Characterization of Brown Seaweed (Ascophyllum nodosum) and Sugar Kelp (Saccharina latissima) Extracts Using Temporal Check-All-That-Apply
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. SPME Seaweed Headspace Analysis
2.3. Participants
2.4. Sensory Procedure
2.5. Statistical Analysis
3. Results
3.1. Analysis of Volatile Compounds
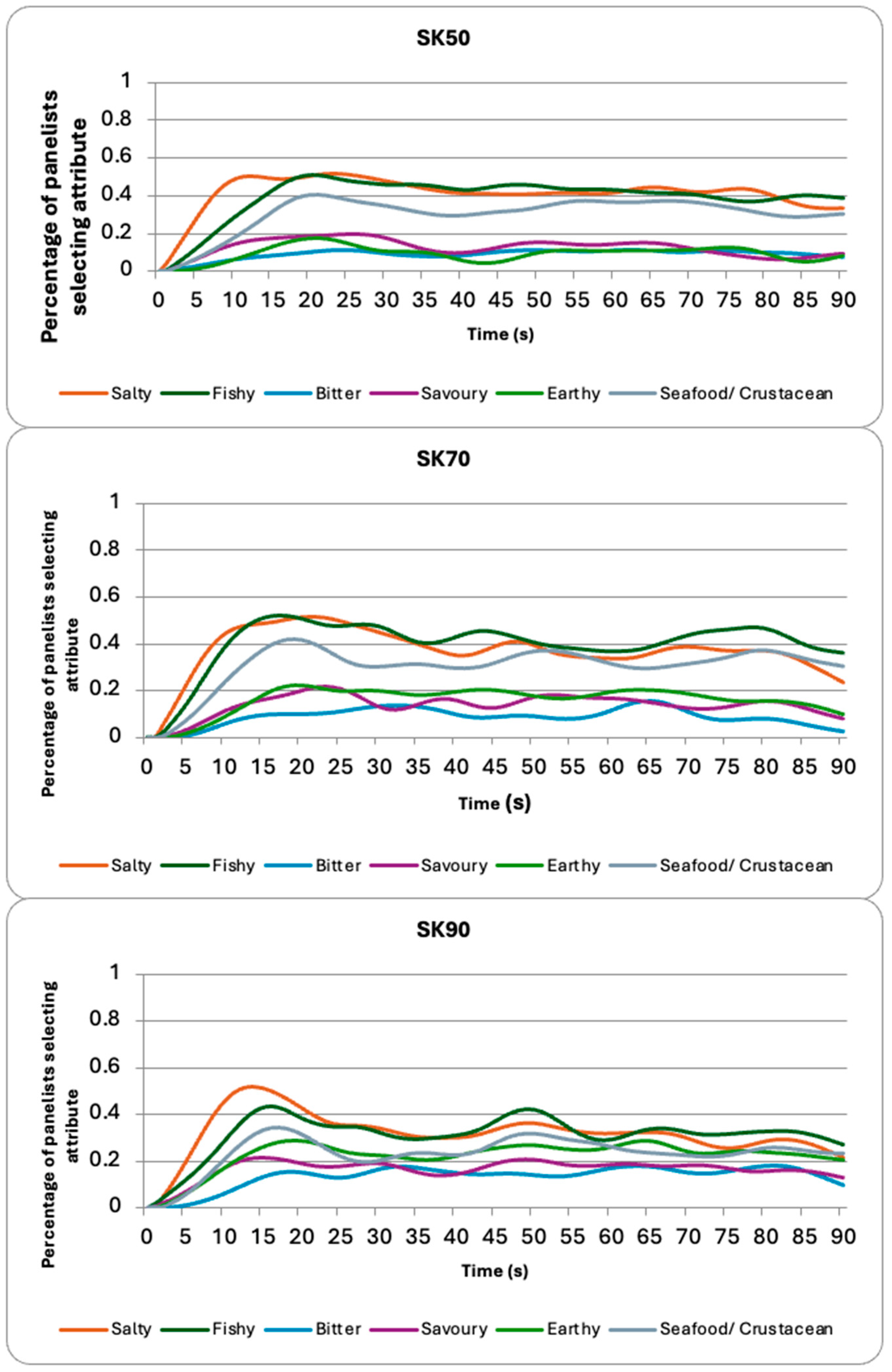
3.2. TCATA
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Shitanaka, T.; Surendra, K.C.; Khanal, S.K. Macroalgae-Derived Bioactive Compounds for Functional Food and Pharmaceutical Applications—A Critical Review. Crit. Rev. Food Sci. Nutr. 2024, 65, 1–23. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Harwanto, D.; Choi, J.-S. Seaweed Exhibits Therapeutic Properties against Chronic Diseases: An Overview. Appl. Sci. 2022, 12, 2638. [Google Scholar] [CrossRef]
- Ali, M.Q.; Azhar, M.A.; Munaim, M.S.A.; Ruslan, N.F.; Alsubhi, L.M.; Ahmad, N.; Noman, A.E. Seaweed Organic Compounds Source of Hydrocolloids and Sustainable Food Packaging: Properties, Application, and Future Direction. Discov Food 2024, 4, 101. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Thien, V.Y.; Rupert, R.; Rodrigues, K.F. Seaweed: A Potential Climate Change Solution. Renew. Sustain. Energy Rev. 2022, 159, 112222. [Google Scholar] [CrossRef]
- Young, M.; Paul, N.; Birch, D.; Swanepoel, L. Factors Influencing the Consumption of Seaweed amongst Young Adults. Foods 2022, 11, 3052. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; McSweeney, M.B. Do Consumers Want Seaweed in Their Food? A Study Evaluating Emotional Responses to Foods Containing Seaweed. Foods 2021, 10, 2737. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.; Moreira-Leite, B.; Afonso, A.; Infante, P.; Mata, P. Chocolates Enriched with Seaweed: Sensory Profiling and Consumer Segmentation. Int. J. Gastron. Food Sci. 2023, 33, 100747. [Google Scholar] [CrossRef]
- Li, T.; Ahsanuzzaman; Messer, K.D. Is There a Potential US Market for Seaweed-Based Products? A Framed Field Experiment on Consumer Acceptance. Mar. Resour. Econ. 2021, 36, 255–268. [Google Scholar] [CrossRef]
- Lamont, T.; McSweeney, M. Consumer Acceptability and Chemical Composition of Whole-Wheat Breads Incorporated with Brown Seaweed (Ascophyllum nodosum) or Red Seaweed (Chondrus crispus). J. Sci. Food Agric. 2021, 101, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.S.; Stévant, P.; Larssen, W.E. Food or Fad? Challenges and Opportunities for Including Seaweeds in a Nordic Diet. Bot. Mar. 2015, 58, 423–433. [Google Scholar] [CrossRef]
- Rabitti, N.S.; Bayudan, S.; Laureati, M.; Neugart, S.; Schouteten, J.J.; Apelman, L.; Dahlstedt, S.; Sandvik, P. Snacks from the Sea: A Cross-National Comparison of Consumer Acceptance for Crackers Added with Algae. Eur. Food Res. Technol. 2024, 250, 2193–2209. [Google Scholar] [CrossRef]
- Vilar, E.G.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. A Chemometric Approach to Characterize the Aroma of Selected Brown and Red Edible Seaweeds / Extracts. J. Sci. Food Agric. 2021, 101, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, V.; Bunger, A.; Ortiz, J.; Aguilera, J.M. Sensory Descriptors for Three Edible Chilean Seaweeds and Their Relations to Umami Components and Instrumental Texture. J. Appl. Phycol. 2022, 34, 3141–3156. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Camire, M.E.; Skonberg, D.I.; Perry, J.J. Effect of Dry Salting and Brining on the Consumer Acceptance of Saccharina latissima (Sugar Kelp). Phycology 2024, 4, 330–339. [Google Scholar] [CrossRef]
- Moss, R.; Dabas, T.; Stright, A.; Caya, E.; Baxter, L.; Dolan, E.; Gorman, M.; McSweeney, M.B. The Use of Sugar Kelp (Saccharina Latissima) as a Seasoning for Popcorn: An Investigation of Consumer Acceptance, Sensory Perception and Emotional Response. Food Humanit. 2024, 3, 100382. [Google Scholar] [CrossRef]
- Garicano Vilar, E.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Volatile Compounds of Six Species of Edible Seaweed: A Review. Algal Res. 2020, 45, 101740. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Williams, L.; Bjerregaard, R.; Duelund, L. Seaweeds for Umami Flavour in the New Nordic Cuisine. Flavour 2012, 1, 4. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A Traditional Ingredients for New Gastronomic Sensation. Food Hydrocoll. 2017, 68, 255–265. [Google Scholar] [CrossRef]
- Yan, X.; Chen, F.; Liu, Q.; Sun, C.; Yu, Q.; Wen, R. Metabolic and Sensory Profiling of Edible Seaweeds: Unraveling the Biochemical Basis of Taste Profile Complexity. Food Res. Int. 2025, 211, 116447. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Stright, A.; Nicolle, L.; Richelle, E.; Baxter, L.; Frampton, K.; Dabas, T.; Gorman, M.; McSweeney, M.B. Impact of Information about Nutritional Benefits, Sustainability and Consumption on Consumer Acceptance and Emotional Response to Smoothies Containing Brown Seaweed, Ascophyllum nodosum. Food Humanit. 2024, 3, 100373. [Google Scholar] [CrossRef]
- Chua, W.C.L.; Yeo, A.Y.Y.; Yuan, W.; Lee, Y.Y.; Ikasari, L.; Dharmawan, J.; Delahunty, C.M. Flavour Characterization of Twelve Species of Edible Algae. Algal Res. 2024, 80, 103540. [Google Scholar] [CrossRef]
- Jensen, S.; Ólafsdóttir, A.; Einarsdóttir, B.; Hreggviðsson, G.Ó.; Guðmundsson, H.; Jónsdóttir, L.B.; Friðjónsson, Ó.H.; Jónsdóttir, R. New Wave of Flavours–On New Ways of Developing and Processing Seaweed Flavours. Int. J. Gastron. Food Sci. 2022, 29, 100566. [Google Scholar] [CrossRef]
- Hung, Y.-H.R.; Peng, C.-Y.; Huang, M.-Y.; Lu, W.-J.; Lin, H.-J.; Hsu, C.-L.; Fang, M.-C.; Lin, H.-T.V. Monitoring the Aroma Compound Profiles in the Microbial Fermentation of Seaweeds and Their Effects on Sensory Perception. Fermentation 2023, 9, 135. [Google Scholar] [CrossRef]
- Lafeuille, B.; Francezon, N.; Goulet, C.; Perreault, V.; Turgeon, S.L.; Beaulieu, L. Impact of Temperature and Cooking Time on the Physicochemical Properties and Sensory Potential of Seaweed Water Extracts of Palmaria palmata and Saccharina Longicruris. J. Appl. Phycol. 2022, 34, 1731–1747. [Google Scholar] [CrossRef]
- Ugarte, R.; Sharp, G. Management and Production of the Brown Algae Ascophyllum nodosum in the Canadian Maritimes. J. Appl. Phycol. 2012, 24, 409–416. [Google Scholar] [CrossRef]
- Moy, F.E.; Christie, H. Large-Scale Shift from Sugar Kelp (Saccharina latissima) to Ephemeral Algae along the South and West Coast of Norway. Mar. Biol. Res. 2012, 8, 309–321. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A Concise Review of the Brown Macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Fradinho, P.; Raymundo, A.; Sousa, I.; Domínguez, H.; Torres, M.D. Edible Brown Seaweed in Gluten-Free Pasta: Technological and Nutritional Evaluation. Foods 2019, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.O.; O’Grady, M.N.; O’Sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. Acceptable Inclusion Levels for Selected Brown and Red Irish Seaweed Species in Pork Sausages. Foods 2022, 11, 1522. [Google Scholar] [CrossRef] [PubMed]
- Losada-Lopez, C.; Dopico, D.C.; Faína-Medín, J.A. Neophobia and Seaweed Consumption: Effects on Consumer Attitude and Willingness to Consume Seaweed. Int. J. Gastron. Food Sci. 2021, 24, 100338. [Google Scholar] [CrossRef]
- Heidkamp, C.P.; Krak, L.V.; Kelly, M.M.R.; Yarish, C. Geographical Considerations for Capturing Value in the U.S. Sugar Kelp (Saccharina latissima) Industry. Mar. Policy 2022, 144, 105221. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The Seasonal Variation in the Chemical Composition of the Kelp Species Laminaria Digitata, Laminaria Hyperborea, Saccharina latissima and Alaria Esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Jönsson, M.; Merkel, A.; Fredriksson, C.; Karlsson, E.N.; Wendin, K. Nutritional, Physicochemical, and Sensory Characterization of Four Common Northern European Seaweed Species Intended for Food. Algal Res. 2023, 75, 103258. [Google Scholar] [CrossRef]
- Castura, J.C.; Antúnez, L.; Giménez, A.; Ares, G. Temporal Check-All-That-Apply (TCATA): A Novel Dynamic Method for Characterizing Products. Food Qual. Prefer. 2016, 47, 79–90. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Alcaire, F.; Hunter, D.C.; Jin, D.; Castura, J.C.; Ares, G. Number of Terms to Use in Temporal Check-All-That-Apply Studies (TCATA and TCATA Fading) for Sensory Product Characterization by Consumers. Food Qual. Prefer. 2018, 64, 154–159. [Google Scholar] [CrossRef]
- Ares, G.; Alcaire, F.; Antúnez, L.; Vidal, L.; Giménez, A.; Castura, J.C. Identification of Drivers of (Dis)Liking Based on Dynamic Sensory Profiles: Comparison of Temporal Dominance of Sensations and Temporal Check-All-That-Apply. Food Res. Int. 2017, 92, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, D.; Punta-Sánchez, I.; Calle, J.L.P.; Setyaningsih, W.; Lideman; Palma, M.; Ningrum, A. Manikharda Optimization of HS-SPME Combined with GC–MS for Key Marker Volatile Organic Compound Analysis in Kappaphycus Alvarezii with a Chemometric Approach. Food Meas. 2024, 18, 3510–3526. [Google Scholar] [CrossRef]
- Gorman, M.; Moss, R.; Barker, S.; Falkeisen, A.; Knowles, S.; McSweeney, M.B. Consumer Perception of Salt-Reduced Bread with the Addition of Brown Seaweed Evaluated under Blinded and Informed Conditions. J. Sci. Food Agric. 2023, 103, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, C.; Jönsson, M.; Merkel, A.; Nordberg Karlsson, E.; Wendin, K. Sensing Seaweed Settings: Making Sense of a Mixed-Method Design for Sensory Analysis. Int. J. Gastron. Food Sci. 2023, 33, 100762. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical Composition, Antioxidant Activity and Sensory Evaluation of Five Different Species of Brown Edible Seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- McMahon, K.M.; Diako, C.; Aplin, J.; Mattinson, D.S.; Culver, C.; Ross, C.F. Trained and Consumer Panel Evaluation of Sparkling Wines Sweetened to Brut or Demi Sec Residual Sugar Levels with Three Different Sugars. Food Res. Int. 2017, 99, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-15083-8. [Google Scholar]
- López-Pérez, O.; Picon, A.; Nuñez, M. Volatile Compounds and Odour Characteristics of Seven Species of Dehydrated Edible Seaweeds. Food Res. Int. 2017, 99, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, F.; Mirzayeva, A.; Roldán, A.; Castro, R.; Palacios, V.; García-Barroso, C.; Durán-Guerrero, E. Evolution of Volatile Compounds and Sensory Characteristics of Edible Green Seaweed (Ulva Rigida) during Storage at Different Temperatures. J. Sci. Food Agric. 2019, 99, 5475–5482. [Google Scholar] [CrossRef] [PubMed]
- Stright, A.; Frampton, K.; McSweeney, M.B. An Investigation into Soup with the Addition of Brown Seaweed (Ascophyllum nodosum) and Red Seaweed (Chondrus crispus) Using Nonconsumers of Seaweed. J. Sens. Stud. 2025, 40, e70012. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Franco, D.; Campagnol, P.C.B.; Lorenzo, J.M. Inclusion of Seaweeds as Healthy Approach to Formulate New Low-Salt Meat Products. Curr. Opin. Food Sci. 2021, 40, 20–25. [Google Scholar] [CrossRef]
- Palczak, J.; Blumenthal, D.; Rogeaux, M.; Delarue, J. Sensory Complexity and Its Influence on Hedonic Responses: A Systematic Review of Applications in Food and Beverages. Food Qual. Prefer. 2019, 71, 66–75. [Google Scholar] [CrossRef]
- Varela, P.; Antúnez, L.; Carlehög, M.; Alcaire, F.; Castura, J.C.; Berget, I.; Giménez, A.; Næs, T.; Ares, G. What Is Dominance? An Exploration of the Concept in TDS Tests with Trained Assessors and Consumers. Food Qual. Prefer. 2018, 64, 72–81. [Google Scholar] [CrossRef]
- Dietz, C.; Cook, D.; Yang, Q.; Wilson, C.; Ford, R. A TCATA by Modality Approach to Study the Multisensory Temporal Profile of Hop Bitter and Flavour Products Applied in Lager. Food Qual. Prefer. 2022, 97, 104470. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Walczak, M.; Skrzypczak-Zielińska, M.; Jeleń, H.H. Bitter Taste of Brassica Vegetables: The Role of Genetic Factors, Receptors, Isothiocyanates, Glucosinolates, and Flavor Context. Crit. Rev. Food Sci. Nutr. 2018, 58, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Schiener, P.; Zhao, S.; Theodoridou, K.; Carey, M.; Mooney-McAuley, K.; Greenwell, C. The Nutritional Aspects of Biorefined Saccharina latissima, Ascophyllum nodosum and Palmaria palmata. Biomass Conv. Bioref. 2017, 7, 221–235. [Google Scholar] [CrossRef]
- Borgogno, M.; Favotto, S.; Corazzin, M.; Cardello, A.V.; Piasentier, E. The Role of Product Familiarity and Consumer Involvement on Liking and Perceptions of Fresh Meat. Food Qual. Prefer. 2015, 44, 139–147. [Google Scholar] [CrossRef]


| Chemicals | RT | Brown | Sugar Kelp | ID | Odours 1 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 50 °C | 70 °C | 90 °C | 50 °C | 70 °C | 90 °C | ||||
| hexanal | 5.015 | S | Green, fruity | ||||||
| 2-hexenal, (E) | 6.128 | S | Green, fruity | ||||||
| heptanal | 7.056 | S | Fatty, pungent | ||||||
| pentane, 1-iodo | 7.406 | NIST | Petroleum | ||||||
| 2-heptenal, (Z) | 8.075 | NIST | Pungent, green | ||||||
| benzaldehyde | 8.151 | S | Bitter almond | ||||||
| 2-octen-1-ol, (Z) | 8.490 | NIST | Fatty, green, nutty | ||||||
| 6-octen-2-one | 8.623 | NIST | Green, earthy | ||||||
| furan, 2-pentyl | 8.728 | NIST | Earthy, vegetable | ||||||
| octanal | 8.915 | S | Fatty, citrus | ||||||
| o-cymene | 9.315 | S | Citrus | ||||||
| D-limonene | 9.387 | S | Lemon, orange | ||||||
| cyclohexanone, 2,2,6-trimethyl | 9.508 | NIST | Sweet, honey, tobacco | ||||||
| 2-octenal, (E) | 9.877 | NIST | Green, cognac | ||||||
| terpinolene | 10.368 | S | Sweet, piney, oily | ||||||
| nonanal | 10.638 | S | Rose, orange | ||||||
| heptane, 1-iodo | 11.075 | NIST | Slightly sweet, oily | ||||||
| 2-nonenal, (E) | 11.512 | NIST | Orris, waxy | ||||||
| 1,6-dimethylhepta-1,3,5-triene | 12.029 | NIST | None | ||||||
| decanal | 12.204 | S | Fatty, citrus | ||||||
| octane, 1-iodo | 12.676 | NIST | Green | ||||||
| 2-decenal, (E) | 13.037 | S | Waxy, fatty | ||||||
| 2,4-decadienal, (E,Z) | 13.497 | S | Fatty, chicken-like, citrus-like | ||||||
| 2,6-octadiene, 2,6-dimethyl | 14.284 | NIST | Citrus-like, tea | ||||||
| alpha-fenchene | 14.698 | NIST | Herbal, sweet, woody | ||||||
| trans -beta-ionone | 16.113 | S | Floral, woody, fruity | ||||||
| pentadecane | 16.17 | S | Waxy | ||||||
| Sample | Salty | Fishy | Bitter | Savory | Earthy | Seafood/Crustacean |
|---|---|---|---|---|---|---|
| BS50 | 0.159 d 1 | 0.390 a | 0.265 a | 0.094 c | 0.271 a | 0.279 ab |
| BS70 | 0.154 d | 0.357 ab | 0.257 a | 0.088 c | 0.260 a | 0.264 bc |
| BS90 | 0.180 d | 0.342 b | 0.276 a | 0.084 c | 0.269 a | 0.252 bc |
| SK50 | 0.417 a | 0.391 a | 0.091 c | 0.125 b | 0.090 d | 0.304 a |
| SK70 | 0.373 b | 0.402 a | 0.087 c | 0.161 c | 0.161 c | 0.306 a |
| SK90 | 0.321 c | 0.314 b | 0.133 b | 0.164 c | 0.221 b | 0.233 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, Z.; Faraone, N.; McSweeney, M.B. Characterization of Brown Seaweed (Ascophyllum nodosum) and Sugar Kelp (Saccharina latissima) Extracts Using Temporal Check-All-That-Apply. Foods 2025, 14, 2565. https://doi.org/10.3390/foods14152565
Adams Z, Faraone N, McSweeney MB. Characterization of Brown Seaweed (Ascophyllum nodosum) and Sugar Kelp (Saccharina latissima) Extracts Using Temporal Check-All-That-Apply. Foods. 2025; 14(15):2565. https://doi.org/10.3390/foods14152565
Chicago/Turabian StyleAdams, Zach, Nicoletta Faraone, and Matthew B. McSweeney. 2025. "Characterization of Brown Seaweed (Ascophyllum nodosum) and Sugar Kelp (Saccharina latissima) Extracts Using Temporal Check-All-That-Apply" Foods 14, no. 15: 2565. https://doi.org/10.3390/foods14152565
APA StyleAdams, Z., Faraone, N., & McSweeney, M. B. (2025). Characterization of Brown Seaweed (Ascophyllum nodosum) and Sugar Kelp (Saccharina latissima) Extracts Using Temporal Check-All-That-Apply. Foods, 14(15), 2565. https://doi.org/10.3390/foods14152565





