Unlocking the Sublime: A Review of Native Australian Citrus Species
Abstract
1. Introduction
1.1. Native Australian Flora and Foodplants
1.2. Citrus: Classification
| Subfamily | Tribe | Subtribe | Group | Genus |
|---|---|---|---|---|
| Aurantioideae | Clauseneae | Micromelinae | - | Micromelum |
| Clauseninae | - | Clausena Glycosmis Murraya | ||
| Merrilliinae | - | Merrillia | ||
| Citreae | Triphasiinae | - | Luvunga Merope Monanthocitrus Oxanthera Pamburus Paramignya Triphasia Wenzelia | |
| Balsamocitrinae | - | Aegle Aeglopsis Afraegle Balsamocitrus Feronia Feroniella Swinglea | ||
| Citrinae | Group A (‘Primitive Citrus) | Burkillanthus Hesperethusa Limnocitrus Pleiospermium Severinia | ||
| Group B (‘Near Citrus’) | Atalantia Citropsis | |||
| Group C (‘True Citrus’) | Citrus Clymenia Eremocitrus Fortunella Microcitrus Poncirus |
1.3. Commercial Citrus Production: A Global Overview
1.4. Commercial Citrus Production in Australia
1.5. Challenges to the Australian Citrus Industry: The Role of Native Citrus
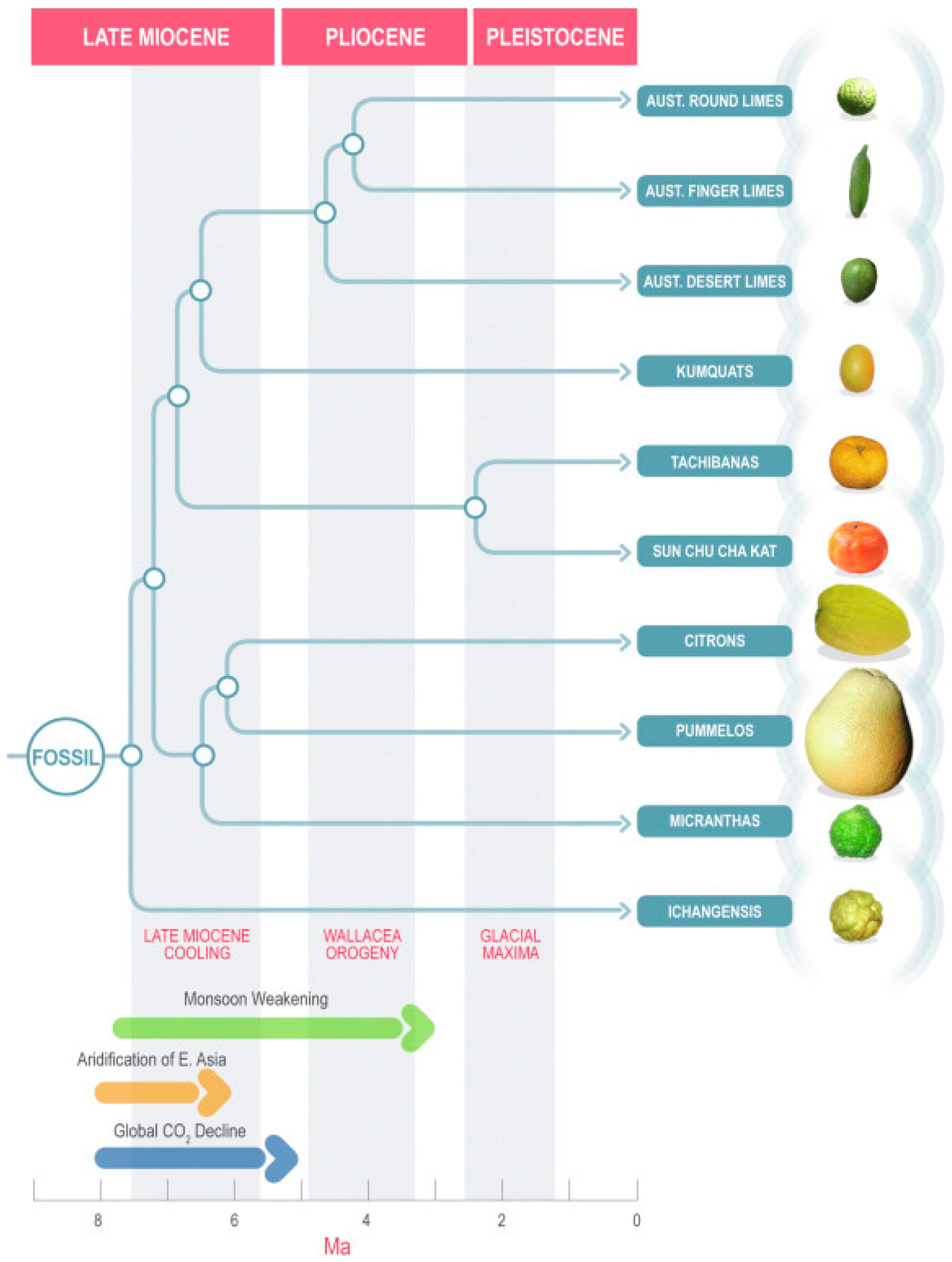
2. Introduction to Native Australian Citrus Species
3. Distribution
4. Description
5. Physiology
5.1. Frost and Cold Tolerance
5.2. Other Abiotic Stresses
5.3. Huanglongbing Resistance
5.4. Resistance to Psyllids and Other Insects
5.5. Citrus Canker Resistance
5.6. Resistance to Viroids and Viruses
5.7. Resistance to Fungi and Nematodes
6. Traditional and Contemporary Use
7. Taxonomy and Hybrids
7.1. C. australasica
7.2. C. australis
7.3. C. garrawayi
7.4. C. glauca
7.5. C. gracilis
7.6. C. inodora
7.7. Phylogeny
7.8. Genomic Characteristics
7.9. Hybrids
8. Flavour and Aroma
9. Pulp Composition of Commercial and Native Citrus
9.1. Nutritional Composition
| Species | C. australasica | C. australis | C. glauca | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variety/Details | Unspecified (DW) | var. australis (FW) | var. sanguinea (FW) | ‘Green’ (DW) | ‘Pink’ (DW) | Unspecified (FW) | Unspecified FW) | Frozen/ Fresh (FW) | Freeze-Dried (DW) |
| Moisture (%) | 78.5 ± 1.9 | 65.5 | 76.7 | - | - | 84.2 | 74.8, 75.4 | 56.5–78.1, 80.4 | - |
| Energy (kJ) | - | 411 | - | - | - | 176.6 | 277 | 198 | - |
| Protein (g) | - | 2.5 | - | - | - | 0.83 | 2.2 | 0.1 | - |
| Total fat (g) | - | 4.9 | 1.7 | - | - | 0.63 | BDL | 2.7 | - |
| Total saturated fatty acids (g) | - | - | - | - | - | - | 1.0 | - | |
| Carbohydrates (g) | - | 11.7 | 8.7 | - | - | 13.7 | 15.0 | 4.0 | - |
| Dietary fibre (g) | - | 14.0 | 12.6 | - | - | 6.7 | - | ||
| Sugar (g) | - | - | - | 4.0 | - | ||||
| Ash (g) | - | 0.7 | 0.7 | - | - | 0.57 | 0.8 | - | - |
| Sodium (mg) | 11.3 ± 0.1 | 9 | 3 | 11.1 | 8.7 | 4 | - | 2.2 | |
| Potassium (mg) | 669.7 ± 9.9 | 290 | 200 | 1459.6 | 1242.6 | 270 | - | 1287.8 | |
| Magnesium (mg) | 57.7 ± 0.5 | 31 | 15 | 139.5 | 111.1 | 24 | - | 94.5 | |
| Calcium (mg) | 139.0 ± 3.6 | 50 | 40 | 352.7 | 334.1 | 46 | - | 384.2 | |
| Iron (mg) | 1.24 ± 0.10 | 0.8 | 0.6 | 7.290 | 3.670 | 0.5 | - | 4.740 | |
| Zinc (mg) | 0.38 ± 0.10 | 0.3 | 0.2 | 0.848 | 0.780 | 0.1 | - | 1.060 | |
| Copper (mg) | 0.83 ± 0.05 | 0.4 | 0.3 | 0.715 | 1.31 | 0.2 | - | 0.641 | |
| Manganese (mg) | 0.26 ± 0.02 | - | - | 0.450 | 0.400 | - | - | 0.877 | |
| Phosphorus (mg) | 87.1 ± 2.4 | - | - | 166.9 | 141.7 | - | - | 127.8 | |
| Sulphur (mg) | 85.7 ± 7.8 | - | - | - | - | - | - | - | |
| Arsenic (mg) | 1.87 ± 0.69 | - | - | - | - | - | - | - | |
| Aluminium (mg) | 0.72 ± 0.17 | - | - | 0.405 | 0.644 | - | - | 3.875 | |
| Nickel (µg) | BDL | - | - | 34.9 | 56.3 | - | - | 48 | |
| Molybdenum (µg) | 130 ± 230 | - | - | 10.4 | 8.3 | - | - | 7.7 | |
| Cadmium (µg) | 170 ± 280 | - | - | 5 | 4 | - | - | 5.5 | |
| Lead (µg) | 140 ± 70 | - | - | 4 | 4 | - | - | 4 | |
| Cobalt (µg) | 150 ± 60 | - | - | 2 | 3 | - | - | 4 | |
| Chromium (µg) | 120 ± 80 | - | - | - | - | - | - | - | |
| Selenium (µg) | - | - | - | <1 | <1 | - | - | <1 | |
| References | [171,224] | [3] | [3] | [226] | [226] | [227] | [3,171] | [67,171,228] | [226] |
9.2. Minerals
9.3. Sugars and Organic Acids
| Species | Variety | Growing Location | Sucrose | Glucose | Fructose | Sorbitol | Reference |
|---|---|---|---|---|---|---|---|
| C. australasica | Pulp | ||||||
| XiangBin | Hainan, China | 2.25 ± 0.14 | 3.34 ± 0.26 | 4.39 ± 0.31 | 1.02 ± 0.08 | [251] | |
| LiSiKe | Hainan, China | 2.77 ± 0.20 | 3.15 ± 0.18 | 4.16 ± 0.28 | 1.66 ± 0.12 | [251] | |
| Unspecified | Victoria, Australia | ND | 1.6–2.6 # | 2.4–5.0 # | - | [227] | |
| ‘Red pulp’ (sanguinea type) 1 | Florida, USA | 9.65 (mg/mL) | 8.48 (mg/mL) | 10.10 (mg/mL) | - | [252] | |
| ‘White pulp’ 1 | Florida, USA | 7.54 (mg/mL) | 4.37 (mg/mL) | 4.22 (mg/mL) | - | [252] | |
| ‘Low-seeded, red pulp, large-leaved’ hybrid 1 | Florida, USA | 2.28 (mg/mL) | 0.73 (mg/mL) | 2.23 (mg/mL) | - | [252] | |
| Sanguinea type 50–36 cultivar 1 | Florida, USA | 9.90 (mg/mL) | 9.85 (mg/mL) | 8.46 (mg/mL) | - | [252] | |
| Peel | |||||||
| XiangBin | Hainan, China | 0.68 ± 0.06 | 2.08 ± 0.16 | 2.56 ± 0.22 | 0.59 ± 0.04 | [251] | |
| LiSiKe | Hainan, China | 2.27 ± 0.10 | 0.71 ± 0.13 | 0.89 ± 0.19 | 0.96 ± 0.04 | [251] | |
| Pulp | |||||||
| C. australis | Unspecified | Australia | 2.4 | 4.2 | 4.3 | - | [166] |
| C. × aurantiifolia | Unspecified | Australia | 5.2 | 7.4 | 5.8 | - | [166] |
| Species | Variety | Growing Location | Malic Acid | Citric Acid | Quinic Acid | Succinic Acid | Reference |
|---|---|---|---|---|---|---|---|
| C. australasica | Pulp | ||||||
| XiangBin | Hainan, China | 4.08 ± 0.27 | 73.49 ± 4.10 | 0.58 ± 0.05 | - | [251] | |
| LiSiKe | Hainan, China | 4.80 ± 0.34 | 71.50 ± 6.47 | 1.13 ± 0.10 | [251] | ||
| ‘Red pulp’ (sanguinea type) | Florida, USA | 5.0 ± 1.1 | 36.2 ± 3.6 | - | - | [252] | |
| ‘White pulp’ | Florida, USA | 38.0 ± 0.8 | 37.3 ± 4.2 | - | - | [252] | |
| ‘Low-seeded, red pulp, large-leaved’ hybrid | Florida, USA | 10.0 ± 3.8 | 14.6 ± 1.9 | - | - | [252] | |
| Sanguinea type 50–36 cultivar | Florida, USA | 15.0 ± 3.2 | 38.8 ± 3.3 | - | - | [252] | |
| ‘Green’ | QLD, Australia | BDL | 46.8 ± 0.5 | - | - | [253] | |
| ‘Pink’ | QLD, Australia | BDL | 58.8 ± 1.7 | - | - | [253] | |
| Unspecified | Australia | - | - | - | - | [171] | |
| Peel | |||||||
| XiangBin | Hainan, China | 2.23 ± 0.17 | 20.75 ± 1.57 | 4.39 ± 0.34 | - | [251] | |
| LiSiKe | Hainan, China | 1.35 ± 0.05 | 8.26 ± 0.22 | 5.48 ± 0.22 | - | [251] | |
| Unspecified | Valencia, Spain | - | 8.11 ± 0.18 ^ | - | - | [254] | |
| Pulp | |||||||
| C. australis | Unspecified | Australia | 12.0 | 50.3 | - | 2.2 | [166] |
| C. glauca | Unspecified | QLD, Australia | 25.2 ± 0.5 | 4.61 ± 0.19 | - | - | [253] |
| C. × aurantiifolia | Unspecified | Australia | 18.6 | 41.4 | - | 1.1 | [166] |
9.4. Pectin and Other Carbohydrates
9.5. Vitamins
9.5.1. Commercial Citrus
| Citrus Variety | Origin | α-Carotene | β-Carotene | β-Cryptoxanthin | Lutein + Zeaxanthin | Reference |
|---|---|---|---|---|---|---|
| Grapefruit, pink and red | USA | 5 | 603 | 12 | 13 | [269] |
| Grapefruit, red | Spain | - | 570 ± 20 | 10 ± 10 | 30 ± 30 | [276] |
| Grapefruit, white | USA | 8 | 14 | - | - | [269] |
| Grapefruit, white | Spain | - | ND | ND | ND | [276] |
| Mandarin (tangerine) | USA | 14 | 71 | 485 | 243 | [269] |
| Mandarin | Spain | - | 0–240 | 310–1830 | 40–90 | [276] |
| Orange, blood | USA | ND | 120 | 69 | - | [269] |
| Orange, blood | Spain | - | 40 ± 10 | 30 ± 10 | 60 ± 10 | [276] |
| Orange, blood | Spain | - | 17 | 21 | Trace | [277] |
| Orange | USA | 16 | 51 | 122 | 187 | [269] |
| Orange | Spain | - | ND | 60 ± 20 | 70 ± 20 | [276] |
| Orange | Spain | - | ND | 57 | 8 | [277] |
| Pumelo, pink | USA | 14 | 320 | 103 | 0 | [269] |
| Pumelo | USA | ND | ND | 10 | - | [269] |
9.5.2. Native Australian Citrus
9.6. Anti-Nutrients
| Analyte | C. australasica | C. australis | C. garrawayi | C. glauca |
|---|---|---|---|---|
| Oxalic acid | 0.11 ± 0.04, 0.8, 2.0 | 0.09 | 0.13 | <0.8, 1.04 ± 0.10, 1.7 |
| Cyanogens | BDL (<0.1) | BDL (<0.1) | BDL (<0.1) | BDL (<0.1) |
| Alkaloids | +ve (>0.04) | Strongly +ve | Slight +ve (>0.04) | Sometimes +ve (>0.04) |
| Saponins | +ve (in 1 of 4 samples) | BDL | BDL | +ve, BDL |
| Safrole | BDL | - | - | - |
| Cardiac glycosides | - | - | - | Slight +ve |
| References | [171,253] | [171] | [171] | [171,253,286] |
9.7. Phytochemical Composition
9.7.1. Commercial Citrus
9.7.2. Native Australian Citrus
9.8. Volatiles
9.9. Bioactive Properties
10. Peel Composition
10.1. Vitamins
10.2. Pectin and Anti-Nutrients
10.3. Phytochemical Composition
10.4. Volatiles
10.4.1. Commercial Citrus Species
10.4.2. Native Australian Citrus Species
| Variety | Most Abundant Compound | Second-Most Abundant Compound | Third-Most Abundant Compound | Reference |
|---|---|---|---|---|
| Alstonville | d-limonene (61.7%) | sabinene (20.6%) | oxypeucedanin (5.7%) | [352] |
| Chartreuse | d-limonene (61.4%) | β-citronellol (6.7%) | citronellal (6.5%) | [284] |
| Collette # | d-limonene (42.4%) | γ-terpinene (14.2%) | terpinen-4-ol (8.4%) | [319] |
| Durham’s Emerald | d-limonene (66.3%) | citronellal (9.3%) | citronellol (5.2%) | [352] |
| Durham’s Emerald | d-limonene (73.8%) | β-citronellol (5.8%) | citronellal (5.6%) | [284] |
| Hybrid ‘P1f2-10’ | d-limonene (83.7%) | bicyclogermacrene (2.9%) | γ-terpinene (2.5%) | [284] |
| Judy’s Everbearing | d-limonene (64.4%) | citronellal (9.0%) | isomenthone (7.3%) | [352] |
| Pink Ice # | d-limonene (37.7%) | sabinene (33.3%) | α-pinene (5.6%) | [331] |
| Pink Ice # | Terpinen-4-ol (38.3%) | limonene (26.5%) | γ-terpinene (7.3%) | [319] |
| Pink Pearl # | d-limonene (63.2%) | sabinene (9.5%) | bicyclogermacrene (7.2%) | [332] |
| ‘Red’ # | d-limonene (73.6%) | bicyclogermacrene (6.9%) | β-bisabolene (2.0%) | [319] |
| Red Champagne | d-limonene (87.5%) | bicyclogermacrene (4.1%) | β-myrcene (2.2%) | [284] |
| Rhyne Red | d-limonene (65.0%) | γ-terpinene (16.8%) | citronellal (2.4%) | [284] |
| Unspecified | d-limonene (6.9%) 1 | α-pinene (5.5%) | furfural (4.3%) | [224] |
| Unspecified | d-limonene (51.6%) | isomenthone (9.7%) | linalool (7.5%) | [330] |
| Unspecified | d-limonene (73.5%) | isomenthone (7.5%) | citronellal (2.6%) | [354] |
| Unspecified # | d-limonene (51.1%) | sabinene (19.6%) | β-pinene (7.9%) | [359] |
| Unspecified # | d-limonene (24.5–38.9%) | citronellal (7.2–23.7%) 2 | β-phellandrene (13.2–18.2%) 2 | [357] |
| Unspecified # | d-limonene (62.6%) | β-pinene (32.4%) | α-pinene (1.5%) | [333] |
| var. sanguinea # | bicyclogermacrene (25.9%) | α-pinene (10.2%) | spathulenol (9.8%) | [360] |
| var. sanguinea # | d-limonene (65.7%) | γ-terpinene (8.8%) | bicyclogermacrene (7.0%) | [332] |
| var. sanguinea # | d-limonene (48.2%) | sabinene (37.2%) | α-pinene (4.3%) | [331] |
| Yellow Sunshine # | d-limonene (40.0%) | bicyclogermacrene (39.8%) | globulol (2.9%) | [319] |
| C. australasica × C. inodora (‘Minnie finger lime’) | d-limonene (82.4%) | β-myrcene (6.5%) | α-pinene (2.1%) | [361] |
| Faustrime (C. australasica × C. × aurantiifolia) # | d-limonene (43.2%) | citronellal (16.3%) | γ-terpinene (11.8%) | [362] |
| Faustrime (C. australasica × C. × aurantiifolia) # | citronellal (22.2%) | β-phellandrene (17.7%) | limonene (17.2%) | [363] |
| Faustrime (C. australasica × C. × aurantiifolia) # | d-limonene (31.5%) | γ-terpinene (11.6%) | citronellal (9.4%) | [319] |
| Faustrime (C. australasica × C. × aurantiifolia) # | d-limonene (29.3%) | β-phellandrene (21.1%) | γ-terpinene (9.5%) | [332] |
| Faustrime (C. australasica × C. × aurantiifolia) # | d-limonene (27.8%) | citronellal (10.5%) | γ-terpinene (10.0%) | [331] |
| Faustrime (C. australasica × C. × aurantiifolia) # | citronellal (23.5%) | d-limonene (13.0%) | citronellol (10.7%) | [334] |
| Citrus australis | ||||
| Citrus australis | d-limonene (38.2%) | β-pinene (14.3%) | γ-terpinene (12.2%) | [166] |
| Citrus australis | d-limonene (35.1%) | β-pinene (13.1%) | γ-terpinene (11.2%) | [358] |
| Commercial Tahitian Lime (Citrus × latifolia) | d-limonene (40.3%) | γ-terpinene (13.4%) | β-pinene (10.9%) | [284] |
| Compound. | C. australis (%) | C. australis (%) | C. × aurantiifolia (%) | Odour Description 1 |
|---|---|---|---|---|
| α-thujene | <0.5 | 0.2 | <0.5 | Sweet, rose, spicy |
| α-pinene | 1.41 | 1.3 | 1.45 | Lemon |
| sabinene | 2.45 | 2.2 | 1.41 | Off-lemon, faint lemon |
| β-pinene | 14.28 | 13.1 | 8.4 | Lime |
| β-myrcene | 0.99 | 1.0 | 0.97 | - |
| α-terpinene | <0.5 | 0.1 | <0.5 | Rose, lemon |
| p-cymene | BDL | 0.1 | BDL | - |
| d-limonene | 38.22 | 35.1 | 31.65 | Grassy, leafy |
| 1,8-cineole | BDL | BDL | <0.5 | Menthol |
| (E)-β-ocimene | <0.5 | 0.2 | BDL | - |
| γ-terpinene | 12.17 | 11.2 | 20.4 | Menthol |
| terpinolene | <0.5 | 0.4 | 0.87 | Plastic |
| linalool | >0.5 | 0.3 | <0.5 | Lime |
| isoborneol | BDL | BDL | <0.5 | Floral |
| α-terpineol | <0.5 | 0.7 | <0.5 | Lemon orange, rose, lemongrass |
| decanal | <0.5 | 0.8 | <0.5 | - |
| neral | 4.91 | 4.5 | 4.02 | - |
| geranial | 7.93 | 7.3 | 6.31 | Faint orange |
| δ-elemene | 1.25 | 1.2 | <0.5 | Faint lemongrass |
| neryl acetate | <0.5 | 0.1 | 2.3 | - |
| geranyl acetate | <0.5 | 0.4 | <0.5 | Grassy |
| β-elemene | <0.5 | 0.7 | <0.5 | - |
| Unidentified | <0.5 | 0.3 | BDL | - |
| (E)-caryophyllene | <0.5 | 1.0 | <0.5 | - |
| γ-elemene | <0.5 | 0.2 | <0.5 | - |
| α-trans bergamotene | 1.76 | 1.6 | 1.84 | - |
| germacrene D | <0.5 | 0.5 | <0.5 | - |
| α-garnesene (E,E) | 3.57 | 3.3 | BDL | - |
| (Z)-α-bisabolene | BDL | 0.1 | <0.5 | Faint lemon |
| unidentified | BDL | BDL | <0.5 | - |
| β-bisabolene | 3.04 | 2.8 | 2.99 | - |
| germacrene B | <0.5 | 0.9 | <0.5 | - |
| 7-methoxy coumarin | <0.5 | 1.1 | 3.48 | - |
| unidentified | BDL | BDL | <0.5 | - |
| 5,7-dimethoxy coumarin | 3.66 | 3.4 | 7.08 | - |
| iso-bergaptene | <0.5 | 0.2 | 3.04 | - |
| bergaptene | <0.5 | 0.4 | <0.5 | - |
| isopimpinellin | 3.78 | 3.5 | 1.94 | - |
| Reference | [166] | [358] | [166] | [166] |
10.5. Bioactive Components
11. Leaf Composition
11.1. Phytochemical Composition
11.2. Volatiles
12. Seed Composition
13. Commercial Production
13.1. C. australasica
13.2. C. australis
13.3. C. garrawayi
13.4. C. glauca
- (1)
- Ease of growing;
- (2)
- Tolerant of frost and heat, so can be grown over a wide climatic area. Cultivated trees have reportedly been grown in all Australian states, including Tasmania [42];
- (3)
- Appealing flavour;
- (4)
- Retains its structure and flavour when frozen;
- (5)
- The fruit is ‘nonbrowning’, unlike most commercial citrus [389];
- (6)
- Wide range of uses. These include puree for food processing, a garnish for fish and chicken, as an ingredient in syrup, jams, curds, chutney, aioli, apple sauce, relish, paste, cordial, cider/liqueur, slush drinks, candied peel/glacé fruit ice cream, and yoghurt. It can also be dried into a powder to use in herb and spice mixtures and yoghurt;
- (7)
- (8)
- The market demand for seasonal fresh fruit;
- (9)
- The year-round supply of fruit possible in dried or frozen form.
- (1)
- The lack of consumer awareness, which can limit the potential market;
- (2)
- The potential for market oversupply (due to a lack of existing demand);
- (3)
- Agronomy is relatively unknown and must be carefully managed;
- (4)
- Labour-intensive, requires further innovation in mechanising processes;
- (5)
- High cost for frozen storage;
- (6)
- Yield can fluctuate depending on the year.
13.5. C. gracilis
13.6. C. inodora
14. Future Research and Directions
14.1. Uses of Australian Citrus
- (1)
- Drought tolerance (or high rainfall tolerance);
- (2)
- Heat tolerance;
- (3)
- Cold tolerance;
- (4)
- Dwarfing;
- (5)
- Short flowering-to-fruiting periods;
- (6)
- Resistance to Phytophthora root rot;
- (7)
- Tolerance to low soil fertility;
- (8)
- Nematode resistance;
- (9)
- Increased levels of potentially beneficial phytochemicals, such as flavonoids.
14.2. Ecology and Morphology
14.3. Nutritional Value and Chemical Composition
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Australian National Herbarium. Australian Flora Statistics. Available online: https://www.anbg.gov.au/aust-veg/australian-flora-statistics.html (accessed on 10 March 2025).
- Nazzaro, C.; Uliano, A.; Lerro, M.; Stanco, M. From Claims to Choices: How Health Information Shapes Consumer Decisions in the Functional Food Market. Foods 2025, 14, 699. [Google Scholar] [CrossRef] [PubMed]
- Brand Miller, J.; James, K.W.; Maggiore, P.M. Tables of Composition of Australian Aboriginal Foods; Aboriginal Studies Press: Canberra, Australia, 1993. [Google Scholar]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits—A novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Wahlqvist, M.L. Koori Nutrition and Health: A Victorian Review; National Better Health Program (Victoria): Melbourne, Australia, 1992. [Google Scholar]
- Kubitzki, K.; Kallunki, J.A.; Duretto, M.; Wilson, P.G. Rutaceae. In Flowering Plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 276–356. [Google Scholar]
- Morton, C.M.; Grant, M.; Blackmore, S. Phylogenetic relationships of the Aurantioideae inferred from chloroplast DNA sequence data. Am. J. Bot. 2003, 90, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Swingle, W.T. Botany of Citrus and Its Wild Relatives of the Orange Subfamily (Family Rutaceae, Subfamily Aurantioideae); University of California Press: Oakland, CA, USA, 1943. [Google Scholar]
- Mabberley, D.J. A classification for edible Citrus (Rutaceae). Telopea 1997, 7, 167–172. [Google Scholar] [CrossRef]
- Tanaka, T. Species Problem in Citrus; Japanese Society for the Promotion of Science: Tokyo, Japan, 1954. [Google Scholar]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Penjor, T.; Yamamoto, M.; Uehara, M.; Ide, M.; Matsumoto, N.; Matsumoto, R.; Nagano, Y. Phylogenetic Relationships of Citrus and Its Relatives Based on matK Gene Sequences. PLoS ONE 2013, 8, e62574. [Google Scholar] [CrossRef]
- Goh, R.M.V.; Pua, A.; Luro, F.; Ee, K.H.; Huang, Y.; Marchi, E.; Liu, S.Q.; Lassabliere, B.; Yu, B. Distinguishing citrus varieties based on genetic and compositional analyses. PLoS ONE 2022, 17, e0267007. [Google Scholar] [CrossRef]
- Zhang, D.; Mabberley, D.J. Citrus. Flora China 2008, 11, 90–96. [Google Scholar]
- Swingle, W.T. Microcitrus, a new genus of Australian citrous fruits. J. Wash. Acad. Sci. 1915, 5, 569–578. [Google Scholar]
- Mabberley, D.J. Australian Citreae with notes on other Aurantioideae (Rutaceae). Telopea 1998, 7, 333–344. [Google Scholar] [CrossRef]
- Borredá Fernández, C. A Genomic Approach to the Evolution, Diversification and Domestication of the Genus Citrus. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2021. [Google Scholar]
- Luro, F.; Curk, F.; Froelicher, Y.; Ollitrault, P. Recent insights on Citrus diversity and phylogeny. In Agrumed. Archaeology and History of Citrus Fruit in the Mediterranean: Acclimatization, Diversifications, Uses; Publications du Centre Jean Bérard: Naples, Italy, 2017; pp. 16–28. [Google Scholar]
- Bayer, R.J.; Mabberley, D.J.; Morton, C.; Miller, C.H.; Sharma, I.K.; Pfeil, B.E.; Rich, S.; Hitchcock, R.; Sykes, S. A molecular phylogeny of the orange subfamily (Rutaceae: Aurantioideae) using nine cpDNA sequences. Am. J. Bot. 2009, 96, 668–685. [Google Scholar] [CrossRef] [PubMed]
- Talon, M.; Wu, G.A.; Gmitter, F.G.; Rokhsar, D.S. Chapter 2—The origin of citrus. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 9–31. [Google Scholar]
- Chen, Y.; Barzee, T.J.; Zhang, R.; Pan, Z. Chapter 9—Citrus. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 217–242. [Google Scholar]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- FAO. International Treaty on Plant Genetic Resources for Food and Agriculture; Food and Agricultural Organisation of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef] [PubMed]
- Rampersaud, G.C. A Comparison of Nutrient Density Scores for 100% Fruit Juices. J. Food Sci. 2007, 72, S261–S266. [Google Scholar] [CrossRef]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- FAOSTAT. Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 May 2024).
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Hort Innovation. Australian Horticulture Statistics Handbook 2021/22; Horticulture Innovation Australia Limited: Sydney, Australia, 2023. [Google Scholar]
- Hogan, L.; Duver, A.; Qin, S.; Eather, J. Australia’s Biosecurity Market Access and Agricultural Exports: Case Study of Citrus Exports to ASEAN Countries; ABARES, Australian Government: Canberra, Australia, 2022.
- Wang, W.; de Silva, D.D.; Moslemi, A.; Edwards, J.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Colletotrichum Species Causing Anthracnose of Citrus in Australia. J. Fungi 2021, 7, 47. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Vincent, C.I.; Urbaneja, A.; Machado, M.A. Editorial: Unravelling Citrus Huanglongbing Disease. Front. Plant Sci. 2020, 11, 609655. [Google Scholar] [CrossRef]
- Wang, N. The Citrus Huanglongbing Crisis and Potential Solutions. Mol. Plant 2019, 12, 607–609. [Google Scholar] [CrossRef]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-Term Field Evaluation Reveals Huanglongbing Resistance in Citrus Relatives. Plant Dis. 2016, 100, 1858–1869. [Google Scholar] [CrossRef]
- Vincent, C.; Morillon, R.; Arbona, V.; Gómez-Cadenas, A. Chapter 13—Citrus in changing environments. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 271–289. [Google Scholar]
- Canning, A.D. Rediscovering wild food to diversify production across Australia’s agricultural landscapes. Front. Sustain. Food Syst. 2022, 6, 865580. [Google Scholar] [CrossRef]
- Delort, E.; Yuan, Y.-M. Finger lime/The Australian Caviar—Citrus australasica. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 203–210. [Google Scholar]
- Shaw, P.E.; Moshonas, M.G.; Bowman, K.D. Volatile constituents in juice and oil of Australian wild lime (Microcitrus inodora). Phytochemistry 2000, 53, 1083–1086. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, M.; Wang, N.; Xu, Q.; Deng, X.; Chai, L. Reproduction in woody perennial Citrus: An update on nucellar embryony and self-incompatibility. Plant Reprod. 2018, 31, 43–57. [Google Scholar] [CrossRef]
- Mabberley, D. Robert Mudie (1777–1842) and Australian botany, or The saga of the Black Bean. Aust. Syst. Bot. Soc. Newsl. 1992, 70, 13–15. [Google Scholar]
- Mueller, F. Citrus Australasica. Fragm. Phytographiae Aust. 1858, 1, 26. [Google Scholar]
- Douglas, J. Cultivation of Desert Limes (Citrus glauca). In Australian Native Plants: Cultivation and Uses in the Health and Food Industries; Sultanbawa, Y., Sultanbawa, F., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 69–80. [Google Scholar]
- Mabberley, D. Citrus reunited. Aust. Plants 2000, 21, 52–55. [Google Scholar]
- Fensham, R.; Bean, A.; Dowe, J.; Dunlop, C. This disastrous event staggered me: Reconstructing the botany of Ludwig Leichhardt on the expedition from Moreton Bay to Port Essington, 1844–1845. Cunninghamia 2006, 9, 451–506. [Google Scholar]
- Henderson, R. Names and Distribution of Queensland Plants, Algae, and Lichens; Queensland Herbarium, Environmental Protection Agency: Brisbane, Australia, 2002.
- Hitchcock, L.F.; Jones, T.G.H. Essential oil from the Queensland flora—Part Vl. Eremocitrus glauca. Proc. Roy. Soc. Qld 1936, 47, 85–88. [Google Scholar] [CrossRef]
- Bradley, M.; House, A.; Robertson, M.; Wild, C. Vegetation succession and recovery of ecological values in the southern Queensland Brigalow Belt. Ecol. Manag. Restor. 2010, 11, 113–118. [Google Scholar] [CrossRef]
- Cope, R.; Ossedryver, S. Chapter 63—Poisonous Plants of Australia and New Zealand. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 911–934. [Google Scholar]
- Cribb, A.B.; Cribb, J.W. Wild food in Australia, 2nd ed.; Angus & Robertson: North Ryde, Australia, 1990. [Google Scholar]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. The Leaf Oils of the Australian Species of Citrus (Rutaceae). J. Essent. Oil Res. 2001, 13, 264–268. [Google Scholar] [CrossRef]
- Hearn, J.C.; Hutchison, D.J.; Barrett, H.C. Breeding Citrus Rootstocks. HortScience 1974, 9, 357–358. [Google Scholar] [CrossRef]
- White, C.T. An Australian Citrus Relative: Notes on the Russel River Lime. J. Hered. 1922, 13, 119–121. [Google Scholar] [CrossRef]
- Holmes, J.; Bisa, D.; Hill, A.; Crase, B. A Guide to Threatened, Near Threatened and Data Deficient Plants in the Litchfield Shire of the Northern Territory; WWF-Australia: Ultimo, Australia, 2005. [Google Scholar]
- Guilfoyle, W.R. Australian Plants Suitable for Gardens, Parks, Timber Reserves, etc.; Whitcombe and Tombs Limited: Melbourne, Australia, 1911. [Google Scholar]
- Saalfeld, M. Home Citrus Growers. Available online: http://www.homecitrusgrowers.co.uk/ (accessed on 1 August 2024).
- Bowman, K.D. Segregation for Double Spine Trait in Hybrids of Microcitrus inodora. HortScience 1998, 33, 473. [Google Scholar] [CrossRef]
- Swingle, W.T.; Reece, P.; Reuther, W.; Webber, H.; Batchelor, L. The citrus industry. In The Citrus Industry. Vol. 1. History, World Distribution, Botany and Varieties; Reuther, W., Webber, H., Batchelor, L., Eds.; University of California, Berkeley, Division of Agricultural Sciences: Berkeley, CA, USA, 1967; Volume 1, pp. 190–430. [Google Scholar]
- Forster, P.I.; Smith, M.W. Citrus wakonai P.I.Forst. & M.W.Sm. (Rutaceae), a new species from Goodenough Island, Papua New Guinea. Austrobaileya 2010, 8, 133–138. [Google Scholar]
- Bailey, F.M. Contributions to the flora of Queensland. Qld. Agric. J. 1904, 15, 491–495. [Google Scholar]
- Aleza, P. Caracterización de dos especies ornamentales pertenecientes a la subfamilia de los Aurantioideas. Murraya paniculata (L). Jack y Microcitrus inodora. Levante Agrícola 1998, 344, 271–283. [Google Scholar]
- Clarke, K.; Prakash, N. Floral morphology and embryology of two Australian species of Citrus (Rutaceae). Aust. J. Bot. 2001, 49, 199–207. [Google Scholar] [CrossRef]
- Swingle, W.T.; Reece, P. The botany of Citrus and its wild relatives. In The Citrus Industry. Vol. 1. History, World Distribution, Botany and Varieties, 2nd ed.; Reuther, W., Webber, H., Batchelor, L., Eds.; University of California, Berkeley, Division of Agricultural Sciences: Berkeley, CA, USA, 1967; Volume 1, pp. 190–430. [Google Scholar]
- Swingle, W.T. Clymenia and Burkillanthus, new genera also three new species of Pleiospermium (Rutaceae—Aurantioideae). J. Arnold Arbor. 1939, 20, 250–263. [Google Scholar] [CrossRef]
- Morrison, W.F. The Aldine History of Queensland: Embracing Sketches and Portraits of Her Noted People; the Rise and Progress of Her Varied Enterprises; and Illustrations of Her Boundless Wealth, Together with Maps of Latest Survey; Aldine Publishing Company: London, UK, 1888; Volume 5262. [Google Scholar]
- Cooper, W.; Cooper, T. Fruits of the Australian Tropical Rainforest; Nokomis Editions: Melbourne, Australia, 2004. [Google Scholar]
- Ramadugu, C.R.C.; Razi, M.; Keremane, M.; Scora, R.; Roose, M. Systematic Classification, Distribution and Botany; CABI International: Wallingford, UK, 2017; pp. 12–36. [Google Scholar]
- Carey, D.; Deuter, P.; Zull, A.; Taylor, H.; White, N. High Value Horticulture Value Chains for the Queensland Murray-Darling Basin Project: Activity 1—Assessing Horticulture Crop Suitability for the Queensland Murray Darling Basin Study Area report; Queensland Government Department of Agriculture and Fisheries: Brisbane, Australia, 2017.
- Chapman, M.; Nelson, P.; Nicholson, H.; Dunphy, M.; McAlpin, S. Australian Rainforest Seeds: A Guide to Collecting, Processing and Propagation; Csiro publishing: Melbourne, Australia, 2020. [Google Scholar]
- Macintosh, H. Native Citrus. In The Newcrop Industries Handbook; Robins, J., Ed.; AgriFutures Australia: Wagga, Australia, 2004; pp. 358–365. [Google Scholar]
- Rennie, S. Cultivation of Australian Finger Lime (Citrus australasica). In Australian Native Plants: Cultivation and Uses in the Health and Food Industries; Sultanbawa, Y., Sultanbawa, F., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 81–87. [Google Scholar]
- Sykes, S. Australian Native Limes (Eremocitrus and Microcitrus); a citrus breeder’s viewpoint. Aust. Bush Foods Mag. 1997, 3, 12–15. [Google Scholar]
- Tisserat, B.; Galletta, P.; Jones, D. Carpel Polymorphism in Citrus Fruit. Bot. Gaz. 1990, 151, 54–63. [Google Scholar] [CrossRef]
- Swingle, W.T. Eremocitrus, a new genus of hardy, drought resistant citrous fruits from Australia. J. Agric. Res. 1914, 2, 85. [Google Scholar]
- Anderson, E. Plants of Central Queensland: Identification and Uses of Native and Introduced Species; CSIRO Publishing: Victoria, Australia, 2016. [Google Scholar]
- Catalano, C.; Ciacciulli, A.; Salonia, F.; Russo, M.P.; Caruso, P.; Caruso, M.; Russo, G.; Distefano, G.; Licciardello, C. Target-Genes Reveal Species and Genotypic Specificity of Anthocyanin Pigmentation in Citrus and Related Genera. Genes 2020, 11, 807. [Google Scholar] [CrossRef]
- Hamilton, K.N.; Ashmore, S.E.; Drew, R.A.; Pritchard, H.W. Seed morphology and ultrastructure in Citrus garrawayi (Rutaceae) in relation to germinability. Aust. J. Bot. 2007, 55, 618–627. [Google Scholar] [CrossRef]
- Young, R.; Barrett, H.C.; Hearn, C.J.; Hutchison, D.J. New Sources of Cold Hardiness for Citrus Breeding. HortScience 1982, 17, 866. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Martínez-Cuenca, M.-R.; Forner-Giner, M.Á. Cold Stress in Citrus: A Molecular, Physiological and Biochemical Perspective. Horticulturae 2021, 7, 340. [Google Scholar] [CrossRef]
- Inch, S.; Stover, E.; Driggers, R.; Lee, R.F. Freeze Response of Citrus and Citrus-related Genotypes in a Florida Field Planting. HortScience 2014, 49, 1010–1016. [Google Scholar] [CrossRef]
- Nolte, K.D.; Hanson, A.D.; Gage, D.A. Proline Accumulation and Methylation to Proline Betaine in Citrus: Implications for Genetic Engineering of Stress Resistance. J. Am. Soc. Hortic. Sci. 1997, 122, 8–13. [Google Scholar] [CrossRef]
- Hamilton, K.N.; Ashmore, S.E.; Pritchard, H.W. Thermal Analysis and Cryopreservation of Seeds of Australian Wild Citrus Species (Rutaceae): Citrus australasica, C. inodora and C. garrawayi. Cryoletters 2009, 30, 268–279. [Google Scholar]
- Hele, A. Australian Native Citrus—Wild Species, Cultivars and Hybrids; Primary Industries and Resources SA, Government of South Australia: Adelaide, Australia, 2001.
- Yelenosky, G.; Barrett, H.; Young, R. Cold Hardiness of Young Hybrid Trees of Eremocitrus glauca (Lindl.) Swing. HortScience 1978, 13, 257–258. [Google Scholar] [CrossRef]
- Lastinger, J.Q., Jr.; Barrett, H.C.; Tatum, J.H. Flavonone Contribution of Parent Plants to Eremocitrus glauca (Lindl.) Swing. Hybrids. Proc. Fla. State Hortic. Soc. 1978, 91, 198–200. [Google Scholar]
- Tworney, A.; Midmore, D.; Ashwath, N. Integrating Australian Nut and Fruit Tree Species with Crops or Pasture; Rural Industries Research and Development Corporation: Barton, Australia, 2009.
- Yelenosky, G.; Young, R.; Hearn, C.; Barrett, H.; Hutchison, D. Cold hardiness of citrus trees during 1981 freeze in Florida. Proc. Fla. State Hortic. Soc. 1981, 94, 46–51. [Google Scholar]
- Scora, R.W.; Ahmed, M. Essential Leaf Oil Composition of Eremocitrus glauca (Lindl.) Swing., an Aurantioid Xerophyte. J. Essent. Oil Res. 1995, 7, 579–584. [Google Scholar] [CrossRef]
- Bitters, W.; Brusca, J.; Cole, D. The search for new citrus rootstocks. Citrograph 1964, 49, 443–448. [Google Scholar]
- Goell, A. Salinity effects on citrus trees. In Proceedings of the First International Citrus Symposium, UC Riverside, Riverside, CA, USA, 16–26 March 1968; pp. 1819–1824. [Google Scholar]
- Sykes, S. Breeding with Indigenous Citrus Species. In Proceedings of the Combined Proceedings—International Plant Propagators Society, Mildura, Australia, 22–24 April 2005; pp. 120–123. [Google Scholar]
- Hirano, E. Relative Abundance of Stomata in Citrus and Some Related Genera. Bot. Gaz. 1931, 92, 296–310. [Google Scholar] [CrossRef]
- Khairi, M.M.A.; Hall, A.E. Comparative Studies of Net Photosynthesis and Transpiration of Some Citrus Species and Relatives. Physiol. Plant. 1976, 36, 35–39. [Google Scholar] [CrossRef]
- Alves, M.N.; Lopes, S.A.; Raiol-Junior, L.L.; Wulff, N.A.; Girardi, E.A.; Ollitrault, P.; Peña, L. Resistance to ‘Candidatus Liberibacter asiaticus,’ the Huanglongbing Associated Bacterium, in Sexually and/or Graft-Compatible Citrus Relatives. Front. Plant Sci. 2021, 11, 617664. [Google Scholar] [CrossRef]
- Mabberley, D.J. A classification for edible citrus: An update, with a note on Murraya (Rutaceae). Telopea 2022, 25, 271–284. [Google Scholar] [CrossRef]
- Weber, K.C.; Mahmoud, L.M.; Stanton, D.; Welker, S.; Qiu, W.; Grosser, J.W.; Levy, A.; Dutt, M. Insights into the mechanism of Huanglongbing tolerance in the Australian finger lime (Citrus australasica). Front. Plant Sci. 2022, 13, 1019295. [Google Scholar] [CrossRef]
- Gonzalez-Ibeas, D.; Ibanez, V.; Perez-Roman, E.; Borredá, C.; Terol, J.; Talon, M. Shaping the biology of citrus: I. Genomic determinants of evolution. Plant Genome 2021, 14, e20104. [Google Scholar] [CrossRef]
- Nakandala, U.; Furtado, A.; Masouleh, A.K.; Smith, M.W.; Mason, P.; Williams, D.C.; Henry, R.J. The genomes of Australian wild limes. Plant Mol. Biol. 2024, 114, 102. [Google Scholar] [CrossRef]
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the Responses of Different Genotypes of Citrus to Huanglongbing (Citrus Greening) Under Different Conditions. Phytopathology 2009, 99, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.N.; Raiol-Junior, L.L.; Girardi, E.A.; Miranda, M.; Wulff, N.A.; Carvalho, E.V.; Lopes, S.A.; Ferro, J.A.; Ollitrault, P.; Peña, L. Insight into resistance to ‘Candidatus Liberibacter asiaticus,’ associated with Huanglongbing, in Oceanian citrus genotypes. Front. Plant Sci. 2022, 13, 1009350. [Google Scholar] [CrossRef]
- Boscariol-Camargo, R.L.; Cristofani-Yaly, M.; Malosso, A.; Coletta Filho, H.D.; Machado, M.A. Evaluation of different genotypes of citrus to Candidatus Liberibacter asiaticus infection. Citrus Res. Technol. 2010, 31, 85–90. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Araujo, K.; Sánchez, J.N.; Kund, G.; Trumble, J.; Roper, C.; Godfrey, K.E.; Jin, H. A stable antimicrobial peptide with dual functions of treating and preventing citrus Huanglongbing. Proc. Natl. Acad. Sci. USA 2021, 118, e2019628118. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Liu, N.; Fu, Z.Q. Dual Functions of a Stable Peptide against Citrus Huanglongbing Disease. Trends Plant Sci. 2021, 26, 668–670. [Google Scholar] [CrossRef]
- Dutt, M.; Mahmoud, L.M.; Chamusco, K.; Stanton, D.; Chase, C.D.; Nielsen, E.; Quirico, M.; Yu, Q.; Gmitter, F.G., Jr.; Grosser, J.W. Utilization of somatic fusion techniques for the development of HLB tolerant breeding resources employing the Australian finger lime (Citrus australasica). PLoS ONE 2021, 16, e0255842. [Google Scholar] [CrossRef]
- Nakandala, U.; Furtado, A.; Masouleh, A.K.; Smith, M.W.; Williams, D.C.; Henry, R.J. The genome of Citrus australasica reveals disease resistance and other species specific genes. BMC Plant Biol. 2024, 24, 260. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Deol, J.K.; Grosser, J.W.; Killiny, N.; Dutt, M. Transcriptomic and biochemical analysis of pummelo x finger lime hybrids in response to Huanglongbing (HLB). BMC Plant Biol. 2025, 25, 235. [Google Scholar] [CrossRef]
- Liu, J.; Singh, K.; Huff, M.; Gottschalk, C.; Do, M.; Staton, M.; Keremane, M.L.; Krueger, R.; Ramadugu, C.; Dardick, C. Deep R-gene discovery in HLB resistant wild Australian limes uncovers evolutionary features and potentially important loci for hybrid breeding. Front. Plant Sci. 2025, 15, 1503030. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, H.; Sun, Y.; Zhang, J.; Gao, K.; Wu, J.; Zhu, C.; Yin, C.; Chen, X.; Liu, Q.; et al. Targeted MYC2 stabilization confers citrus Huanglongbing resistance. Science 2025, 388, 191–198. [Google Scholar] [CrossRef]
- Beattie, G.A. Hosts of the Asian citrus psyllid. In Asian Citrus Psyllid: Biology, Ecology and Managementof the Huanglongbing Vector; Qureshi, J., Stansly, P., Eds.; CABI International: Wallingford, UK, 2020; pp. 67–87. [Google Scholar]
- Westbrook, C.J.; Hall, D.G.; Stover, E.; Duan, Y.P.; Lee, R.F. Colonization of Citrus and Citrus-related Germplasm by Diaphorina citri (Hemiptera: Psyllidae). HortScience Horts 2011, 46, 997–1005. [Google Scholar] [CrossRef]
- Koizumi, M.; Prommintara, M.; Ohtsu, Y. Wood apple, Limonia acidissima L.: A new host for the huanglongbing (greening) vector, Diaphorina citri. Int. Organ. Citrus Virol. Conf. Proc. 1996, 13, 271–275. [Google Scholar] [CrossRef]
- Beattie, G.A.; Barkley, P. Huanglongbing and Its Vectors: A Pest-Specific Contingency Plan for the Citrus and Nursery and Garden Industries; Horticulture Australia Ltd.: Sydney, Australia, 2009. [Google Scholar]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Eduardo, W.I.; Miranda, M.P.; Volpe, H.X.L.; Garcia, R.B.; Girardi, E.A.; Alquezar, B.; Ruiz, A.E.; Peña, L. Resistance of True Citrus species to Diaphorina citri. Pest Manag. Sci. 2022, 78, 4783–4792. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health; Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.-A.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; et al. Pest categorisation of Diaphorina citri. EFSA J. 2021, 19, e06357. [Google Scholar] [CrossRef] [PubMed]
- CAB UK. Bactrocera Tryoni ((Froggatt)), Queensland Fruit Fly. [Pest/Pathogen]; CAB International: Wallingford, UK, 2014; pp. 1–39. [Google Scholar]
- Follett, P.A.; Asmus, G.; Hamilton, L.J. Poor Host Status of Australian Finger Lime, Citrus australasica, to Ceratitis capitata, Zeugodacus cucurbitae, and Bactrocera dorsalis (Diptera: Tephritidae) in Hawai’i. Insects 2022, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Jessup, A. Host Status of Fresh Australian Finger Limes (Citrus australasica, [F. Muell.] Swingle: Rutaceae) to Queensland Fruit Fly (Bactrocera tryoni [Froggatt] Diptera: Tephtitidae); NSW Department of Primary Industries: Orange, Australia, 2013; p. 39.
- Cant, R.G.; Spooner-Hart, R.N.; Beattie, G.A.C.; Meats, A. The biology and ecology of the bronze orange bug, “Musgraveia sulciventris”, (Stal)—A literature review: Part I—Description, biology, host species and distribution. Gen. Appl. Entomol. J. Entomol. Soc. New South Wales 1996, 27, 19–29. [Google Scholar]
- Sinclair, D.P. A generic revision of the Oncomerinae (Heteroptera: Pentatomoidea: Tessaratomidae). Mem. Qld. Mus. 2000, 46, 307–330. [Google Scholar]
- Ryder, M.H.; Latham, Y.; Hawke, B. Cultivation and Harvest Quality of Native Food Crops; 1741516080; Rural Industries Research and Development Corporation Canberra: Barton, Australian, 2008.
- Smith, D.; Papacek, D.F. Studies of the predatory mite Amblyseius victoriensis (Acarina: Phytoseiidae) in citrus orchards in south-east Queensland: Control of Tegolophus australis and Phyllocoptruta oleivora (Acarina: Eriophyidae), effect of pesticides, alternative host plants and augmentative release. Exp. Appl. Acarol. 1991, 12, 195–217. [Google Scholar] [CrossRef]
- CAB International. Trioza Erytreae (African Citrus Psyllid); CABI Compendium: Wallingford, UK, 2022. [Google Scholar]
- Mo, J.; Falivene, S. Citrus Gall Wasp in Southern Australia; NSW Department of Primary Industries: Orange, Australia, 2014.
- Bowman, K.D.; Shapiro, J.P.; Lapointe, S.L. Sources of Resistance to Diaprepes Weevil in Subfamily Aurantiodeae, Rutaceae. HortScience 2001, 36, 332–336. [Google Scholar] [CrossRef]
- Peltier, G.L.; Frederich, W.J. Relative susceptibility to citrus-canker of different species and hybrids of the genus Citrus, including the wild relatives. J. Agric. Res. 1920, 19, 339–362. [Google Scholar]
- Peltier, G.L.; Frederich, W.J. Further studies on the relative susceptibility to Citrus canker of different species and hybrids of the genus Citrus, including the wild relatives. J. Agric. Res. 1924, 28, 227–239. [Google Scholar]
- Hailstones, D.; Weinert, M.; Smith, M.; Ghalayini, A.; Gambley, C. Evaluating potential alternative hosts of citrus canker. In Proceedings of the 2nd International Citrus Canker and Huanglongbing Research Workshop, Orlando, FL, USA, 7–11 November 2005; p. 71. [Google Scholar]
- Licciardello, G.; Pruvost, O.; Robene, I.; Cubero, J.; Redondo, C.; Caruso, A.; Licciardello, C.; Caruso, P.; Catara, V. Assessment of the host status of ornamental rutaceous species to Xanthomonas citri pathovars causing Citrus Bacterial Canker. [O.20]. In Proceedings of the 15th Congress of the Mediterranean Phytopathological Union, Cordoba, Spain, 20–23 June 2017; p. 57. [Google Scholar]
- Licciardello, G.; Caruso, P.; Bella, P.; Boyer, C.; Smith, M.W.; Pruvost, O.; Robene, I.; Cubero, J.; Catara, V. Pathotyping Citrus Ornamental Relatives with Xanthomonas citri pv. citri and X. citri pv. aurantifolii Refines Our Understanding of Their Susceptibility to These Pathogens. Microorganisms 2022, 10, 986. [Google Scholar] [CrossRef]
- Gambley, C.F.; Miles, A.K.; Ramsden, M.; Doogan, V.; Thomas, J.E.; Parmenter, K.; Whittle, P.J.L. The distribution and spread of citrus canker in Emerald, Australia. Australas. Plant Pathol. 2009, 38, 547–557. [Google Scholar] [CrossRef]
- Stover, E.; Driggers, R.; Richardson, M.L.; Hall, D.G.; Duan, Y.; Lee, R.F. Incidence and Severity of Asiatic Citrus Canker on Diverse Citrus and Citrus-related Germplasm in a Florida Field Planting. HortScience Horts 2014, 49, 4–9. [Google Scholar] [CrossRef]
- Lee, H.A. Further data on the susceptibility of Rutaceous plants to citrus-canker. J. Agric. Res. 1918, 15, 661–665. [Google Scholar]
- Peltier, G.L. Susceptibility and resistance to citrus-canker of the wild relatives, citrus fruits, and hybrids of the genus Citrus. J. Agric. Res. 1918, 14, 337–358. [Google Scholar]
- Reddy, M.R.S. Sources of resistance to bacterial canker in citrus. J. Mycol. Plant Pathol. 1997, 27, 80–81. [Google Scholar]
- Bové, J.; Navarro, L.; Bonnet, P.; Zreik, L.; Garnier, M. Reaction of citrus cultivars to graft-inoculation of Phytoplasma aurantifolia-infected lime shoots. Int. Organ. Citrus Virol. Conf. Proc. 1996, 13, 249–251. [Google Scholar] [CrossRef]
- Barbosa, C.; Pina, J.A.; Navarro, L.; Durán-Vila, N. Replication/accumulation and symptom expression of citrus viroids on some species of citrus and related genera. Int. Organ. Citrus Virol. Conf. Proc. 2002, 15, 264–271. [Google Scholar] [CrossRef]
- Barbosa, C.D.J. Comportamiento de Especies de Cítricos, Híbridos y Géneros Afines Frente a la Infección con Viroides. Evaluación del Impacto de la Transmisión Mecánica. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2004. [Google Scholar]
- Bani Hashemian, S.M.; Barbosa, C.J.; Serra, P.; Duran-Vila, N. Effects of resistance of Eremocitrus glauca and Microcitrus australis to viroid infection: Replication, accumulation and long-distance movement of six citrus viroids. Plant Pathol. 2010, 59, 413–421. [Google Scholar] [CrossRef]
- Iwanami, T.; Omura, M.; Ieki, H. Susceptibility of several citrus relatives to Satsuma dwarf virus. Int. Organ. Citrus Virol. Conf. Proc. 1993, 12, 352–356. [Google Scholar] [CrossRef]
- Yoshida, T. Graft compatibility of Citrus with plants in the Aurantioideae and their susceptibility to citrus tristeza virus. Plant Dis. 1996, 80, 414–417. [Google Scholar] [CrossRef]
- Caruso, P.; Massimino Coccuzza, E.; Di Silvestro, S.; Licciardello, C.; Puglisi, D.; Bazzano, M.; Scuderi, G.; Catara, A.; Licciardello, G. Inoculation of Citrus relatives ornamental rutaceous and rootstocks highlights a different replication rate of local CTV isolates. J. Plant Pathol. 2022, 104, 1224. [Google Scholar]
- Mestre, P.F.; Asíns, M.J.; Pina, J.A.; Navarro, L. Efficient search for new resistant genotypes to the citrus tristeza closterovirus in the orange subfamily Aurantioideae. Theor. Appl. Genet. 1997, 95, 1282–1288. [Google Scholar] [CrossRef]
- Müller, G.; Garnsey, S. Susceptibility of citrus varieties, species, citrus relatives, and non-rutaceous plants to slash-cut mechanical inoculation with citrus tristeza virus (CTV). Int. Organ. Citrus Virol. Conf. Proc. 1984, 9, 33–40. [Google Scholar] [CrossRef]
- Habili, N. Detection of Australian Field Isolates of Citrus Tristeza Virus by Double-Stranded RNA Analysis. J. Phytopathol. 1993, 138, 308–316. [Google Scholar] [CrossRef]
- Caruso, M.; Smith, M.W.; Froelicher, Y.; Russo, G.; Gmitter, F.G. Chapter 7—Traditional breeding. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 129–148. [Google Scholar]
- Velázquez, K.; Alba, L.; Zarza, O.; Vives, M.C.; Pina, J.A.; Juárez, J.; Navarro, L.; Moreno, P.; Guerri, J. The response of different genotypes of citrus and relatives to Citrus psorosis virus inoculation. Eur. J. Plant Pathol. 2016, 144, 73–81. [Google Scholar] [CrossRef]
- Broadbent, P. Obervations on the mode of infection of Phytophora citrophthora in resistant and suspectible citrus roots. In Proceedings of the 1st International Citrus Symposium, Riverside, CA, USA, 16–26 March 1968; pp. 1207–1210. [Google Scholar]
- Miles, A.K.; Smith, M.W.; Tran, N.T.; Shuey, T.A.; Dewdney, M.M.; Drenth, A. Identification of Resistance to Citrus Black Spot Using a Novel In-field Inoculation Assay. HortScience Horts 2019, 54, 1673–1681. [Google Scholar] [CrossRef]
- Winston, J.R.; Bowman, J.J.; Bach, W.J. Citrus Melanose and Its Control; US Department of Agriculture: Washington, DC, USA, 1927.
- Miles, A.K.; Tan, Y.P.; Shivas, R.G.; Drenth, A. Novel Pathotypes of Elsinoë australis Associated with Citrus australasica and Simmondsia chinensis in Australia. Trop. Plant Pathol. 2015, 40, 26–34. [Google Scholar] [CrossRef]
- Shivas, R.G.; Smith, M.W.; Marney, T.S.; Newman, T.K.; Hammelswang, D.L.; Cooke, A.W.; Pegg, K.G.; Pascoe, I.G. First record of Nematospora coryli in Australia and its association with dry rot of Citrus. Australas. Plant Pathol. 2005, 34, 99–101. [Google Scholar] [CrossRef]
- Broadbent, P. Quarantine in relation to Australian citrus imports and exports. Australas. Plant Pathol. 1995, 24, 145–156. [Google Scholar] [CrossRef]
- Ravichandra, N.G. Nematode Diseases of Horticultural Crops. In Horticultural Nematology; Springer: New Delhi, India, 2014; pp. 127–205. [Google Scholar]
- Mourao-Filho, F.d.A.A. Protoplast Fusion of Citrus for Rootstock and Scion Improvement with Emphasis on Wide Hybridization. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1995. [Google Scholar]
- Baines, R.C.; Bitters, W.P.; Clarke, O.F. Susceptibility of some species and varieties of citrus and some other rutaceous plants to the citrus nematode. Plant Dis. Report. 1960, 44, 281–285. [Google Scholar]
- Hatte, E. An Assessment of the Cultural Heritage Vaues of the Proposed Caval Ridge Mine Area, BMA Bowen Basin Coal Growth Project; BM Alliance Coal Operations Pty Ltd.: City East, Australia, 2008. [Google Scholar]
- Roth, W.E. Food: Its Search, Capture and Preparation; George Arthur Vaughan: Brisbane, Australia, 1901; Volume 3. [Google Scholar]
- Richmond, R.; Bowyer, M.; Vuong, Q. Australian native fruits: Potential uses as functional food ingredients. J. Funct. Foods 2019, 62, 103547. [Google Scholar] [CrossRef]
- Packer, J.; Brouwer, N.; Harrington, D.; Gaikwad, J.; Heron, R.; Yaegl Community, E.; Ranganathan, S.; Vemulpad, S.; Jamie, J. An ethnobotanical study of medicinal plants used by the Yaegl Aboriginal community in northern New South Wales, Australia. J. Ethnopharmacol. 2012, 139, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Leichhardt, L. Journal of an Overland Expedition in Australia 1844-5; T&W Boone: London, UK, 1847. [Google Scholar]
- Bailey, F.M. Phanerogamic Flora of Queensland. Proc. Australas. Assoc. Adv. Sci. 1895, 6, 389–405. [Google Scholar]
- Maiden, J.H. The Useful Native Plants of Australia (Including Tasmania); Turner and Henderson: Sydney, Australia, 1889. [Google Scholar]
- Cooper, W.; Cooper, W. Fruits of the Rain Forest; RD Press: Sydney, Australia, 1994. [Google Scholar]
- Irvine, F.R. Wild and Emergency Foods of Australian and Tasmanian Aborigins. Oceania 1957, 28, 113–142. [Google Scholar] [CrossRef]
- Gorst, J.R. Indigenous Fruits of Australia. In Proceedings of the International Symposium on Tropical and Subtropical Fruits, Cairns, Australia, 30 April 2002; pp. 555–561. [Google Scholar]
- Forbes-Smith, M.; Paton, J. Innovative Products from Australian Native Foods; Rural Industries Research and Development Corporation: Barton, Australia, 2002.
- Hilton, S. Australian native food plants. ReNew Technol. A Sustain. Future 1997, 58, 20–23. [Google Scholar]
- Lim, T.K. Citrus garrawayi. In Edible Medicinal And Non-Medicinal Plants: Volume 4, Fruits; Springer: Dordrecht, The Netherlands, 2012; pp. 631–633. [Google Scholar]
- Floyd, A.G. Rainforest Trees of Mainland South-Eastern Australia; lnkata Press: Melbourne, Australia; Sydney, Australia, 1989. [Google Scholar]
- Bailey, F.M. Botany: Contributions to Queensland Flora. Dep. Agr. Queensland Bot. Bull. 1892, 18, 8. [Google Scholar]
- Hegarty, M.; Hegarty, E.; Wills, R. Food Safety of Australian Plant Bushfoods; Rural Industries Research and Development Corporation: Barton, Australian, 2001.
- Glover, R.; Taylor, T.; Redman, M.; Trainer, P. Australian Native Finger Lime RD&E Plan (2023–2028); AgriFutures Australia: Wagga, Australia, 2021.
- Planchon, J.E. Hortus Donatensis: Catalogue des Plantes Cultivées Dans les Serres de S. Ex. le Prince A. de Démidoff à San Donato, près Florence; W. Remquet: Paris, France, 1854. [Google Scholar]
- Forster, P. Microcitrus garrawayae (Rutaceae) and its distribution in New Guinea and Australia. Telopea 1991, 4, 357–358. [Google Scholar] [CrossRef]
- Mitchell, T.L. Journal of an Expedition into the Interior of Tropical Australia, in Search of a Route from Sydney to the Gulf of Carpentaria; Longman, Brown, Green and Longmans: London, UK, 1848. [Google Scholar]
- Bentham, G. Flora Australiensis; John Edward Taylor: London, UK, 1863; Volume 1, pp. 371–372. [Google Scholar]
- Swingle, W.T. A new taxonomic arrangement of the orange subfamily, Aurantioideae. J. Wash. Acad. Sci. 1938, 28, 530–533. [Google Scholar]
- Clarke, K.; Prakash, N. Studies on the Australian Rutaceae with particular reference to subtribe Citrinae. Malay. Nat. J. 2001, 55, 231–240. [Google Scholar]
- Ghada, B.; Amel, O.; Aymen, M.; Aymen, A.; Amel, S.H. Phylogenetic patterns and molecular evolution among ‘True citrus fruit trees’ group (Rutaceae family and Aurantioideae subfamily). Sci. Hortic. 2019, 253, 87–98. [Google Scholar] [CrossRef]
- Oueslati, A.; Ollitrault, F.; Baraket, G.; Salhi-Hannachi, A.; Navarro, L.; Ollitrault, P. Towards a molecular taxonomic key of the Aurantioideae subfamily using chloroplastic SNP diagnostic markers of the main clades genotyped by competitive allele-specific PCR. BMC Genet. 2016, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.; Nylinder, S.; Ramadugu, C.; Antonelli, A.; Pfeil, B.E. The Origin of Oranges: A Multi-locus Phylogeny of Rutaceae Subfamily Aurantioideae. Syst. Bot. 2016, 40, 1053–1062. [Google Scholar] [CrossRef]
- Rang-Jin, X.; Zhi-Qin, Z.; Lie, D.; Rang-Jin, X.; Zhi-Qin, Z.; Lie, D. Taxonomic and phylogenetic relationships among the genera of the True Citrus Fruit Trees Group (Aurantioideae, Rutaceae) based on AFLP markers. J. Syst. Evol. 2008, 46, 682. [Google Scholar] [CrossRef]
- Keremane, M.L.; McCollum, T.G.; Roose, M.L.; Lee, R.F.; Ramadugu, C. An Improved Reference Gene for Detection of “Candidatus Liberibacter asiaticus” Associated with Citrus Huanglongbing by qPCR and Digital Droplet PCR Assays. Plants 2021, 10, 2111. [Google Scholar] [CrossRef]
- Morton, C.M. Phylogenetic relationships of the Aurantioideae (Rutaceae) based on the nuclear ribosomal DNA ITS region and three noncoding chloroplast DNA regions, atpB-rbcL spacer, rps16, and trnL-trnF. Org. Divers. Evol. 2009, 9, 52–68. [Google Scholar] [CrossRef]
- Kalita, B.; Roy, A.; Annamalai, A.; Ptv, L. A molecular perspective on the taxonomy and journey of Citrus domestication. Perspect. Plant Ecol. Evol. Syst. 2021, 53, 125644. [Google Scholar] [CrossRef]
- Barrett, H.C.; Rhodes, A.M. A Numerical Taxonomic Study of Affinity Relationships in Cultivated Citrus and Its Close Relatives. Syst. Bot. 1976, 1, 105–136. [Google Scholar] [CrossRef]
- Gmitter, F.G.; Wu, G.A.; Rokhsar, D.S.; Talon, M. Chapter 1—The citrus genome. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 1–8. [Google Scholar]
- Wang, F.; Wang, S.; Wu, Y.; Jiang, D.; Yi, Q.; Zhang, M.; Yu, H.; Yuan, X.; Li, M.; Li, G.; et al. Haplotype-resolved genome of a papeda provides insights into the geographical origin and evolution of Citrus. J. Integr. Plant Biol. 2025, 67, 276–293. [Google Scholar] [CrossRef]
- Yamamoto, M.; Abkenar, A.A.; Matsumoto, R.; Kubo, T.; Tominaga, S. CMA Staining Analysis of Chromosomes in Citrus Relatives, Clymenia, Eremocitrus and Microcitrus. J. Jpn. Soc. Hortic. Sci. 2008, 77, 24–27. [Google Scholar] [CrossRef]
- Gamage, H.K.; Schmidt, S. Short Communication. A robust method for chromosome quantification and ploidy determination in woody species. Aust. J. Bot. 2009, 57, 87–93. [Google Scholar] [CrossRef]
- Garcia-Lor, A.; Curk, F.; Luro, F.; Navarro, L.; Ollitrault, P. Nuclear and maternal phylogeny within Citrus and four related genera based on nuclear genes sequence SNPs and mitochondrial InDels. In Proceedings of the Current Opinion Conferences on Plant Genome Evolution, Amsterdam, The Netherlands, 4–6 September 2011; p. 2. [Google Scholar]
- Iwamasa, M.; Nito, N.; Ling, J. Intra-and intergeneric hybridization in the orange subfamily, Aurantioideae. In Proceedings of the Citriculture: Proceedings of the Sixth International Citrus Congress: Middle-East, Tel Aviv, Israel, 6–11 March 1988. [Google Scholar]
- Wooldridge, S. Cross-Compatibility, Graft-Compatibility, and Phylogenetic Relationships in the Aurantioideae: New Data from the Balsamocitrinae. Ph.D. Thesis, UC Riverside, Riverside, CA, USA, 2016. [Google Scholar]
- Vanarelli, S.; Rizzo, D.; Stefani, L.; Paoli, M. Cancro Batterico Degli Agrumi (Citrus Bacterial Canker); Rivista di Frutticoltura: Bologna, Italy, 2017; pp. 40–45. [Google Scholar]
- de Lange, J.H.; Vincent, A.P. Studies on Citrus pollination using gamma-irradiated pollen. South Afr. J. Bot. 1988, 54, 257–264. [Google Scholar] [CrossRef]
- Abkenar, A.; Isshiki, S.; Tashiro, Y. Maternal inheritance of chloroplast DNA in intergeneric sexual hybrids of “true citrus fruit trees” revealed by PCR-RFLP analysis. J. Hortic. Sci. Biotechnol. 2004, 79, 360–363. [Google Scholar] [CrossRef]
- Huang, C.Y.; Niu, D.; Kund, G.; Jones, M.; Albrecht, U.; Nguyen, L.; Bui, C.; Ramadugu, C.; Bowman, K.D.; Trumble, J.; et al. Identification of citrus immune regulators involved in defence against Huanglongbing using a new functional screening system. Plant Biotechnol. J. 2021, 19, 757–766. [Google Scholar] [CrossRef]
- O’Bannon, J.; Esser, R. Evaluation of citrus, hybrids, and relatives as hosts of Pratylenchus coffeae, with cpmments on other hosts. Nematol. Mediterr. 1975, 3, 113–122. [Google Scholar]
- Mabberley, D.J. Citrus (Rutaceae): A Review of Recent Advances in Etymology, Systematics and Medical Applications. Blumea Biodivers. Evol. Biogeogr. Plants 2004, 49, 481–498. [Google Scholar] [CrossRef]
- Ashmore, S.E. Wild citrus in Oceania: Harnessing the diversity. In XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): IV International Symposium on Plant Genetic Resources; ISHS Acta Horticulturae: Bierbeek, Belgium, 2015; pp. 191–198. [Google Scholar]
- Winters, H.F. Microcitrus papuana, a new species from Papua New Guinea (Rutaceae). Baileya 1976, 20, 19–24. [Google Scholar]
- Barrett, H.C.; Rhodes, A.M. Intergeneric hybridization of Citrus and other genera in citrus cultivar improvement. In Proceedings of the International Society of Citriculture, Orlando, FL, USA, 1–8 May 1977. [Google Scholar]
- Webber, H.J. Rootstocks: Their character and reactions. In The Citrus Industry; Batchelor, L.D., Webber, H.J., Eds.; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1948; Volume 2, pp. 69–168. [Google Scholar]
- Smith, M.W.; Gultzow, D.L.; Newman, T.K.; Parfitt, S.C. Lime bush (Citrus glauca) hybrids resistant to CTV. In Proceedings of the International Citrus Congress, Valencia, Spain, 18 November 2012; p. Poster SO2P12. [Google Scholar]
- Cimen, B.; Yeşiloğlu, T. Rootstock breeding for abiotic stress tolerance in citrus. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; IntechOpen: London, UK, 2016; pp. 527–563. [Google Scholar]
- Swingle, W.T. The history, botany and breeding. In The Citrus Industry; Webber, H.J., Batchelor, L.D., Eds.; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1943; Volume 1, p. 1028. [Google Scholar]
- Lovell, C.J. Concerning the ‘Ugly Fruit’ and Other Citrus Hybrids. Am. Speech 1951, 26, 35–37. [Google Scholar] [CrossRef]
- Rieger, M.; Krewer, G.; Lewis, P.; Linton, M.; McClendon, T. Field Evaluation of Cold Hardy Citrus in Coastal Georgia. HortTechnology Horttech 2003, 13, 540–544. [Google Scholar] [CrossRef]
- Russo, M.P.; Russo, G. Potted trees of Rutaceae hybrids from CREA-OFA breeding program. Citrus Res. Technol. 2017, 38, 88–94. [Google Scholar] [CrossRef]
- Smyth, H.E.; Sanderson, J.E.; Sultanbawa, Y. Lexicon for the Sensory Description of Australian Native Plant Foods and Ingredients. J. Sens. Stud. 2012, 27, 471–481. [Google Scholar] [CrossRef]
- Bronzi, P.; Rosenthal, H. Present and future sturgeon and caviar production and marketing: A global market overview. J. Appl. Ichthyol. 2014, 30, 1536–1546. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Perry, K.R.; Abbott, J.A.; King, J.M.; Hoffman, E.W. Physical, colour, and mechanical properties of pearls (citrus caviar) from three finger lime (Citrus australasica) varieties: Implications for economic value, quality grading, and benchmarking. Food Biophys. 2024, 19, 784–794. [Google Scholar] [CrossRef]
- Bailey, F.M. The Queensland Flora. Part 1; H. J. Diddams & Co.: Brisbane, Australia, 1899. [Google Scholar]
- von Mueller, F. Select Plants Readily Eligible for Industrial Culture or Naturalisation in Victoria, with Indications of Their Native Countries and Some of Their Uses; McCarron, Bird & Company: Melbourne, Australia, 1876. [Google Scholar]
- Benson, A.H. Fruits of Queensland; AJ Cumming, Government Printer: Brisbane, Australia, 1914. [Google Scholar]
- Von Mueller, F. Select Extra-Tropical Plants: Readily Eligible for Industrial Culture or Naturalisation, with Indications of Their Native Countries and Some of Their Uses; Government Printer: Pretoria, South Africa, 1888. [Google Scholar]
- Olusegun, A.M.; Passy, O.G.; Terwase, D.S. Effects of Waxing Materials, Storage Conditions on Protein, Sugar and ash Contents of Citrus Fruits Stored at Room and Refrigerated Temperatures. J. Asian Sci. Res. 2012, 2, 913–926. [Google Scholar]
- USDA. USDA National Nutrient Database for Standard Reference. Available online: https://fdc.nal.usda.gov/ (accessed on 31 January 2025).
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of Citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Matsuo, Y.; Miura, L.A.; Araki, T.; Yoshie-Stark, Y. Proximate composition and profiles of free amino acids, fatty acids, minerals and aroma compounds in Citrus natsudaidai peel. Food Chem. 2019, 279, 356–363. [Google Scholar] [CrossRef]
- Ranganna, S.; Govindarajan, V.S.; Ramana, K.V.R.; Kefford, J.F. Citrus fruits—Varieties, chemistry, technology, and quality evaluation. Part II. Chemistry, technology, and quality evaluation. A. Chemistry. C R C Crit. Rev. Food Sci. Nutr. 1983, 18, 313–386. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of phytochemical and nutritional characteristics and food applications of Citrus L. fruits. Front. Nutr. 2022, 9, 968604. [Google Scholar] [CrossRef] [PubMed]
- Lim, V.; Gorji, S.G.; Daygon, V.D.; Fitzgerald, M. Untargeted and Targeted Metabolomic Profiling of Australian Indigenous Fruits. Metabolites 2020, 10, 114. [Google Scholar] [CrossRef]
- Lim, T.K. Citrus inodora. In Edible Medicinal and Non-Medicinal Plants: Volume 4, Fruits; Springer: Dordrecht, The Netherlands, 2012; pp. 644–646. [Google Scholar]
- Konczak, I.; Roulle, P. Nutritional properties of commercially grown native Australian fruits: Lipophilic antioxidants and minerals. Food Res. Int. 2011, 44, 2339–2344. [Google Scholar] [CrossRef]
- Michalski, P.; Nur-A-Tomal, M.S.; Crawford, S.; Rudman, M.; van ‘t Hag, L. Advancement in fruit drying through the analysis of moisture sorption isotherms: Processing effects on Australian native fruits in comparison to apple. J. Food Eng. 2025, 395, 112526. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P.; Roulfe, P.; Pavan, A. Health Benefits of Australian Native Foods: An Evaluation of Health-Enhancing Compounds; Rural Industries Research and Development Corporation: Barton, Australia, 2009.
- Ladaniya, M.S. (Ed.) 6—Fruit biochemistry. In Citrus Fruit; Academic Press: San Diego, CA, USA, 2008; pp. 125–190. [Google Scholar]
- Barros, H.R.d.M.; Ferreira, T.A.P.d.C.; Genovese, M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Hussain, S.; Ali, M.A.; Minhas, A.; Waheed, W.; Danish, S.; Fahad, S.; Ghafoor, U.; Baig, K.S.; Sultan, H.; et al. Correlation of Soil Characteristics and Citrus Leaf Nutrients Contents in Current Scenario of Layyah District. Horticulturae 2022, 8, 61. [Google Scholar] [CrossRef]
- Potortì, A.G.; Di Bella, G.; Mottese, A.F.; Bua, G.D.; Fede, M.R.; Sabatino, G.; Salvo, A.; Somma, R.; Dugo, G.; Lo Turco, V. Traceability of Protected Geographical Indication (PGI) Interdonato lemon pulps by chemometric analysis of the mineral composition. J. Food Compos. Anal. 2018, 69, 122–128. [Google Scholar] [CrossRef]
- Toplu, C.; Uygur, V.; Kaplankıran, M.; Demirkeser, T.H.; Yıldız, E. Effect of citrus rootstocks on leaf mineral composition of ‘Okitsu’, ‘Clausellina’, and ‘Silverhill’ mandarin cultivars. J. Plant Nutr. 2012, 35, 1329–1340. [Google Scholar] [CrossRef]
- Sau, S.; Ghosh, S.N.; Sarkar, S.; Gantait, S. Effect of rootstocks on growth, yield, quality, and leaf mineral composition of Nagpur mandarin (Citrus reticulata Blanco.), grown in red lateritic soil of West Bengal, India. Sci. Hortic. 2018, 237, 142–147. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef]
- Kafkas, E.; Polatöz, S.; Koç, N. Quantification and comparison of sugars, carboxylic acids and vitamin C components of various citrus species by HPLC techniques. Nong Ye Ke Xue Yu Ji Shu 2011, 5, 175–180. [Google Scholar]
- Zhang, J.; Ritenour, M.A. Sugar composition analysis of commercial citrus juice products. Proc. Fla. State Hortic. Soc. 2016, 129, 178–180. [Google Scholar]
- Rampersaud, G.C.; Valim, M.F. 100% citrus juice: Nutritional contribution, dietary benefits, and association with anthropometric measures. Crit. Rev. Food Sci. Nutr. 2017, 57, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Asencio, A.D.; Serrano, M.; García-Martínez, S.; Pretel, M.T. Organic acids, sugars, antioxidant activity, sensorial and other fruit characteristics of nine traditional Spanish Citrus fruits. Eur. Food Res. Technol. 2018, 244, 1497–1508. [Google Scholar] [CrossRef]
- Canan, İ.; Gündoğdu, M.; Seday, U.; Oluk, C.A.; Karaşahin, Z.; Eroğlu, E.Ç.; Yazici, E.; ÜNLÜ, M. Determination of antioxidant, total phenolic, total carotenoid, lycopene, ascorbic acid, and sugar contents of Citrus species and mandarin hybrids. Turk. J. Agric. For. 2016, 40, 894–899. [Google Scholar] [CrossRef]
- Sun, X.-H.; Xiong, J.-J.; Zhu, A.-D.; Zhang, L.; Ma, Q.-L.; Xu, J.; Cheng, Y.-J.; Deng, X.-X. Sugars and organic acids changes in pericarp and endocarp tissues of pumelo fruit during postharvest storage. Sci. Hortic. 2012, 142, 112–117. [Google Scholar] [CrossRef]
- Pan, T.; Ali, M.M.; Gong, J.; She, W.; Pan, D.; Guo, Z.; Yu, Y.; Chen, F. Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China. Agronomy 2021, 11, 2393. [Google Scholar] [CrossRef]
- Clements, R.L. Organic Acids in Citrus Fruits. I. Varietal Differences. J. Food Sci. 1964, 29, 276–280. [Google Scholar] [CrossRef]
- Ting, S.V.; Deszyck, E.J. Isolation of I-Quinic Acid in Citrus Fruit. Nature 1959, 183, 1404–1405. [Google Scholar] [CrossRef]
- Yu, K.; Xu, Q.; Da, X.; Guo, F.; Ding, Y.; Deng, X. Transcriptome changes during fruit development and ripening of sweet orange (Citrus sinensis). BMC Genom. 2012, 13, 10. [Google Scholar] [CrossRef]
- Shaw, P.E.; Wilson III, C.W. Organic acids in orange, grapefruit and cherry juices quantified by high-performance liquid chromatography using neutral resin or propylamine columns. J. Sci. Food Agric. 1983, 34, 1285–1288. [Google Scholar] [CrossRef]
- Vandercook, C. Organic acids. In Citrus Science and Technology; Nagy, S., Shaw, P.E., Veldhuis, M.K., Eds.; AVI Publishing Company: Westport, CT, USA, 1977; Volume 1, pp. 209–227. [Google Scholar]
- Hewson, L.; Hollowood, T.; Chandra, S.; Hort, J. Taste–aroma interactions in a citrus flavoured model beverage system: Similarities and differences between acid and sugar type. Food Qual. Prefer. 2008, 19, 323–334. [Google Scholar] [CrossRef]
- Hussain, S.B.; Shi, C.-Y.; Guo, L.-X.; Kamran, H.M.; Sadka, A.; Liu, Y.-Z. Recent Advances in the Regulation of Citric Acid Metabolism in Citrus Fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in Organic Acid Profiles During Fruit Development and Ripening: Correlation or Causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, S.; Zang, W.; Wang, N.; Cao, J.; Li, X.; Sun, C. Identification of phenolic compounds from a unique citrus species, finger lime (Citrus australasica) and their inhibition of LPS-induced NO-releasing in BV-2 cell line. Food Chem. Toxicol. 2019, 129, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Dutt, M.; Vashisth, T. Comparative phytochemical analysis of the fruits of four Florida-grown finger lime (Citrus australasica) selections. LWT 2021, 135, 110003. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Aznar, R.; Rodríguez-Pérez, C.; Rai, D.K. Comprehensive Characterization and Quantification of Antioxidant Compounds in Finger Lime (Citrus australasica L.) by HPLC-QTof-MS and UPLC-MS/MS. Appl. Sci. 2022, 12, 1712. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes; National Health and Medical Research Council: Canberra, Australia, 2006.
- Bermejo, A.; Cano, A. Analysis of nutritional constituents in twenty citrus cultivars from the Mediterranean area at different stages of ripening. Food Nutr. Sci. 2012, 3, 639–650. [Google Scholar] [CrossRef]
- Di Matteo, A.; Di Rauso Simeone, G.; Cirillo, A.; Rao, M.A.; Di Vaio, C. Morphological characteristics, ascorbic acid and antioxidant activity during fruit ripening of four lemon (Citrus limon (L.) Burm. F.) cultivars. Sci. Hortic. 2021, 276, 109741. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Opara, U.L. Postharvest factors affecting vitamin C content of citrus fruits: A review. Sci. Hortic. 2017, 218, 95–104. [Google Scholar] [CrossRef]
- Vanderslice, J.T.; Higgs, D.J.; Hayes, J.M.; Block, G. Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J. Food Compos. Anal. 1990, 3, 105–118. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Xie, J.-X.; Wang, F.-F.; Zhong, J.; Liu, Y.-Z.; Li, G.-H.; Peng, S.-A. Comparison of ascorbate metabolism in fruits of two citrus species with obvious difference in ascorbate content in pulp. J. Plant Physiol. 2011, 168, 2196–2205. [Google Scholar] [CrossRef]
- Mitchell, G.E.; McLauchlan, R.L.; Isaacs, A.R.; Williams, D.J.; Nottingham, S.M. Effect of low dose irradiation on composition of tropical fruits and vegetables. J. Food Compos. Anal. 1992, 5, 291–311. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Al-Ansi, W.; Ahmed, M.I.; Xiaoyun, C.; Mohammed, J.K.; Sulieman, A.A.; Mushtaq, B.S.; Harimana, Y.; Wang, H. Microwave assisted extraction of the bioactive compounds from peel/pulp of Citrus medica L. var. sarcodactylis Swingle along with its nutritional profiling. J. Food Meas. Charact. 2020, 14, 283–292. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef] [PubMed]
- Chanson-Rolle, A.; Braesco, V.; Chupin, J.; Bouillot, L. Nutritional composition of orange juice: A comparative study between French commercial and home-made juices. Food Nutr. Sci. 2016, 7, 252. [Google Scholar] [CrossRef]
- Öhrvik, V.; Witthöft, C. Orange juice is a good folate source in respect to folate content and stability during storage and simulated digestion. Eur. J. Nutr. 2008, 47, 92–98. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Tang, L.; Tang, J.; Liu, D.; Geng, F. Quantitative metabolomic analysis reveals the fractionation of active compounds during lemon fruit juicing. Food Res. Int. 2023, 169, 112829. [Google Scholar] [CrossRef]
- Dadwal, V.; Joshi, R.; Gupta, M. A comparative metabolomic investigation in fruit sections of Citrus medica L. and Citrus maxima L. detecting potential bioactive metabolites using UHPLC-QTOF-IMS. Food Res. Int. 2022, 157, 111486. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.M.; Eldridge, A.L.; Beecher, G.R.; Marilyn Buzzard, I.; Bhagwat, S.; Davis, C.S.; Douglass, L.W.; Gebhardt, S.; Haytowitz, D.; Schakel, S. Carotenoid Content of U.S. Foods: An Update of the Database. J. Food Compos. Anal. 1999, 12, 169–196. [Google Scholar] [CrossRef]
- Jiao, Y.; Reuss, L.; Wang, Y. β-Cryptoxanthin: Chemistry, Occurrence, and Potential Health Benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar] [CrossRef]
- Fanciullino, A.-L.; Dhuique-Mayer, C.; Luro, F.; Casanova, J.; Morillon, R.; Ollitrault, P. Carotenoid Diversity in Cultivated Citrus Is Highly Influenced by Genetic Factors. J. Agric. Food Chem. 2006, 54, 4397–4406. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, Q.; Huang, Y.; Ye, J.; Xu, Q.; Deng, X. Genome-wide Characterization of cis-acting Elements in the Promoters of Key Carotenoid Pathway Genes from the Main Species of Genus Citrus. Hortic. Plant J. 2020, 6, 385–395. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.; Kato, M.; Yamawaki, K.; Takagi, T.; Kiriiwa, Y.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Nesumi, H. Regulation of carotenoid accumulation and the expression of carotenoid metabolic genes in citrus juice sacs in vitro. J. Exp. Bot. 2011, 63, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ikoma, Y.; Kato, M.; Kuniga, T.; Nakajima, N.; Yoshida, T. Quantification of Carotenoids in Citrus Fruit by LC-MS and Comparison of Patterns of Seasonal Changes for Carotenoids among Citrus Varieties. J. Agric. Food Chem. 2007, 55, 2356–2368. [Google Scholar] [CrossRef]
- Xu, C.-J.; Fraser, P.D.; Wang, W.-J.; Bramley, P.M. Differences in the Carotenoid Content of Ordinary Citrus and Lycopene-Accumulating Mutants. J. Agric. Food Chem. 2006, 54, 5474–5481. [Google Scholar] [CrossRef]
- Zacarías-García, J.; Rey, F.; Gil, J.-V.; Rodrigo, M.J.; Zacarías, L. Antioxidant capacity in fruit of Citrus cultivars with marked differences in pulp coloration: Contribution of carotenoids and vitamin C. Food Sci. Technol. Int. 2021, 27, 210–222. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Cilla, A.; Barberá, R.; Zacarías, L. Carotenoid bioaccessibility in pulp and fresh juice from carotenoid-rich sweet oranges and mandarins. Food Funct. 2015, 6, 1950–1959. [Google Scholar] [CrossRef]
- Rey, F.; Zacarias, L.; Rodrigo, M.J. Regulation of Tocopherol Biosynthesis During Fruit Maturation of Different Citrus Species. Front. Plant Sci. 2021, 12, 743993. [Google Scholar] [CrossRef]
- Rey, F.; Rodrigo, M.J.; Zacarias, L. Accumulation of tocopherols and transcriptional regulation of their biosynthesis during cold storage of mandarin fruit. Postharvest Biol. Technol. 2021, 180, 111594. [Google Scholar] [CrossRef]
- Assefa, A.D.; Saini, R.K.; Keum, Y.-S. Fatty acids, tocopherols, phenolic and antioxidant properties of six citrus fruit species: A comparative study. J. Food Meas. Charact. 2017, 11, 1665–1675. [Google Scholar] [CrossRef]
- Dismore, M.L.; Haytowitz, D.B.; Gebhardt, S.E.; Peterson, J.W.; Booth, S.L. Vitamin K content of nuts and fruits in the US diet. J. Am. Diet. Assoc. 2003, 103, 1650–1652. [Google Scholar] [CrossRef]
- Vieira, E.F.; Souza, S. Formulation Strategies for Improving the Stability and Bioavailability of Vitamin D-Fortified Beverages: A Review. Foods 2022, 11, 847. [Google Scholar] [CrossRef]
- Tangpricha, V.; Koutkia, P.; Rieke, S.M.; Chen, T.C.; Perez, A.A.; Holick, M.F. Fortification of orange juice with vitamin D: A novel approach for enhancing vitamin D nutritional health. Am. J. Clin. Nutr. 2003, 77, 1478–1483. [Google Scholar] [CrossRef]
- Johnson, J.B.; Batley, R.; Manson, D.; White, S.; Naiker, M. Volatile compounds, phenolic acid profiles and phytochemical content of five Australian finger lime (Citrus australasica) cultivars. LWT 2022, 154, 112640. [Google Scholar] [CrossRef]
- McDonald, J.; Caffin, N.; Sommano, S.; Cocksedge, R. The Effect of Post Harvest Handling on Selected Native Food Plants; Rural Industries Research and Development Corporation: Barton, Australia, 2008.
- Wright, M.H.; Matthews, B.; Arnold, M.S.J.; Greene, A.C.; Cock, I.E. The prevention of fish spoilage by high antioxidant Australian culinary plants: Shewanella putrefaciens growth inhibition. Int. J. Food Sci. Technol. 2016, 51, 801–813. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Chemistry of the Antioxidant Effect of Polyphenols. Ann. New York Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Peleg, H.; Naim, M.; Rouseff, R.L.; Zehavi, U. Distribution of bound and free phenolic acids in oranges (Citrus sinensis) and Grapefruits (Citrus paradisi). J. Sci. Food Agric. 1991, 57, 417–426. [Google Scholar] [CrossRef]
- García-Nicolás, M.; Ledesma-Escobar, C.A.; Priego-Capote, F. Spatial Distribution and Antioxidant Activity of Extracts from Citrus Fruits. Antioxidants 2023, 12, 781. [Google Scholar] [CrossRef]
- Huang, D.; Yuan, Y.; Tang, Z.; Huang, Y.; Kang, C.; Deng, X.; Xu, Q. Retrotransposon promoter of Ruby1 controls both light- and cold-induced accumulation of anthocyanins in blood orange. Plant Cell Environ. 2019, 42, 3092–3104. [Google Scholar] [CrossRef]
- Habibi, F.; García-Pastor, M.E.; Puente-Moreno, J.; Garrido-Auñón, F.; Serrano, M.; Valero, D. Anthocyanin in blood oranges: A review on postharvest approaches for its enhancement and preservation. Crit. Rev. Food Sci. Nutr. 2022, 63, 12089–12101. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.L.; Arigò, A.; Cacciola, F.; De Gara, L.; Dugo, P.; Mondello, L. Blood orange (Citrus sinensis) as a rich source of nutraceuticals: Investigation of bioactive compounds in different parts of the fruit by HPLC-PDA/MS. Nat. Prod. Res. 2021, 35, 4606–4610. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent For Obesity. Drug Des. Dev. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crops Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Lemos da Silva, L.A.; de Athayde, A.E.; Moreira, M.A.; Tizziani, T.; Gkionis, S.V.; da Silva, L.V.; Biavatti, M.W.; de Moraes, A.C.R.; dos Santos Nascimento, M.V.P.; Dalmarco, E.M.; et al. Anti-inflammatory and anti-aggregating effects of rangpur in the first trimester of growth: Ultra-performance liquid chromatography–electrospray mass spectrometry profile and quantification of hesperidin. J. Sci. Food Agric. 2022, 102, 4151–4161. [Google Scholar] [CrossRef]
- Kim, Y.D.; Ko, W.J.; Koh, K.S.; Jeon, Y.J.; Kim, S.H. Composition of Flavonoids and Antioxidative Activity from Juice of Jeju Native Citrus Fruits during Maturation. Kjn 2009, 42, 278–290. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid Composition and Antioxidant Activity of Juices from Chinotto (Citrus × myrtifolia Raf.) Fruits at Different Ripening Stages. J. Agric. Food Chem. 2010, 58, 3031–3036. [Google Scholar] [CrossRef]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: An update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef]
- Razavi, B.M.; Hosseinzadeh, H. Chapter 34—A Review of the Effects of Citrus paradisi (Grapefruit) and Its Flavonoids, Naringin, and Naringenin in Metabolic Syndrome. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 515–543. [Google Scholar]
- Gupta, A.K.; Das, S.; Sahu, P.P.; Mishra, P. Design and development of IDE sensor for naringin quantification in pomelo juice: An indicator of citrus maturity. Food Chem. 2022, 377, 131947. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.-L.; Li, P.; Liu, E.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, R.; Chen, J.; Cao, J.; Xiao, J.; Li, X.; Sun, C. Tangeretin maintains antioxidant activity by reducing CUL3 mediated NRF2 ubiquitination. Food Chem. 2021, 365, 130470. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Wei, C.-C.; Huang, J.; Pan, M.-H.; Shahidi, F.; Ho, C.-T. Anti-inflammatory effects of polymethoxyflavones from citrus peels: A review. J. Food Bioact. 2018, 3, 76–86. [Google Scholar] [CrossRef]
- Lai, C.-S.; Ho, M.-H.; Tsai, M.-L.; Li, S.; Badmaev, V.; Ho, C.-T.; Pan, M.-H. Suppression of Adipogenesis and Obesity in High-Fat Induced Mouse Model by Hydroxylated Polymethoxyflavones. J. Agric. Food Chem. 2013, 61, 10320–10328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, S.; Ho, C.-T.; Huang, Q. Citrus polymethoxyflavones as regulators of metabolic homoeostasis: Recent advances for possible mechanisms. Trends Food Sci. Technol. 2021, 110, 743–753. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, H.; Chen, J.; Cao, J.; Chen, Q.; Li, X.; Sun, C. Polymethoxyflavones from citrus inhibited gastric cancer cell proliferation through inducing apoptosis by upregulating RARβ, both in vitro and in vivo. Food Chem. Toxicol. 2020, 146, 111811. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lo, C.-Y.; Ho, C.-T. Hydroxylated Polymethoxyflavones and Methylated Flavonoids in Sweet Orange (Citrus sinensis) Peel. J. Agric. Food Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef]
- Stohs, S.J. Safety, Efficacy, and Mechanistic Studies Regarding Citrus aurantium (Bitter Orange) Extract and p-Synephrine. Phytother. Res. 2017, 31, 1463–1474. [Google Scholar] [CrossRef]
- Arbo, M.D.; Larentis, E.R.; Linck, V.M.; Aboy, A.L.; Pimentel, A.L.; Henriques, A.T.; Dallegrave, E.; Garcia, S.C.; Leal, M.B.; Limberger, R.P. Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem. Toxicol. 2008, 46, 2770–2775. [Google Scholar] [CrossRef]
- Dragull, K.; Breksa Iii, A.P.; Cain, B. Synephrine Content of Juice from Satsuma Mandarins (Citrus unshiu Marcovitch). J. Agric. Food Chem. 2008, 56, 8874–8878. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, H.; Agar, O.T.; Imran, A.; de Souza, T.S.P.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Phytochemicals in finger lime and their potential health benefits: A review. Food Rev. Int. 2023, 40, 2167–2187. [Google Scholar] [CrossRef]
- Cioni, E.; Migone, C.; Ascrizzi, R.; Muscatello, B.; De Leo, M.; Piras, A.M.; Zambito, Y.; Flamini, G.; Pistelli, L. Comparing Metabolomic and Essential Oil Fingerprints of Citrus australasica F. Muell (Finger Lime) Varieties and Their In Vitro Antioxidant Activity. Antioxidants 2022, 11, 2047. [Google Scholar] [CrossRef]
- Cáceres-Vélez, P.R.; Ali, A.; Fournier-Level, A.; Dunshea, F.R.; Jusuf, P.R. Phytochemical and Safety Evaluations of Finger Lime, Mountain Pepper, and Tamarind in Zebrafish Embryos. Antioxidants 2022, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Singh, A.; Reddell, P.; Münch, G. Anti-inflammatory activity of prenyl and geranyloxy furanocoumarins from Citrus garrawayi (Rutaceae). Phytochem. Lett. 2018, 27, 197–202. [Google Scholar] [CrossRef]
- Dugrand, A.; Olry, A.; Duval, T.; Hehn, A.; Froelicher, Y.; Bourgaud, F. Coumarin and Furanocoumarin Quantitation in Citrus Peel via Ultraperformance Liquid Chromatography Coupled with Mass Spectrometry (UPLC-MS). J. Agric. Food Chem. 2013, 61, 10677–10684. [Google Scholar] [CrossRef] [PubMed]
- Hayat, F.; Li, J.; Liu, W.; Li, C.; Song, W.; Iqbal, S.; Khan, U.; Umer Javed, H.; Ahsan Altaf, M.; Tu, P.; et al. Influence of Citrus Rootstocks on Scion Growth, Hormone Levels, and Metabolites Profile of ’Shatangju’ Mandarin (Citrus reticulata Blanco). Horticulturae 2022, 8, 608. [Google Scholar] [CrossRef]
- Zhao, J.; Agboola, S.O. Functional Properties of Australian Bushfoods: A Report for the Rural Industries Research and Development Corporation; 1741514290; Rural Industries Research and Development Corporation: Canberra, Australia, 2007.
- Sirdaarta, J.; Maen, A.; Rayan, P.; Matthews, B.; Cock, I.E. High performance liquid chromatography-mass spectrometry analysis of high antioxidant australian fruits with antiproliferative activity against cancer cells. Pharmacogn. Mag. 2016, 12, S181. [Google Scholar] [CrossRef]
- Berhow, M.; Tisserat, B.; Kanes, K.; Vandercook, C. Survey of Phenolic Compounds Produced in Citrus; United States Department of Agriculture: Peoria, IL, USA, 1998.
- Kanes, K.; Tisserat, B.; Berhow, M.; Vandercook, C. Phenolic composition of various tissues of rutaceae species. Phytochemistry 1993, 32, 967–974. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Huey, P.F. Coumarins in Eremocitrus glauca. Phytochemistry 1974, 13, 1237–1239. [Google Scholar] [CrossRef]
- Bashir, R. Bio-Analytical Screening and Characterization of Antioxidant Compounds Using Online Liquid Chromatography Techniques and Mass Spectrometry. Ph.D. Thesis, Swinburne University of Technology, Melbourne, Australia, 2017. [Google Scholar]
- Harada, K. Volatile Constituents Identified from Finger Lime (Citrus australasica). Fragrances 2017, 273, 81–86. [Google Scholar]
- D’Auria, M.; Racioppi, R. Volatile organic compounds from Citrus australasica growing in Basilicata (Southern Italy). Nat. Prod. Res. 2022, 37, 3302–3305. [Google Scholar] [CrossRef]
- Cozzolino, R.; Câmara, J.S.; Malorni, L.; Amato, G.; Cannavacciuolo, C.; Masullo, M.; Piacente, S. Comparative Volatilomic Profile of Three Finger Lime (Citrus australasica) Cultivars Based on Chemometrics Analysis of HS-SPME/GC–MS Data. Molecules 2022, 27, 7846. [Google Scholar] [CrossRef]
- de Sousa, I.M.P. Desenvolvimento de Metodologias de Biologia Molecular Para a Deteção de Ephedra Sinica e Citrus Aurantium em Infusões e Suplementos Alimentares à Base de Plantas. Master’s Thesis, Universidade do Porto, Porto, Portugal, 2016. [Google Scholar]
- Cannavacciuolo, C.; Pagliari, S.; Giustra, C.M.; Carabetta, S.; Guidi Nissim, W.; Russo, M.; Branduardi, P.; Labra, M.; Campone, L. LC-MS and GC-MS Data Fusion Metabolomics Profiling Coupled with Multivariate Analysis for the Discrimination of Different Parts of Faustrime Fruit and Evaluation of Their Antioxidant Activity. Antioxidants 2023, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.S.M.; Srzednicki, G.; Craske, J.D. Aroma compounds in minor citrus species grown in Australia. In Proceedings of the Southeast Asia Symposium on Quality and Safety of Fresh and Fresh-Cut Produce, Bangkok, Thailand, 3–5 August 2009; pp. 341–350. [Google Scholar]
- Zibaee, E.; Kamalian, S.; Tajvar, M.; Amiri, M.S.; Ramezani, M.; Moghadam, A.T.; Emami, S.A.; Sahebkar, A. Citrus species: A Review of Traditional Uses, Phytochemistry and Pharmacology. Curr. Pharm. Des. 2020, 26, 44–97. [Google Scholar] [CrossRef]
- Briskey, D.; Malfa, G.A.; Rao, A. Effectiveness of "Moro" Blood Orange Citrus sinensis Osbeck (Rutaceae) Standardized Extract on Weight Loss in Overweight but Otherwise Healthy Men and Women—A Randomized Double-Blind Placebo-Controlled Study. Nutrients 2022, 14, 427. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Liu, F.; Cui, Y.; Li, X. Dietary citrus and/or its extracts intake contributed to weight control: Evidence from a systematic review and meta-analysis of 13 randomized clinical trials. Phytother. Res. 2020, 34, 2006–2022. [Google Scholar] [CrossRef]
- Santos, K.G.d.; Yoshinaga, M.Y.; Glezer, I.; Chaves-Filho, A.d.B.; Santana, A.A.d.; Kovacs, C.; Magnoni, C.D.; Lajolo, F.M.; Miyamoto, S.; Aymoto Hassimotto, N.M. Orange juice intake by obese and insulin-resistant subjects lowers specific plasma triglycerides: A randomized clinical trial. Clin. Nutr. ESPEN 2022, 51, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Q.; Kuriyama, S.; Li, Q.; Nagai, M.; Hozawa, A.; Nishino, Y.; Tsuji, I. Citrus consumption and cancer incidence: The Ohsaki cohort study. Int. J. Cancer 2010, 127, 1913–1922. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef]
- Visvanathan, R.; Williamson, G. Effect of citrus fruit and juice consumption on risk of developing type 2 diabetes: Evidence on polyphenols from epidemiological and intervention studies. Trends Food Sci. Technol. 2021, 115, 133–146. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Cornara, L.; Xiao, J.; Smeriglio, A.; Trombetta, D.; Burlando, B. Emerging Exotic Fruits: New Functional Foods in the European Market. Efood 2020, 1, 126–139. [Google Scholar] [CrossRef]
- Ryan, M.; Maguire, G. Adult stem cell released molecules in combination with microneedling restore hair growth. Int. J. Sci. Res. 2022, 11, 5–7. [Google Scholar] [CrossRef]
- Lee, C.J.; Wright, M.H.; Jean Arnold, M.S.; Greene, A.C.; Cock, I.E. Inhibition of Streptococcus pyogenes growth by native Australian plants: New approaches towards the management of impetigo, pharyngitis and rheumatic heart disease. Pharmacogn. Commun. 2016, 6, 164–173. [Google Scholar]
- Wright, M.H.; Matthews, B.; Greene, A.C.; Cock, I.E. Growth inhibition of the zoonotic bacteria Bacillus anthracis by high antioxidant Australian plants: New leads for the prevention and treatment of anthrax. Pharmacogn. Commun. 2015, 5, 173–189. [Google Scholar] [CrossRef]
- Vélez, P.R.C.; Ali, A.; Fournier-Level, A.; Dunshea, F.; Jusuf, P.R. P06-04 Antioxidant activity and embryotoxicity of Citrus australasica, Tasmannia lanceolata and Diploglottis australis extracts in zebrafish. Toxicol. Lett. 2022, 368, S114. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Citrus Fruit-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2021, 40, 5S–38S. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-N.; Tai, Y.-N.; Dong, M.; Shao, J.-H.; Yang, S.-Z.; Pan, S.-Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Delort, E.; Jaquier, A.; Decorzant, E.; Chapuis, C.; Casilli, A.; Frérot, E. Comparative analysis of three Australian finger lime (Citrus australasica) cultivars: Identification of unique citrus chemotypes and new volatile molecules. Phytochemistry 2015, 109, 111–124. [Google Scholar] [CrossRef]
- Killiny, N.; Jones, S.E.; Nehela, Y.; Hijaz, F.; Dutt, M.; Gmitter, F.G.; Grosser, J.W. All roads lead to Rome: Towards understanding different avenues of tolerance to huanglongbing in citrus cultivars. Plant Physiol. Biochem. 2018, 129, 1–10. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A. Novel terpenyl esters from Australian finger lime (Citrus australasica) peel extract. Flavour Fragr. J. 2009, 24, 123–132. [Google Scholar] [CrossRef]
- Kvittingen, L.; Sjursnes, B.J.; Schmid, R. Limonene in Citrus: A String of Unchecked Literature Citings? J. Chem. Educ. 2021, 98, 3600–3607. [Google Scholar] [CrossRef]
- Bonaccorsi, I.; Sciarrone, D.; Cotroneo, A.; Mondello, L.; Dugo, P.; Dugo, G. Enantiomeric distribution of key volatile components in Citrus essential oils. Rev. Bras. De Farmacogn. 2011, 21, 841–849. [Google Scholar] [CrossRef]
- Cucinotta, L.; Giovanna, C.; Filippo, A.; Marina, R.; Danilo, S.; Luigi, M.; Mondello, M. Characterization of oxygen heterocyclic compounds and volatile fraction of Citrus australasica finger lime peel essential oil exploiting a multi-technique chromatographic approach. J. Essent. Oil Res. 2025, 37, 46–55. [Google Scholar] [CrossRef]
- Craske, J.D.; Suryadi, N.; Wootton, M. A comparison of the peel oil components of Australian native lime (Microcitrus australe) and Mexican lime (Citrus aurantifolia Swingle). J. Sci. Food Agric. 2005, 85, 522–525. [Google Scholar] [CrossRef]
- Lota, M.-L.; de Rocca Serra, D.; Tomi, F.; Jacquemond, C.; Casanova, J. Volatile Components of Peel and Leaf Oils of Lemon and Lime Species. J. Agric. Food Chem. 2002, 50, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Rocco, C.; Rapisarda, P. Chemical Composition of the Peel Essential Oil of Microcitrus australasica var. sanguinea (F.M. Bail) Swing. J. Essent. Oil Res. 2000, 12, 379–382. [Google Scholar] [CrossRef]
- Bowman, K.D.; McCollum, G.; Plotto, A.; Bai, J. Minnie Finger Lime: A New Novelty Citrus Cultivar. HortScience Horts 2019, 54, 1425–1428. [Google Scholar] [CrossRef]
- Trozzi, A.; Verzera, A.; d’Alcontres, I.S. Constituents of the Cold-Pressed Oil of Faustrime, A Trigeneric Hybrid of Monocitrus australasica x Fortunella sp. x Citrus aurantifolia. J. Essent. Oil Res. 1993, 5, 97–100. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Zappia, G.; Bonaccorsi, I.; Cotroneo, A.; Russo, M.T. The Composition of the Volatile Fraction and the Enantiomeric Distribution of Five Volatile Components of Faustrime Oil (Monocitrus australatica x Fortunella sp. x Citrus urantifolia). J. Essent. Oil Res. 2004, 16, 328–333. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Citrus Peel-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2021, 40, 77S–99S. [Google Scholar] [CrossRef]
- Grieve, C.M.; Scora, R.W. Flavonoid Distribution in the Aurantioideae (Rutaceae). Syst. Bot. 1980, 5, 39–53. [Google Scholar] [CrossRef]
- Bar-Peled, M. Purification and Characterization of 1-2-Rhamnosyl Transferases Catalyzing the Production of Bitter Flavonoids, and Their Juvenile-Specific Accumulation in Foliage, Flower, and Citrus Fruit. Ph.D. Thesis, The Weizmann Institute of Science (Israel), Rehovot, Israel, 1993. [Google Scholar]
- Mpala, L.; Chikowe, G.; Cock, I.E. Citrus australasica F. Muell. Leaf Extracts are Ineffective Inhibitors of the Growth of a Panel of Bacterial Pathogens. Pharmacogn. Commun. 2022, 12, 126–131. [Google Scholar] [CrossRef]
- Webb, L.J. An Australian Phytochemical Survey. II. Alkaloids in Queensland Flowering Plants; CSIRO: Melbourne, Australia, 1952.
- Collins, D.J.; Culvenor, C.C.J.; A., L.J.; Loder, J.W.; Price, J.R. Plants for Medicines; CSIRO: Melbourne, Australia, 1990.
- Katayama, Y.; Nito, N.; Machino, J. Essential Oils in Genus Microcitrus. Bull. Fac. Agric. Saga Univ. 1994, 77, 47–51. [Google Scholar]
- Lin, Z.-k.; Hua, Y.-f.; Gu, Y.-h. The Chemical Constituents of the Essential Oil from the Flowers, Leaves and Peels of Citrus aurantium. Act. Bot. Sin 1986, 28, 641–645. [Google Scholar]
- Azam, M.; Jiang, Q.; Zhang, B.; Xu, C.; Chen, K. Citrus Leaf Volatiles as Affected by Developmental Stage and Genetic Type. Int. J. Mol. Sci. 2013, 14, 17744–17766. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.L. Citrus bitter principles—V.: Botanical distribution and chemotaxonomy in the rutaceae. Phytochemistry 1966, 5, 367–378. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Citrus Plant- and Seed-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2021, 40, 39S–52S. [Google Scholar] [CrossRef]
- Altman, A.; Goell, A. Chloride Content of Citrus Seeds and its Distribution in Various Fractions. J. Am. Soc. Hortic. Sci. 1970, 95, 193–195. [Google Scholar] [CrossRef]
- Nand, N. Application of Biotechnology to Davidsonia Species: An Australian Native Plum. Ph.D. Thesis, Griffith University, Brisbane, Australia, 2008. [Google Scholar]
- Oliver, G.W. The Seedling-Inarch and Nurse-Plant Methods of Propagation; US Government Printing Office: Washington, DC, USA, 1911.
- Sykes, S.R.; Broadbent, P.; Bevington, K.B. Conservation and evaluation of citrus germplasm from the Peoples’ Republic of China and Vietnam. Int. Symp. Trop. Subtrop. Fruits 2002, 575, 231–236. [Google Scholar] [CrossRef]
- Singh, A.; Evans, E.A.; Wasielewski, J.; Dutt, M.; Grosser, J. Finger Lime: An Alternative Crop with Great Potential in South Florida. EDIS 2018, 1. [Google Scholar] [CrossRef]
- de Melo Virginio Filho, E.; Somarriba, E.; Cerda, R.; Casanoves, F.; Cordero, C.A.; Avelino, J.; Roupsard, O.; Rapidel, B.; Vaast, P.; Harmand, J.-M.; et al. Aportes a la investigación, fortalecimiento de capacidades y formulación de políticas para el sector cafetalero en 20 años de ensayos de sistemas agroforestales con café. AgroForestería En Las Américas 2021, 51, 107–151. [Google Scholar]
- Zhu, Z.; Johnson, J.; Zaman, Q.U.; Wang, H. Challenges and opportunities to improve tropical fruits in Hainan, China. Trop. Plants 2022, 1, 13. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Perry, K.R.; Alagappan, S.; Kolobaric, A.; King, J.M.; Hoffman, E.W.; Fitzgerald, M.A.; Cozzolino, D. Exploring the use of portable infrared spectroscopy as a quality assurance tool for the Australian Native/Traditional food industry: A case study on finger lime (Citrus australasica). Int. J. Food Sci. Technol. 2024, 59, 7570–7579. [Google Scholar] [CrossRef]
- Hardy, S.; Wilk, P.; Viola, J.; Rennie, S. Growing Australian native finger limes. PrimeFacts 2010, 979, 1–11. [Google Scholar]
- Drew, R.A.; Bailey, R. Australian tropical fruits: A source of distinctive flavours. In II International Symposium on Beverage Crops; ISHS Acta Horticulturae: Bierbeek, Belgium, 2020; pp. 41–56. [Google Scholar]
- Tryon, H. Report on Insect and Fungus Pests No. 1; Government Printer: Pretoria, South Africa, 1889. [Google Scholar]
- Lim, T.K. Citrus australis. In Edible Medicinal and Non-Medicinal Plants: Volume 4, Fruits; Springer: Dordrecht, The Netherlands, 2012; pp. 629–630. [Google Scholar]
- Phelps, D.D.G. Feasibility of a Sustainable Bush Food Industry in Western Queensland; Rural Industries Research and Development Corporation: Wagga, Australia, 1997.
- Ahmed, A.K.; Johnson, K.A. Horticultural development of Australian native edible plants. Aust. J. Bot. 2000, 48, 417–426. [Google Scholar] [CrossRef]
- Esen, A.; Scora, R.W.; Soost, R.K. A Simple and Rapid Screening Procedure for Identification of Zygotic Citrus Seedlings Among Crosses of Certain Taxa. J. Am. Soc. Hortic. Sci. 1975, 100, 558–561. [Google Scholar] [CrossRef]
- Hallaby, S.F. Further Investigation of Australian Native Orange (Capparis Mitchellii) and Native Lime (Citrus Glauca) Micropropagation. Master’s Thesis, University of Adelaide, Adelaide, Australia, 2012. [Google Scholar]
- Gasic, K.; Preece, J.E.; Karp, D. Register of New Fruit and Nut Cultivars List 50. HortScience Horts 2020, 55, 1164–1201. [Google Scholar] [CrossRef]
- Herrero, R.; Asíns, M.J.; Pina, J.A.; Carbonell, E.A.; Navarro, L. Genetic diversity in the orange subfamily Aurantioideae. II. Genetic relationships among genera and species. Theor. Appl. Genet. 1996, 93, 1327–1334. [Google Scholar] [CrossRef]
- Nakandala, U.; Masouleh, A.K.; Smith, M.W.; Furtado, A.; Mason, P.; Constantin, L.; Henry, R.J. Haplotype resolved chromosome level genome assembly of Citrus australis reveals disease resistance and other citrus specific genes. Hortic. Res. 2023, 10, uhad058. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Xiao, J.; Cornara, L.; Burlando, B.; Trombetta, D. New insights into Citrus genus: From ancient fruits to new hybrids. Food Front. 2020, 1, 305–328. [Google Scholar] [CrossRef]
- Hamilton, K.N.; Ashmore, S.E.; Drew, R.A. Morphological characterization of seeds of three Australian wild Citrus species (Rutaceae): Citrus australasica F. Muell., C. inodora F.M. Bailey and C. garrawayi F.M. Bailey. Genet. Resour. Crop Evol. 2008, 55, 683–693. [Google Scholar] [CrossRef]
- Hamilton, K.N.; Ashmore, S.E.; Drew, R.A. Development of conservation strategies for Citrus species of importance to Australia. In Proceedings of the International Symposium on Harnessing the Potential of Horticulture in the Asian-Pacific Region, Coolum, Australia, 1–3 September 2005; pp. 111–115. [Google Scholar]
- Finlay, K.J.; Yen, A.L.; Aurambout, J.P.; Beattie, G.A.C.; Barkley, P.; Luck, J.E. Consequences for Australian biodiversity with establishment of the Asiatic Citrus Psyllid, Diaphorina citri, under present and future climates. Biodiversity 2009, 10, 25–32. [Google Scholar] [CrossRef]
- Hamilton, K.N. Ex Situ Conservation of Australian Citrus Species: Investigations on Seed Biology, Cryopreservation and in Vitro Culture. Ph.D. Thesis, Griffith University, Brisbane, Australia, 2007. [Google Scholar]
- Citrus Australia. Regions. Available online: https://citrusaustralia.com.au/growers-industry/regions/ (accessed on 21 June 2025).
- Maiden, J.H. The chemistry of the Australian indigenous vegetation. Proc. Australas. Assoc. Adv. Sci. 1895, 6, 25–57. [Google Scholar]

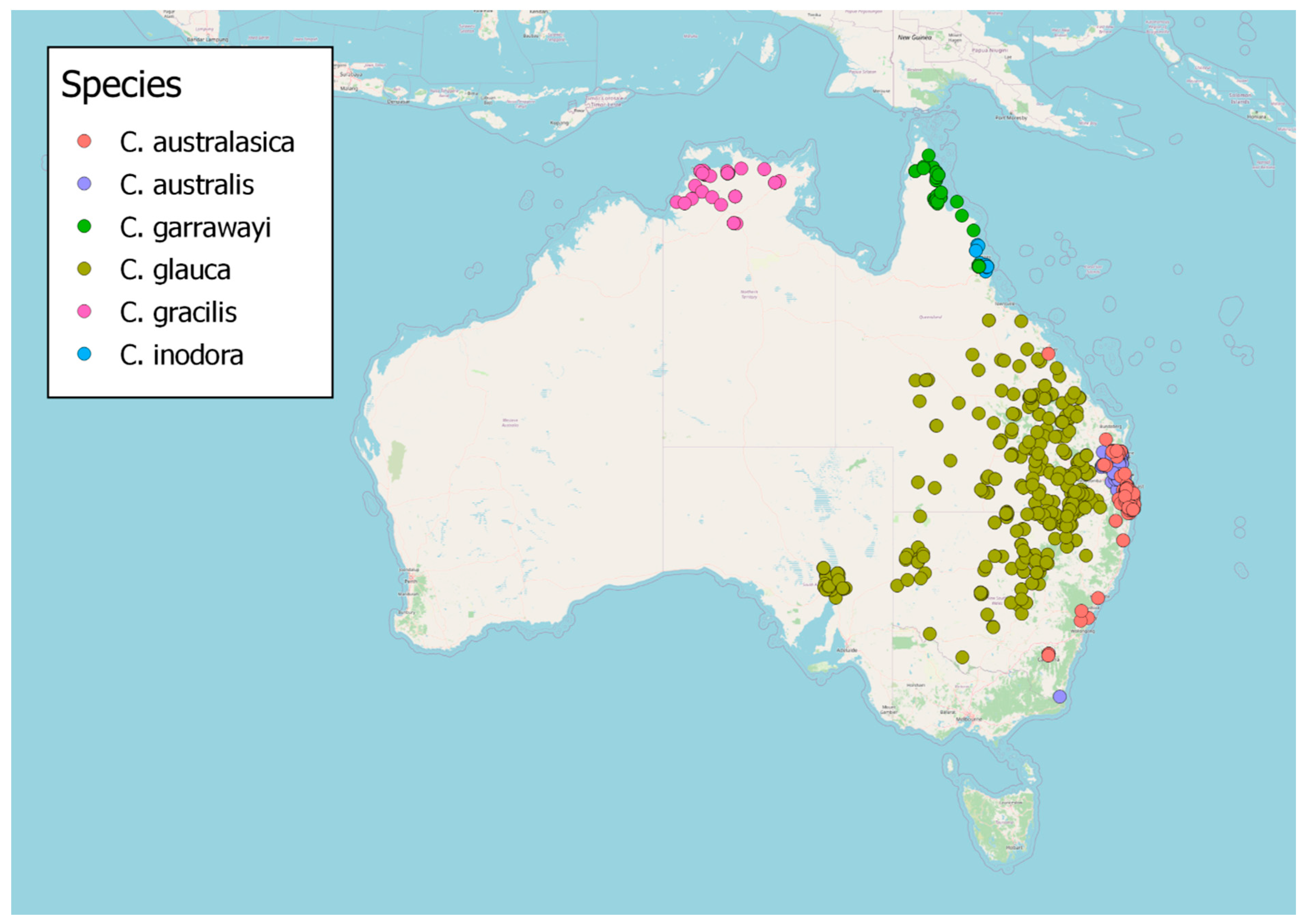
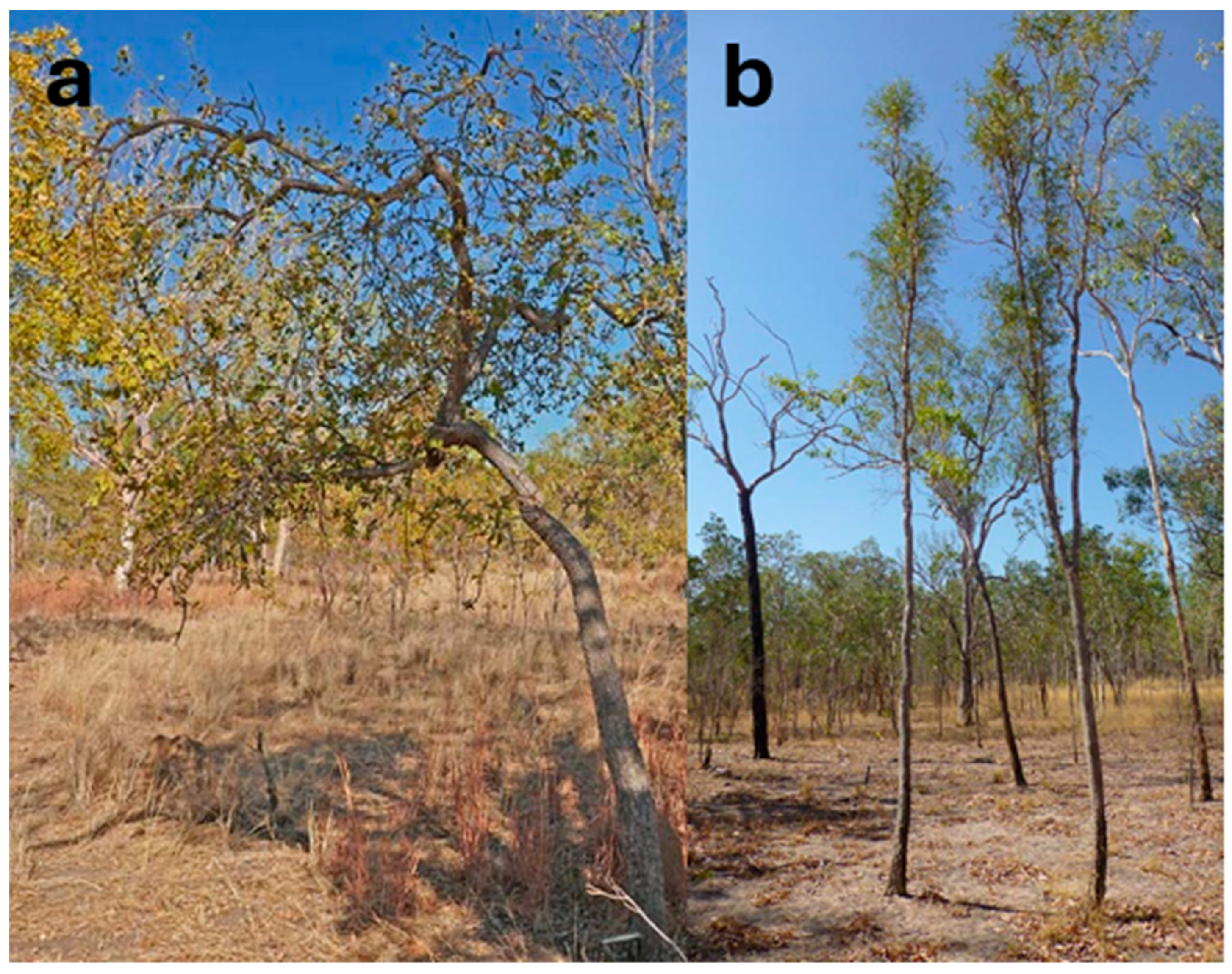
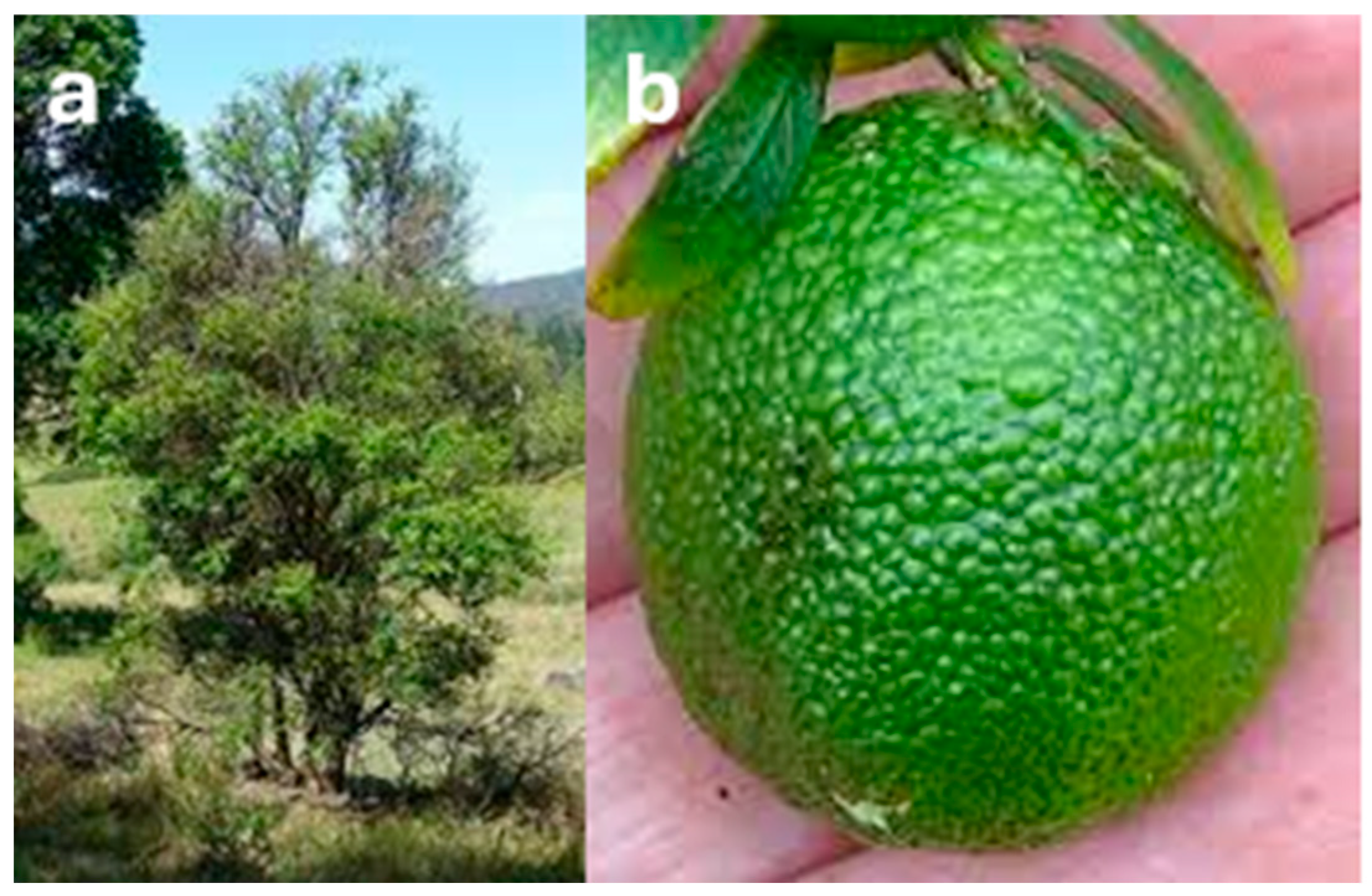

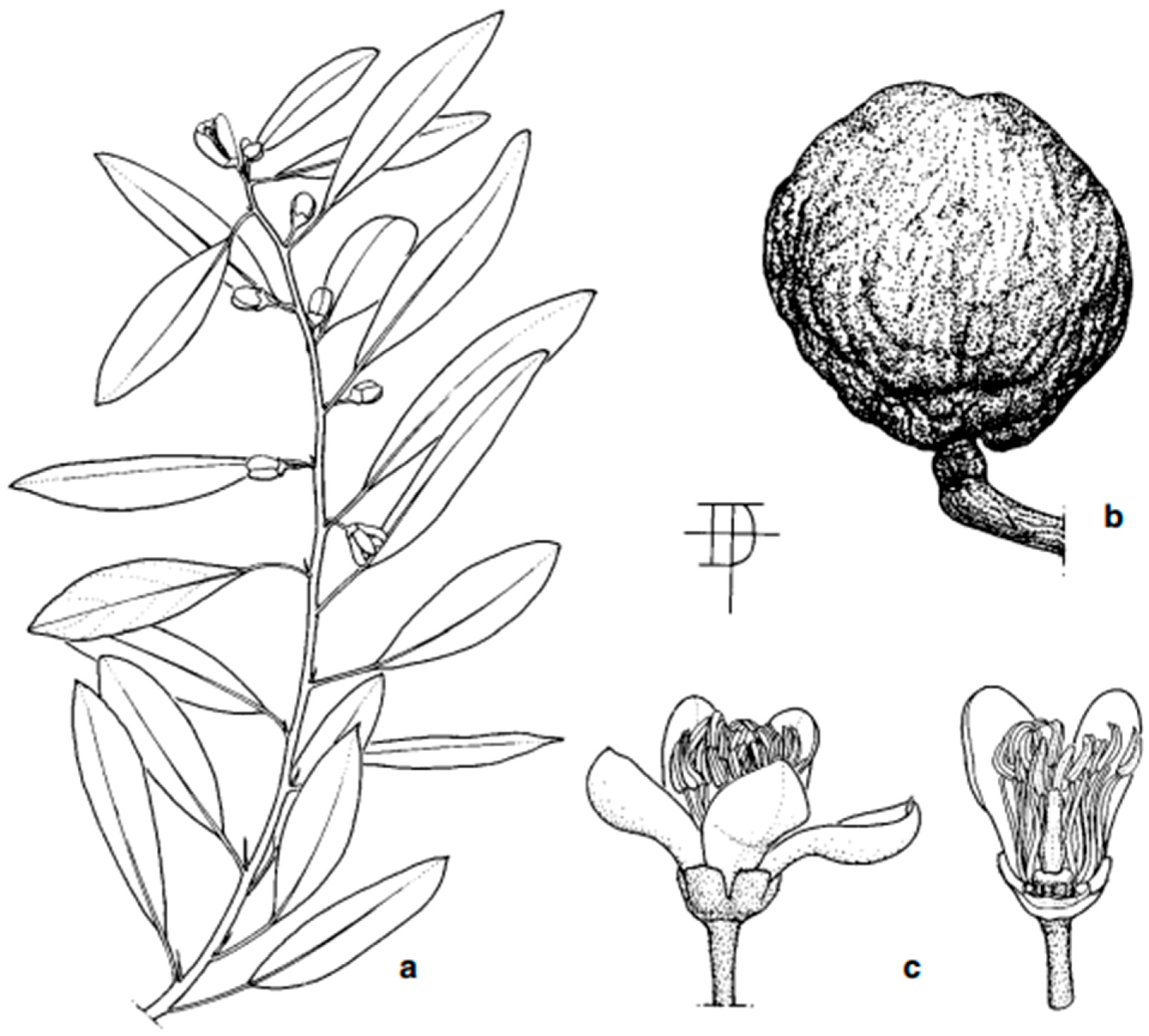
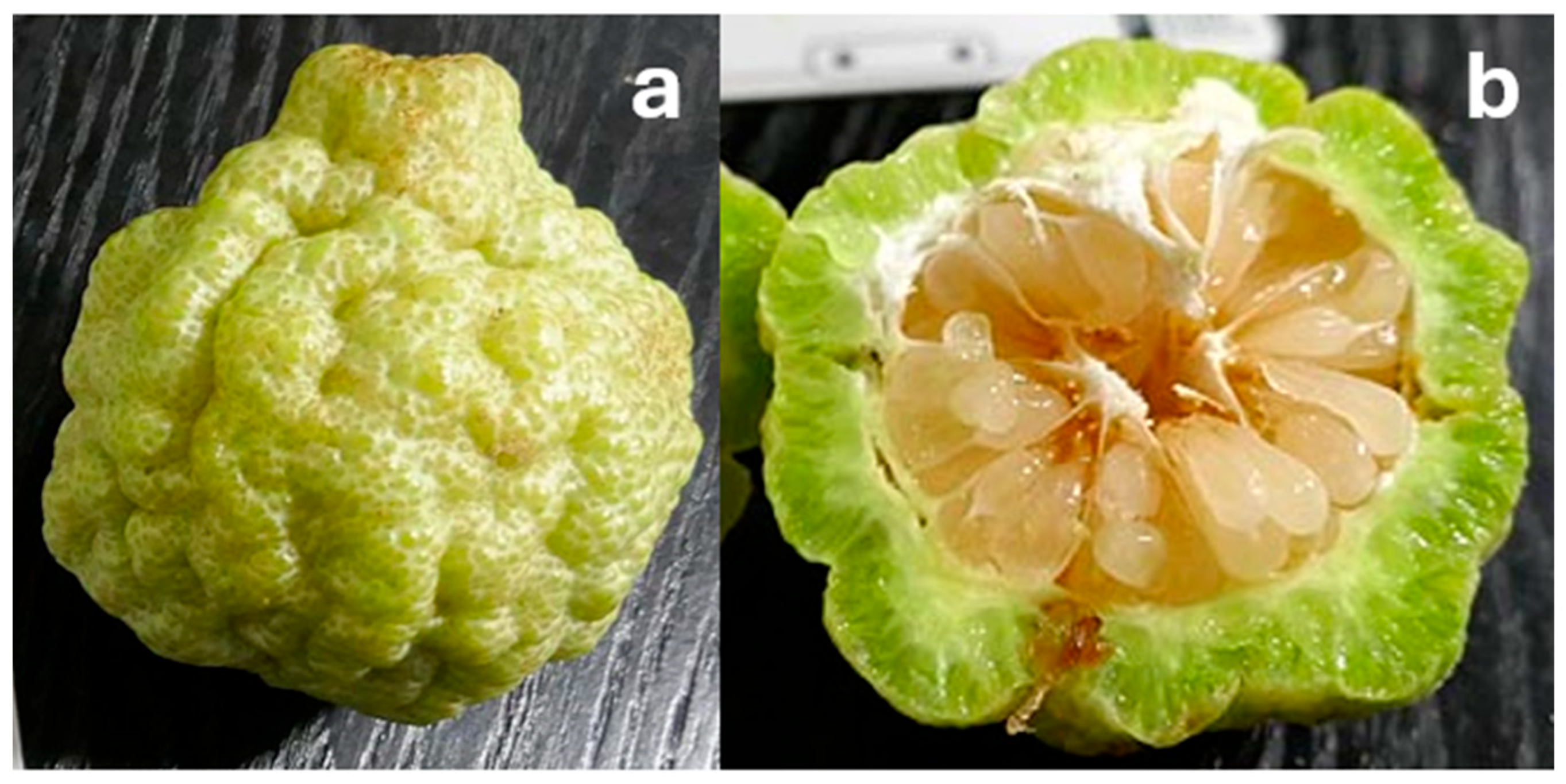



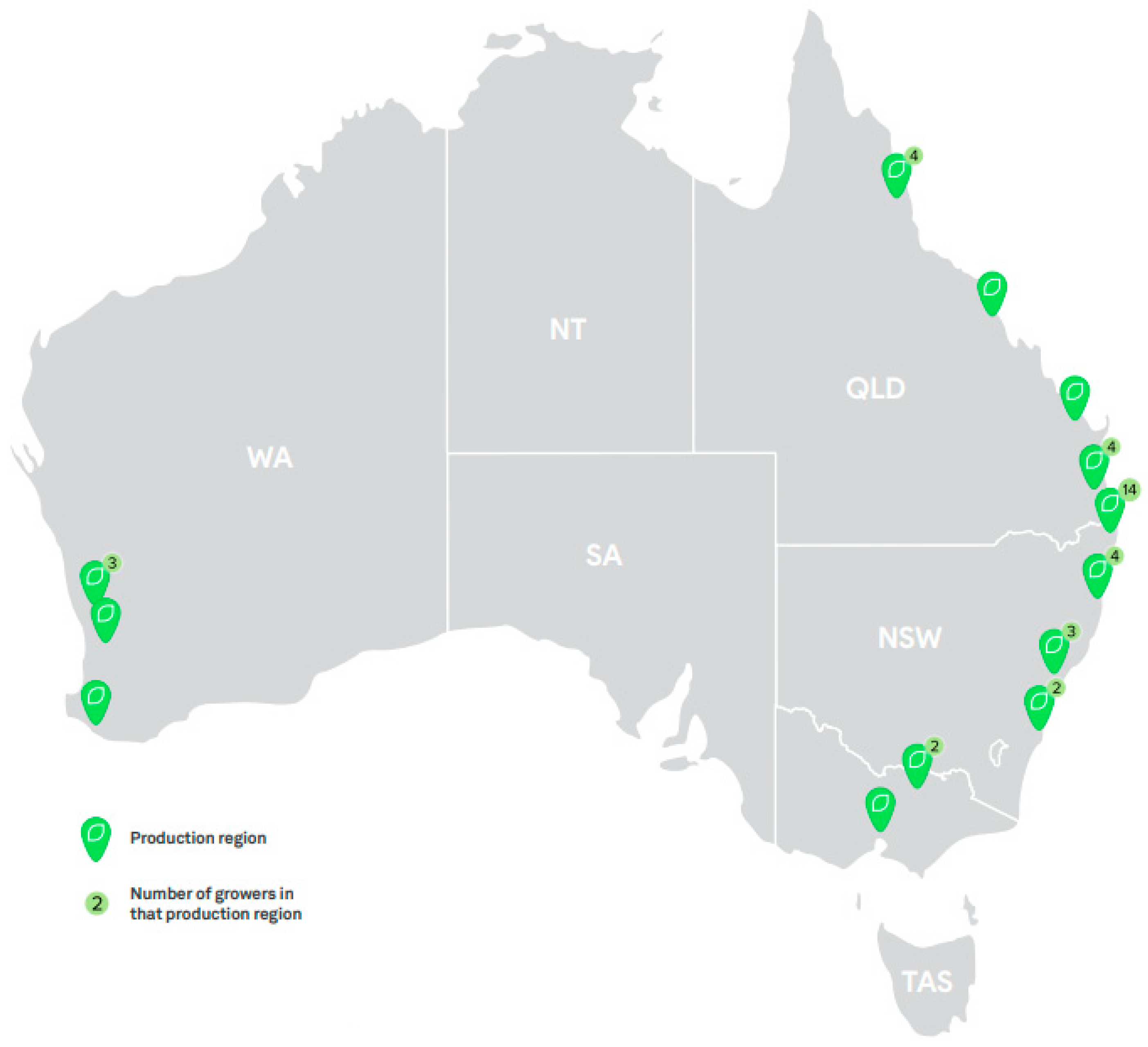
| Citrus Type | 1961 Production (t) | 2021 Production (t) | % Increase |
|---|---|---|---|
| Oranges | 15,976,472 | 75,567,952 | 373% |
| Tangerines, mandarins, clementines | 2,835,347 | 41,950,302 | 1380% |
| Pomelos and grapefruits | 2,163,741 | 9,556,999 | 342% |
| Other citrus | 1,470,554 | 13,896,889 | 845% |
| Total | 22,446,114 | 140,972,142 | 528% |
| Citrus Variety | Growing Location | Glucose | Fructose | Sucrose | Reference |
|---|---|---|---|---|---|
| Grapefruit | Spain | 1.7 | 2.4 | 3.5 | [239] |
| Grapefruit | Turkey | 2.4 | 2.3 | 4.6 | [240] |
| Grapefruit | China | 1.1–3.9 | 1.3–4.8 | 1.9–6.6 | [241] |
| Grapefruit | China | 1.6–2.4 | 1.6–2.4 | 2.0–5.5 | [242] |
| Lemon | Spain | 0.5–4 | 2.7–3.0 | 0.2–2.1 | [239] |
| Lime | Spain | 2.4 | 0.2 | 0.3 | [239] |
| Mandarin | Spain | 0.4–0.6 | 1.8–2.4 | 9.5–11.9 | [239] |
| Mandarin | Turkey | 2.3–3.5 | 2.5–3.4 | 2.6–8.8 | [240] |
| Mandarin | USA | 1.0–1.9 | 1.4–2.4 | 4.5–7.1 | [218] |
| Orange | Turkey | 1.5 | 1.6 | 3.5 | [240] |
| Orange | USA | 1.0–2.6 | 1.9–2.7 | 3.3–5.2 | [218] |
| Citrus Variety | Origin | L-Ascorbic Acid | Dehydroascorbic Acid | Total Vitamin C | Reference |
|---|---|---|---|---|---|
| Grapefruit | USA | 21.3 | 2.3 | 24 | [259] |
| Mandarin, Satsuma | China | 30.5 | 1.2 | 32 | [260] |
| Mandarin, Ellendale | QLD, Australia | 34.0 | 3.7 | 38 | [261] |
| Orange Juice | USA | 32.6 | 8.7 | 41 | [259] |
| Orange | China | 57.8 | 5.0 | 63 | [260] |
| Orange, Florida | Florida, USA | 54.7 | 8.3 | 63 | [259] |
| Lemon | QLD, Australia | 50.4 | 23.9 | 74 | [261] |
| Orange, Navel | California, USA | 75.0 | 8.2 | 83 | [259] |
| Citrus Variety | Thiamine (B1) | Riboflavin (B2) | Niacin (B3) | Pantothenic Acid (B5) | Pyridoxine (B6) | Total Folate (B9) |
|---|---|---|---|---|---|---|
| Orange | 68 | 51 | 425 | 261 | 79 | 25 |
| Lemon | 40 | 20 | 100 | 190 | 80 | 11 |
| Tangerine | 58 | 36 | 376 | 216 | 78 | 16 |
| Lime | 30 | 20 | 200 | 217 | 43 | 8 |
| Grapefruit (white) | 40 | 20 | 200 | 283 | 42 | 9 |
| RDI (men) | 1200 | 1300 | 16,000 | 5000 | 1700 | 400 |
| RDI (women) | 1100 | 1100 | 14,000 | 5000 | 1300 | 400 |
| Species/Variety | Growing Location | Vitamin C | Reference |
|---|---|---|---|
| C. australasica | |||
| Chartreuse | Bundaberg, QLD | 23.0 ± 1.3 | [284] |
| Durham’s Emerald | Bundaberg, QLD | 34.6 ± 1.3 | [284] |
| Hybrid ‘P1f2-10’ | Bundaberg, QLD | 31.0 ± 0.5 | [284] |
| Red Champagne | Bundaberg, QLD | 53.8 ± 0.7 | [284] |
| Rhyne Red | Bundaberg, QLD | 33.0 ± 0.7 | [284] |
| ‘Red pulp’ (sanguinea type) | Florida, USA | 80 | [252] |
| ‘White pulp’ | Florida, USA | 35 | [252] |
| ‘Low-seeded, red pulp, large-leaved’ hybrid | Florida, USA | 57 | [252] |
| Sanguinea type 50–36 cultivar | Florida, USA | 115 | [252] |
| ‘Green’ | QLD, Australia | 26 ± 1 | [253] |
| ‘Pink’ | QLD, Australia | 91 ± 2 | [253] |
| ‘Red’ | Teven, NSW | 40.9 | [4] |
| ‘Yellow’ | Teven, NSW | 59.5 | [4] |
| var. sanguinea | Australia | 82 | [3] |
| Unknown | Lismore, NSW | 87.7 ± 5.5 | [285] |
| C. glauca | Unknown | 188 ± 5 | [85] |
| Tahitian lime | Bundaberg, QLD | 19.7 ± 0.2 | [284] |
| Species | C. australasica | C. glauca | |
|---|---|---|---|
| Cultivar | ‘Green’ | ‘Pink’ | |
| Vitamin E (total) | 0.521 ± 0.033 | 2.360 ± 0.235 | 0.783 ± 0.194 |
| α-tocopherol | 0.517 ± 0.033 | 2.335 ± 0.233 | 0.701 ± 0.177 |
| β-tocopherol | - | - | 0.081 ± 0.017 |
| γ-tocopherol | 0.004 ± 0.0004 | 0.025 ± 0.002 | BDL |
| δ-tocopherol | BDL | BDL | - |
| Folate | - | - | 0.082 |
| Lutein (provitamin A) | 0.401 ± 0.027 | 0.139 ± 0.011 | 0.295 ± 0.013 |
| Chlorophyll a | Trace | Trace | Trace |
| Chlorophyll b | Trace | Trace | 1.350 ± 0.044 |
| References | [226] | [226] | [226,228,253] |
| Variety | Most Abundant Compound | Second-Most Abundant Compound | Third-Most Abundant Compound | Reference |
|---|---|---|---|---|
| Collette # | β-caryophyllene (21.4%) | terpinen-4-ol (12.0%) | bicyclogermacrene (10.9%) | [319] |
| Pink Ice # | sabinene (31.7%) | d-limonene (20.7%) | bicyclogermacrene (14.2%) | [331] |
| Pink Ice # | terpinen-4-ol (19.3%) | caryophyllene oxide (15.6%) | β-caryophyllene (14.1%) | [319] |
| Pink Pearl # | d-limonene (32.2%) | γ-terpinene (15.8%) | terpinen-4-ol (15.2%) | [332] |
| ‘Red’ # | bicyclogermacrene (28.0%) | β-bisabolene (24.6%) | viridiflorol (10.9%) | [319] |
| Unspecified | d-limonene (71.1%) | β-bisabolene (5.2%) | β-phellandrene (3.4%) | [330] |
| Unspecified # | d-limonene (41.5%) | trans-sabinene hydrate (38.4%) | γ-terpinene (14.8%) | [333] |
| var. sanguinea # | d-limonene (45.6%) | sabinene (22.7%) | bicyclogermacrene (10.6%) | [331] |
| var. sanguinea # | d-limonene (66.8%) | γ-terpinene (5.5%) | ledene (4.1%) | [332] |
| Yellow Sunshine # | bicyclogermacrene (20.3%) | viridiflorol (19.9%) | globulol (14.5%) | [319] |
| Faustrime (C. australasica × C. × aurantiifolia) # | d-limonene (48.3%) | γ-terpinene (10.8%) | α-phellandrene (7.2%) | [319] |
| Faustrime (C. australasica × C. × aurantiifolia) # | d-limonene (35.7%) | β-phellandrene (23.5%) | γ-terpinene (12.6%) | [332] |
| Faustrime (C. australasica × C. × aurantiifolia) # | d-limonene (51.5%) | γ-terpinene (10.0%) | α-bergamotene (7.5%) | [331] |
| Faustrime (C. australasica × C. × aurantiifolia) # | linalyl acetate (18.2%) | d-limonene (11.4%) | citronellol (8.6%) | [334] |
| Commercial Tahitian lime (Citrus × latifolia) # | d-limonene (41.8%) | γ-terpinene (14.8%) | α-terpineol (10.6%) | [335] |
| Variety | Growing Location | Vitamin C | Reference |
|---|---|---|---|
| Chartreuse | Bundaberg, QLD | 26.0 ± 6.8 | [284] |
| Durham’s Emerald | Bundaberg, QLD | 34.2 ± 1.5 | [284] |
| Hybrid ‘P1f2-10’ | Bundaberg, QLD | 49.0 ± 5.9 | [284] |
| Red Champagne | Bundaberg, QLD | 42.2 ± 3.8 | [284] |
| Rhyne Red | Bundaberg, QLD | 21.3 ± 0.6 | [284] |
| Tahitian Lime | Bundaberg, QLD | 36.7 ± 4.6 | [284] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, J.B.; Hungerford, N.L.; Sultanbawa, Y.; Netzel, M.E. Unlocking the Sublime: A Review of Native Australian Citrus Species. Foods 2025, 14, 2425. https://doi.org/10.3390/foods14142425
Johnson JB, Hungerford NL, Sultanbawa Y, Netzel ME. Unlocking the Sublime: A Review of Native Australian Citrus Species. Foods. 2025; 14(14):2425. https://doi.org/10.3390/foods14142425
Chicago/Turabian StyleJohnson, Joel B., Natasha L. Hungerford, Yasmina Sultanbawa, and Michael E. Netzel. 2025. "Unlocking the Sublime: A Review of Native Australian Citrus Species" Foods 14, no. 14: 2425. https://doi.org/10.3390/foods14142425
APA StyleJohnson, J. B., Hungerford, N. L., Sultanbawa, Y., & Netzel, M. E. (2025). Unlocking the Sublime: A Review of Native Australian Citrus Species. Foods, 14(14), 2425. https://doi.org/10.3390/foods14142425