact on the product from the teeth, tongue, and palate (pressing, crushing, and chewing). It forms the “body” of the product. | 0.20 |
| Taste | The presence of flavor, per se, perceived by the receptors initially upon contact with the product. | 0.10 |
| Aftertaste | A set of residual receptor responses after exposure to a product on the oral cavity, tongue, and palate. | 0.05 |
| Smell | The presence of an odor, per se, perceived by the receptors initially upon contact with the product. | 0.10 |
| Flavor | The totality of all the elements that form the overall perception of aroma-forming sensations by the senses of touch. | 0.05 |
| General acceptability | The overall assessment of consumer properties of the product according to the totality of all indicators. | 0.15 |
| Indicator | Evaluation Criteria | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Appearance | unsatisfactory | poor | satisfactory | good | excellent |
| Consistency | not thickened | not thickened | thickened | viscous, homogeneous | thick, homogeneous |
| Color | neutral | neutral | typical of this raw material | pleasant, with a beige shade | pleasant, pronounced |
| Texture | watery | watery | light, not watery | elastic, velvety | elastic, gummy, enveloping |
| Taste | not pronounced | weak | weakly pronounced | pronounced | pleasant, pronounced |
| Aftertaste | insignificant | insignificant | significant | developed | long aftertaste, harmonious |
| Smell | neutral | weak | pleasant | pronounced, typical | pronounced, strong |
| Flavor | not pronounced | weak | significant | developed | intense, balanced |
| General Acceptability | dislike extremely | dislike | like moderately | like very much | like extremely |
| Parameter | Before | After |
|---|---|---|
| pH | 7.04 ± 0.03 | 6.52 ± 0.03 |
| Titrable acidity, °T | 8.5 ± 0.05 | 14.0 ± 0.06 |
| Protein, % | 2.16 ± 0.01 | 2.56 ± 0.01 |
| Fat, % | 0.99 ± 0.01 | 1.03 ± 0.01 |
| Dry matter, % | 12.57 ± 0.05 | 13.95 ± 0.08 |
| Total sugar, % | 2.73 ± 0.02 | 8.26 ± 0.05 |
| Glucose, mmol/L | 0.1 ± 0.01 | 12.8 ± 0.02 |
| Sample | Protein, % | Fat, % | Dry Matter, % | |||
|---|---|---|---|---|---|---|
| No Pectin | +Pectin | No Pectin | +Pectin | No Pectin | +Pectin | |
| Control | 1.95 ± 0.05 | 2.19 ± 0.05 ab | 0.93 ± 0.03 | 1.03 ± 0.01 | 12.16 ± 0.22 | 13.64 ± 0.24 a |
| Control_h | 1.94 ± 0.06 | 2.27 ± 0.06 a | 0.95 ± 0.01 | 1.10 ± 0.02 ab | 13.14 ± 0.21 b | 13.38 ± 0.22 a |
| Unhomog | 1.93 ± 0.06 | 2.24 ± 0.06 a | 0.91 ± 0.01 | 1.10 ± 0.02 ab | 12.74 ± 0.21 b | 13.43 ± 0.22 a |
| Homog | 1.85 ± 0.06 | 2.16 ± 0.07 a | 0.90 ± 0.02 | 1.11 ± 0.02 ab | 12.16 ± 0.23 | 14.39 ± 0.24 ab |
| Sample | Apparent Viscosity, cP | Lƞ, % | CMR | |||
|---|---|---|---|---|---|---|
| No Pectin | +Pectin | No Pectin | +Pectin | No Pectin | +Pectin | |
| Control | ND | 114.9 ± 2.0 ab | - | 14.2 a | - | 0.88 a |
| Control_h | ND | 180.3 ± 2.0 ab | - | 11.3 a | - | 0.92 a |
| Unhomog | 165.2 ± 1.3 a | 285.1 ± 2.2 ab | 2.9 b | 1.5 b | 0.98 | 0.98 b |
| Homog | 283.0 ± 3.4 a | 376.1 ± 7.3 ab | 1.04 b | 1.0 b | 0.99 | 0.99 b |
| Sample | Syn, % | Δ Syn, % to No Pectin | WHC, % | Δ WHC, % to No Pectin | ||
| No Pectin | +Pectin | No Pectin | +Pectin | |||
| Control | 52.9 ± 2.3 ab | 11.0 ± 1.4 ab | −79.2 | 30.3 ± 1.2 ab | 55.2 ± 1.6 ab | +82.2 |
| Control_h | 45.4 ± 2.3 ab | 9.0 ± 1.4 ab | −80.2 | 36.3 ± 1.2 ab | 58.2 ± 1.6 ab | +60.3 |
| Unhomog | 24.3 ± 1.8 ab | 1.5 ± 0.7 ab | −96.0 | 47.5 ± 1.4 ab | 98.0 ± 1.0 ab | +108.6 |
| Homog | 3.1 ± 0.6 ab | 0.5 ± 0.1 ab | −84.1 | 93.0 ± 1.4 ab | 98.0 ± 1.0 ab | +7.0 |
| Sample | Hardness, g | Cohesiveness, % | ||
|---|---|---|---|---|
| No Pectin | +Pectin | No Pectin | +Pectin | |
| Control | 11.22 ± 0.56 | 12.11 ± 0.61 | 94.34 ± 4.72 | 97.61 ± 4.88 |
| Control_h | 11.58 ± 0.56 | 12.47 ± 0.61 | 96.34 ± 4.82 | 98.15 ± 4.91 |
| Unhomog | 12.53 ± 0.63 | 13.21 ± 0.69 | 75.90 ± 3.80 | 81.51 ± 4.08 |
| Homog | 11.92 ± 0.60 | 13.12 ± 0.66 a | 92.68 ± 4.63 | 96.37 ± 4.92 |
| Sample | Gumminess, g | Adhesiveness, g·mm | ||
| No Pectin | +Pectin | No Pectin | +Pectin | |
| Control | 10.60 ± 0.53 | 11.81 ± 0.59 a | 45.44 ± 2.27 | 51.53 ± 2.58 a |
| Control_h | 11.15 ± 0.56 | 11.91 ± 0.59 a | 44.24 ± 2.21 | 52.49 ± 2.62 a |
| Unhomog | 9.5 ± 0.48 b | 11.16 ± 0.56 a | 52.2 ± 2.61 b | 56.85 ± 2.84 a |
| Homog | 11.72 ± 0.59 b | 12.12 ± 0.61 a | 49.63 ± 2.48 b | 54.86 ± 2.74 a |
| Indicator | Sample | Mean | ||
|---|---|---|---|---|
| Appearance | Unhomog | 0.40 ± 0.02 | ||
| Homog | 0.40 ± 0.02 | |||
| Unhomog+ | 0.45 ± 0.02 ab | |||
| Homog+ | 0.48 ± 0.02 ab | |||
| Color | Unhomog | 0.41 ± 0.02 | ||
| Homog | 0.42 ± 0.02 | |||
| Unhomog+ | 0.43 ± 0.02 | |||
| Homog+ | 0.43 ± 0.02 | |||
| Consistency | Unhomog | 0.51 ± 0.03 ab | ||
| Homog | 0.63 ± 0.03 ab | |||
| Unhomog+ | 0.68 ± 0.03 ab | |||
| Homog+ | 0.74 ± 0.04 ab | |||
| Texture | Unhomog | 0.64 ± 0.04 ab | ||
| Homog | 0.82 ± 0.04 ab | |||
| Unhomog+ | 0.92 ± 0.05 ab | |||
| Homog+ | 0.98 ± 0.05 ab | |||
| Taste | Unhomog | 0.42 ± 0.02 ab | ||
| Homog | 0.45 ± 0.02 ab | |||
| Unhomog+ | 0.47 ± 0.02 ab | |||
| Homog+ | 0.49 ± 0.02 ab | |||
| Aftertaste | Unhomog | 0.11 ± 0.01 ab | ||
| Homog | 0.17 ± 0.01 ab | |||
| Unhomog+ | 0.21 ± 0.01 ab | |||
| Homog+ | 0.24 ± 0.01 ab | |||
| Smell | Unhomog | 0.31 ± 0.02 | ||
| Homog | 0.31 ± 0.02 | |||
| Unhomog+ | 0.43 ± 0.02 ab | |||
| Homog+ | 0.45 ± 0.02 ab | |||
| Flavor | Unhomog | 0.14 ± 0.01 | ||
| Homog | 0.16 ± 0.01 | |||
| Unhomog+ | 0.22 ± 0.01 ab | |||
| Homog+ | 0.23 ± 0.01 ab | |||
| General acceptability | Unhomog | 0.50 ± 0.03 ab | ||
| Homog | 0.57 ± 0.03 ab | |||
| Unhomog+ | 0.67 ± 0.03 ab | |||
| Homog+ | 0.70 ± 0.03 ab | |||
| Total | Unhomog | Homog | Unhomog+ | Homog+ |
| 3.4 | 3.9 | 4.5 | 4.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khrundin, D.V.; Nikitina, E.V. Effect of Homogenization and Pectin on Chemical, Textural, Antioxidant and Sensory Characteristics of L. bulgaricus-Fermented Oat-Based Product. Foods 2025, 14, 2615. https://doi.org/10.3390/foods14152615
Khrundin DV, Nikitina EV. Effect of Homogenization and Pectin on Chemical, Textural, Antioxidant and Sensory Characteristics of L. bulgaricus-Fermented Oat-Based Product. Foods. 2025; 14(15):2615. https://doi.org/10.3390/foods14152615
Chicago/Turabian StyleKhrundin, Dmitrii V., and Elena V. Nikitina. 2025. "Effect of Homogenization and Pectin on Chemical, Textural, Antioxidant and Sensory Characteristics of L. bulgaricus-Fermented Oat-Based Product" Foods 14, no. 15: 2615. https://doi.org/10.3390/foods14152615
APA StyleKhrundin, D. V., & Nikitina, E. V. (2025). Effect of Homogenization and Pectin on Chemical, Textural, Antioxidant and Sensory Characteristics of L. bulgaricus-Fermented Oat-Based Product. Foods, 14(15), 2615. https://doi.org/10.3390/foods14152615





