Metabolic Master Switch: Pyruvate Carboxylase Fuels Antimicrobial Resistance and Virulence in Foodborne Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
2.2. Mutated Strain Construction
2.3. Minimum Inhibitory Concentration Determination
2.4. Time-Kill Curve Assay
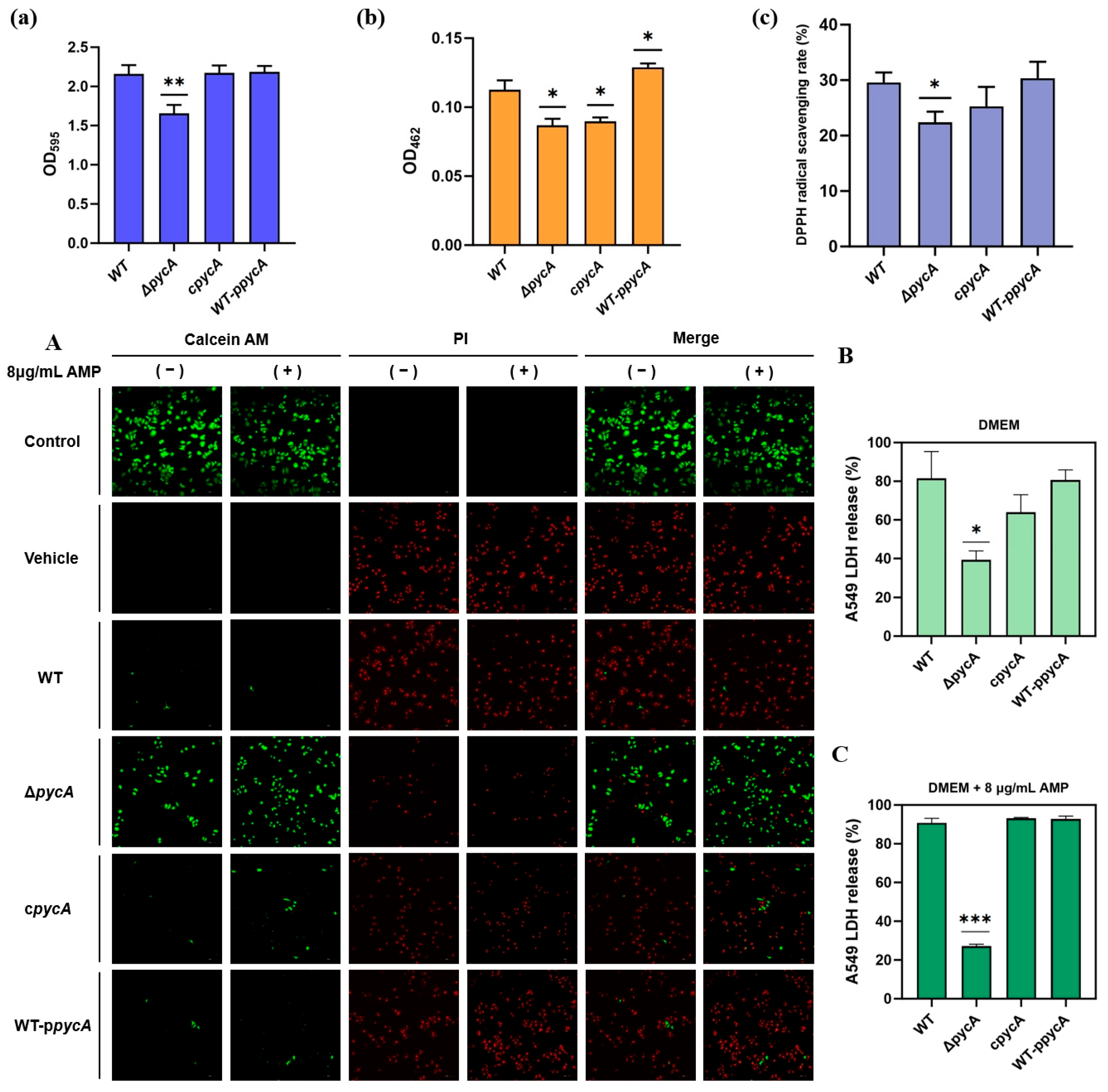
2.5. Biofilm Quantification
2.6. Staphyloxanthin Quantification
2.7. 2,2-Diphenyl-1-Picrylhydrazyl Free Radical Scavenging Activity Assay
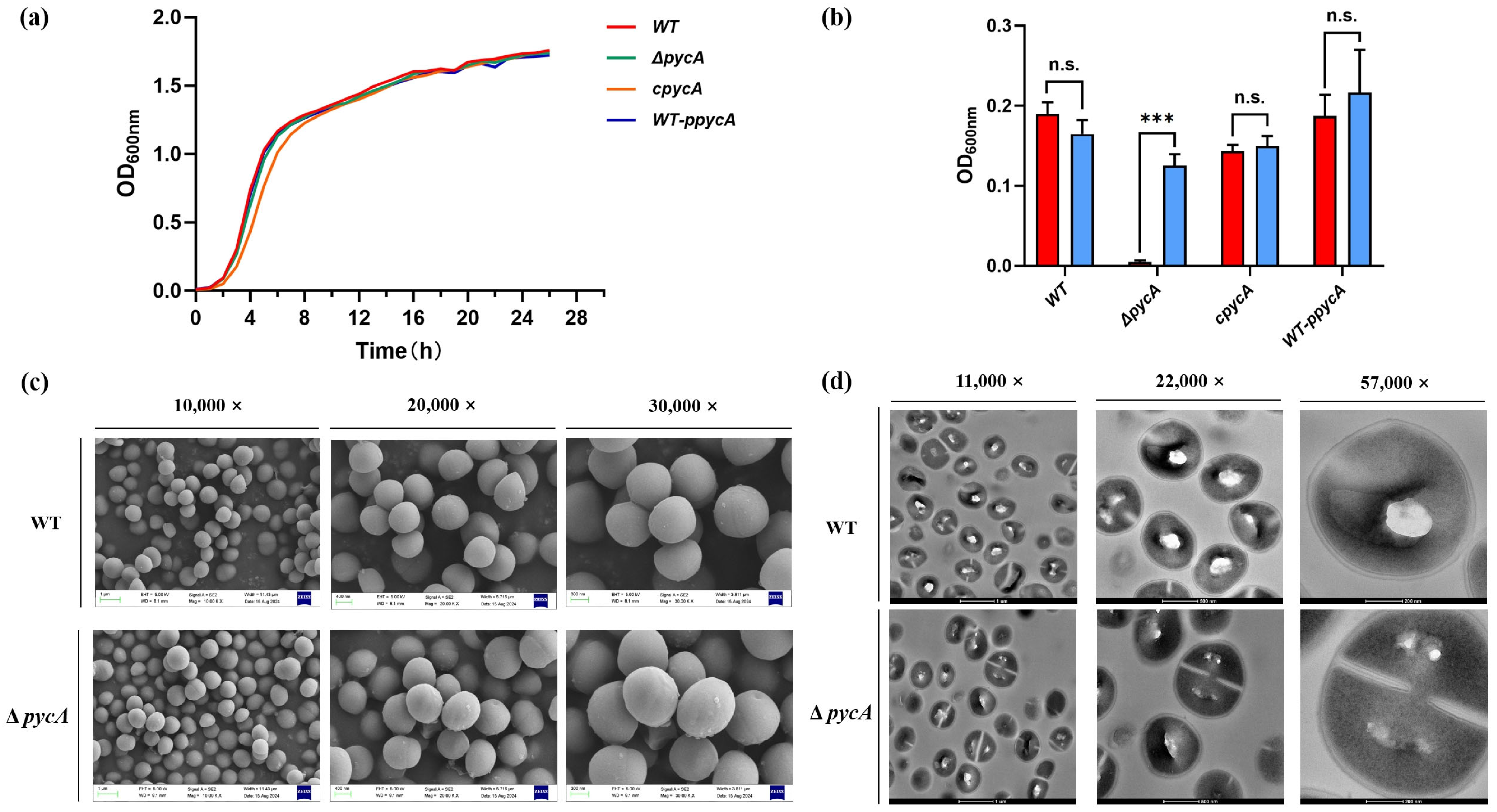
2.8. L-Aspartate Auxotrophy Assay
2.9. Growth Curve Measurement
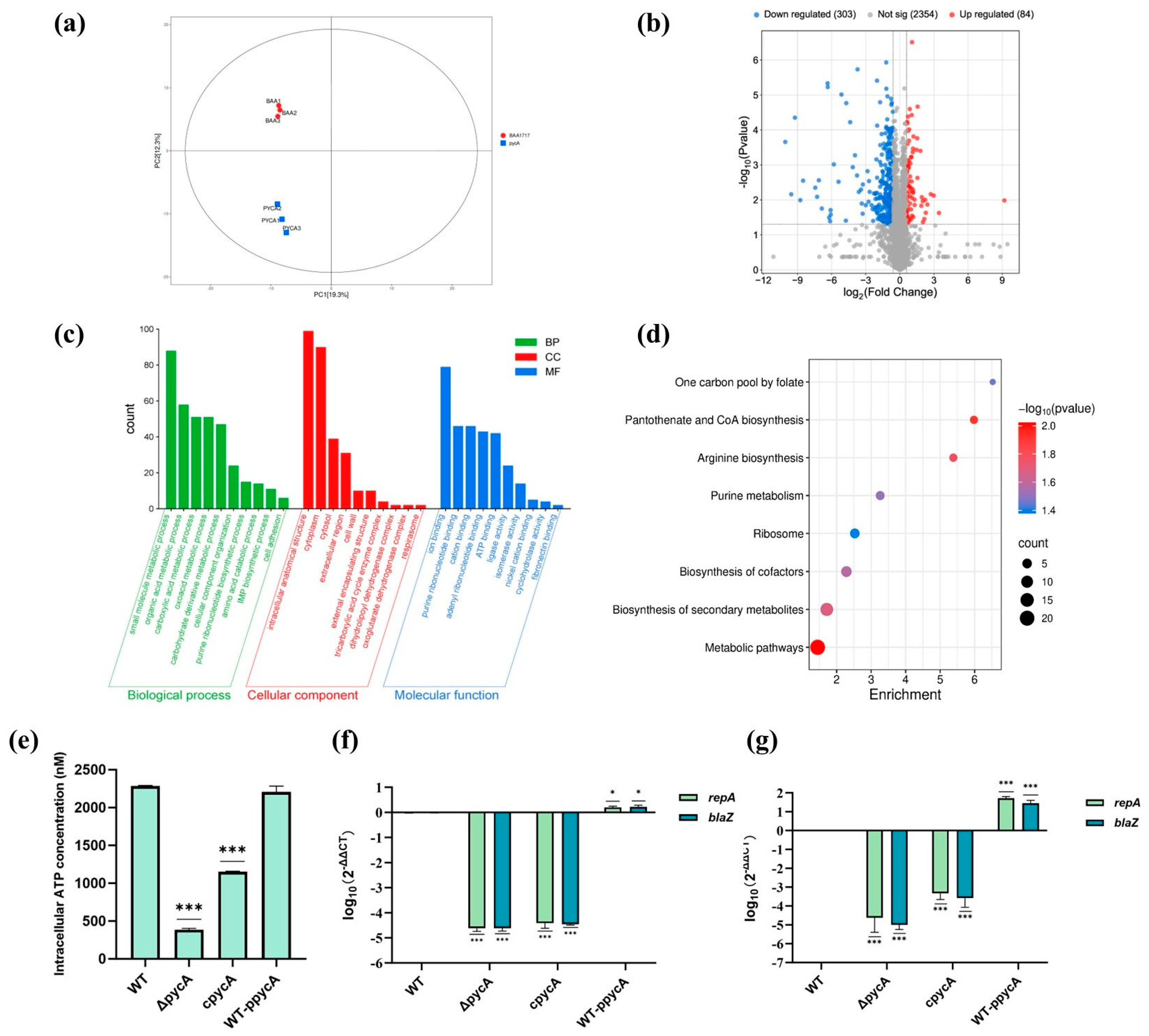
2.10. Real-Time PCR Analysis
2.11. Scanning Electron Microscopy Analysis
2.12. Transmission Electron Microscopy Analysis
2.13. Intracellular ATP Analysis
2.14. Proteomic Analysis
2.15. A549 Cells Infection Assay
2.16. Statistical Analyses
3. Results
3.1. Deletion of pycA Increases Antimicrobial Susceptibility in S. aureus
3.2. PycA Is Dispensable for Growth Under Nutrient-Rich Conditions but Essential in the Absence of L-Aspartate
3.3. Impaired Virulence isConfirmed in the ΔpycA Mutant
3.3.1. Deletion of pycA Inhibits Biofilm Formation by S. aureus In Vitro
3.3.2. Deletion of pycA Inhibits STX Production and Reduces Total Antioxidant Capacity in S. aureus
3.3.3. Deletion of pycA Reduces the Cytotoxicity of S. aureus and Its Survival Under Antimicrobial Pressure
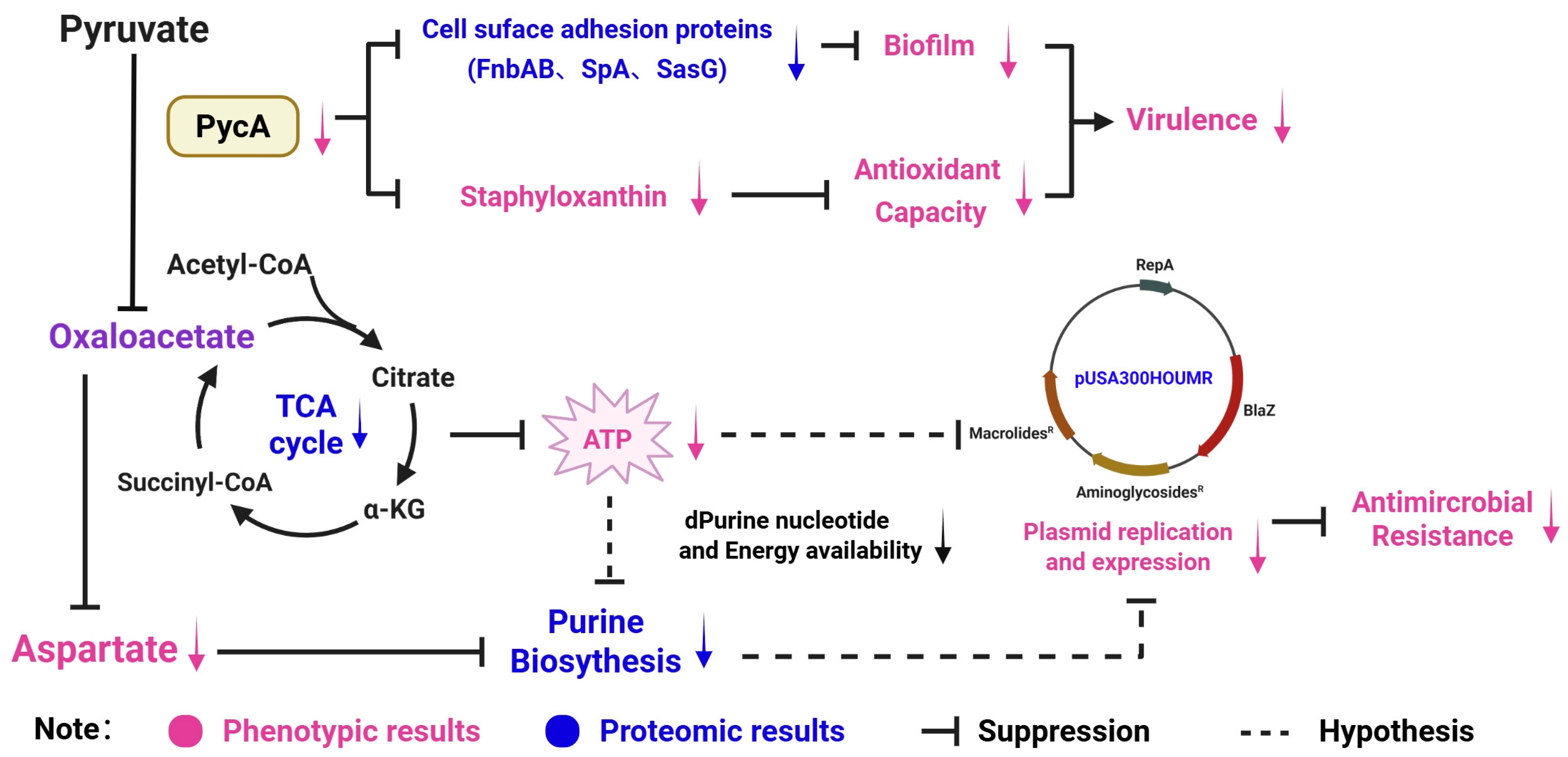
3.4. Key Proteins Involved in the Regulation of Virulence and Antimicrobial Resistance via Proteomic Analysis
3.4.1. Deletion of pycA Affects the Overall Metabolism of S. aureus
3.4.2. Deletion of pycA Downregulates Plasmid-Associated Antimicrobial Resistance Proteins
3.4.3. Deletion of pycA Downregulates the Expression of Cell Adhesion-Associated Proteins
3.5. Outlook: PycA as a Target for Food Safety Interventions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PycA | Pyruvate Carboxylase |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| TCA | Tricarboxylic Acid |
| TSB | Tryptic Soy Broth |
| BHI | Brain Heart Infusion |
| PBS | Phosphate-Buffered Saline |
| STX | Staphyloxanthin |
| MIC | Minimum Inhibitory Concentration |
| CLSI | Clinical and Laboratory Standards Institute |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| MEM | Minimum Essential Medium |
| OD | Optical Density |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| LDH | Lactate Dehydrogenase |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| MOI | Multiplicity of Infection |
| PCA | Principal Component Analysis |
| BCA | Bicinchoninic Acid |
| iRT | Indexed Retention Time |
| nano-UPLC | Nano-scale Ultra Performance Liquid Chromatography |
| DIA | Data-Independent Acquisition |
| FDR | False Discovery Rate |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ppGpp | Guanosine Tetraphosphate |
References
- Wakabayashi, Y.; Umeda, K.; Yonogi, S.; Nakamura, H.; Yamamoto, K.; Kumeda, Y.; Kawatsu, K. Staphylococcal food poisoning caused by Staphylococcus argenteus harboring staphylococcal enterotoxin genes. Int. J. Food Microbiol. 2018, 265, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.A.; Hurford, I.M.; Cassat, J.E. Antivirulence Strategies for the Treatment of Staphylococcus aureus Infections: A Mini Review. Front. Microbiol. 2021, 11, 632706. [Google Scholar] [CrossRef] [PubMed]
- Mama, O.M.; Gómez-Sanz, E.; Ruiz-Ripa, L.; Gómez, P.; Torres, C. Diversity of staphylococcal species in food producing animals in Spain, with detection of PVL-positive MRSA ST8 (USA300). Vet. Microbiol. 2019, 233, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Dubal, Z.B.; Kumar, A.; Bhilegaonkar, K.; Vinodh Kumar, O.R.; Kumar, S.; Kadwalia, A.; Shagufta, B.; Grace, M.R.; Ramees, T.P.; et al. Virulent methicillin resistant Staphylococcus aureus (MRSA) in street vended foods. J. Food Sci. Technol. 2019, 56, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Noskin, G.A.; Rubin, R.J.; Schentag, J.J.; Kluytmans, J.; Hedblom, E.C.; Smulders, M.; Lapetina, E.; Gemmen, E. The Burden of Staphylococcus aureus Infections on Hospitals in the United States: An Analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch. Intern. Med. 2005, 165, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-B.; Pan, Q.; Ye, B.-C. Glucose-Induced Cyclic Lipopeptides Resistance in Bacteria via ATP Maintenance through Enhanced Glycolysis. iScience 2019, 21, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Vitko, N.P.; Spahich, N.A.; Richardson, A.R. Glycolytic Dependency of High-Level Nitric Oxide Resistance and Virulence in Staphylococcus aureus. mBio 2015, 6, e00045-15. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Nakashima, T.; Cho, O.; Ohkubo, T.; Kato, J.; Sugita, T. Pyruvate-triggered TCA cycle regulation in Staphylococcus aureus promotes tolerance to betamethasone valerate. Biochem. Biophys. Res. Commun. 2020, 528, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Nabb, D.L.; Song, S.; Kluthe, K.E.; Daubert, T.A.; Luedtke, B.E.; Nuxoll, A.S. Polymicrobial Interactions Induce Multidrug Tolerance in Staphylococcus aureus Through Energy Depletion. Front. Microbiol. 2019, 10, 2803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bojer, M.S.; George, S.E.; Wang, Z.; Jensen, P.R.; Wolz, C.; Ingmer, H. Inactivation of TCA cycle enhances Staphylococcus aureus persister cell formation in stationary phase. Sci. Rep. 2018, 8, 10849. [Google Scholar] [CrossRef] [PubMed]
- Benton, B.M.; Zhang, J.P.; Bond, S.; Pope, C.; Christian, T.; Lee, L.; Winterberg, K.M.; Schmid, M.B.; Buysse, J.M. Large-Scale Identification of Genes Required for Full Virulence of Staphylococcus aureus. J. Bacteriol. 2004, 186, 8478–8489. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-L.; Hooven, T.A.; Norambuena, J.; Li, B.; Boyd, J.M.; Yang, J.H.; Parker, D. Growth and Stress Tolerance Comprise Independent Metabolic Strategies Critical for Staphylococcus aureus Infection. mBio 2021, 12, e0081421. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Yamada-Mabuchi, M.; Zhao, G.; Li, L.; Liu, G.; Ou, H.Y.; Deng, Z.; Zheng, Y.; He, X. Recognition and cleavage of 5-methylcytosine DNA by bacterial SRA-HNH proteins. Nucleic Acids Res. 2015, 43, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Guo, Y.; Liu, X.; Zhang, J.; Niu, X.; Yu, Q.; Deng, X.; Wang, J. Theaflavin-3,3′-digallate increases the antibacterial activity of β-lactam antibiotics by inhibiting metallo-β-lactamase activity. J. Cell. Mol. Med. 2019, 23, 6955–6964. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhai, D.; Wu, Z.; Zhao, Y.; Qiao, D.; Zhao, X. Impairment of the Cell Wall Ligase, LytR-CpsA-Psr Protein (LcpC), in Methicillin Resistant Staphylococcus aureus Reduces Its Resistance to Antibiotics and Infection in a Mouse Model of Sepsis. Front. Microbiol. 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Karinou, E.; Schuster, C.F.; Pazos, M.; Vollmer, W.; Gründling, A. Inactivation of the Monofunctional Peptidoglycan Glycosyltransferase SgtB Allows Staphylococcus aureus To Survive in the Absence of Lipoteichoic Acid. J. Bacteriol. 2019, 201, e00574-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Duan, J.; Jin, Y.; Zhan, Q.; Xu, Y.; Zhao, H.; Wang, X.; Rao, L.; Guo, Y.; Yu, F. Functional Insights of MraZ on the Pathogenicity of Staphylococcus aureus. Infect. Drug Resist. 2021, 14, 4539–4551. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Valverde, M.; Valiente-Mendez, A.; Torres, E.; Almirante, B.; Gómez-Zorrilla, S.; Borrell, N.; Aller-García, A.I.; Gurgui, M.; Almela, M.; Sanz, M.; et al. MIC of amoxicillin/clavulanate according to CLSI and EUCAST: Discrepancies and clinical impact in patients with bloodstream infections due to Enterobacteriaceae. J. Antimicrob. Chemother. 2017, 72, 1478–1487. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gan, C.; Langa, E.; Valenzuela, A.; Ballestero, D.; Pino-Otín, M.R. Synergistic Activity of Thymol with Commercial Antibiotics against Critical and High WHO Priority Pathogenic Bacteria. Plants 2023, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Machado, H.; Weng, L.L.; Dillon, N.; Seif, Y.; Holland, M.; Pekar, J.E.; Monk, J.M.; Nizet, V.; Palsson, B.O.; Feist, A.M. Strain-Specific Metabolic Requirements Revealed by a Defined Minimal Medium for Systems Analyses of Staphylococcus aureus. Appl. Environ. Microbiol. 2019, 85, e01773-19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Qu, L.B.; Bi, Y.F.; Pan, C.X.; Yang, R.; Zeng, H.J. Antibacterial activity and mechanism of chloroform fraction from aqueous extract of mugwort leaves (Artemisia argyi L.) against Staphylococcus aureus. Lett. Appl. Microbiol. 2022, 74, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Evaluation of Ultrasound-Induced Damage to Escherichia coli and Staphylococcus aureus by Flow Cytometry and Transmission Electron Microscopy. Appl. Environ. Microbiol. 2016, 82, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, L.; Liu, X.; Wang, B.; Guo, X.; Wang, Y.; Xu, Y.; Guan, J.; Zhao, Y. Elucidating the potential of isorhapontigenin in targeting the MgrA regulatory network: A paradigm shift for attenuating MRSA virulence. Antimicrob. Agents Chemother. 2024, 68, e00611–e00624. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Fan, L.; Zhong, Y.; Guo, J.; Dong, X.; Xu, X.; Wang, C.; Su, Y. Quantitative proteomics reveals reduction in central carbon and energy metabolisms contributes to gentamicin resistance in Staphylococcus aureus. J. Proteom. 2023, 277, 104849. [Google Scholar] [CrossRef] [PubMed]
- Collatz, E.; Carlier, C.; Courvalin, P. The chromosomal 3′,5″-aminoglycoside phosphotransferase in Streptococcus pneumoniae is closely related to its plasmid-coded homologs in Streptococcus faecalis and Staphylococcus aureus. J. Bacteriol. 1983, 156, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Miklasińska-Majdanik, M. Mechanisms of Resistance to Macrolide Antibiotics Among Staphylococcus aureus. Antibiotics 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, D.; Zhou, B.; Li, Z.; Liu, G.; Li, H.; Hu, X.; Wang, X. Fine-Regulating the Carbon Flux of l-Isoleucine Producing Corynebacterium glutamicum WM001 for Efficient l-Threonine Production. ACS Synth. Biol. 2024, 13, 3446–3460. [Google Scholar] [CrossRef] [PubMed]
- Algawwam, G.; Mnaather, A.A.; Al-Mahdawi, A.M.; Fawzi, H.A.; Shareef, L.G. Serum Aspartic Acid as a Marker of Epilepsy. Glob. J. Health Sci. 2019, 11, 142. [Google Scholar] [CrossRef]
- Nuzzo, T.; Sekine, M.; Punzo, D.; Miroballo, M.; Katane, M.; Saitoh, Y.; Galbusera, A.; Pasqualetti, M.; Errico, F.; Gozzi, A.; et al. Dysfunctional d-aspartate metabolism in BTBR mouse model of idiopathic autism. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140531. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.D.; Butrico, C.E.; Ford, C.A.; Curry, J.M.; Trenary, I.A.; Tummarakota, S.S.; Hendrix, A.S.; Young, J.D.; Cassat, J.E. Host nutrient milieu drives an essential role for aspartate biosynthesis during invasive Staphylococcus aureus infection. Proc. Natl. Acad. Sci. USA 2020, 117, 12394–12401. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Yang, S.; Lim, Y. Antibiotic susceptibility of Staphylococcus aureus with different degrees of biofilm formation. J. Anal. Sci. Technol. 2021, 12, 41. [Google Scholar] [CrossRef]
- Tran, N.N.; Morrisette, T.; Jorgensen, S.C.J.; Orench-Benvenutti, J.M.; Kebriaei, R. Current therapies and challenges for the treatment of Staphylococcus aureus biofilm-related infections. Pharmacotherapy 2023, 43, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tong, Y.; Cheng, J.; Abbas, Z.; Li, Z.; Wang, J.; Zhou, Y.; Si, D.; Zhang, R. Biofilm and Small Colony Variants—An Update on Staphylococcus aureus Strategies toward Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1241. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.W.; Yang, J.; Guo, H.; Ji, Y. The Staphylococcus aureus AirSR Two-Component System Mediates Reactive Oxygen Species Resistance via Transcriptional Regulation of Staphyloxanthin Production. Infect. Immun. 2017, 85, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Braungardt, H.; Singh, V.K. Impact of Deficiencies in Branched-Chain Fatty Acids and Staphyloxanthin in Staphylococcus aureus. BioMed Res. Int. 2019, 2019, 2603435. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.N.; Liu, G.Y.; Yeaman, M.R.; Nast, C.C.; Proctor, R.A.; McKinnell, J.; Bayer, A.S. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 2011, 55, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Schär, J.; Stoll, R.; Schauer, K.; Loeffler, D.I.; Eylert, E.; Joseph, B.; Eisenreich, W.; Fuchs, T.M.; Goebel, W. Pyruvate carboxylase plays a crucial role in carbon metabolism of extra- and intracellularly replicating Listeria monocytogenes. J. Bacteriol. 2010, 192, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Chatterjee, S.S.; Villaruz, A.E.; Dickey, S.W.; Tan, V.Y.; Chen, Y.; Sturdevant, D.E.; Ricklefs, S.M.; Otto, M. Mechanism of Gene Regulation by a Staphylococcus aureus Toxin. mBio 2016, 7, e01579-16. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.; Gedeon, A.; Munier-Lehmann, H. A journey into the regulatory secrets of the de novo purine nucleotide biosynthesis. Front. Pharmacol. 2024, 15, 1329011. [Google Scholar] [CrossRef] [PubMed]
- Zalkin, H.; Dixon, J.E. De Novo Purine Nucleotide Biosynthesis. Prog. Nucleic Acid Res. Mol. Biol. 1992, 42, 259–287. [Google Scholar] [CrossRef] [PubMed]
- Gélinas, M.; Museau, L.; Milot, A.; Beauregard, P.B. The de novo Purine Biosynthesis Pathway Is the Only Commonly Regulated Cellular Pathway during Biofilm Formation in TSB-Based Medium in Staphylococcus aureus and Enterococcus faecalis. Microbiol. Spectr. 2021, 9, e0080421. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Guo, L.; Dong, Y.; Bao, T.; Wang, H.; Xu, T.; Zhang, Y.; Han, J. PurN Is Involved in Antibiotic Tolerance and Virulence in Staphylococcus aureus. Antibiotics 2022, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Abdelhady, W.; Donegan, N.P.; Seidl, K.; Cheung, A.; Zhou, Y.-F.; Yeaman, M.R.; Bayer, A.S.; Xiong, Y.Q. Role of Purine Biosynthesis in Persistent Methicillin-Resistant Staphylococcus aureus Infection. J. Infect. Dis. 2018, 218, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.D.; Porcella, S.F.; Martens, C.; Whitney, A.R.; Braughton, K.R.; Chen, L.; Craig, C.T.; Tenover, F.C.; Kreiswirth, B.N.; Musser, J.M.; et al. Complete Nucleotide Sequence Analysis of Plasmids in Strains of Staphylococcus aureus Clone USA300 Reveals a High Level of Identity among Isolates with Closely Related Core Genome Sequences. J. Clin. Microbiol. 2010, 48, 4504–4511. [Google Scholar] [CrossRef] [PubMed]
- San Millan, A.; MacLean, R.C. Fitness Costs of Plasmids: A Limit to Plasmid Transmission. Microbiol. Spectr. 2017, 5, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Gries, C.M.; Biddle, T.; Bose, J.L.; Kielian, T.; Lo, D.D. Staphylococcus aureus Fibronectin Binding Protein A Mediates Biofilm Development and Infection. Infect. Immun. 2020, 88, e00859-19. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Ito, R.; Tanaka, Y.; Yao, M.; Matoba, K.; Saito, S.; Tanaka, I.; Ohta, T. Staphylococcus aureus surface protein SasG contributes to intercellular autoaggregation of Staphylococcus aureus. Biochem. Biophys. Res. Commun. 2008, 377, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.B.; Maciag, J.J.; Wang, C.; Crawford, J.A.; Enroth, T.J.; Keim, K.C.; Dufrêne, Y.F.; Robinson, D.A.; Fey, P.D.; Herr, A.B.; et al. Staphylococcus aureus skin colonization is mediated by SasG lectin variation. Cell Rep. 2023, 43, 114022. [Google Scholar] [CrossRef] [PubMed]
- Speziale, P.; Pietrocola, G.; Rindi, S.; Provenzano, M.; Provenza, G.; Di Poto, A.; Visai, L.; Arciola, C.R. Structural and functional role of Staphylococcus aureus surface components recognizing adhesive matrix molecules of the host. Future Microbiol. 2009, 4, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.K.; Kuzmicheva, G.A.; Lin, J.; Sunley, K.M.; Bowling, R.A., Jr.; Kwan, T.-Y.; Mays, H.R.; Rambhadran, A.; Zhang, Y.; Martin, R.L.; et al. A natural human monoclonal antibody targeting Staphylococcus Protein A protects against Staphylococcus aureus bacteremia. PLoS ONE 2018, 13, e0190537. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Emolo, C.; Holtfreter, S.; Wiles, S.; Kreiswirth, B.; Missiakas, D.; Schneewind, O. Staphylococcal Protein A Contributes to Persistent Colonization of Mice with Staphylococcus aureus. J. Bacteriol. 2018, 200, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, Y.; Zhang, L.; Liu, F.; Duan, G.; Chen, S.; Long, J.; Jin, Y.; Yang, H. Recent advances in the use of resveratrol against Staphylococcus aureus infections (Review). Med. Int. 2024, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, W.A.; Zhu, R.; Peng, W.; Al-Hariri, M.; Kobeissy, F.; Tran, P.; Hamood, A.N.; Hegazy, M.F.; Paré, P.W.; Mechref, Y. Multitargeted Flavonoid Inhibition of the Pathogenic Bacterium Staphylococcus aureus: A Proteomic Characterization. J. Proteome Res. 2017, 16, 2579–2586. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Liu, X.; Liu, L.; Zhong, K.; Huang, Y.; Gao, H. Antibacterial effect of 3-p-trans-coumaroyl-2-hydroxyquinic acid, a phenolic compound from needles of Cedrus deodara, on cellular functions of Staphylococcus aureus. RSC Adv. 2018, 8, 4969–4975. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.A.; Jupudi, S. Structure-based virtual screening to identify inhibitors against Staphylococcus aureus MurD enzyme. Struct. Chem. 2019, 30, 2123–2133. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Zhang, J.; Liu, Q.; Wang, B.; Liu, D.; Gao, F.; Lanzi, G.; Zhao, Y.; Shi, Y. Thwarting resistance: MgrA inhibition with methylophiopogonanone a unveils a new battlefront against S. aureus. npj Biofilms Microbiomes 2024, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Burkett, D.J.; Wyatt, B.N.; Mews, M.; Bautista, A.; Engel, R.; Dockendorff, C.; Donaldson, W.A.; Maurice, M.S. Evaluation of α-hydroxycinnamic acids as pyruvate carboxylase inhibitors. Bioorganic Med. Chem. 2019, 27, 4041–4047. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Satiaputra, J.; Sternicki, L.M.; Paparella, A.S.; Feng, Z.; Lee, K.J.; Blanco-Rodriguez, B.; Tieu, W.; Eijkelkamp, B.A.; Shearwin, K.E.; et al. Advanced Resistance Studies Identify Two Discrete Mechanisms in Staphylococcus aureus to Overcome Antibacterial Compounds that Target Biotin Protein Ligase. Antibiotics 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Mei, J.; Liu, F.; Chen, L.; Li, F.; Zeng, Q.; Wang, J.J. Preparation and characterization of carvacrol essential oil-loaded halloysite nanotubes and their application in antibacterial packaging. Food Packag. Shelf Life 2022, 34, 100972. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Preparation and antibacterial activity of Litsea cubeba essential oil/dandelion polysaccharide nanofiber. Ind. Crops Prod. 2019, 140, 111739. [Google Scholar] [CrossRef]
| Name | Description | Source or Reference |
|---|---|---|
| Strains | ||
| E. coli JTU006 | Based on the E. coli DH10B lineage and engineered to be restriction deficient. | Han et al. [13] |
| S. aureus ATCC BAA1717 | A standard MRSA strain originating from the USA300 lineage. | Teng et al. [14] |
| ΔpycA | BAA1717 mutant strain with pycA deletion. | This study |
| cpycA | ΔpycA complemented with pCL55-pycA, cmR. | This study |
| WT-ppycA | BAA1717 overexpressing pycA with pCL55-pycA, cmR. | This study |
| Plasmids | ||
| pKZ2 | Thermosensitive E. coli–S. aureus shuttle plasmid for gene deletion; AmpR/E. coli, CmR/S. aureus. | Li et al. [15] |
| pKZ2-pycA | pKZ2-pycA homologous arm sequence. | This study |
| pCL55 | Anhydrotetracycline-inducible E. coli–S. aureus shuttle plasmid; AmpR/E. coli, CmR/S. aureus. | Karinou et al. [16] |
| pCL55-pycA | pCL55-pycA CDS | This study |
| Class of Antimicrobials | Antimicrobial | MIC (μg/mL) | Foldchange | ||||
|---|---|---|---|---|---|---|---|
| WT | ΔpycA | cpycA | WT-ppycA | WT/ΔpycA | WT-ppycA/WT | ||
| β-lactams | Oxacillin | 16 | 0.25 | 32 | 64 | 64 | 4 |
| Amoxicillin | 4096 | 0.5 | 32 | 8192 | 8192 | 2 | |
| Ampicillin | 1024 | 1 | 32 | 2048 | 1024 | 2 | |
| Carbenicillin disodium | 1024 | 1 | 128 | 4096 | 1024 | 4 | |
| Penicillin potassium | 2048 | 4 | 16 | 4096 | 512 | 2 | |
| Imipenem | 1 | 0.25 | 4 | 8 | 4 | 8 | |
| Meropenem | 4 | 1 | 4 | 8 | 4 | 2 | |
| Cefoxitin | 64 | 64 | 64 | 64 | 1 | 1 | |
| Cephalosporins | Cefotaxime | 16 | 4 | 32 | 128 | 4 | 8 |
| Cephalothin sodium | 32 | 0.5 | 16 | 32 | 64 | 1 | |
| Aminoglycosides | Kanamycin sulfate | 4096 | 2 | 8 | 8192 | 2048 | 2 |
| Amikacin | 16 | 0.25 | 16 | 64 | 64 | 4 | |
| Macrolides | Erythromycin | 32 | 0.25 | 1 | 64 | 128 | 2 |
| Azithromycin | 64 | 0.5 | 1 | 128 | 128 | 2 | |
| Function | Protein | Description | Accession No. | Foldchange | p-Value |
|---|---|---|---|---|---|
| TCA cycle | FumC | Fumarate hydratase class II | Q5HES4 | 0.605 | 0.0225 |
| SucC | Succinyl-CoA synthetase subunit beta | A5ISD0 | 0.587 | 0.0003 | |
| SucD | Succinyl-CoA synthetase subunit alpha | P66866 | 0.565 | 0.0001 | |
| Mqo | Malate quinone oxidoreductase | Q5HDJ0 | 0.623 | 0.0251 | |
| Purine metabolism | PurC | Phosphoribosylaminoimidazole-succinocarboxamide synthase | A5IRV3 | 0.178 | 0.0140 |
| PurM | Phosphoribosylformylglycinamidine cyclo-ligase | A5IRV8 | 0.300 | 0.0040 | |
| PurL | Phosphoribosylformylglycinamidine synthase | A6QFS7 | 0.223 | 0.0160 | |
| PurQ | Phosphoribosylaminoimidazole carboxylase catalytic subunit | P65904 | 0.225 | 0.0060 | |
| PurH | IMP cyclohydrolase | Q2FI05 | 0.299 | 0.0080 | |
| PurD | Phosphoribosylamine-glycine ligase | Q5HH10 | 0.305 | 0.0020 | |
| PurN | Phosphoribosylglycinamide formyltransferase | Q5HH12 | 0.227 | 0.0060 | |
| PurF | Amidophosphoribosyltransferase | Q5HH14 | 0.240 | 0.0006 | |
| PurK | Phosphoribosylaminoimidazole carboxylase non-catalytic subunit | Q5HH19 | 0.252 | 0.0090 | |
| PurS | Phosphoribosylformylglycinamidine synthase subunit | A0A0E1VKY1 | 0.248 | 0.0060 | |
| PurE | Phosphoribosylaminoimidazole carboxylase | A0A5F0HIJ8 | 0.472 | 0.0820 | |
| Antimicrobial resistance | BlaZ | Beta-lactamase | D2J684 | 0.028 | 9.67 × 10−6 |
| BlaI | Beta-lactamase repressor | P0A042 | 0.065 | 0.0005 | |
| BlaR1 | Beta-lactamase sensor–transducer protein | P18357 | 0.001 | 0.0069 | |
| Cell adhesion | FnbA | Fibronectin-binding protein A | P14738 | 0.491 | 0.0105 |
| FnbB | Fibronectin-binding protein B | A0A0H2XKG3 | 0.397 | 0.0125 | |
| Fib | Fibrinogen-binding protein | A6QG59 | 0.471 | 0.0006 | |
| SasG | Surface-anchored protein G | Q2G2B2 | 0.418 | 0.0049 | |
| SpA | Immunoglobulin G-binding protein A | Q70AB9 | 0.153 | 0.0029 | |
| IsaA | Immunodominant staphylococcal antigen A | A6QK59 | 0.596 | 0.0001 | |
| IsaB | Immunodominant staphylococcal antigen B | Q2FDM1 | 0.477 | 0.0019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, Z.; Li, J.; Zhan, Z.; Tian, X.; Hou, W.; He, M.; Shi, C. Metabolic Master Switch: Pyruvate Carboxylase Fuels Antimicrobial Resistance and Virulence in Foodborne Staphylococcus aureus. Foods 2025, 14, 2566. https://doi.org/10.3390/foods14152566
Mai Z, Li J, Zhan Z, Tian X, Hou W, He M, Shi C. Metabolic Master Switch: Pyruvate Carboxylase Fuels Antimicrobial Resistance and Virulence in Foodborne Staphylococcus aureus. Foods. 2025; 14(15):2566. https://doi.org/10.3390/foods14152566
Chicago/Turabian StyleMai, Zifeng, Jiahui Li, Zeqiang Zhan, Xiaorong Tian, Wanwan Hou, Mu He, and Chunlei Shi. 2025. "Metabolic Master Switch: Pyruvate Carboxylase Fuels Antimicrobial Resistance and Virulence in Foodborne Staphylococcus aureus" Foods 14, no. 15: 2566. https://doi.org/10.3390/foods14152566
APA StyleMai, Z., Li, J., Zhan, Z., Tian, X., Hou, W., He, M., & Shi, C. (2025). Metabolic Master Switch: Pyruvate Carboxylase Fuels Antimicrobial Resistance and Virulence in Foodborne Staphylococcus aureus. Foods, 14(15), 2566. https://doi.org/10.3390/foods14152566







