Physicochemical, Nutritional, and Structural Characterization of a Novel Meat-Based Hummus
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Processing
2.2. Hummus Preparation
2.3. Product Evaluation
2.3.1. pH
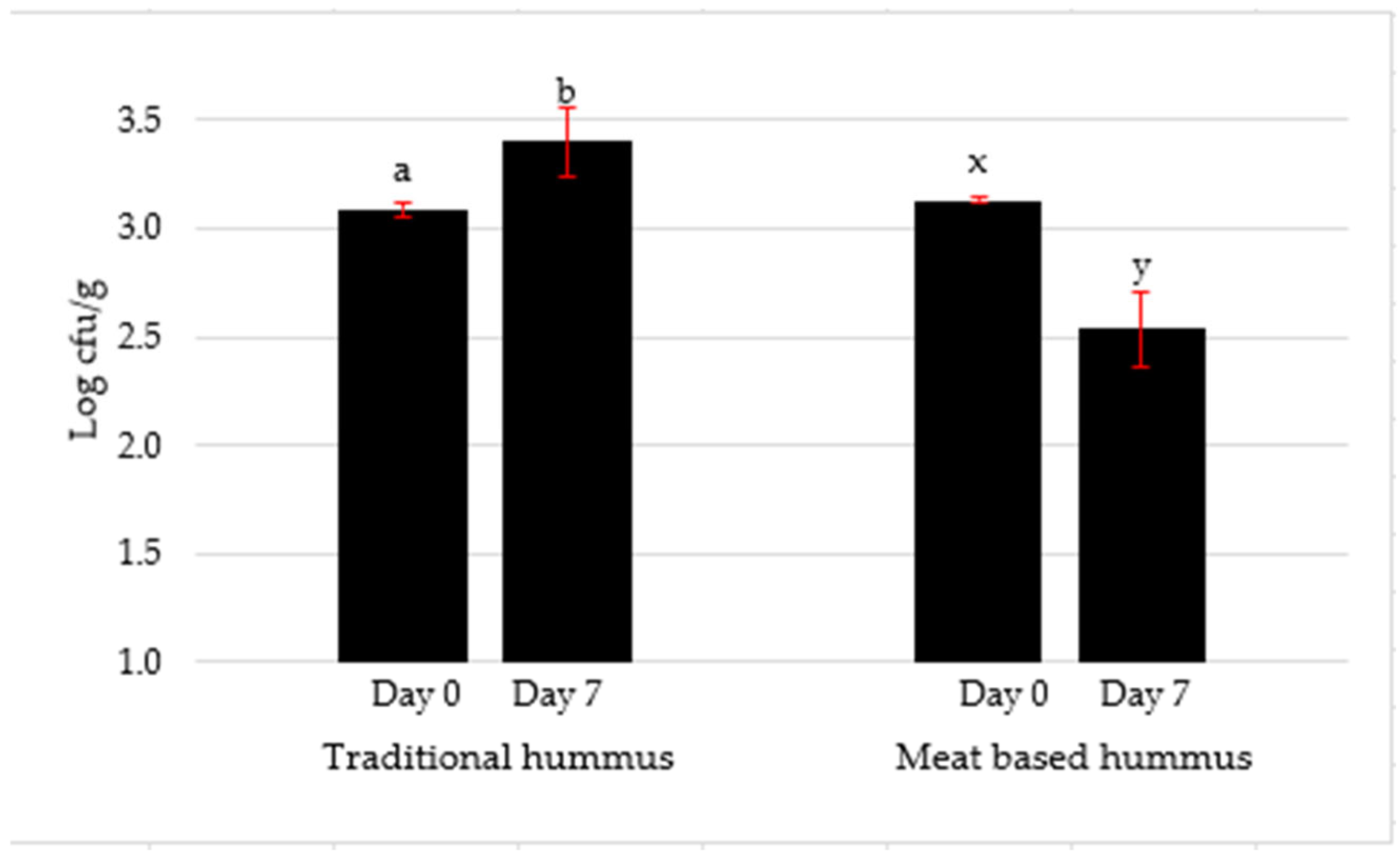
2.3.2. Total Plate Count
2.3.3. Instrumental Color Analysis
2.3.4. Nutritional Fact Panel
2.3.5. Scanning Electron Microscopy
2.3.6. Sensory Evaluation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Nutritional Fact Panel
3.3. Scanning Electron Microscopy
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, T.; Murray, R.; Zelman, K. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Chatziharalambous, D.; Sarris, D.; Gkatzionis, K.; Koutelidakis, A. Bioactive Compounds from Chickpea, Olive, and Grape By-Products for Human Health: A Systematic Review. Curr. Top. Nutraceutical Res. 2022, 20, 675–684. [Google Scholar] [CrossRef]
- Reister, E.J.; Belote, L.N.; Leidy, H.J. The Benefits of Including Hummus and Hummus Ingredients into the American Diet to Promote Diet Quality and Health: A Comprehensive Review. Nutrients 2020, 12, 3678. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.Y.; Ivens, K.O.; Nowatzke, W.L.; Robotham, J.; Samadpour, M.; Grace, T.; Oliver, K.G.; Garber, E.A.E. Extension of xMAP Food Allergen Detection Assay to Include Sesame. J. Food Prot. 2020, 83, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghian, S.; McClements, D.J.; Khalesi, M.; Garcia-Vaquero, M.; Mirzapour-Kouhdasht, A. Digestibility and bioavailability of plant-based proteins intended for use in meat analogues: A review. Trends Food Sci. Technol. 2022, 129, 646–656. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kamenju, P.; Madzorera, I.; Hertzmark, E.; Urassa, W.; Fawzi, W.W. Higher Dietary Intake of Animal Protein Foods in Pregnancy Is Associated with Lower Risk of Adverse Birth Outcomes. J. Nutr. 2022, 152, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Al-Nabulsi, A.A.; Taybeh, A.O.; Olaimat, A.N.; Taha, S.; Karam, L.; Ayyash, M.; Hasan, F.; Al Dabbas, M.M.; Bamigbade, G.B.; et al. Garlic and Chitosan Improve the Microbial Quality of Hummus and Reduce Lipid Oxidation. Foods 2024, 13, 4074. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, C.; Teng, X.M.; Jaroni, D.; Jadeja, R. Investigation of the antimicrobial mode of action of sodium acid sulfate and potassium acid sulfate. LWT 2021, 148, 111719. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Nabulsi, A.A.; Osaili, T.M.; Al-Holy, M.; Ghoush, M.A.; Alkhalidy, H.; Jaradat, Z.W.; Ayyash, M.; Holley, R.A. Inactivation of stressed Salmonella enterica, Escherichia coli O157:H7, and Listeria monocytogenes in hummus using low dose gamma irradiation. J. Food Sci. 2022, 87, 845–855. [Google Scholar] [CrossRef] [PubMed]
- CDC, Centers for Disease Control and Prevention (CDC). National Outbreak Reporting System (NORS). 2017. Available online: https://www.cdc.gov/nors/reports/index.html (accessed on 2 February 2025).
- EPA, U. Safer Chemical Ingredients List. 2021. Available online: https://www.epa.gov/saferchoice/safer-ingredients#pop7681381 (accessed on 2 February 2025).
- Dittoe, D.K.; Atchley, J.A.; Feye, K.M.; Lee, J.A.; Knueven, C.J.; Ricke, S.C. The efficacy of sodium bisulfate salt (SBS) alone and combined with peracetic acid (PAA) as an antimicrobial on whole chicken drumsticks artificially inoculated with Salmonella Enteritidis. Front Vet. Sci. 2019, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Slavik, M.F.; Walker, J.T.; Xiong, H. Pre-chill spray of chicken carcasses to reduce Salmonella typhimurium. J. Food Sci. 1997, 62, 605–607. [Google Scholar]
- Weerarathne, P.; Payne, J.; Saha, J.; Kountoupis, T.; Jadeja, R.; Jaroni, D. Evaluating the efficacy of sodium acid sulfate to reduce Escherichia coli O157:H7 and its biofilms on food-contact surfaces. LWT 2021, 139, 110501. [Google Scholar] [CrossRef]
- CIE. Recommendations on Uniform Color Spaces-Color Difference Equations, Psychometric Color Terms. In Supplement No. 2 to CIE Publication No. 15 (E-1.3.1.) 1978, 1971/(TC-1-3); Commission Internationale de l’Eclairage: Paris, France, 1976. [Google Scholar]
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.H.B.; Lindahl, G.; Mancini, R.A.; Nair, M.N.; et al. American Meat Science Association Guidelines for Meat Color Measurement. Meat Muscle Biol. 2023, 6, 1–81. [Google Scholar] [CrossRef]
- Stewart, M.; Zimmer, J. A High Fiber Cookie Made with Resistant Starch Type 4 Reduces Post-Prandial Glucose and Insulin Responses in Healthy Adults. Nutrients 2017, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Harper, T.; Clarke, A.D. Utilization of Psyllium Husk Powder as a Dietary Fiber Source for Improving the Quality of a Processed Turkey Product. Meat Muscle Biol. 2018, 2, 75. [Google Scholar] [CrossRef]
- Gourineni, V.; Stewart, M.L.; Wilcox, M.L.; Maki, K.C. Nutritional Bar with Potato-Based Resistant Starch Attenuated Post-Prandial Glucose and Insulin Response in Healthy Adults. Foods 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- American Meat Science Association. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat; American Meat Science Association: Kearney, MO, USA, 2016; Available online: https://meatscience.org/publications-resources/pri (accessed on 2 February 2025).
- Goswami, M.; Kumar, R.; Teng, X.; Jadeja, R.; Pfeiffer, M.; Mafi, G.; Pathak, V.; Ramanathan, R. Optimization of formulation and processing techniques for the development of meat-based hummus using response surface methodology. Abstr. Present. Am. Chem. Soc. 2024, 4098388. Available online: https://acs.digitellinc.com/p/s/optimization-of-formulation-and-processing-techniques-for-the-development-of-meat-based-hummus-using-response-surface-methodology-603655 (accessed on 2 February 2025).
- Denzer, M.L.; Mafi, G.G.; VanOverebeke, D.L.; Ramanathan, R. Effects of glucono delta-lactone enhancement and nitrite-embedded packaging on fresh color, cooked color, and sensory attributes of dark-cutting beef. Appl. Food Res. 2022, 2, 100189. [Google Scholar] [CrossRef]
- Fan, X.; Annous, B.A.; Keskinen, L.A.; Mattheis, J.P. Use of chemical sanitizers to reduce microbial populations and maintain quality of whole and fresh-cut cantaloupe. J. Food Prot. 2009, 72, 2453. [Google Scholar] [CrossRef] [PubMed]
- Scott-Bullard, B.R.; Geornaras, I.; Delmore, R.J.; Woerner, D.R.; Reagan, J.O.; Morgan, J.B.; Belk, K.E. Efficacy of a Blend of Sulfuric Acid and Sodium Sulfate against Shiga Toxin–Producing Escherichia coli, Salmonella, and Nonpathogenic Escherichia coli Biotype I on Inoculated Prerigor Beef Surface Tissue. J. Food Prot. 2017, 80, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Mao, B.; Beniwal, A.S.; Abhilasha; Kaur, R.; Chian, F.M.; Singh, J. Alternative proteins vs animal proteins: The influence of structure and processing on their gastro-small intestinal digestion. Trends Food Sci. Technol. 2022, 122, 275–286. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose–response meta-analysis of prospective cohort studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.M.; Babiker, E.E.; Eltinay, A.H. Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food Chem. 2004, 84, 207–212. [Google Scholar] [CrossRef]
- Singh, N. Functional and physicochemical properties of pulse starch. In Pulse Foods; Elsevier: Amsterdam, The Netherlands, 2021; pp. 87–112. [Google Scholar] [CrossRef]
- Ramanathan, R.; Mancini, R.A.; Konda, M.R.; Bailey, K.; More, S.; Mafi, G.G. Evaluating the failure to bloom in dark-cutting and lactate-enhanced beef longissimus steaks. Meat Sci. 2022, 184, 108684. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A. Food Protein Functionality—A New Model. J. Food Sci. 2015, 80, C2670–C2677. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic Amines in Meat and Meat Products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–599. [Google Scholar] [CrossRef] [PubMed]
- Harr, K.M.; Jewell, N.; Mafi, G.G.; Pfeiffer, M.M.; Ramanathan, R. Nontargeted Metabolomics to Understand the Impact of Modified Atmospheric Packaging on Metabolite Profiles of Cooked Normal-pH and Atypical Dark-Cutting Beef. Metabolites 2024, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiong, Y.L. Oxidation-initiated myosin subfragment cross-linking and structural instability differences between white and red muscle fiber types. J. Food Sci. 2015, 80, C288–C297. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (%) | Traditional Hummus (%) | Meat-Based Hummus (%) |
|---|---|---|
| Boiled chickpea paste | 70.0 | 35.0 |
| Steam-cooked ground lamb meat | 0.0 | 35.0 |
| Tahini paste (70% toasted sesame seeds + 30% olive oil) | 10.0 | 10.0 |
| Water | 6.0 | 6.0 |
| Olive oil | 7.0 | 7.0 |
| Salt | 1.0 | 1.0 |
| Fresh garlic paste | 1.0 | 1.0 |
| Fresh lemon juice | 5.0 | 4.6 |
| Sodium acid sulfate | 0.0 | 0.4 |
| Total | 100 | 100 |
| Parameter | Traditional Hummus | Meat-Based Hummus |
|---|---|---|
| pH | 5.65 ± 0.01 | 4.76 ± 0.01 |
| L* | 77.97 ± 0.31 | 63.80 ± 0.48 |
| a* | 10.06 ± 0.32 | 9.90 ± 0.05 |
| b* | 29.67 ± 0.07 | 22.88 ± 0.24 |
| Traditional Hummus | % Daily Value | Meat-Based Hummus | % Daily Value | |
|---|---|---|---|---|
| Calorie | 80 | 90 | ||
| Total fat | 5 g | 6 | 6 g | 8 |
| Saturated fat | 0.5 g | 3 | 1 g | 5 |
| Cholesterol | 0 mg | 0 | 10 mg | 3 |
| Sodium | 130 mg | 6 | 150 mg | 7 |
| Total carbohydrates | 7 g | 3 | 4 g | 1 |
| Dietary fiber | 2 g | 7 | 1 g | 4 |
| Protein | 3 g | 5 g | ||
| Calcium | 17 mg | 2 | 14 mg | 2 |
| Iron | 1 mg | 6 | 1 mg | 6 |
| Potassium | 68 mg | 2 | 77 mg | 2 |
| Traditional Hummus | Meat-Based Hummus | |
|---|---|---|
| Calorie | 100.00 | 100.00 |
| Total fat (g) | 6.25 | 6.66 |
| Saturated fat (g) | 0.63 | 1.11 |
| Cholesterol (mg) | 0.00 | 11.10 |
| Sodium (mg) | 162.50 | 166.50 |
| Total carbohydrates (g) | 8.75 | 4.44 |
| Dietary fiber (g) | 2.50 | 1.11 |
| Protein (g) | 3.75 | 5.55 |
| Calcium (mg) | 21.25 | 15.54 |
| Iron (mg) | 1.25 | 1.11 |
| Potassium g | 85.00 | 85.47 |
| Attribute | Traditional Hummus | Meat-Based Hummus |
|---|---|---|
| Color and appearance | 6.70 ± 0.31 | 6.82 ± 0.42 |
| Flavor | 6.23 ± 0.63 | 7.59 ± 0.34 |
| Creaminess | 6.81 ± 0.40 | 7.46 ± 0.32 |
| Grain properties | 5.63 ± 0.42 | 7.73 ± 0.37 |
| Mouth coating | 6.42 ± 0.34 | 7.76 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, M.; Kumar, R.; Teng, X.M.; Jadeja, R.; Scott, D.; Pfeiffer, M.; Mafi, G.G.; Pathak, V.; Ramanathan, R. Physicochemical, Nutritional, and Structural Characterization of a Novel Meat-Based Hummus. Foods 2025, 14, 2507. https://doi.org/10.3390/foods14142507
Goswami M, Kumar R, Teng XM, Jadeja R, Scott D, Pfeiffer M, Mafi GG, Pathak V, Ramanathan R. Physicochemical, Nutritional, and Structural Characterization of a Novel Meat-Based Hummus. Foods. 2025; 14(14):2507. https://doi.org/10.3390/foods14142507
Chicago/Turabian StyleGoswami, Meena, Rishav Kumar, Xin M. Teng, Ravi Jadeja, Darren Scott, Morgan Pfeiffer, Gretchen G. Mafi, Vikas Pathak, and Ranjith Ramanathan. 2025. "Physicochemical, Nutritional, and Structural Characterization of a Novel Meat-Based Hummus" Foods 14, no. 14: 2507. https://doi.org/10.3390/foods14142507
APA StyleGoswami, M., Kumar, R., Teng, X. M., Jadeja, R., Scott, D., Pfeiffer, M., Mafi, G. G., Pathak, V., & Ramanathan, R. (2025). Physicochemical, Nutritional, and Structural Characterization of a Novel Meat-Based Hummus. Foods, 14(14), 2507. https://doi.org/10.3390/foods14142507







