The Global Prevalence of Bacillus spp. in Milk and Dairy Products: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Meta-Analysis and Statistical Analyses
3. Results and Discussion
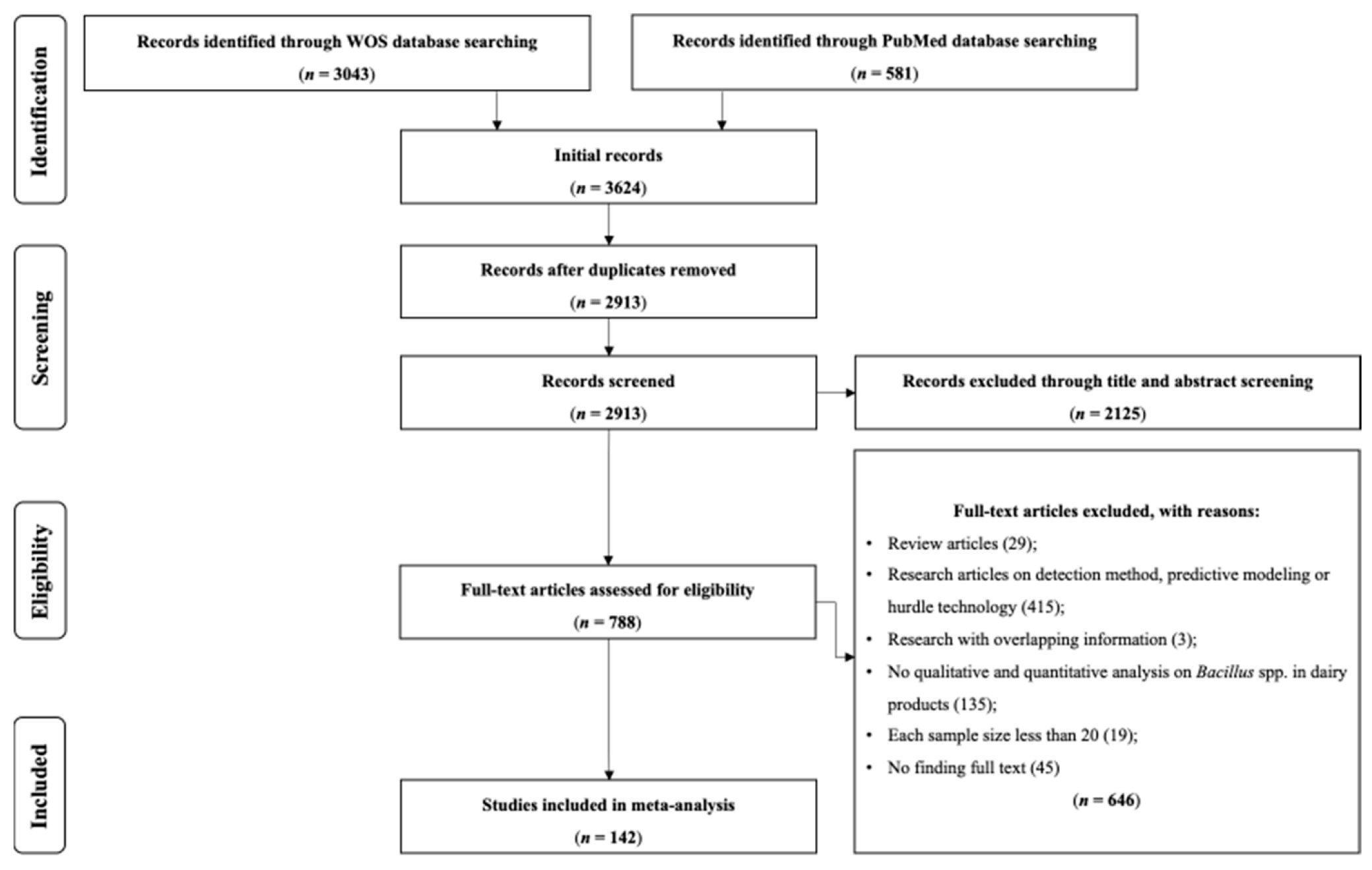
3.1. Systematic Review Process
3.2. Overall Prevalence of Bacillus spp.
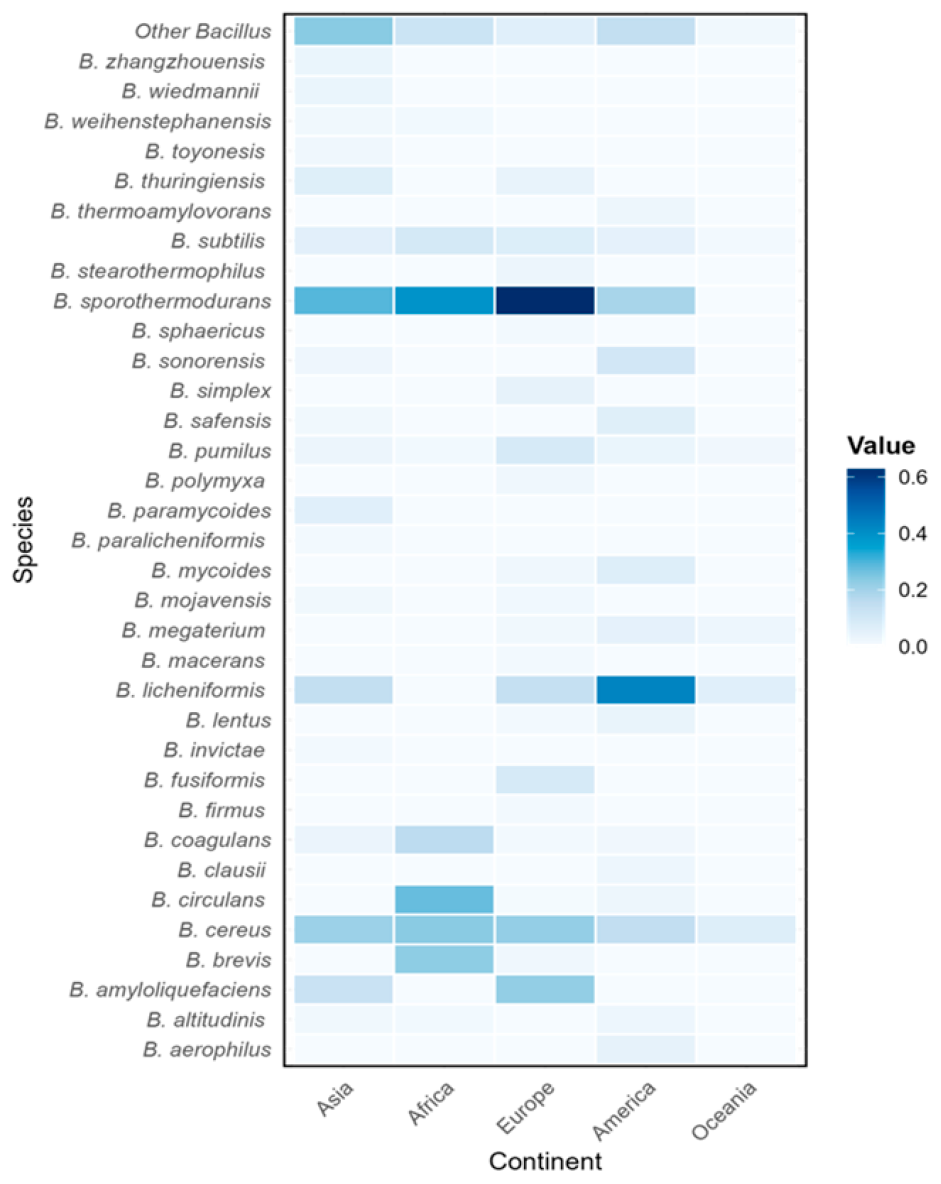
3.3. Prevalence of Bacillus spp. Considering Geographic Regions
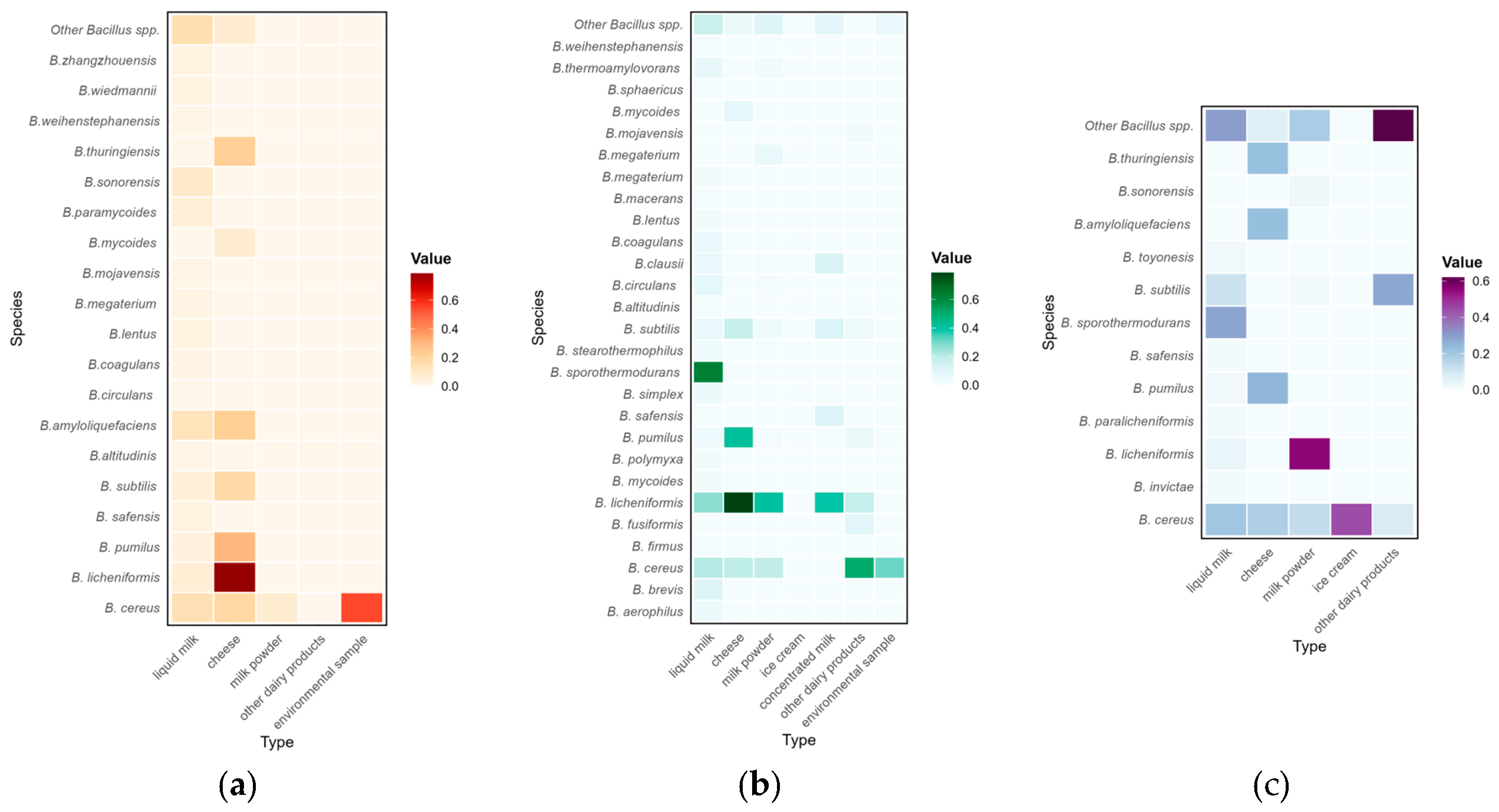
3.4. Prevalence of Bacillus spp. Considering the Source of Sampling and Type of Samples
3.5. Distribution of Bacillus Species
3.6. Prevalence of Bacillus spp. by Detection Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and Dairy Products and Their Nutritional Contribution to the Average Polish Diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Bhargava, N.; O’Connor, A.; Gibney, E.R.; Feeney, E.L. Dairy Consumption in Adults in China: A Systematic Review. BMC Nutr. 2023, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Saha, A.; Aggarwal, K. Natural Biopreservatives for Dairy Products: Microbial and Plant-Derived Solutions and Recent Advancements. Int. Dairy J. 2025, 166, 106256. [Google Scholar] [CrossRef]
- Pereira, A.P.M.; Sant’Ana, A.S. Diversity and Fate of Spore Forming Bacteria in Cocoa Powder, Milk Powder, Starch and Sugar during Processing: A Review. Trends Food Sci. Technol. 2018, 76, 101–118. [Google Scholar] [CrossRef]
- Quintieri, L.; Caputo, L.; Brasca, M.; Fanelli, F. Recent Advances in the Mechanisms and Regulation of QS in Dairy Spoilage by Pseudomonas spp. Foods 2021, 10, 3088. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.; Vieira, A.R.; Perez, E.; Wong, D.L.F.; Hald, T. Attributing Human Foodborne Illness to Food Sources and Water in Latin America and the Caribbean Using Data from Outbreak Investigations. Int. J. Food Microbiol. 2012, 152, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Grace, D.; Wu, F.; Havelaar, A.H. MILK Symposium Review: Foodborne Diseases from Milk and Milk Products in Developing Countries—Review of Causes and Health and Economic Implications. J. Dairy Sci. 2020, 103, 9715–9729. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Anand, S.K. Characterization of Constitutive Microflora of Biofilms in Dairy Processing Lines. Food Microbiol. 2002, 19, 627–636. [Google Scholar] [CrossRef]
- Ostrov, I.; Sela, N.; Belausov, E.; Steinberg, D.; Shemesh, M. Adaptation of Bacillus Species to Dairy Associated Environment Facilitates Their Biofilm Forming Ability. Food Microbiol. 2019, 82, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Cempírková, R. Psychrotrophic vs. Total Bacterial Counts in Bulk Milk Samples. Vet. Med. 2002, 47, 227–233. [Google Scholar] [CrossRef]
- Flint, S.; Gonzaga, Z.J.; Good, J.; Palmer, J. Bacillus Thermoamylovorans—A New Threat to the Dairy Industry—A Review. Int. Dairy J. 2017, 65, 38–43. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Rahnama, H.; Azari, R.; Yousefi, M.H.; Berizi, E.; Mazloomi, S.M.; Hosseinzadeh, S.; Derakhshan, Z.; Ferrante, M.; Conti, G.O. A Systematic Review and Meta-Analysis of the Prevalence of Bacillus Cereus in Foods. Food Control 2023, 143, 109250. [Google Scholar] [CrossRef]
- Faille, C.; Bénézech, T.; Midelet-Bourdin, G.; Lequette, Y.; Clarisse, M.; Ronse, G.; Ronse, A.; Slomianny, C. Sporulation of Bacillus spp. within Biofilms: A Potential Source of Contamination in Food Processing Environments. Food Microbiol. 2014, 40, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Fogele, B.; Granta, R.; Valciņa, O.; Bērziņš, A. Occurrence and Diversity of Bacillus Cereus and Moulds in Spices and Herbs. Food Control 2018, 83, 69–74. [Google Scholar] [CrossRef]
- Lücking, G.; Stoeckel, M.; Atamer, Z.; Hinrichs, J.; Ehling-Schulz, M. Characterization of Aerobic Spore-Forming Bacteria Associated with Industrial Dairy Processing Environments and Product Spoilage. Int. J. Food Microbiol. 2013, 166, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Logan, N. Bacillus and relatives in foodborne illness. J. Appl. Microbiol. 2012, 112, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Ortuzar, J.; Martinez, B.; Bianchini, A.; Stratton, J.; Rupnow, J.; Wang, B. Quantifying Changes in Spore-Forming Bacteria Contamination along the Milk Production Chain from Farm to Packaged Pasteurized Milk Using Systematic Review and Meta-Analysis. Food Control 2018, 86, 319–331. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Butler, F. The Use of Meta-Analytical Tools in Risk Assessment for Food Safety. Food Microbiol. 2011, 28, 823–827. [Google Scholar] [CrossRef] [PubMed]
- den Besten, H.M.W.; Zwietering, M.H. Meta-Analysis for Quantitative Microbiological Risk Assessments and Benchmarking Data. Trends Food Sci. Technol. 2012, 25, 34–39. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Cadavez, V.; Sheridan, J.J.; Butler, F. Modelling the Effect of Chilling on the Occurrence of Salmonella on Pig Carcasses at Study, Abattoir and Batch Levels by Meta-Analysis. Int. J. Food Microbiol. 2013, 163, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Barron, U.; Gonçalves-Tenório, A.; Rodrigues, V.; Cadavez, V. Foodborne Pathogens in Raw Milk and Cheese of Sheep and Goat Origin: A Meta-Analysis Approach. Curr. Opin. Food Sci. 2017, 18, 7–13. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Sun, T.; Gorris, L.G.M.; Wang, X.; Liu, B.; Dong, Q. The Prevalence of Listeria Monocytogenes in Meat Products in China: A Systematic Literature Review and Novel Meta-Analysis Approach. Int. J. Food Microbiol. 2020, 312, 108358. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, Y.; Aspridou, Z.; Zheng, J.; Wang, X.; Li, Z.; Dong, Q. The Prevalence and Epidemiology of Salmonella in Retail Raw Poultry Meat in China: A Systematic Review and Meta-Analysis. Foods 2021, 10, 2757. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Explanation and Elaboration: Updated Guidance and Examples for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- PRISMA 2020 Flow Diagram. Available online: https://www.prisma-statement.org/prisma-2020-flow-diagram (accessed on 5 September 2024).
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, Y.; Gasser, R.B.; Rostami, A.; Fan, C.K.; Ghasemi, S.M.; Javanian, M.; Bayani, M.; Armoon, B.; Moradi, B. Toxocara Eggs in Public Places Worldwide—A Systematic Review and Meta-Analysis. Environ. Pollut. 2018, 242, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Dadar, M.; Fakhri, Y.; Shahali, Y.; Mousavi Khaneghah, A. Contamination of Milk and Dairy Products by Brucella Species: A Global Systematic Review and Meta-Analysis. Food Res. Int. 2020, 128, 108775. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Nematollahi, A.; Bashiry, M.; Javanmardi, F.; Mousavi, M.; Hosseini, H. The Global Prevalence of Campylobacter spp. in Milk: A Systematic Review and Meta-Analysis. Int. Dairy J. 2022, 133, 105423. [Google Scholar] [CrossRef]
- El-Zamkan, M.A.; Abdel Hameed, K.G. Prevalence of Campylobacter jejuni and Campylobacter coli in Raw Milk and Some Dairy Products. Vet World 2016, 9, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Bertasi, B.; Losio, M.N.; Daminelli, P.; Finazzi, G.; Serraino, A.; Piva, S.; Giacometti, F.; Massella, E.; Ostanello, F. Seasonal Variability of Thermophilic Campylobacter spp. in Raw Milk Sold by Automatic Vending Machines in Lombardy. Ital. J. Food Saf. 2016, 5, 5848. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Firstenberg-Eden, R.; Rosen, B.; Mannheim, C.H. Seasonal and Processing Influences on Bacterial Count of Raw and Processed Milk. J. Food Prot. 1979, 42, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, P.; Zhao, X.; He, L.; Qu, T.; Chen, Y. Single-Molecule Real-Time Sequencing Reveals Differences in Bacterial Diversity in Raw Milk in Different Regions and Seasons in China. J. Dairy Sci. 2022, 105, 5669–5684. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Hu, Q.; Xu, F.; Ding, S.-Y.; Zhu, K. Characterization of Bacillus cereus in Dairy Products in China. Toxins 2020, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Rezende-Lago, N.C.M.; Rossi, O.D., Jr.; Vidal-Martins, A.M.C.; Amaral, L.A. Occurrence of Bacillus Cereus in Whole Milk and Enterotoxigenic Potential of the Isolated Strains. Arq. Bras. Med. Vet. Zootec. 2007, 59, 1563–1569. [Google Scholar] [CrossRef]
- Kumari, S.; Sarkar, P.K. Bacillus Cereus Hazard and Control in Industrial Dairy Processing Environment. Food Control 2016, 69, 20–29. [Google Scholar] [CrossRef]
- Lesley, M.B.; Velnetti, L.; Yousr, A.N.; Kasing, A.; Samuel, L. Presence of Bacillus Cereus s.l. from Ready-to-Eat Cereals (RTE) Products in Sarawak. Int. Food Res. J. 2013, 20, 1031–1034. [Google Scholar]
- Kim, J.-B.; Choi, O.-K.; Kwon, S.-M.; Cho, S.-H.; Park, B.-J.; Jin, N.Y.; Yu, Y.M.; Oh, D.-H. Prevalence and Toxin Characteristics of Bacillus thuringiensis Isolated from Organic Vegetables. J. Microbiol. Biotechnol. 2017, 27, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; McSweeney, P.L.H. Cheese: An Overview. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; General Aspects; Academic Press: Cambridge, MA, USA, 2004; Volume 1, pp. 1–18. [Google Scholar] [CrossRef]
- Button, J.E.; Dutton, R.J. Cheese Microbes. Curr. Biology 2012, 22, R587–R589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ning, C.; Chen, L.; Zhao, Y.; Yang, G.; Wang, C.; Chen, N.; Zhang, Z.; Li, S. Impacts of Manufacture Processes and Geographical Regions on the Microbial Profile of Traditional Chinese Cheeses. Food Res. Int. 2021, 148, 110600. [Google Scholar] [CrossRef] [PubMed]
- FSANZ. Microbiological Risk Assessmet of Raw Cow Milk. Risk Assessment Microbiology Section. Available online: https://www.foodstandards.gov.au/sites/default/files/food-standards-code/proposals/Documents/P1007%20PPPS%20for%20raw%20milk%201AR%20SD1%20Cow%20milk%20Risk%20Assessment.pdf (accessed on 5 June 2025).
- Ruwer, C.M.; de Moura, J.F.; Gonçalves, M.J.F. Outbreaks of Foodborne Diseases in Manaus, Amazonas (2005–2009): The Problem of Cheese Curd. Segurança Aliment. E Nutr. 2011, 18, 60–66. [Google Scholar] [CrossRef]
- Amenu, K.; Wieland, B.; Szonyi, B.; Grace, D. Milk Handling Practices and Consumption Behavior among Borana Pastoralists in Southern Ethiopia. J. Health Popul. Nutr. 2019, 38, 6. [Google Scholar] [CrossRef] [PubMed]
- Keba, A.; Rolon, M.L.; Tamene, A.; Dessie, K.; Vipham, J.; Kovac, J.; Zewdu, A. Review of the Prevalence of Foodborne Pathogens in Milk and Dairy Products in Ethiopia. Int. Dairy J. 2020, 109, 104762. [Google Scholar] [CrossRef]
- Zastempowska, E.; Grajewski, J.; Twarużek, M. Food-Borne Pathogens and Contaminants in Raw Milk—A Review. Ann. Anim. Sci. 2016, 16, 623–639. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Y.; Yan, L.; Yang, S.; Yang, D. Correlation Analysis of Microbial Contamination and Alkaline Phosphatase Activity in Raw Milk and Dairy Products. Int. J. Environ. Res. Public Health 2023, 20, 1825. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, B.; Djekic, I.; Miocinovic, J.; Miloradovic, Z.; Savic-Radovanovic, R.; Zdravkovic, N.; Smigic, N. The Hygienic Assessment of Dairy Products’ Selling Places at Open Markets. Food Control 2023, 148, 109628. [Google Scholar] [CrossRef]
- Mitchell, R. Aseptic and other designs that extend the shelf life of dairy products are driving functionality and efficiency. Dairy Foods 2023, 124, 12. [Google Scholar]
- Bennett, S.D.; Walsh, K.A.; Gould, L.H. Foodborne Disease Outbreaks Caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus—United States, 1998–2008. Clin. Infect. Dis. 2013, 57, 425–433. [Google Scholar] [CrossRef]
- Liu, J.; Bai, L.; Li, W.; Han, H.; Fu, P.; Ma, X.; Bi, Z.; Yang, X.; Zhang, X.; Zhen, S.; et al. Trends of Foodborne Diseases in China: Lessons from Laboratory-Based Surveillance since 2011. Front. Med. 2018, 12, 48–57. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2013. EFSA J. 2015, 13, 3991. [Google Scholar] [CrossRef]
- Schoeni, J.L.; Lee Wong, A.C. Bacillus Cereus Food Poisoning and Its Toxins. J. Food Prot. 2005, 68, 636–648. [Google Scholar] [CrossRef] [PubMed]
- From, C.; Hormazabal, V.; Granum, P.E. Food Poisoning Associated with Pumilacidin-Producing Bacillus Pumilus in Rice. Int. J. Food Microbiol. 2007, 115, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Apetroaie-Constantin, C.; Mikkola, R.; Andersson, M.A.; Teplova, V.; Suominen, I.; Johansson, T.; Salkinoja-Salonen, M. Bacillus subtilis and B. mojavensis Strains Connected to Food Poisoning Produce the Heat Stable Toxin Amylosin. J. Appl. Microbiol. 2009, 106, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Scheldeman, P.; Herman, L.; Foster, S.; Heyndrickx, M. Bacillus sporothermodurans and Other Highly Heat-resistant Spore Formers in Milk. J. Appl. Microbiol. 2006, 101, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Huemer, I.A.; Klijn, N.; Vogelsang, H.W.J.; Langeveld, L.P.M. Thermal Death Kinetics of Spores of Bacillus sporothermodurans Isolated from UHT Milk. Int. Dairy J. 1998, 8, 851–855. [Google Scholar] [CrossRef]
- Alonso, V.P.P.; de Oliveira Morais, J.; Kabuki, D.Y. Incidence of Bacillus cereus, Bacillus sporothermodurans and Geobacillus stearothermophilus in Ultra-High Temperature Milk and Biofilm Formation Capacity of Isolates. Int. J. Food Microbiol. 2021, 354, 109318. [Google Scholar] [CrossRef] [PubMed]
- Lazcka, O.; Campo, F.J.D.; Muñoz, F.X. Pathogen Detection: A Perspective of Traditional Methods and Biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Baraketi, A.; Salmieri, S.; Lacroix, M.; Baraketi, A.; Salmieri, S.; Lacroix, M. Foodborne Pathogens Detection: Persevering Worldwide Challenge. In Biosensing Technologies for the Detection of Pathogens—A Prospective Way for Rapid Analysis; Rinken, T., Kivirand, K., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Razzuoli, E.; Vencia, W.; Fedele, V.; Mignone, G.; Lazzara, F.; Rubini, D.; Vito, G.; Porcario, C.; Bozzetta, E.; Ferrari, A. Evaluation and Validation of an Alternative Method to Detect Campylobacter spp. in Dairy Products. Ital. J. Food Safety 2018, 7, 89–94. [Google Scholar] [CrossRef] [PubMed]
| Total Included Study | Total Inputs | Total Sample Size | Pooled Prevalence (95% CI) a | τ2 b | I2 c |
|---|---|---|---|---|---|
| 142 | 417 | 54,772 | 11.8% (10.1–13.7%) | 2.9600 | 94.80% |
| Continents | Total Inputs | Total Sample Size | Pooled Prevalence (95% CI) a | τ2 b | I2 c |
|---|---|---|---|---|---|
| Asia | 134 | 13,953 | 15.4% (12.3–19.1%) | 2.1207 | 92.30% |
| Africa | 31 | 2252 | 13.9% (9.3–20.3%) | 1.4542 | 86.20% |
| Europe | 110 | 12,841 | 11.4% (8.2–15.6%) | 3.4255 | 94.90% |
| America | 123 | 19,096 | 10.2% (7.5–13.7%) | 3.1815 | 95.60% |
| Oceania | 13 | 2877 | 3.5% (3.3–3.7%) | 5.4501 | 96.00% |
| Unknown | 6 | 3753 | 3.1% (0.5–16.0%) | 4.8592 | 98.80% |
| Sampling Site | Sample Type | Total Inputs | Total Sample Size | Pooled Prevalence(95% CI) a | τ2 b | I2 c |
|---|---|---|---|---|---|---|
| Farm | Liquid milk | 77 | 9606 | 7.6% (5.1–11.2%) | 3.3014 | 94.40% |
| Cheese | 2 | 105 | 20.8% (7.3–46.7%) | 0.6157 | 90.30% | |
| Milk powder | 1 | 30 | 73.3% (55.0–86.1%) | - | - | |
| Other dairy products | 1 | 27 | 0.0% | - | - | |
| Environmental sample | 12 | 906 | 45.5% (20.1–72.8%) | 4.0493 | 94.00% | |
| Total | / | 93 | 10,674 | 10.3% (6.9–15.0%) | 4.1614 | 94.60% |
| Dairy plant | Liquid milk | 76 | 9492 | 8.6% (6.0–12.2%) | 2.4552 | 94.70% |
| Cheese | 15 | 865 | 24.2% (10.7–46.0%) | 3.5303 | 94.60% | |
| Milk powder | 49 | 13,356 | 5.9% (3.3–10.5%) | 4.3748 | 97.10% | |
| Ice cream | 1 | 24 | 0.0% | - | - | |
| Concentrated milk | 9 | 209 | 14.6% (8.2–24.4%) | 0.5367 | 60.50% | |
| Other dairy products | 11 | 584 | 19.7% (9.1–37.6%) | 2.0681 | 90.30% | |
| Environmental sample | 6 | 321 | 13.4% (3.9–37.0%) | 2.3417 | 93.20% | |
| Total | / | 167 | 24,851 | 9.2% (7.1–12.0%) | 3.2256 | 95.40% |
| Retail | Liquid milk | 60 | 4171 | 16.4% (12.0–22.1%) | 1.8553 | 88.90% |
| Cheese | 35 | 2853 | 17.6% (12.7–23.7%) | 1.1223 | 85.50% | |
| Milk powder | 26 | 4472 | 12.9% (7.0–22.4%) | 2.7663 | 96.70% | |
| Ice cream | 6 | 1012 | 44.2% (28.3–61.4%) | 0.6456 | 88.70% | |
| Other dairy products | 15 | 2708 | 10.9% (4.7–23.2%) | 3.0115 | 93.20% | |
| Total | / | 142 | 15,216 | 16.1% (13.0–19.7%) | 2.0203 | 93.50% |
| Species | Total Inputs | Total Sample Size | Pooled Prevalence (95% CI) a | τ2 b | I2 c |
|---|---|---|---|---|---|
| Bacillus cereus | 210 | 21,837 | 19.8% (16.6–23.3%) | 2.2394 | 93.50% |
| Bacillus licheniformis | 32 | 6027 | 26.3% (16.0–40.1%) | 3.1015 | 94.40% |
| Bacillus pumilus | 25 | 4165 | 3.1% (1.6–5.9%) | 2.2891 | 88.10% |
| Bacillus safensis | 5 | 311 | 3.6% (1.4–9.2%) | 0.6948 | 64.60% |
| Bacillus subtilis | 31 | 5184 | 5.3% (3.4–8.2%) | 1.4281 | 91.40% |
| Bacillus altitudinis | 3 | 232 | 1.7% (0.7–4.5%) | 0.0000 | 0.00% |
| Bacillus mojavensis | 2 | 135 | 1.5% (0.4–5.7%) | 0.0000 | 0.00% |
| Bacillus wiedmannii | 1 | 66 | 3.0% (0.8–11.3%) | - | - |
| Bacillus weihenstephanensis | 1 | 66 | 1.5% (0.2–1.0%) | - | - |
| Bacillus zhangzhouensis | 1 | 66 | 3.0% (0.7–11.3%) | - | - |
| Bacillus paramycoides | 1 | 66 | 6.1% (2.3–15.1%) | - | - |
| Bacillus sonorensis | 3 | 906 | 5.7% (2.4–12.9%) | 0.4974 | 90.30% |
| Bacillus circulans | 6 | 1212 | 3.4% (0.7–14.6%) | 3.3753 | 95.10% |
| Bacillus coagulans | 7 | 1102 | 3.1% (1.0–9.0%) | 1.7724 | 88.90% |
| Bacilus weithenstephanensis | 1 | 83 | 1.2% (0.2–8.1%) | - | - |
| Bacillus amyloliquefaciens | 2 | 58 | 17.2% (9.5–29.2%) | 0.0000 | 0.00% |
| Bacillus thuringiensis | 3 | 321 | 4.52% (0.65–25.5%) | 2.5422 | 88.40% |
| Bacillus mycoides | 2 | 169 | 3.6% (1.4–8.8%) | 0.1241 | 60.10% |
| Bacillus megaterium | 4 | 759 | 2.9% (1.8–4.7%) | 0.0449 | 32.10% |
| Bacillus lentus | 3 | 195 | 2.1% (0.8–5.3%) | 0.0000 | 0.00% |
| Bacillus sporothermodurans | 4 | 483 | 36.5% (21.8–54.3%) | 0.5024 | 95.00% |
| Bacillus clausii | 7 | 1570 | 2.4% (1.0–5.8%) | 1.0418 | 70.70% |
| Bacillus brevis | 3 | 240 | 10.2% (2.2–37.1%) | 1.8577 | 92.20% |
| Bacillus paralicheniformis | 1 | 114 | 0.9% (0.1–6.0%) | - | - |
| Bacillus toyonesis | 1 | 114 | 1.8% (0.4–6.7%) | - | - |
| Bacillus invictae | 1 | 114 | 0.9% (0.1–6.0%) | - | - |
| Bacillus thermoamylovorans | 5 | 1523 | 2.5% (0.5–11.3%) | 2.6330 | 88.00% |
| Bacillus simplex | 1 | 22 | 4.6% (0.6–26.2%) | - | - |
| Bacillus fusiformis | 1 | 69 | 8.7% (4.0–18.0%) | - | - |
| Bacillus sphaericus | 1 | 111 | 0.9% (0.1–6.1%) | - | - |
| Bacillus macerans | 1 | 111 | 0.9% (0.1–6.1%) | - | - |
| Bacillus firmus | 1 | 111 | 0.9% (0.1–6.1%) | - | - |
| Bacillus polymyxa | 1 | 111 | 1.8% (0.5–7.0%) | - | - |
| Bacillus stearothermophilus | 1 | 111 | 2.7% (0.9–8.0%) | - | - |
| Bacillus aerophilus | 1 | 22 | 4.6% (0.6–26.2%) | - | - |
| Other Bacillus | 44 | 6986 | 11.9% (7.8–17.6%) | 2.1392 | 95.30% |
| Detection Method | Total Inputs | Total Sample Size | Pooled Prevalence (95% CI) a | τ2 b | I2 c |
|---|---|---|---|---|---|
| Culture–biochemical | 222 | 34,572 | 9.2% (7.4–11.3%) | 2.8320 | 95.50% |
| Culture–molecular | 165 | 17,428 | 16.6% (13.1–20.8%) | 3.0342 | 93.70% |
| Culture–biochemical–molecular | 30 | 2772 | 10.8% (6.6–17.0%) | 1.9548 | 91.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Wang, R.; Sun, Y.; Zhang, X.; Ge, C.; Li, Y. The Global Prevalence of Bacillus spp. in Milk and Dairy Products: A Systematic Review and Meta-Analysis. Foods 2025, 14, 2599. https://doi.org/10.3390/foods14152599
Sun T, Wang R, Sun Y, Zhang X, Ge C, Li Y. The Global Prevalence of Bacillus spp. in Milk and Dairy Products: A Systematic Review and Meta-Analysis. Foods. 2025; 14(15):2599. https://doi.org/10.3390/foods14152599
Chicago/Turabian StyleSun, Tianmei, Ran Wang, Yanan Sun, Xiaoxu Zhang, Chongtao Ge, and Yixuan Li. 2025. "The Global Prevalence of Bacillus spp. in Milk and Dairy Products: A Systematic Review and Meta-Analysis" Foods 14, no. 15: 2599. https://doi.org/10.3390/foods14152599
APA StyleSun, T., Wang, R., Sun, Y., Zhang, X., Ge, C., & Li, Y. (2025). The Global Prevalence of Bacillus spp. in Milk and Dairy Products: A Systematic Review and Meta-Analysis. Foods, 14(15), 2599. https://doi.org/10.3390/foods14152599





