Punicic Acid: A Potential Nutraceutical Compound in Pomegranate Seed Oil and Its Cardiovascular Benefits
Abstract
1. Introduction
2. Methodology of Literature Review
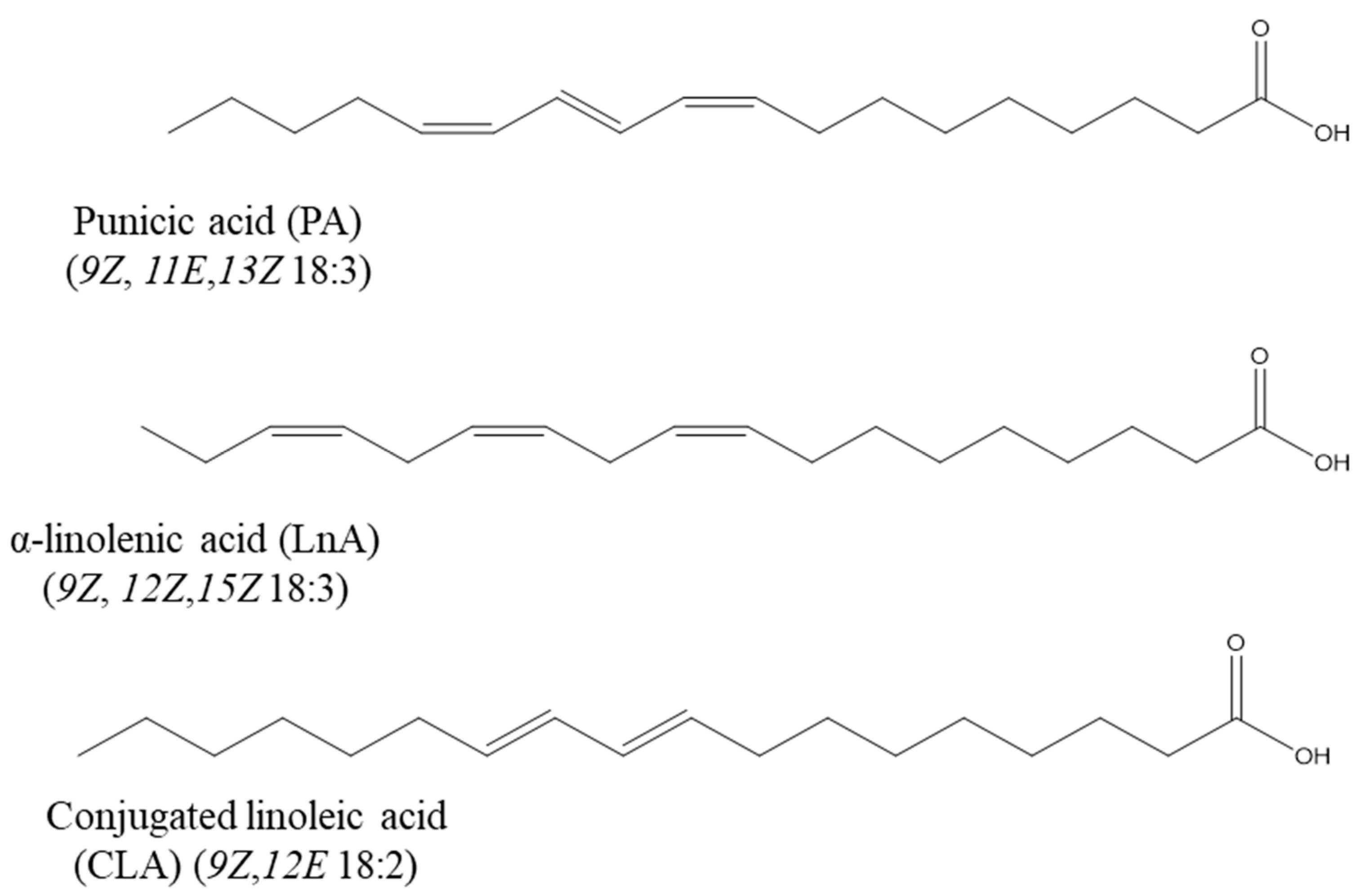
3. Structure, Biosynthesis, and Natural Sources of PA
4. Metabolism of Punicic Acid
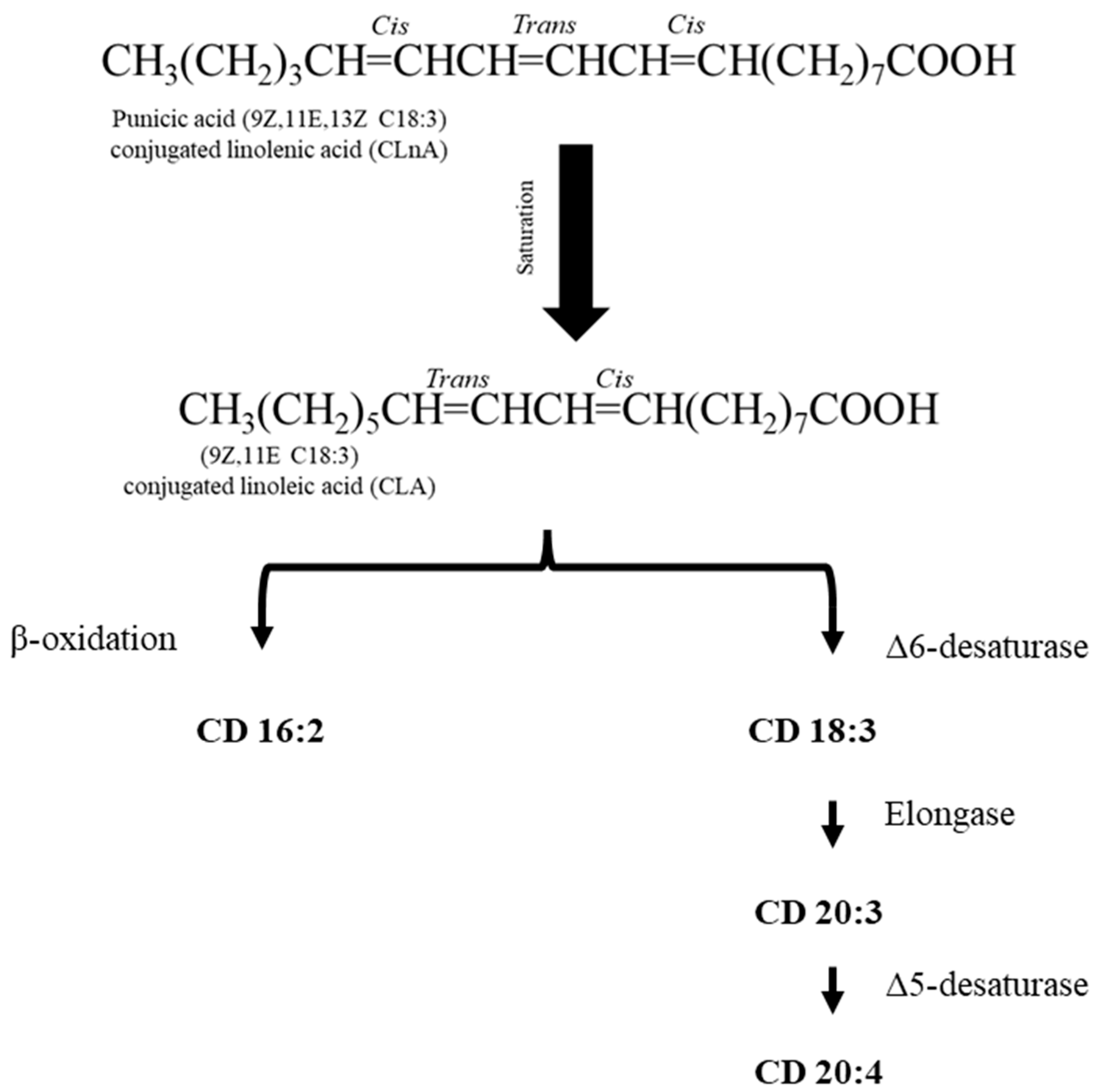
5. Cardiovascular Benefits of PA
5.1. Cardiovascular Protection
5.2. Lipid Reduction Effects
5.3. Antidiabetic Effects
5.4. Anti-Inflammatory Effects
| Reference | Source | N (M/F), Age (y) | Subject Group | Study Design | Duration | Treatment | Comparison | Treatment Effect |
|---|---|---|---|---|---|---|---|---|
| [49] | Trichosanthes kirilowii (TK) seed (3 g PA) | 30 (24 M, 6 F), aged 21–35 | Healthy young humans | Randomised controlled trial | 28 days | 3 g/day | Sunflower seed | ↔ lipid profile |
| [71] | PSO | 51 (both), > 20 | Hyperlipidaemic subjects (BMI ≥ 35 kg/m2, TC < 5.2 mmol/L, TAG > 1.65 mmol/L) | Parallel, randomised, double-blind, and placebo-controlled | 4 weeks | 400 mg × 2 | Placebo | ↓ TG: HDL-C ratio ↓ TG ↔ cholesterol and LDL-C |
| [72] | PSO | 51 (both), >20 | Hyperlipidaemic | Randomised, double-blind, placebo-controlled | 4 weeks | 400 mg × 2 | Placebo | ↔ TNF-α levels↓TG |
| [88] | PSO | 80 (28 M/52 F), 52 ± 6.8 | Type 2 diabetes; BMI 20–30 | Randomised, double-blind, placebo-controlled | 8 weeks | 1000 mg × 2 | Placebo | ↔ FBS, insulin resistance, and lipid profile |
| [59] | PSO | 46 (F) | Patients with breast cancer + overweight; BMI < 35 kg/m2 | Randomised, double-blind, placebo-controlled | 13 weeks | 1 g/day | Placebo (350 mg sunflower oil, 350 mg palm olein, and 300 mg corn oil) | ↓ SBP and DBP |
| [87] | PSO | 52 (both), 30–50 | Patients with obesity and type 2 diabetes | Randomised, double-blind, placebo-controlled | 8 weeks | 1 g × 3/day | Placebo (paraffin) | ↑ Gene expression of GLUT-4 ↓ FBS |
| [100] | PSO | 52 (both), 30–50 | Patients with obesity and type 2 diabetes | Randomised, double-blind, placebo-controlled | 8 weeks | 1 g × 3/day | Placebo (paraffin) | ↓ FBS ↓ IL-6 and TNF-α ↔ lipid profile levels |
| [86] | PSP (as a tea bag in hot water for [10 min]) | 60 (both), 30–60 | Type 2 diabetes BMI > 35 | Prospective, double-blind, randomised, placebo-controlled clinical trial | 8 weeks | 5 g × 2/day | Placebo (n = 30) HMWPG | ↓ HbA1c ↓ FBS |
5.5. Antioxidant Effects
6. Clinical Relevance and Limitations of PA
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ApoB100 | Apolipoprotein B100 |
| BMI | Body Mass Index |
| Caco-2 | Human Epithelial Colorectal Adenocarcinoma Cells (commonly used as a model of the intestinal barrier) |
| CD | Conjugated Diene |
| CFA | Conjugated Fatty Acid |
| CLA | Conjugated Linoleic Acid |
| CLnA | Conjugated Linolenic Acid |
| CVD | Cardiovascular Disease |
| GPx | Glutathione Peroxidase |
| HDL-C | High-Density Lipoprotein Cholesterol |
| ICR | Institute of Cancer Research (mouse strain) |
| IL | Interleukin |
| LA | Linoleic Acid |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| NF-κB | Nuclear Factor Kappa B |
| PA | Punicic Acid |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor Gamma |
| PS | Pomegranate Seed |
| PSO | Pomegranate Seed Oil |
| PSP | Pomegranate Seed Powder |
| PUFA | Polyunsaturated Fatty Acid |
| SOD | Superoxide Dismutase |
| TAG | Triacylglycerol |
| TC | Total Cholesterol |
| TFAs | Trans-Fatty Acids |
| TG | Triglycerides |
| TNF-α | Tumour Necrosis Factor Alpha |
| α-ESA | Alpha-Eleostearic Acid |
| α-LnA | Alpha-Linolenic Acid |
References
- World Health Organization. Cardiovascular Diseases Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 4 January 2025).
- British Heart Foundation. Heart and Circulatory Disease Statistics. 2023. Available online: https://www.bhf.org.uk/-/media/files/for-professionals/research/heart-statistics/bhf-cvd-statistics-uk-factsheet.pdf (accessed on 4 January 2025).
- World Heart Federation. Risk factors—Cardiovascular Risk Factors. 2019. Available online: https://www.world-heart-federation.org/resources/risk-factors/ (accessed on 8 January 2025).
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Kenneth, R.; Feingold, M.D. The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels. In Endotext. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570127/ (accessed on 10 January 2025).
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, L.; Liu, J.; Zhang, X.; Lu, Y. Analysis of the volatile flavor compounds of pomegranate seeds at different processing temperatures by GC-IMS. Molecules 2023, 28, 2717. [Google Scholar] [CrossRef]
- Wang, J.; Sun, M.; Yu, J.; Wang, J.; Cui, Q. Pomegranate seeds: A comprehensive review of traditional uses, chemical composition, and pharmacological properties. Front. Pharmacol. 2024, 15, 1401826. [Google Scholar] [CrossRef] [PubMed]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste: A review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef] [PubMed]
- Fourati, M.; Smaoui, S.; Hlima, H.B.; Elhadef, K.; Braïek, O.B.; Ennouri, K.; Mtibaa, A.C.; Mellouli, L. Bioactive compounds and pharmacological potential of pomegranate (Punica granatum) seeds—A review. Plant Foods Hum. Nutr. 2020, 75, 477–486. [Google Scholar] [CrossRef]
- Guzmán-Lorite, M.; Marina, M.L.; García, M.C. Pressurized liquids vs. high intensity focused ultrasounds for the extraction of proteins from a pomegranate seed waste. Innov. Food Sci. Emerg. Technol. 2022, 77, 102958. [Google Scholar] [CrossRef]
- Kang, S.J.; Choi, B.R.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Kim, D.C.; Choi, S.H.; Han, C.H.; Park, S.J.; Song, C.H.; et al. Dried Pomegranate Potentiates Anti-Osteoporotic and Anti-Obesity Activities of Red Clover Dry Extracts in Ovariectomized Rats. Nutrients 2015, 7, 2622–2647. [Google Scholar] [CrossRef]
- Almoraie, M.; Spencer, J.; Wagstaff, C. Fatty acid profile, tocopherol content, and phenolic compounds of pomegranate (Punica granatum L.) seed oils. J. Food Compost. Anal. 2025, 145, 107788. [Google Scholar] [CrossRef]
- Bedel, H.; Turgut, N.; Kurtoglu, A.; Usta, C. Effects of the nutraceutical, punicic acid. Indian. J. Pharm. Sci. 2017, 79, 328–334. [Google Scholar]
- Bouroshaki, M.T.; Sadeghnia, H.R.; Banihasan, M.; Yavari, S. Protective Effect of Pomegranate Seed Oil on Hexachlorobutadiene-Induced Nephrotoxicity in Rat Kidneys. Renal Fail. 2010, 32, 612–617. [Google Scholar] [CrossRef]
- Boroushaki, M.T.; Mollazadeh, H.; Afshari, A.R. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Res. 2016, 7, 430. [Google Scholar]
- Aruna, P.; Venkataramanamma, D.; Singh, A.K.; Singh, R.P. Health Benefits of Punicic Acid: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Franczyk-Żarów, M.; Tarko, T.; Drahun-Misztal, A.; Czyzynska-Cichon, I.; Kus, E.; Kostogrys, R.B. Pomegranate seed oil as a source of conjugated linolenic acid (CLnA) has no effect on atherosclerosis development but improves lipid profile and affects the expression of lipid metabolism genes in apoE/LDLR−/− mice. Int. J. Mol. Sci. 2023, 24, 1737. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Boussetta, N.; Marina, M.L.; García, M.C.; Vorobiev, E. High voltage electrical discharges followed by deep eutectic solvents extraction for the valorization of pomegranate seeds (Punica granatum L). Innov. Food Sci. Emerg. Technol. 2022, 79, 103055. [Google Scholar] [CrossRef]
- Iriti, G.; Bonacci, S.; Lopreiato, V.; Frisina, M.; Oliverio, M.; Procopio, A. Functional compounds of cold-pressed pomegranate seed oil: Fatty acids and phytosterols profile as quality biomarkers for origin discrimination. Foods 2023, 12, 2599. [Google Scholar] [CrossRef]
- Yuan, G.F.; Chen, X.E.; Li, D. Conjugated linolenic acids and their bioactivities: A review. Food Funct. 2014, 5, 1360–1368. [Google Scholar] [CrossRef]
- Zielińska, A.; Wójcicki, K.; Klensporf-Pawlik, D.; Marzec, M.; Lucarini, M.; Durazzo, A.; Fonseca, J.; Santini, A.; Nowak, I.; Souto, E.B. Cold-pressed pomegranate seed oil: Study of punicic acid properties by coupling of GC/FID and FTIR. Molecules 2022, 27, 5863. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef]
- Dhar Dubey, K.K.; Sharma, G.; Kumar, A. Conjugated linolenic acids: Implication in cancer. J. Agric. Food Chem. 2019, 67, 6091–6101. [Google Scholar] [CrossRef]
- Grossmann, M.E.; Mizuno, N.K.; Schuster, T.; Cleary, M.P. Punicic acid is an omega-5 fatty acid capable of inhibiting breast cancer proliferation. Int. J. Oncol. 2010, 36, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Vroegrijk, I.O.C.M.; van Diepen, J.A.; van den Berg, S.; Westbroek, I.; Keizer, H.; Gambelli, L.; Hontecillas, R.; Bassaganya-Riera, J.; Zondag, G.C.M.; Romijn, J.A.; et al. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem. Toxicol. 2011, 49, 1426–1430. [Google Scholar] [CrossRef]
- Saha, S.S.; Ghosh, M. Comparative study of antioxidant activity of α-eleostearic acid and punicic acid against oxidative stress generated by sodium arsenite. Food Chem. Toxicol. 2009, 47, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Viladomiu, M.; Hontecillas, R.; Yuan, L.; Lu, P.; Bassaganya-Riera, J. Nutritional protective mechanisms against gut inflammation. J. Nutr. Biochem. 2013, 24, 929–939. [Google Scholar] [CrossRef]
- Holic, R.; Xu, Y.; Caldo, K.M.P.; Singer, S.D.; Field, C.J.; Weselake, R.J.; Chen, G. Bioactivity and biotechnological production of punicic acid. Appl. Microbiol. Biotechnol. 2018, 102, 3537–3549. [Google Scholar] [CrossRef]
- Koba, K.; Belury, M.A.; Sugano, M. Potential health benefits of conjugated trienoic acids. Lipid Technol. 2007, 19, 200–203. [Google Scholar] [CrossRef]
- Carvalho, E.B.T.d.; Melo, I.L.P.d.; Mancini-Filho, J. Chemical and physiological aspects of isomers of conjugated fatty acids. Ciênc. Tecnol. Aliment. 2010, 30, 295–307. [Google Scholar] [CrossRef]
- Tulloch, A.P.; Bergter, L. Analysis of the Conjugated Trienoic Acid Containing Oil from Fevillea trilobata by13C Nuclear Magnetic Resonance Spectroscopy. Lipids 1979, 14, 996–1002. [Google Scholar] [CrossRef]
- Spilmont, M.; Léotoing, L.; Davicco, M.J.; Lebecque, P.; Mercier, S.; Miot-Noirault, E.; Pilet, P.; Rios, L.; Wittrant, Y.; Coxam, V. Pomegranate Seed Oil Prevents Bone Loss in a Mice Model of Osteoporosis, through Osteoblastic Stimulation, Osteoclastic Inhibition and Decreased Inflammatory Status. J. Nutr. Biochem. 2013, 24, 1840–1848. [Google Scholar] [CrossRef]
- Gaydou, E.M.; Miralles, J.; Rasoazanakolona, V. Analysis of Conjugated Octadecatrienoic Acids in Momordica balsamina Seed Oil by GLC and13C NMR Spectroscopy. J. Am. Oil Chem. Soc. 1987, 64, 997–1000. [Google Scholar] [CrossRef]
- Mukherjee, C.; Bhattacharyya, S.; Ghosh, S.; Bhattacharyya, D.K. Dietary effects of punicic acid on the composition and peroxidation of rat plasma lipid. J. Oleo Sci. 2002, 51, 513–522. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, C.; Yuan, G.; Li, D. Effects of Geographical Origin on the Conjugated Linolenic Acid of Trichosanthes kirilowii Maxim Seed Oil. J. Am. Oil Chem. Soc. 2012, 89, 401–407. [Google Scholar] [CrossRef]
- Tulloch, A.P. 13C Nuclear Magnetic Resonance Spectroscopic Analysis of Seed Oils Containing Conjugated Unsaturated Acids. Lipids 1982, 17, 544–550. [Google Scholar] [CrossRef]
- Dulf, F.V.; Pamfil, D.; Baciu, A.D.; Pintea, A. Fatty Acid Composition of Lipids in Pot Marigold (Calendula officinalis L.) Seed Genotypes. Chem. Cent. J. 2013, 7, 8. [Google Scholar] [CrossRef]
- Takagi, T.; Itabashi, Y. Occurrence of Mixtures of Geometrical Isomers of Conjugated Octadecatrienoic Acids in Some Seed Oils: Analysis by Open-Tubular Gas Liquid Chromatography and High Performance Liquid Chromatography. Lipids 1981, 16, 546–551. [Google Scholar] [CrossRef]
- Özgül-Yücel, S. Determination of Conjugated Linolenic Acid Content of Selected Oil Seeds Grown in Turkey. J. Am. Oil Chem. Soc. 2005, 82, 893–897. [Google Scholar] [CrossRef]
- Lerch, S.; Shingfield, K.J.; Ferlay, A.; Vanhatalo, A.; Chilliard, Y. Rapeseed or Linseed in Grass-Based Diets: Effects on Conjugated Linoleic and Conjugated Linolenic Acid Isomers in Milk Fat from Holstein Cows over 2 Consecutive Lactations. J. Dairy. Sci. 2012, 95, 7269–7287. [Google Scholar] [CrossRef]
- Mapiye, C.; Aalhus, J.L.; Turner, T.D.; Rolland, D.C.; Basarab, J.A.; Baron, V.S.; McAllister, T.A.; Block, H.C.; Uttaro, B.; Lopez-Campos, O.; et al. Effects of Feeding Flaxseed or Sunflower-Seed in High-Forage Diets on Beef Production, Quality and Fatty Acid Composition. Meat Sci. 2013, 95, 98–109. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Rajion, M.A.; Goh, Y.M. Effects of Oils Rich in Linoleic and α-Linolenic Acids on Fatty Acid Profile and Gene Expression in Goat Meat. Nutrients 2014, 6, 3913–3928. [Google Scholar] [CrossRef]
- Gong, M.; Hu, Y.; Wei, W.; Jin, Q.; Wang, X. Production of conjugated fatty acids: A review of recent advances. Biotechnol. Adv. 2019, 37, 107454. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.C.; Cannelli, G.; Carta, G.; Cordeddu, L.; Melis, M.P.; Murru, E.; Stanton, C.; Banni, S. Metabolism of c9,t11-conjugated linoleic acid (CLA) in humans. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 115–119. [Google Scholar] [CrossRef]
- Yuan, G.F.; Yuan, J.Q.; Li, D. Punicic acid from Trichosanthes kirilowii seed oil is rapidly metabolized to conjugated linoleic acid in rats. J. Med. Food 2009, 12, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Kawakami, Y.; Abe, R.; Nakagawa, K.; Koba, K.; Imamura, J.; Iwata, T.; Ikeda, I.; Miyazawa, T. Conjugated linolenic acid is slowly absorbed in rat intestine, but quickly converted to conjugated linoleic acid. J. Nutr. 2006, 136, 2153–2159. [Google Scholar] [CrossRef]
- Yuan, G.F.; Wahlqvist, M.L.; Yuan, J.Q.; Wang, Q.M.; Li, D. Effect of punicic acid naturally occurring in food on lipid peroxidation in healthy young humans. J. Sci. Food Agric. 2009, 89, 2331–2335. [Google Scholar] [CrossRef]
- Schneider, A.C.; Mignolet, E.; Schneider, Y.J.; Larondelle, Y. Uptake of conjugated linolenic acids and conversion to cis-9, trans-11-or trans-9, trans-11-conjugated linoleic acids in Caco-2 cells. Br. J. Nutr. 2013, 109, 57–64. [Google Scholar] [CrossRef]
- Stiti, N.; Chandrasekar, B.; Strubl, L.; Mohammed, S.; Bartels, D.; van der Hoorn, R.A.L. Nicotinamide cofactors suppress active-site labeling of aldehyde dehydrogenases. ACS Chem. Biol. 2016, 11, 1578–1586. [Google Scholar] [CrossRef]
- Plourde, M.; Sergiel, J.P.; Chardigny, J.M.; Grégoire, S.; Angers, P.; Sébédio, J.L. Absorption and metabolism of conjugated α-linolenic acid given as free fatty acids or triacylglycerols in rats. Nutr. Metab. 2006, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Vázquez, C.M.; Martínez-Ávila, M.; Guajardo-Flores, D.; Antunes-Ricardo, M. Punicic Acid and Its Role in the Prevention of Neurological Disorders: A Review. Foods 2022, 11, 252. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Yılmaz, B.; Usta, C.; Taşatargil, A.; Özdemir, S. Punicic acid induces endothelium-dependent vasorelaxation in rat thoracic aortic rings. Akd. Med. J. 2015, 1, 43–49. [Google Scholar] [CrossRef]
- Yilmaz, B.; Tasatargil, A.; Ozdemir, S. Pomegranate seed oil, a rich source of punicic acid, induces endothelium-dependent vasorelaxation in rat thoracic aortic rings. Atherosclerosis 2014, 235, e123–e124. [Google Scholar]
- Radjabian, T.; Fallah Husseini, H.; Karami, M.; Rasooli, I.; Faghihzadeh, S. Effect of pomegranate fruit juice and seed oil on serum lipid levels and atherosclerosis development in hypercholesterolemic rabbits. J. Med. Plants 2008, 7, 93–104. [Google Scholar]
- Bihamta, M.; Hosseini, A.; Ghorbani, A.; Boroushaki, M.T. Protective effect of pomegranate seed oil against H2O2 -induced oxidative stress in cardiomyocytes. Avicenna J. Phytomed. 2017, 7, 46–53. [Google Scholar]
- Rezvani, N.; Montazeri, V.; Baradaran, B.; Taghizadeh, M.; Pirouzpanah, S. Effects of conjugated fatty acid supplementation on central obesity and blood pressure in women with benign breast disease: A randomized controlled-clinical trial. Prog. Nutr. 2018, 20, 163–172. [Google Scholar]
- Manemann, S.M.; Bielinski, S.J.; Moser, E.D.; St Sauver, J.L.; Takahashi, P.Y.; Roger, V.L.; Olson, J.E.; Chamberlain, A.M.; Remaley, A.T.; Decker, P.A.; et al. Variability in lipid levels and risk for cardiovascular disease: An electronic health record–based population cohort study. J. Am. Heart Assoc. 2023, 12, e027639. [Google Scholar] [CrossRef]
- Elbandy, M.; Ashoush, I. Phytochemicals in pomegranate seeds and their effect as hypolipidemic agent in hypercholesterolemic rats. World J. Dairy. Food Sci. 2012, 7, 85–92. [Google Scholar]
- Yang, L.; Leung, K.Y.; Cao, Y.; Huang, Y.; Ratnayake, W.M.N.; Chen, Z.Y. α-linolenic acid but not conjugated linolenic acid is hypocholesterolaemic in hamsters. Br. J. Nutr. 2005, 93, 433–438. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kitagawa, T.; Koyanagi, N.; Chujo, H.; Maeda, H.; Kohno-Murase, J.; Imamura, J.; Tachibana, H.; Yamada, K. Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition 2006, 22, 54–59. [Google Scholar] [CrossRef]
- Arao, K.; Wang, Y.M.; Inoue, N.; Hirata, J.; Cha, J.Y.; Nagao, K.; Yanagita, T. Dietary effect of pomegranate seed oil rich in 9cis, 11trans, 13cis conjugated linolenic acid on lipid metabolism in obese, hyperlipidemic OLETF rats. Lipids Health Dis. 2004, 3, 24. [Google Scholar] [CrossRef]
- Teh, H.E.; Yokoyama, W.H.; German, J.B.; McHugh, T.H.; Pan, Z. Hypocholesterolemic Effects of Expeller-Pressed and Solvent-Extracted Fruit Seed Oils and Defatted Pomegranate Seed Meals. J. Agric. Food Chem. 2019, 67, 6150–6159. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Costa, E.M.; Silva, S.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Pomegranate oil’s potential as an anti-obesity ingredient. Molecules 2022, 27, 4958. [Google Scholar] [CrossRef]
- Manterys, A.; Franczyk-Zarow, M.; Czyzynska-Cichon, I.; Drahun, A.; Kus, E.; Szymczyk, B.; Kostogrys, R.B. Haematological parameters, serum lipid profile, liver function and fatty acid profile of broiler chickens fed on diets supplemented with pomegranate seed oil and linseed oil. Br. Poult. Sci. 2016, 57, 771–779. [Google Scholar] [CrossRef]
- Arao, K.; Yotsumoto, H.; Han, S.Y.; Nagao, K.; Yanagita, T. The 9cis,11trans,13cis isomer of conjugated linolenic acid reduces apolipoprotein B100 secretion and triacylglycerol synthesis in HepG2 cells. Biosci. Biotechnol. Biochem. 2004, 68, 2643–2645. [Google Scholar] [CrossRef]
- Guo, Q.; Yu, Z.Y.; Chen, J.; Wang, H.; Wang, J.H. Effects of pomegranate seed oil on body weight and UCP1 expression of mice fed with high fat diet. J. Dalian Polytech. Univ. 2019, 38, 244–247. [Google Scholar]
- McFarlin, B.K.; Strohacker, K.A.; Kueht, M.L. Pomegranate seed oil consumption during a period of high-fat feeding reduces weight gain and reduces type 2 diabetes risk in CD-1 mice. Br. J. Nutr. 2009, 102, 54–59. [Google Scholar] [CrossRef]
- Mirmiran, P.; Fazeli, M.R.; Asghari, G.; Shafiee, A.; Azizi, F. Effect of pomegranate seed oil on hyperlipidaemic subjects: A double-blind placebo-controlled clinical trial. Br. J. Nutr. 2010, 104, 402–406. [Google Scholar] [CrossRef]
- Asghari, G.; Sheikholeslami, S.; Mirmiran, P.; Chary, A.; Hedayati, M.; Shafiee, A.; Azizi, F. Effect of pomegranate seed oil on serum TNF-α level in dyslipidemic patients. Int. J. Food Sci. Nutr. 2012, 63, 368–371. [Google Scholar] [CrossRef]
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Litvinova, L.; Poggio, P.; Sukhorukov, V.N.; Orekhov, A.N. Effect of Glucose Levels on Cardiovascular Risk. Cells 2022, 11, 3034. [Google Scholar] [CrossRef]
- Shabbir, M.A.; Khan, M.R.; Saeed, M.; Pasha, I.; Khalil, A.A.; Siraj, N. Punicic acid: A striking health substance to combat metabolic syndromes in humans. Lipids Health Dis. 2017, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Alipour, B. Punicic acid: A potential compound of pomegranate seed oil in Type 2 diabetes mellitus management. J. Cell Physiol. 2019, 234, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Mandal, S.C.; Banerjee, S.K.; Sinha, S.; Saha, B.P.; Pal, M. Studies on the hypoglycaemic activity of Punica granatum seed in streptozotocin induced diabetic rats. Phytother. Res. 2001, 15, 628–629. [Google Scholar] [CrossRef]
- Nekooeian, A.A.; Eftekhari, M.H.; Adibi, S.; Rajaeifard, A. Effects of pomegranate seed oil on insulin release in rats with type 2 diabetes. Iran. J. Med. Sci. 2014, 39, 130–135. [Google Scholar]
- Hontecillas, R.; O’Shea, M.; Einerhand, A.; Diguardo, M.; Bassaganya-Riera, J. Activation of PPARγ and α by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J. Am. Coll. Nutr. 2009, 28, 184–195. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Guri, A.J.; Hontecillas, R. Treatment of Obesity-Related Complications with Novel Classes of Naturally Occurring PPAR Agonists. J. Obes. 2011, 2011, 897894. [Google Scholar] [CrossRef]
- Harzallah, A.; Hammami, M.; Kępczyńska, M.A.; Hislop, D.C.; Arch, J.R.S.; Cawthorne, M.A.; Zaibi, M.S. Comparison of potential preventive effects of pomegranate flower, peel and seed oil on insulin resistance and inflammation in high-fat and high-sucrose diet-induced obesity mice model. Arch. Physiol. Biochem. 2016, 122, 75–87. [Google Scholar] [CrossRef]
- Miranda, J.; Aguirre, L.; Fernández-Quintela, A.; Macarulla, M.T.; Martínez-Castaño, M.G.; Ayo, J.; Bilbao, E.; Portillo, M.P. Effects of pomegranate seed oil on glucose and lipid metabolism–related organs in rats fed an obesogenic diet. J. Agric. Food Chem. 2013, 61, 5089–5096. [Google Scholar] [CrossRef]
- Anusree, S.S.; Nisha, V.M.; Priyanka, A.; Raghu, K.G. Insulin resistance by TNF-α is associated with mitochondrial dysfunction in 3T3-L1 adipocytes and is ameliorated by punicic acid, a PPARγ agonist. Mol. Cell. Endocrinol. 2015, 413, 120–128. [Google Scholar] [CrossRef]
- Anusree, S.S.; Priyanka, A.; Nisha, V.M.; Das, A.A.; Raghu, K.G. An in vitro study reveals the nutraceutical potential of punicic acid relevant to diabetes via enhanced GLUT4 expression and adiponectin secretion. Food Funct. 2014, 5, 2590–2601. [Google Scholar] [CrossRef]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef]
- Seyed Hashemi, M.; Namiranian, N.; Tavahen, H.; Dehghanpour, A.; Rad, M.H.; Jam-Ashkezari, S.; Emtiazy, M.; Hashempur, M.H. Efficacy of pomegranate seed powder on glucose and lipid metabolism in patients with type 2 diabetes: A prospective randomized double-blind placebo-controlled clinical trial. Complement. Med. Res. 2021, 28, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Hamishehkar, H.; Mobasseri, M.; Ebrahimzadeh, V.; Alipour, M.; Alipour, B. Effect of pomegranate seed oil supplementation on the GLUT-4 gene expression and glycemic control in obese people with type 2 diabetes: A randomized controlled clinical trial. J. Cell. Physiol. 2019, 234, 19621–19628. [Google Scholar] [CrossRef]
- Faghihimani, Z.; Mirmiran, P.; Sohrab, G.; Iraj, B.; Faghihimani, E. Effects of pomegranate seed oil on metabolic state of patients with type 2 diabetes mellitus. Int. J. Prev. Med. 2016, 7, 124. [Google Scholar] [CrossRef]
- Potere, N.; Bonaventura, A.; Abbate, A. Novel therapeutics and upcoming clinical trials targeting inflammation in cardiovascular diseases. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2371–2395. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The triglyceride/high-density lipoprotein cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and cardiovascular disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Koyuncu, A.G.; Cumbul, A.; Noval, M.K.A.; Akyüz, E.Y. Pomegranate seed oil alleviates colitis: Therapeutic effects achieved by modulation of oxidative stress and inflammation in a rat model. Prostaglandins Other Lipid Mediat. 2024, 173, 106837. [Google Scholar] [CrossRef]
- Ren, Q.; Yang, B.; Zhang, H.; Ross, R.P.; Stanton, C.; Chen, H.; Chen, W. c9, t11, c15-CLNA and t9, t11, c15-CLNA from Lactobacillus plantarum ZS2058 ameliorate dextran sodium sulfate–induced colitis in mice. J. Agric. Food Chem. 2020, 68, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Chen, X.; Li, D. Modulation of peroxisome proliferator-activated receptor gamma (PPAR γ) by conjugated fatty acid in obesity and inflammatory bowel disease. J. Agric. Food Chem. 2015, 63, 1883–1895. [Google Scholar] [CrossRef]
- Bañares, C.; Carballeda-Sangiao, N.; Chabni, A.; García-Cordero, J.; Reglero, G.; de Pascual-Teresa, S.; Torres, C.F. Anti-inflammatory effect of two pomegranate seed oils obtained by green technologies in Caco-2 cells using the bioaccessible fraction from in vitro gastrointestinal digestion. Food Res. Int. 2023, 165, 112475. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, T.; Raad, H.; Lettéron, P.; Gougerot-Pocidalo, M.A.; Marie, J.C.; Driss, F.; El-Benna, J. Punicic acid, a conjugated linolenic acid, inhibits TNFα-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS ONE 2009, 4, e6458. [Google Scholar] [CrossRef]
- Coursodon-Boyiddle, C.F.; Snarrenberg, C.L.; Adkins-Rieck, C.K.; Bassaganya-Riera, J.; Hontecillas, R.; Lawrence, P.; Brenna, J.T.; Jouni, Z.E.; Dvorak, B. Pomegranate seed oil reduces intestinal damage in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G744–G751. [Google Scholar] [CrossRef]
- Taheri Rouhi, S.Z.; Sarker, M.M.R.; Rahmat, A.; Alkahtani, S.A.; Othman, F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague–Dawley rats. BMC Complement. Altern. Med. 2017, 17, 156. [Google Scholar] [CrossRef]
- Calder, P.C. Long chain fatty acids and gene expression in inflammation and immunity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 425–433. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Hamishehkar, H.; Alivand, M.; Alipour, M.; Solhi, M.; Alipour, B. Effect of pomegranate seed oil on the expression of PPAR-γ and pro-inflammatory biomarkers in obese type 2 diabetic patients. Nutr. Food Sci. 2019, 49, 854–865. [Google Scholar] [CrossRef]
- Dhar, P.; Bhattacharyya, D.; Bhattacharyya, D.K.; Ghosh, S. Dietary comparison of conjugated linolenic acid (9 cis, 11 trans, 13 trans) and α-tocopherol effects on blood lipids and lipid peroxidation in alloxan-induced diabetes mellitus in rats. Lipids 2006, 41, 49–54. [Google Scholar] [CrossRef]
- Amri, Z.; Ghorbel, A.; Turki, M.; Akrout, F.M.; Ayadi, F.; Elfeki, A.; Hammami, M. Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement. Altern. Med. 2017, 17, 339. [Google Scholar] [CrossRef]
- Cairone, F.; Salvitti, C.; Iazzetti, A.; Fabrizi, G.; Troiani, A.; Pepi, F.; Cesa, S. In-depth chemical characterization of Punica granatum L. seed oil. Foods 2023, 12, 1592. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases–Reports from the last 10 years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Wang, S.; Bai, H.; Liu, T.; Yang, J.; Wang, Z. Optimization of concentrations of different n-3PUFAs on antioxidant capacity in mouse hepatocytes. Lipids Health Dis. 2024, 23, 214. [Google Scholar] [CrossRef]
- Fathima, S.; Prokopiou, E.; Georgiou, T. Omega-3 Polyunsaturated Fatty Acids and Their Anti-Oxidant, Anti-Inflammatory and Neuroprotective Effects in Diabetic Retinopathy: A Narrative Review. Front. Biosci.-Landmark 2023, 28, 153. [Google Scholar] [CrossRef]
| Common Name | Isomeric Formula and Chemical Structure | Amount (%) | Reference |
|---|---|---|---|
| Pomegranate seed (Punica granatum L., Lythraceae) and Trichosanthes seed (Trichosanthes kirilowii Maxim., Cucurbitaceae) | 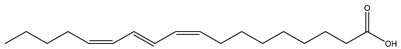 (Punicic acid) C18:3 c9,t11,c13 | >70% >40% | [45] |
| Catalpa seed (Catalpa ovata G. Don, Bignoniaceae) | 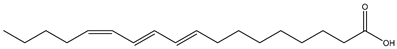 (Catalpic acid) C18:3 t9,t11,c13 | >40% | [40] |
| Tung tree seed (Aleurites fordii Hemsl., Euphorbiaceae) | 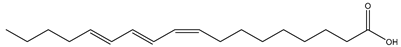 (α-Eleostearic acid) C18:3 c9,t11,t13 | >70% | [45] |
| Marigold seed (Calendula officinalis L., Asteraceae) | 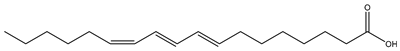 (Calendic acid) C18:3 t8,t10,c12 | >50% | [39] |
| Jacaranda seed (Jacaranda mimosifolia D. Don, Bignoniaceae) | 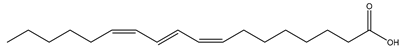 (Jacaric acid) C18:3 c8,t10,c12 | 36% | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almoraie, M.; Spencer, J.; Wagstaff, C. Punicic Acid: A Potential Nutraceutical Compound in Pomegranate Seed Oil and Its Cardiovascular Benefits. Foods 2025, 14, 2412. https://doi.org/10.3390/foods14142412
Almoraie M, Spencer J, Wagstaff C. Punicic Acid: A Potential Nutraceutical Compound in Pomegranate Seed Oil and Its Cardiovascular Benefits. Foods. 2025; 14(14):2412. https://doi.org/10.3390/foods14142412
Chicago/Turabian StyleAlmoraie, Manal, Jeremy Spencer, and Carol Wagstaff. 2025. "Punicic Acid: A Potential Nutraceutical Compound in Pomegranate Seed Oil and Its Cardiovascular Benefits" Foods 14, no. 14: 2412. https://doi.org/10.3390/foods14142412
APA StyleAlmoraie, M., Spencer, J., & Wagstaff, C. (2025). Punicic Acid: A Potential Nutraceutical Compound in Pomegranate Seed Oil and Its Cardiovascular Benefits. Foods, 14(14), 2412. https://doi.org/10.3390/foods14142412







