Improvement of Oxidative Stability and Antioxidative Capacity of Virgin Olive Oil by Flash Thermal Pretreatment—Optimization Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Olive Oil Production
2.3. Basic Quality Parameters
2.4. Oil Yield
2.5. Volatile Components
2.6. Phenolic Compounds
2.7. Tocopherol Content
2.8. Fatty Acid Composition
2.9. Oxidative Stability Index
2.10. Antioxidative Capacity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Basic Quality Parameters and Processing Yield
3.2. Volatile Compounds
3.3. Phenolic Compounds
3.4. Tocopherols
3.5. Fatty Acid Composition
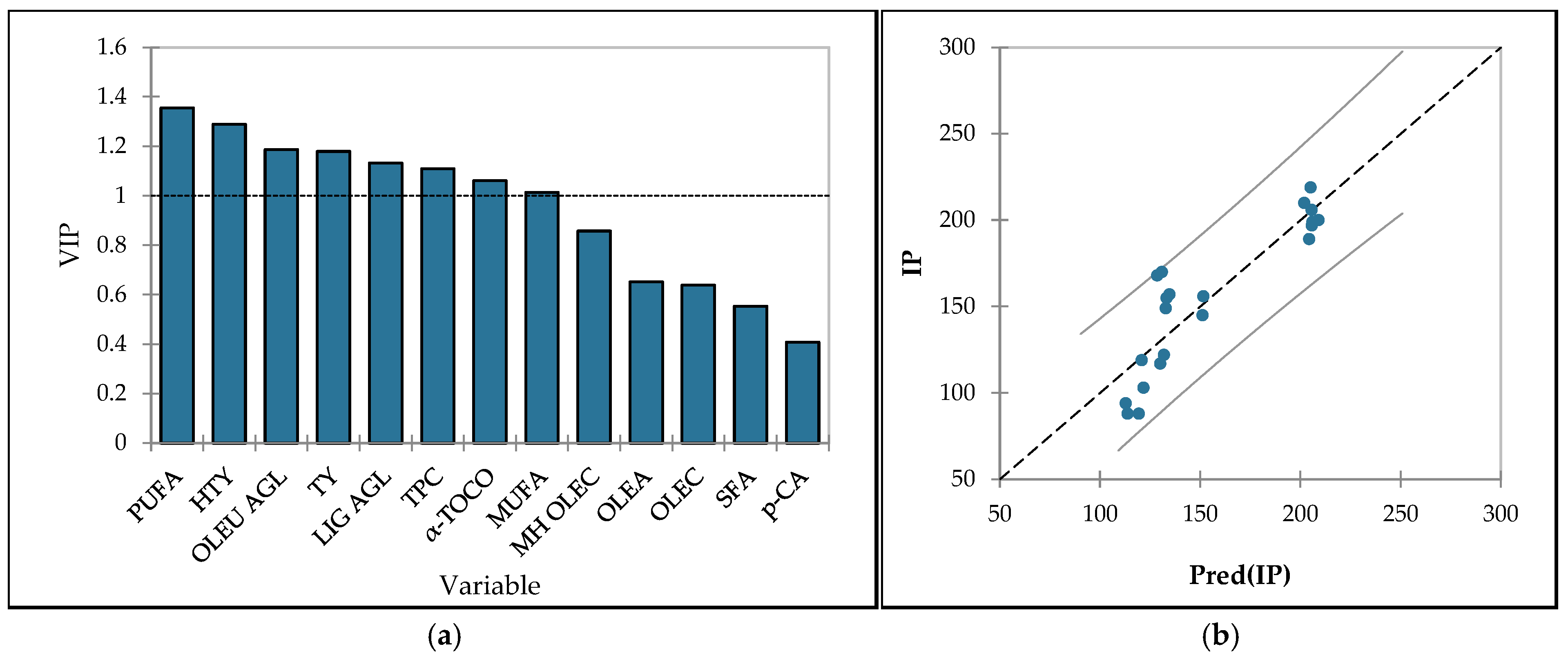
3.6. Oxidative Stability and Antioxidant Capacity
| Sample | IP (min) * | AC (% DPPH˙ Reduction) * |
|---|---|---|
| Istarska Bjelica | p ≤ 0.01 | p = 0.379 |
| Control | 189 ± 8 b | 60.07 ± 0.25 |
| 15 °C | 206 ± 8 ab | 58.77 ± 0.76 |
| 20 °C | 197 ± 6 b | 61.58 ± 2.98 |
| 25 °C | 200 ± 9 ab | 59.87 ± 1.01 |
| 30 °C | 210 ± 5 ab | 60.73 ± 1.54 |
| 35 °C | 199 ± 7 ab | 59.88 ± 1.10 |
| 40 °C | 219 ± 5 a | 59.53 ± 0.59 |
| Levantinka | p = 0.253 | p ≤ 0.001 |
| Control | 168 ± 11 | 50.18 ± 1.33 ab |
| 15 °C | 149 ± 4 | 50.00 ± 0.46 ab |
| 20 °C | 156 ± 10 | 51.77 ± 1.20 ab |
| 25 °C | 155 ± 9 | 44.53 ± 0.38 c |
| 30 °C | 170 ± 27 | 51.12 ± 1.46 ab |
| 35 °C | 145 ± 5 | 48.87 ± 1.53 b |
| 40 °C | 157 ± 7 | 52.53 ± 0.67 a |
| Oblica | p ≤ 0.001 | p ≤ 0.001 |
| Control | 122 ± 6 a | 65.70 ± 1.91 a |
| 15 °C | 119 ± 5 a | 57.73 ± 1.55 b |
| 20 °C | 117 ± 2 a | 63.20 ± 1.18 a |
| 25 °C | 103 ± 9 b | 55.70 ± 2.13 b |
| 30 °C | 94 ± 1 bc | 54.07 ± 0.70 bc |
| 35 °C | 88 ± 0 c | 50.53 ± 0.81 cd |
| 40 °C | 88 ± 3 c | 47.07 ± 1.10 d |
3.7. Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marrero, A.D.; Quesada, A.R.; Martínez-Poveda, B.; Medina, M.Á. Anti-Cancer, Anti-Angiogenic, and Anti-Atherogenic Potential of Key Phenolic Compounds from Virgin Olive Oil. Nutrients 2024, 16, 1283. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, J.; Xin, Q.; Yuan, R.; Miao, Y.; Yang, M.; Mo, H.; Chen, K.; Cong, W. Protective Effects of Oleic Acid and Polyphenols in Extra Virgin Olive Oil on Cardiovascular Diseases. Food Sci. Hum. Wellness 2024, 13, 529–540. [Google Scholar] [CrossRef]
- Gonçalves, M.; Vale, N.; Silva, P. Neuroprotective Effects of Olive Oil: A Comprehensive Review of Antioxidant Properties. Antioxidants 2024, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Tsimihodimos, V.; Psoma, O. Extra Virgin Olive Oil and Metabolic Diseases. Int. J. Mol. Sci. 2024, 25, 8117. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and Biotransformations of Phenol-Conjugated Oleosidic Secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167. [Google Scholar] [CrossRef]
- Salas, J.J.; Sánchez, J. The Decrease of Virgin Olive Oil Flavor Produced by High Malaxation Temperature Is Due to Inactivation of Hydroperoxide. J. Agric. Food Chem. 1999, 47, 809–812. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Romero-Segura, C.; Sanz, C.; Pérez, A.G. Synthesis of Volatile Compounds of Virgin Olive Oil Is Limited by the Lipoxygenase Activity Load during the Oil Extraction Process. J. Agric. Food Chem. 2012, 60, 812–822. [Google Scholar] [CrossRef]
- Kalua, C.M.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D. Changes in Volatile and Phenolic Compounds with Malaxation Time and Temperature during Virgin Olive Oil Production. J. Agric. Food Chem. 2006, 54, 7641–7651. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Bejaoui, M.A.; Sánchez-Ortiz, A.; Aguilera, M.P.; Ruiz-Moreno, M.J.; Sánchez, S.; Jiménez, A.; Beltrán, G. High Power Ultrasound Frequency for Olive Paste Conditioning: Effect on the Virgin Olive Oil Bioactive Compounds and Sensorial Characteristics. Innov. Food Sci. Emerg. Technol. 2018, 47, 136–145. [Google Scholar] [CrossRef]
- Romero-Segura, C.; García-Rodríguez, R.; Sánchez-Ortiz, A.; Sanz, C.; Pérez, A.G. The Role of Olive β-Glucosidase in Shaping the Phenolic Profile of Virgin Olive Oil. Food Res. Int. 2012, 45, 191–196. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Sordini, B.; Servili, M. Flash Thermal Conditioning of Olive Pastes during the Oil Mechanical Extraction Process: Cultivar Impact on the Phenolic and Volatile Composition of Virgin Olive Oil. J. Agric. Food Chem. 2015, 63, 6066–6074. [Google Scholar] [CrossRef]
- Nardella, M.; Moscetti, R.; Bedini, G.; Bandiera, A.; Chakravartula, S.S.N.; Massantini, R. Impact of Traditional and Innovative Malaxation Techniques and Technologies on Nutritional and Sensory Quality of Virgin Olive Oil—A Review. Food Chem. Adv. 2023, 2, 100163. [Google Scholar] [CrossRef]
- Kalogianni, E.P.; Georgiou, D.; Exarhopoulos, S. Olive Oil Droplet Coalescence during Malaxation. J. Food Eng. 2019, 240, 99–104. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on Virgin Olive Oil Quality. Past, Present and Future—An Overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Olmo-Cunillera, A.; Lozano-Castellón, J.; Pérez, M.; Miliarakis, E.; Tresserra-Rimbau, A.; Ninot, A.; Romero-Aroca, A.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Optimizing the Malaxation Conditions to Produce an Arbequina EVOO with High Content of Bioactive Compounds. Antioxidants 2021, 10, 1819. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Zagaria, R.; Sabella, E.; Romaniello, R. Plant Innovation in the Olive Oil Extraction Process: A Comparison of Efficiency and Energy Consumption between Microwave Treatment and Traditional Malaxation of Olive Pastes. J. Food Eng. 2015, 146, 44–52. [Google Scholar] [CrossRef]
- Clodoveo, M.L. New Advances in the Development of Innovative Virgin Olive Oil Extraction Plants: Looking Back to See the Future. Food Res. Int. 2013, 54, 726–729. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Moramarco, V.; Paduano, A.; Sacchi, R.; Di Palmo, T.; Crupi, P.; Corbo, F.; Pesce, V.; Distaso, E.; Tamburrano, P. Engineering Design and Prototype Development of a Full Scale Ultrasound System for Virgin Olive Oil by Means of Numerical and Experimental Analysis. Ultrason. Sonochemistry 2017, 37, 169–181. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Alexandrakis, Z.; Katsaros, G.; Oikonomou, D.; Toepfl, S.; Heinz, V.; Taoukis, P. Shelf-Life Evaluation of Virgin Olive Oil Extracted from Olives Subjected to Nonthermal Pretreatments for Yield Increase. Innov. Food Sci. Emerg. Technol. 2017, 40, 52–57. [Google Scholar] [CrossRef]
- Romaniello, R.; Tamborrino, A.; Leone, A. Use of Ultrasound and Pulsed Electric Fields Technologies Applied to the Olive Oil Extraction Process. Chem. Eng. Trans. 2019, 75, 13–18. [Google Scholar] [CrossRef]
- Amirante, P.; Clodoveo, M.L.; Dugo, G.; Leone, A.; Tamborrino, A. Advance Technology in Virgin Olive Oil Production from Traditional and De-Stoned Pastes: Influence of the Introduction of a Heat Exchanger on Oil Quality. Food Chem. 2006, 98, 797–805. [Google Scholar] [CrossRef]
- Esposto, S.; Veneziani, G.; Taticchi, A.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Sordini, B.; Minnocci, A.; Sebastiani, L.; Servili, M. Flash Thermal Conditioning of Olive Pastes during the Olive Oil Mechanical Extraction Process: Impact on the Structural Modifications of Pastes and Oil Quality. J. Agric. Food Chem. 2013, 61, 4953–4960. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Esposto, S.; Tamborrino, A.; Romaniello, R.; Taticchi, A.; Urbani, S.; Servili, M. Using a Tubular Heat Exchanger to Improve the Conditioning Process of the Olive Paste: Evaluation of Yield and Olive Oil Quality. Eur. J. Lipid Sci. Technol. 2016, 118, 308–317. [Google Scholar] [CrossRef]
- Tamborrino, A.; Veneziani, G.; Romaniello, R.; Perone, C.; Urbani, S.; Leone, A.; Servili, M. Development of an Innovative Rotating Spiral Heat Exchanger with Integrated Microwave Module for the Olive Oil Industry. LWT Food Sci. Technol. 2021, 147, 111622. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Angeloni, G.; Zanoni, B.; Breschi, C.; Calamai, I.; Parenti, A. The Effect of an Increase in Paste Temperature between Malaxation and Centrifugation on Olive Oil Quality and Yield: Preliminary Results. Ital. J. Food Sci. 2019, 31, 451–458. [Google Scholar]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Di Maio, I.; Sordini, B.; Servili, M. Cooling Treatment of Olive Paste during the Oil Processing: Impact on the Yield and Extra Virgin Olive Oil Quality. Food Chem. 2017, 221, 107–113. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Servili, M. Characterization of Phenolic and Volatile Composition of Extra Virgin Olive Oil Extracted from Six Italian Cultivars Using a Cooling Treatment of Olive Paste. LWT Food Sci. Technol. 2018, 87, 523–528. [Google Scholar] [CrossRef]
- Veneziani, G.; Nucciarelli, D.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Tomasone, R.; Pagano, M.; Servili, M. Application of Low Temperature during the Malaxation Phase of Virgin Olive Oil Mechanical Extraction Processes of Three Different Italian Cultivars. Foods 2021, 10, 1578. [Google Scholar] [CrossRef]
- Selvaggini, R.; Esposto, S.; Taticchi, A.; Urbani, S.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Optimization of the Temperature and Oxygen Concentration Conditions in the Malaxation during the Oil Mechanical Extraction Process of Four Italian Olive Cultivars. J. Agric. Food Chem. 2014, 62, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile Compounds in Virgin Olive Oil: Occurrence and Their Relationship with the Quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Fregapane, G.; Salvador, M.D. Effect of Cultivar and Ripening on Minor Components in Spanish Olive Fruits and Their Corresponding Virgin Olive Oils. Food Res. Int. 2008, 41, 433–440. [Google Scholar] [CrossRef]
- IOC—Economic Affairs & Promotion Unit. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 10 May 2025).
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Brkić Bubola, K. Inter-Varietal Diversity of Typical Volatile and Phenolic Profiles of Croatian Extra Virgin Olive Oils as Revealed by GC-IT-MS and UPLC-DAD Analysis. Foods 2019, 8, 565. [Google Scholar] [CrossRef]
- COI/OH/Doc. No 1 2011 Guide for the Determination of the Characteristics of Oil-Olives. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 5 December 2023).
- Camposeo, S.; Vivaldi, G.A.; Gattullo, C.E. Ripening Indices and Harvesting Times of Different Olive Cultivars for Continuous Harvest. Sci. Hortic. 2013, 151, 1–10. [Google Scholar] [CrossRef]
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. Available online: https://www.iso.org/standard/71268.html (accessed on 4 December 2023).
- COI/T.20/Doc. No 34/Rev. 1 2017 Method Determination of Free Fatty Acids, Cold Method. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 5 December 2023).
- COI/T.20/Doc. No 19/Rev. 5 2019 Method of Analysis Spectrophotometric Investigation in the Ultraviolet. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 4 December 2023).
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Laboratory-scale Optimization of Olive Oil Extraction: Simultaneous Addition of Enzymes and Microtalc Improves the Yield. Eur. J. Lipid Sci. Technol. 2014, 116, 1054–1062. [Google Scholar] [CrossRef]
- Kraljić, K.; Stjepanović, T.; Obranović, M.; Pospišil, M.; Balbino, S.; Škevin, D. Influence of Conditioning Temperature on the Quality, Nutritional Properties and Volatile Profile of Virgin Rapeseed Oil. Food Technol. Biotechnol. 2018, 56, 562–572. [Google Scholar] [CrossRef] [PubMed]
- COI/T.20/Doc. No 29/Rev. 2 2022 Document to Declare the Use of IOC Methods for Phenolic Compounds Determination. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 11 December 2023).
- ISO 9936:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. BSI: London, UK, 2016. Available online: https://www.iso.org/standard/69595.html (accessed on 6 December 2023).
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneve, Switzerland, 2017. Available online: https://www.iso.org/standard/72142.html (accessed on 6 December 2023).
- Tan, C.P.; Che Man, Y.B.; Selamat, J.; Yusoff, M.S.A. Comparative Studies of Oxidative Stability of Edible Oils by Differential Scanning Calorimetry and Oxidative Stability Index Methods. Food Chem. 2002, 76, 385–389. [Google Scholar] [CrossRef]
- Markić, F.; Kraljić, K.; Stulić, V.; Pleslić, S.; Pavičić, T.V.; Strmečki, N.M. Improving the Extraction of Tomato Seed Oil and the Retention of Bioactive Substances Using Pulsed Electric Field Technology. Future Foods 2025, 12, 100706. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The Influence of the Malaxation Temperature on the Activity of Polyphenoloxidase and Peroxidase and on the Phenolic Composition of Virgin Olive Oil. Food Chem. 2013, 136, 975–983. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Gómez-Rico, A.; Salvador, M.D.; Fregapane, G. Influence of Malaxation Conditions on Virgin Olive Oil Yield, Overall Quality and Composition. Eur. Food Res. Technol. 2009, 228, 671–677. [Google Scholar] [CrossRef]
- Kraljić, K.; Balbino, S.; Filipan, K.; Herceg, Z.; Ivanov, M.; Vukušić Pavičić, T.; Stuparević, I.; Pavlić, K.; Škevin, D. Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part I—Thermal Techniques. Processes 2023, 11, 1194. [Google Scholar] [CrossRef]
- Commission Delegated Regulation Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union 2022, 65, L 284.
- Fiori, F.; Di Lecce, G.; Boselli, E.; Pieralisi, G.; Frega, N.G. Effects of Olive Paste Fast Preheating on the Quality of Extra Virgin Olive Oil during Storage. LWT Food Sci. Technol. 2014, 58, 511–518. [Google Scholar] [CrossRef]
- Espínola, F.; Moya, M.; Fernández, D.G.; Castro, E. Improved Extraction of Virgin Olive Oil Using Calcium Carbonate as Coadjuvant Extractant. J. Food Eng. 2009, 92, 112–118. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Esposto, S.; Berardi, A.; Servili, M. Investigation on the Effects of a Pulsed Electric Field (PEF) Continuous System Implemented in an Industrial Olive Oil Plant. Foods 2022, 11, 2758. [Google Scholar] [CrossRef]
- Yang, S.; Li, S.; Li, G.; Li, C.; Li, W.; Bi, Y.; Wei, J. Pulsed Electric Field Treatment Improves the Oil Yield, Quality, and Antioxidant Activity of Virgin Olive Oil. Food Chem. X 2024, 22, 101372. [Google Scholar] [CrossRef]
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of Pulsed Electric Fields and High Pressure on Improved Recovery of High-added-value Compounds from Olive Pomace. J. Food Sci. 2020, 85, 1500–1512. [Google Scholar] [CrossRef]
- Andreou, V.; Kourmbeti, E.; Dimopoulos, G.; Psarianos, M.; Katsaros, G.; Taoukis, P. Optimization of Virgin Olive Oil Yield and Quality Applying Nonthermal Processing. Food Bioprocess Technol. 2022, 15, 891–903. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Tamborrino, A.; Romaniello, R.; Caponio, F.; Squeo, G.; Leone, A. Combined Industrial Olive Oil Extraction Plant Using Ultrasounds, Microwave, and Heat Exchange: Impact on Olive Oil Quality and Yield. J. Food Eng. 2019, 245, 124–130. [Google Scholar] [CrossRef]
- Juliano, P.; Gaber, M.A.F.M.; Romaniello, R.; Tamborrino, A.; Berardi, A.; Leone, A. Advances in Physical Technologies to Improve Virgin Olive Oil Extraction Efficiency in High-Throughput Production Plants. Food Eng. Rev. 2023, 15, 625–642. [Google Scholar] [CrossRef]
- Mikrou, T.; Litsa, M.; Papantoni, A.; Kapsokefalou, M.; Gardeli, C.; Mallouchos, A. Effect of Cultivar and Geographical Origin on the Volatile Composition of Greek Monovarietal Extra Virgin Olive Oils. Chemosensors 2023, 11, 80. [Google Scholar] [CrossRef]
- Žanetić, M.; Jukić Špika, M.; Ožić, M.M.; Brkić Bubola, K. Comparative Study of Volatile Compounds and Sensory Characteristics of Dalmatian Monovarietal Virgin Olive Oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Soldo, B.; Jukić Špika, M.; Pasković, I.; Vuko, E.; Polić Pasković, M.; Ljubenkov, I. The Composition of Volatiles and the Role of Non-Traditional LOX on Target Metabolites in Virgin Olive Oil from Autochthonous Dalmatian Cultivars. Molecules 2024, 29, 1696. [Google Scholar] [CrossRef] [PubMed]
- Marx, Í.M.G.; Rodrigues, N.; Veloso, A.C.A.; Casal, S.; Pereira, J.A.; Peres, A.M. Effect of Malaxation Temperature on the Physicochemical and Sensory Quality of Cv. Cobrançosa Olive Oil and Its Evaluation Using an Electronic Tongue. LWT Food Sci. Technol. 2021, 137, 110426. [Google Scholar] [CrossRef]
- Olmo-Cunillera, A.; Casadei, E.; Valli, E.; Lozano-Castellón, J.; Miliarakis, E.; Domínguez-López, I.; Ninot, A.; Romero-Aroca, A.; Lamuela-Raventós, R.M.; Pérez, M.; et al. Aromatic, Sensory, and Fatty Acid Profiles of Arbequina Extra Virgin Olive Oils Produced Using Different Malaxation Conditions. Foods 2022, 11, 3446. [Google Scholar] [CrossRef]
- Servili, M.; Taticchi, A.; Esposto, S.; Urbani, S.; Selvaggini, R.; Montedoro, G.F. Effect of Olive Stoning on the Volatile and Phenolic Composition of Virgin Olive Oil. J. Agric. Food Chem. 2007, 55, 7028–7035. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic Syndrome, Mediterranean Diet, and Polyphenols: Evidence and Perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Emma, M.R.; Augello, G.; Di Stefano, V.; Azzolina, A.; Giannitrapani, L.; Montalto, G.; Cervello, M.; Cusimano, A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 1234. [Google Scholar] [CrossRef]
- Diamantakos, P.; Ioannidis, K.; Papanikolaou, C.; Tsolakou, A.; Rigakou, A.; Melliou, E.; Magiatis, P. A New Definition of the Term “High-Phenolic Olive Oil” Based on Large Scale Statistical Data of Greek Olive Oils Analyzed by QNMR. Molecules 2021, 26, 1115. [Google Scholar] [CrossRef]
- Lukić, I.; Horvat, I.; Godena, S.; Krapac, M.; Lukić, M.; Vrhovsek, U.; Brkić Bubola, K. Towards Understanding the Varietal Typicity of Virgin Olive Oil by Correlating Sensory and Compositional Analysis Data: A Case Study. Food Res. Int. 2018, 112, 78–89. [Google Scholar] [CrossRef]
- Lukić, I.; Krapac, M.; Horvat, I.; Godena, S.; Kosić, U.; Brkić Bubola, K. Three-Factor Approach for Balancing the Concentrations of Phenols and Volatiles in Virgin Olive Oil from a Late-Ripening Olive Cultivar. LWT Food Sci. Technol. 2018, 87, 194–202. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Perica, S.; Žanetić, M.; Škevin, D. Virgin Olive Oil Phenols, Fatty Acid Composition and Sensory Profile: Can Cultivar Overpower Environmental and Ripening Effect? Antioxidants 2021, 10, 689. [Google Scholar] [CrossRef]
- Diamantakos, P.; Giannara, T.; Skarkou, M.; Melliou, E.; Magiatis, P. Influence of Harvest Time and Malaxation Conditions on the Concentration of Individual Phenols in Extra Virgin Olive Oil Related to Its Healthy Properties. Molecules 2020, 25, 2449. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Žanetić, M.; Jukić Špika, M.; Lukić, M.; Koprivnjak, O.; Brkić Bubola, K. Complex Interactive Effects of Ripening Degree, Malaxation Duration and Temperature on Oblica Cv. Virgin Olive Oil Phenols, Volatiles and Sensory Quality. Food Chem. 2017, 232, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, M.P.; Jimenez, A.; Sanchez-Villasclaras, S.; Uceda, M.; Beltran, G. Modulation of Bitterness and Pungency in Virgin Olive Oil from Unripe “Picual” Fruits. Eur. J. Lipid Sci. Technol. 2015, 117, 1463–1472. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Koutsaftakis, A.; Harwood, J.L. Influence of Malaxation Conditions on Characteristic Qualities of Olive Oil. Food Chem. 2011, 127, 1481–1486. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Influence of Malaxation Temperature and Time on the Quality of Virgin Olive Oils. Food Chem. 2001, 72, 19–28. [Google Scholar] [CrossRef]
- Parenti, A.; Spugnoli, P.; Masella, P.; Calamai, L. The Effect of Malaxation Temperature on the Virgin Olive Oil Phenolic Profile under Laboratory-scale Conditions. Eur. J. Lipid Sci. Technol. 2008, 110, 735–741. [Google Scholar] [CrossRef]
- Amirante, R.; Cini, E.; Montel, G.L.; Pasqualone, A. Influence of Mixing and Extraction Parameters on Virgin Olive Oil Quality. Grasas Aceites 2001, 52, 198–201. [Google Scholar] [CrossRef]
- Nucciarelli, D.; Esposto, S.; Veneziani, G.; Daidone, L.; Urbani, S.; Taticchi, A.; Selvaggini, R.; Servili, M. The Use of a Cooling Crusher to Reduce the Temperature of Olive Paste and Improve EVOO Quality of Coratina, Peranzana, and Moresca Cultivars: Impact on Phenolic and Volatile Compounds. Food Bioprocess Technol. 2022, 15, 1988–1996. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Kriško, A.; Valić, S.; Carić, D.; Krapac, M.; Poljuha, D. Antioxidants, Radical-Scavenging and Protein Carbonylation Inhibition Capacity of Six Monocultivar Virgin Olive Oils in Istria (Croatia). Acta Aliment. 2016, 45, 427–433. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Vrhovnik, I.; Hladnik, T.; Prgomet, Ž.; Hlevnjak, B.; Germek, V.M. Characteristics of Nutritive Value of Virgin Olive Oils from Uža, Istarska Bjelica, Leccino and Rosulja Cultivars. Croat. J. Food Technol. Biotechnol. Nutr. 2012, 7, 172–178. [Google Scholar]
- Pérez, A.G.; León, L.; Pascual, M.; de la Rosa, R.; Belaj, A.; Sanz, C. Analysis of Olive (Olea europaea L.) Genetic Resources in Relation to the Content of Vitamin E in Virgin Olive Oil. Antioxidants 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Šarolić, M.; Gugić, M.; Friganović, E.; Tuberoso, C.; Jerković, I. Phytochemicals and Other Characteristics of Croatian Monovarietal Extra Virgin Olive Oils from Oblica, Lastovka and Levantinka Varieties. Molecules 2015, 20, 4395–4409. [Google Scholar] [CrossRef] [PubMed]
- Jukić Špika, M.; Kraljić, K.; Koprivnjak, O.; Škevin, D.; Žanetić, M.; Katalinić, M. Effect of Agronomical Factors and Storage Conditions on the Tocopherol Content of Oblica and Leccino Virgin Olive Oils. J. Am. Oil Chem. Soc. 2015, 92, 1293–1301. [Google Scholar] [CrossRef]
- Pazos, M.; Andersen, M.L.; Medina, I.; Skibsted, L.H. Efficiency of Natural Phenolic Compounds Regenerating α-Tocopherol from α-Tocopheroxyl Radical. J. Agric. Food Chem. 2007, 55, 3661–3666. [Google Scholar] [CrossRef]
- Rastrelli, L.; Passi, S.; Ippolito, F.; Vacca, G.; De Simone, F. Rate of Degradation of α-Tocopherol, Squalene, Phenolics, and Polyunsaturated Fatty Acids in Olive Oil during Different Storage Conditions. J. Agric. Food Chem. 2002, 50, 5566–5570. [Google Scholar] [CrossRef]
- Sánchez, J.; Harwood, J.L. Biosynthesis of Triacylglycerols and Volatiles in Olives. Eur. J. Lipid Sci. Technol. 2002, 104, 564–573. [Google Scholar] [CrossRef]
- Harwood, J.L. Working with Randy: The Diacylglycerol Acyltransferase Story. Lipids 2020, 55, 419–423. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Contrasting Patterns of Fatty Acid Composition and Oil Accumulation during Fruit Growth in Several Olive Varieties and Locations in a Non-Mediterranean Region. Eur. J. Agron. 2014, 52, 237–246. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Shahidi, F. Olive Oil Quality and Its Relation to the Functional Bioactives and Their Properties. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing; Shahidi, F., Kiritsakis, A., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 205–219. [Google Scholar]
- Špika Jukić, M.; Žanetić, M.; Pinatel, C.; Vitanović, E.; Strikić, F. Fatty Acid and Triacylgycerol Profile of Levantinka Virgin Olive Oil. In Proceedings of the 14th Ružička Days: Today Science—Tomorrow Industry, Vukovar, Croatia, 13–15 September 2012; Jukić, A., Ed.; Croatian Society of Chemical Engineers Faculty of Food Technology: Osijek, Croatia, 2013; pp. 213–218. [Google Scholar]
- Jukić Špika, M.; Žanetić, M.; Kraljić, K.; Soldo, B.; Ljubenkov, I.; Politeo, O.; Škevin, D. Differentiation Between Unfiltered and Filtered Oblica and Leccino Cv. Virgin Olive Oils. J. Food Sci. 2019, 84, 877–885. [Google Scholar] [CrossRef]
- Brkic Bubola, K.; Valencic, V.; Bucar-Miklavcic, M.; Krapac, M.; Lukic, M.; Setic, E.; Sladonja, B. Sterol, Triterpen Dialcohol and Fatty Acid Profile of Less- and Well-Known Istrian Monovarietal Olive Oil. Croat. J. Food Technol. Biotechnol. Nutr. 2018, 10, 118–122. [Google Scholar] [CrossRef]
- Morales, M.T.; Przybylski, R. Olive Oil Oxidation. In Handbook of Olive Oil, 2nd ed.; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 479–522. [Google Scholar]
- Velasco, J.; Dobarganes, C. Oxidative Stability of Virgin Olive Oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Arnao, M.B.; Ceron, J.J. Validation of Three Automated Assays for Total Antioxidant Capacity Determination in Canine Serum Samples. J. Vet. Diagn. Investig. 2016, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free Radical-Scavenging Properties of Olive Oil Polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Effect of Fatty Acids and Tocopherols on the Oxidative Stability of Vegetable Oils. Eur. J. Lipid Sci. Technol. 2006, 108, 1051–1061. [Google Scholar] [CrossRef]
- Serrano, A.; De la Rosa, R.; Sánchez-Ortiz, A.; Cano, J.; Pérez, A.G.; Sanz, C.; Arias-Calderón, R.; Velasco, L.; León, L. Chemical Components Influencing Oxidative Stability and Sensorial Properties of Extra Virgin Olive Oil and Effect of Genotype and Location on Their Expression. LWT Food Sci. Technol. 2021, 136, 110257. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernández-Gutiérrez, A. Evaluation of the Antioxidant Capacity of Individual Phenolic Compounds in Virgin Olive Oil. J. Agric. Food Chem. 2005, 53, 8918–8925. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Aprile, A.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; Sabella, E.; De Bellis, L. Phenolic Profile and Antioxidant Activity of Italian Monovarietal Extra Virgin Olive Oils. Antioxidants 2019, 8, 161. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC–ESI–QTOF–MS as a Powerful Analytical Tool for Characterising Phenolic Compounds in Olive-leaf Extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kalogiouri, N.P.; Kritikou, E.; Martakos, I.C.; Lazarou, C.; Pentogennis, M.; Thomaidis, N.S. Characterization of the Phenolic Fingerprint of Kolovi Extra Virgin Olive Oils from Lesvos with Regard to Altitude and Farming System Analyzed by UHPLC-QTOF-MS. Molecules 2021, 26, 5634. [Google Scholar] [CrossRef]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.-L. From Olive Drupes to Olive Oil. An HPLC-Orbitrap-Based Qualitative and Quantitative Exploration of Olive Key Metabolites. Planta Medica 2013, 79, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Medina, V.; Miho, H.; Melliou, E.; Magiatis, P.; Priego-Capote, F.; Luque de Castro, M.D. Quantitative Method for Determination of Oleocanthal and Oleacein in Virgin Olive Oils by Liquid Chromatography–Tandem Mass Spectrometry. Talanta 2017, 162, 24–31. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Golc Wondra, A.; Vrhovšek, U.; Mozetič Vodopivec, B. Phenolic Profiling of Olives and Olive Oil Process-Derived Matrices Using UPLC-DAD-ESI-QTOF-HRMS Analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar] [CrossRef]

| Sample | Acidity (% Oleic Fatty Acid) * | PV (Meq O2/kg) | K Values | Yield (%) * | ||
|---|---|---|---|---|---|---|
| K232 * | K268 * | ΔK * | ||||
| Istarska Bjelica | p ≤ 0.01 | p ≤ 0.001 | p ≤ 0.05 | p = 0.522 | p ≤ 0.001 | p ≤ 0.05 |
| Control | 0.42 ± 0.03 a | 2.9 ± 0.5 d | 1.92 ± 0.12 a | 0.23 ± 0.03 | 0.00 ± 0.00 a | 24.70 ± 1.02 a |
| 15 °C | 0.38 ± 0.01 b | 4.8 ± 0.1 abc | 1.89 ± 0.06 a | 0.19 ± 0.02 | −0.01 ± 0.01 b | 25.18 ± 0.59 a |
| 20 °C | 0.39 ± 0.01 ab | 5.3 ± 0.1 ab | 2.02 ± 0.01 a | 0.23 ± 0.02 | −0.01 ± 0.00 b | 25.72 ± 0.14 a |
| 25 °C | 0.39 ± 0.00 ab | 6.0 ± 0.3 a | 2.00 ± 0.04 a | 0.21 ± 0.01 | −0.01 ± 0.00 b | 26.69 ± 0.58 a |
| 30 °C | 0.39 ± 0.01 ab | 4.5 ± 1.0 bc | 2.06 ± 0.13 a | 0.23 ± 0.08 | −0.01 ± 0.00 b | 25.86 ± 0.30 a |
| 35 °C | 0.42 ± 0.01 ab | 3.4 ± 0.9 cd | 2.10 ± 0.07 a | 0.25 ± 0.03 | −0.01 ± 0.00 b | 25.98 ± 0.39 a |
| 40 °C | 0.42 ± 0.01 a | 3.4 ± 0.1 cd | 2.07 ± 0.07 a | 0.20 ± 0.04 | −0.01 ± 0.00 b | 25.69 ± 0.27 a |
| Levantinka | p = 0.071 | p ≤ 0.05 | p = 0.525 | p ≤ 0.05 | p ≤ 0.001 | p = 0.127 |
| Control | 0.24 ± 0.01 | 3.3 ± 0.2 b | 1.7 ± 0.05 | 0.10 ± 0.01 b | −0.01 ± 0.01 a | 12.22 ± 0.17 |
| 15 °C | 0.24 ± 0.01 | 6.9 ± 0.8 a | 1.67 ± 0.09 | 0.15 ± 0.05 ab | 0.00 ± 0.00 a | 12.44 ± 0.24 |
| 20 °C | 0.25 ± 0.01 | 4.9 ± 1.0 ab | 1.62 ± 0.04 | 0.12 ± 0.01 ab | 0.00 ± 0.00 a | 12.95 ± 0.48 |
| 25 °C | 0.22 ± 0.00 | 3.9 ± 1.6 ab | 1.69 ± 0.06 | 0.16 ± 0.01 a | 0.00 ± 0.00 a | 12.22 ± 0.58 |
| 30 °C | 0.23 ± 0.02 | 4.6 ± 1.6 ab | 1.69 ± 0.09 | 0.13 ± 0.01 ab | 0.00 ± 0.00 a | 12.36 ± 0.27 |
| 35 °C | 0.23 ± 0.01 | 5.3 ± 1.5 ab | 1.66 ± 0.02 | 0.11 ± 0.01 ab | −0.01 ± 0.00 a | 12.43 ± 0.57 |
| 40 °C | 0.24 ± 0.00 | 6.3 ± 0.6 a | 1.71 ± 0.03 | 0.12 ± 0.01 ab | −0.01 ± 0.00 a | 13.03 ± 0.32 |
| Oblica | p ≤ 0.001 | p ≤ 0.001 | p = 0.546 | p = 0.470 | p ≤ 0.001 | p ≤ 0.001 |
| Control | 0.30 ± 0.01 bc | 3.5 ± 0.2 d | 1.98 ± 0.08 | 0.20 ± 0.05 | −0.01 ± 0.00 b | 13.58 ± 0.32 ab |
| 15 °C | 0.30 ± 0.01 c | 4.5 ± 0.4 bcd | 1.93 ± 0.10 | 0.22 ± 0.07 | 0.00 ± 0.01 a | 12.81 ± 0.39 b |
| 20 °C | 0.30 ± 0.00 bc | 4.3 ± 0.7 cd | 1.88 ± 0.06 | 0.19 ± 0.05 | 0.00 ± 0.00 a | 12.89 ± 0.36 b |
| 25 °C | 0.27 ± 0.01 d | 4.3 ± 0.7 cd | 1.80 ± 0.11 | 0.17 ± 0.04 | 0.00 ± 0.00 a | 12.71 ± 0.23 b |
| 30 °C | 0.32 ± 0.00 ab | 6.5 ± 0.3 abc | 2.10 ± 0.06 | 0.19 ± 0.02 | −0.01 ± 0.00 b | 13.67 ± 0.39 ab |
| 35 °C | 0.33 ± 0.01 a | 6.8 ± 1.4 ab | 1.45 ± 1.08 | 0.12 ± 0.09 | −0.01 ± 0.00 b | 13.93 ± 0.15 a |
| 40 °C | 0.32 ± 0.01 abc | 7.7 ± 1.3 a | 2.09 ± 0.02 | 0.19 ± 0.02 | −0.01 ± 0.00 b | 13.94 ± 0.21 a |
| Sample | Phenolic Compound (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hydroxytyrosol * | Tyrosol * | Oleacein * | Oleocanthal * | Methyl Hemiacetal of Oleocanthal * | Σ of Oleuropein Aglycones *§ | Σ of Ligstroside Aglycones *£ | p-Coummaric Acid * | Total Phenolic * | |
| Istarska Bjelica | p = 0.324 | p = 0.368 | p = 0.371 | p = 0.538 | p ≤ 0.05 | p = 0.231 | p = 0.234 | p ≤ 0.05 | p = 0.845 |
| Control | 36 ± 4 | 16 ± 1 | 77 ± 12 | 106 ± 8 | 32 ± 4 a | 330 ± 42 | 169 ± 16 | 7 ± 0 b | 773 ± 85 |
| 15 °C | 39 ± 2 | 18 ± 1 | 70 ± 4 | 107 ± 2 | 24 ± 1 ab | 368 ± 18 | 163 ± 3 | 8 ± 1 ab | 798 ± 25 |
| 20 °C | 41 ± 3 | 20 ± 2 | 57 ± 2 | 102 ± 2 | 21 ± 1 b | 360 ± 16 | 168 ± 6 | 9 ± 1 ab | 778 ± 21 |
| 25 °C | 42 ± 5 | 21 ± 7 | 67 ± 24 | 112 ± 11 | 29 ± 7 ab | 327 ± 20 | 156 ± 1 | 10 ± 0 a | 765 ± 37 |
| 30 °C | 38 ± 2 | 18 ± 1 | 79 ± 15 | 111 ± 8 | 23 ± 3 ab | 344 ± 25 | 157 ± 7 | 8 ± 1 ab | 775 ± 50 |
| 35 °C | 40 ± 3 | 19 ± 1 | 71 ± 4 | 108 ± 2 | 24 ± 1 ab | 360 ± 23 | 167 ± 5 | 9 ± 1 ab | 798 ± 24 |
| 40 °C | 40 ± 1 | 18 ± 1 | 76 ± 2 | 111 ± 1 | 24 ± 0 ab | 366 ± 9 | 165 ± 3 | 9 ± 0 ab | 809 ± 10 |
| Levantinka | p = 0.791 | p = 0.701 | p = 0.421 | p = 0.383 | p = 0.603 | p ≤ 0.05 | p ≤ 0.05 | p = 0.102 | p ≤ 0.01 |
| Control | 15 ± 1 | 5 ± 0 | 83 ± 8 | 83 ± 4 | 15 ± 2 | 69 ± 6 ab | 54 ± 5 ab | 5 ± 0 | 328 ± 20 ab |
| 15 °C | 16 ± 2 | 9 ± 4 | 60 ± 10 | 79 ± 3 | 11 ± 10 | 62 ± 12 ab | 57 ± 12 ab | 5 ± 1 | 299 ± 41 ab |
| 20 °C | 24 ± 14 | 12 ± 10 | 60 ± 26 | 82 ± 13 | 23 ± 9 | 83 ± 11 a | 69 ± 3 a | 5 ± 1 | 356 ± 10 a |
| 25 °C | 16 ± 13 | 10 ± 9 | 63 ± 27 | 72 ± 17 | 17 ± 11 | 52 ± 5 b | 38 ± 13 b | 4 ± 1 | 273 ± 27 b |
| 30 °C | 14 ± 1 | 5 ± 0 | 81 ± 4 | 85 ± 1 | 15 ± 1 | 70 ± 12 ab | 58 ± 3 ab | 4 ± 0 | 333 ± 20 ab |
| 35 °C | 27 ± 25 | 14 ± 4 | 54 ± 37 | 74 ± 17 | 22 ± 15 | 67 ± 10 ab | 57 ± 4 ab | 4 ± 0 | 318 ± 19 ab |
| 40 °C | 17 ± 1 | 7 ± 1 | 82 ± 10 | 91 ± 2 | 13 ± 3 | 73 ± 7 ab | 63 ± 8 a | 5 ± 0 | 351 ± 18 ab |
| Oblica | p ≤ 0.05 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 | p = 0.059 | p = 0.423 | p ≤ 0.001 | p ≤ 0.001 | p = 0.146 |
| Control | 23 ± 2 a | 12 ± 2 a | 56 ± 12 b | 61 ± 5 c | 21 ± 5 | 138 ± 22 | 106 ± 10 a | 12 ± 0 a | 429 ± 50 |
| 15 °C | 19 ± 1 a | 8 ± 1 abc | 57 ± 11 b | 63 ± 6 bc | 20 ± 1 | 123 ± 10 | 96 ± 3 ab | 12 ± 0 a | 397 ± 2 |
| 20 °C | 21 ± 1 a | 9 ± 0 abc | 62 ± 4 b | 62 ± 3 c | 22 ± 1 | 115 ± 5 | 103 ± 2 a | 10 ± 1 ab | 405 ± 6 |
| 25 °C | 17 ± 2 a | 8 ± 1 bc | 65 ± 3 b | 70 ± 1 b | 17 ± 3 | 129 ± 19 | 80 ± 8 ab | 10 ± 1 ab | 395 ± 25 |
| 30 °C | 10 ± 5 a | 6 ± 1 c | 114 ± 15 a | 97 ± 9 a | 18 ± 2 | 103 ± 4 | 74 ± 11 b | 9 ± 0 b | 430 ± 13 |
| 35 °C | 10 ± 5 a | 7 ± 1 c | 106 ± 19 ab | 96 ± 13 ab | 16 ± 2 | 90 ± 7 | 71 ± 6 b | 10 ± 0 b | 405 ± 25 |
| 40 °C | 21 ± 11 a | 10 ± 3 ab | 154 ± 14 a | 132 ± 12 a | 14 ± 5 | 119 ± 64 | 33 ± 20 c | 9 ± 1 b | 496 ± 99 |
| Sample | Tocopherols (mg/kg) | |
|---|---|---|
| α-Tocopherol * | γ-Tocopherol * | |
| Istarska Bjelica | p ≤ 0.05 | ** |
| Control | 75 ± 4 a | nd |
| 15 °C | 73 ± 6 a | nd |
| 20 °C | 83 ± 0 a | nd |
| 25 °C | 78 ± 4 a | nd |
| 30 °C | 76 ± 6 a | nd |
| 35 °C | 85 ± 5 a | nd |
| 40 °C | 82 ± 3 a | nd |
| Levantinka | p ≤ 0.001 | p = 0.433 |
| Control | 273 ± 6 a | 12 ± 1 |
| 15 °C | 237 ± 4 cd | 11 ± 1 |
| 20 °C | 232 ± 13 d | 11 ± 0 |
| 25 °C | 264 ± 9 ab | 11 ± 1 |
| 30 °C | 258 ± 8 abc | 11 ± 0 |
| 35 °C | 248 ± 8 bcd | 11 ± 0 |
| 40 °C | 232 ± 6 d | 11 ± 0 |
| Oblica | p ≤ 0.001 | p ≤ 0.001 |
| Control | 244 ± 7 b | 11 ± 0 a |
| 15 °C | 292 ± 3 a | 9 ± 0 ab |
| 20 °C | 213 ± 36 bc | 7 ± 1 cd |
| 25 °C | 221 ± 2 bc | 8 ± 0 bc |
| 30 °C | 211 ± 1 bc | 7 ± 1 cd |
| 35 °C | 185 ± 1 c | 6 ± 0 d |
| 40 °C | 187 ± 2 c | 6 ± 0 d |
| Variety | Parameters for Optimization | ||||||
|---|---|---|---|---|---|---|---|
| IP min | AC (% DPPH Red.) | Σ LOX mg/kg | Σ OX mg/kg | Σ MBA mg/kg | |||
| Model * | y = a + b × T + c × T2 + d × T3 | ||||||
| Istarska Bjelica * | a | 237.017 | 54.088 | 5.761 | −1.755 | −0.593 | |
| b | −3.128 | 0.484 | 1.549 | 0.264 | 0.059 | ||
| c | 0.065 | −0.009 | 0.029 | −0.004 | −0.001 | ||
| p-Value | Model | 0.035 | 0.362 | 0.210 | 0.146 | 0.040 | |
| Lack of fit | 0.056 | 0.338 | 0.001 | 0.007 | <0.0001 | ||
| R2 | 0.361 | 0.127 | 0.189 | 0.227 | 0.350 | ||
| Optimal temperature | 18.9 | ||||||
| Desirability | 0.481 | ||||||
| Predicted values | 201 | 60.80 | 24.56 | 1.51 | 0.07 | ||
| Levantinka * | a | 119.932 | 62.511 | 39.282 | 3.009 | −0.133 | |
| b | 2.705 | −1.096 | 0.249 | 0.004 | 0.012 | ||
| c | −0.047 | 0.021 | −0.005 | −2.98 × 10−5 | −1.54 × 10−4 | ||
| p-Value | Model | 0.641 | 0.107 | 0.787 | 0.976 | 0.027 | |
| Lack of fit | 0.209 | ≤0.0001 | 0.138 | 0.458 | 0.924 | ||
| R2 | 0.058 | 0.258 | 0.031 | 0.003 | 0.383 | ||
| Optimal temperature | 15.4 | ||||||
| Desirability | 0.518 | ||||||
| Predicted values | 150 | 50.61 | 41.75 | 3.07 | 0.01 | ||
| Oblica | a | 50.898 | 1.54 | 48.997 | 7.564 | −1.316 | |
| b | 10.48 | 7.245 | −1.191 | −0.141 | 0.179 | ||
| c | −0.489 | −0.276 | 0.015 | −0.001 | −0.007 | ||
| d | 0.006 | 0.003 | 0 ** | 0 ** | 7.99 × 10−5 | ||
| p-Value | Model | ≤0.0001 | ≤ 0.0001 | ≤0.0001 | ≤0.0001 | 0.001 | |
| Lack of fit | 0.513 | 0.001 | 0.110 | 0.764 | 0.001 | ||
| R2 | 0.908 | 0.864 | 0.772 | 0.736 | 0.659 | ||
| Optimal temperature | 15.5 | ||||||
| Desirability | 0.639 | ||||||
| Predicted values | 119 | 58.96 | 34.15 | 5.65 | 0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škevin, D.; Balbino, S.; Žanetić, M.; Jukić Špika, M.; Koprivnjak, O.; Filipan, K.; Obranović, M.; Žanetić, K.; Smajić, E.; Radić, M.; et al. Improvement of Oxidative Stability and Antioxidative Capacity of Virgin Olive Oil by Flash Thermal Pretreatment—Optimization Process. Foods 2025, 14, 2564. https://doi.org/10.3390/foods14152564
Škevin D, Balbino S, Žanetić M, Jukić Špika M, Koprivnjak O, Filipan K, Obranović M, Žanetić K, Smajić E, Radić M, et al. Improvement of Oxidative Stability and Antioxidative Capacity of Virgin Olive Oil by Flash Thermal Pretreatment—Optimization Process. Foods. 2025; 14(15):2564. https://doi.org/10.3390/foods14152564
Chicago/Turabian StyleŠkevin, Dubravka, Sandra Balbino, Mirella Žanetić, Maja Jukić Špika, Olivera Koprivnjak, Katarina Filipan, Marko Obranović, Karla Žanetić, Edina Smajić, Mateo Radić, and et al. 2025. "Improvement of Oxidative Stability and Antioxidative Capacity of Virgin Olive Oil by Flash Thermal Pretreatment—Optimization Process" Foods 14, no. 15: 2564. https://doi.org/10.3390/foods14152564
APA StyleŠkevin, D., Balbino, S., Žanetić, M., Jukić Špika, M., Koprivnjak, O., Filipan, K., Obranović, M., Žanetić, K., Smajić, E., Radić, M., Bunić, M., Dilber, M., & Kraljić, K. (2025). Improvement of Oxidative Stability and Antioxidative Capacity of Virgin Olive Oil by Flash Thermal Pretreatment—Optimization Process. Foods, 14(15), 2564. https://doi.org/10.3390/foods14152564










