Abstract
Fermented foods represent an intricate ecosystem that delivers live microbes and numerous metabolites, influencing gut health. In this review, we explore how complex microbial communities and metabolites generated during food fermentation modulate the gut microbiome and affect human health. We discuss fermentation-induced biochemical transformations, including enhanced fiber fermentability; nutrient availability; and the synthesis of bioactive metabolites such as short-chain fatty acids, exopolysaccharides, bacteriocins, and modified polyphenols. We describe the dynamic microbial ecology of fermented foods, influenced by ingredient variations, highlighting its effect on health-related metabolic outcomes. Fermented products when consumed transiently introduce beneficial microbes and bioactive compounds into the gut, thereby boosting microbial diversity, resilience, and barrier function. We review clinical and preclinical studies to substantiate the roles of fermented foods in immune regulation, metabolic homeostasis, cognitive function, and inflammation mitigation. Individual variability in response to fermented foods has been emphasized, underscoring the potential for personalized nutrition strategies informed by advanced omics technologies. By integrating microbial ecology, metabolomics, and clinical evidence, this review positions fermented food intake as a strategic dietary intervention for microbiome modulation and health promotion.
1. Introduction
Fermented foods have been an essential component of human diets since ancient times; although the fermentation process was initially developed for food preservation, it is valued for enhancing flavor, texture, and nutritional benefits [1]. Fermentation products such as yogurt, kefir, kimchi, sauerkraut, tempeh, and miso are an integral part of culinary and medicinal practices across diverse cultures. Beyond preservation, fermentation substantially transforms food matrices, enhancing digestibility, improving nutrient bioavailability, and introducing beneficial microbial communities, diverse enzymes, and various bioactive metabolites [2].
In recent decades, fermented foods have garnered renewed scientific interest, largely driven by the growing evidence of their beneficial effects on the human gut microbiome [3]. The gut microbiome, comprising trillions of diverse microorganisms, including bacteria, archaea, fungi, and viruses, exerts profound effects on human physiology, metabolism, immune regulation, and overall health [4,5]. Dietary approaches aimed at positively modulating the gut microbiome are being recognized as pivotal for health promotion and disease prevention.
In contrast to isolated probiotic supplements, fermented foods contain complex microbial ecosystems that deliver diverse live microorganisms and bioactive metabolites, which uniquely interact with and influence the resident gut microbiota. Clinical evidence suggests that regular consumption of fermented foods can substantially enhance gut microbial diversity, improve intestinal barrier function, and modulate systemic inflammation, thereby positively influencing various chronic health conditions [6,7]. However, there is considerable variability in individual responses to fermented foods, regulated by factors such as genetics, baseline microbiota composition, dietary habits, and environmental exposure. This variability underscores the need for personalized nutritional strategies based on microbiome profiling [8].
In this review, we critically evaluate fermented foods as dynamic systems that shape the human gut microbiome and enhance health outcomes. We first discuss the biochemical transformations that occur during fermentation—including microbial enzyme activity—and their influence on food nutritional quality, such as enhanced fiber accessibility, bioactive compound production, and improved digestibility. Subsequently, we focus on fermented food microbiomes, describing their microbial diversity and ecological dynamics, and examine how these microbes and metabolites interact with the gut microbiota upon consumption, thereby affecting microbial composition, resilience, and metabolic health. Furthermore, we consolidate evidence from current clinical and preclinical studies linking fermented food consumption to improvements in immune function, metabolic regulation, inflammation control, and cognitive health. Recognizing interindividual differences, we discuss the prospects of personalized nutritional approaches based on microbiome profiling and precision fermentation technologies. We highlight key knowledge gaps and propose future research directions to optimize fermented foods as a microbiome-targeted nutritional intervention. Finally, we present an integrated conceptual overview to summarize the multidimensional interactions among fermented foods, the gut microbiome, and systemic health (Figure 1). Although this review does not differentiate between naturally fermented and artificially inoculated foods, it is important to recognize that differences in fermentation methods may influence microbial diversity and metabolite composition. Future studies could compare their distinct impacts on health-related outcomes.
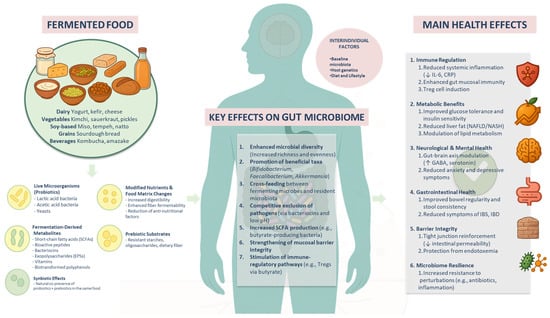
Figure 1.
Overview of the key mechanisms through which fermented foods influence gut microbiota and host health. Fermented foods—including dairy (e.g., yogurt and kefir), vegetables (e.g., kimchi and sauerkraut), soy-based products (e.g., miso and tempeh), grain-based products (e.g., sourdough bread), and beverages (e.g., kombucha and amazake)—deliver live beneficial microbes, fermentation-derived metabolites (e.g., organic acids such as acetic acid, exopolysaccharides, bacteriocins, and vitamins), modified nutrients, and prebiotic substrates. These components interact with the host gut microbiome by enhancing microbial diversity; promoting the growth of beneficial taxa (e.g., Bifidobacterium, Faecalibacterium, and Akkermansia); facilitating cross-feeding; suppressing the growth of pathogens; and promoting gut microbiota-derived short-chain fatty acid (SCFA) production, mucosal integrity, and immune modulation. These microbiome-mediated effects contribute to systemic health outcomes including immune regulation, metabolic improvement, neurological and gastrointestinal health, barrier function, and microbiome resilience. Interindividual factors such as baseline microbiota, host genetics, and lifestyle influence response to fermented food interventions.
2. Fermented Food Characteristics: Beyond Live Microbes
Traditionally, fermented foods have been recognized primarily for their probiotic properties, which are largely attributed to the content of live beneficial microorganisms such as lactic acid bacteria (LAB), bifidobacteria, and yeasts [9]. However, recent research indicates that the health-promoting characteristics of fermented foods are not limited to the live microbial communities. During fermentation, complex biochemical transformations occur within food matrices, profoundly modifying their nutritional composition, enhancing nutrient bioavailability, and producing a diverse array of bioactive metabolites, which collectively play a substantial role in modulating the gut microbiome composition and influencing host health outcomes [10,11].
2.1. Enhanced Dietary Fiber Bioavailability and Nutrient Accessibility
Dietary fibers, particularly complex polysaccharides, are pivotal substrates for the gut microbiota, selectively supporting beneficial bacterial growth. However, the structural complexity of these fibers often limits their fermentability and accessibility to gut microbes. Fermentation processes address this limitation by enzymatically breaking down complex carbohydrates into simpler sugars, oligosaccharides, and more accessible polysaccharides, thereby enhancing the fermentability and prebiotic potential of the fibers [12,13]. For example, during the fermentation of cabbage into sauerkraut or kimchi, the levels of structural polysaccharides and anti-nutritional factors, such as glucosinolates and phytates, are substantially reduced. This biochemical transformation results in an increase in soluble fiber content and improved digestibility, which in turn supports the proliferation and metabolic activity of beneficial gut microbes upon consumption [14,15]. Similarly, in cereal fermentations, such as in sourdough bread production, LAB and yeasts substantially reduce the level of phytic acid, a strong chelator of minerals, thereby enhancing mineral bioavailability and improving overall nutritional quality [16].
2.2. Generation of Bioactive Metabolites During Fermentation
Fermentation processes produce various organic acids such as lactic, acetic, gluconic, and glucuronic acids, particularly in fermented dairy and kombucha beverages. Additionally, fermentation generates bioactive compounds including bacteriocins (e.g., nisin and plantaricin), bioactive peptides—such as antihypertensive peptides (Val-Pro-Pro (VPP) and Ile-Pro-Pro (IPP)) and antimicrobial peptides (lactoferricin and casocidin-I)—extracellular polysaccharides (EPSs) with notable prebiotic and immunomodulatory properties, and modified polyphenolic compounds like gallic acid derivatives. These metabolites significantly enhance the nutritional and health-promoting properties of fermented foods [10,11].
Short-chain fatty acids (SCFAs), predominantly acetate, propionate, and butyrate, are primarily produced in the gut by resident microbiota fermenting dietary fibers. SCFAs serve as energy sources to colonocytes, fortify gut barrier integrity, modulate inflammatory responses, and regulate metabolism and appetite [17,18]. Although fermented foods generally contain modest levels of SCFAs, regular consumption of fermented foods such as kefir [19] and kimchi [20] provides dietary substrates and beneficial microbes that subsequently stimulate the gut microbiota to produce larger quantities of SCFAs, thereby enhancing gut and systemic health.
Bacteriocins are antimicrobial peptides primarily synthesized by LAB. They exert potent antimicrobial effects against pathogens and spoilage microorganisms, helping maintain microbiota homeostasis and enhancing food safety and shelf life. Fermented foods depicted in Figure 1, including yogurt, kefir, cheese, kimchi, and sauerkraut, frequently harbor bacteriocin-producing LAB strains, making these foods valuable for gastrointestinal health and protection against enteric pathogens [21,22].
Exopolysaccharides (EPSs), high-molecular-weight polysaccharides secreted by certain fermentation microbes, have considerable prebiotic and immunomodulatory properties. EPSs produced by LAB in fermented dairy products such as kefir and yogurt enhance mucosal barrier integrity, modulate intestinal immunity, selectively support beneficial gut microbiota, and exert cholesterol-lowering effects [23,24].
Fermentation also leads to the microbial biotransformation of plant-derived polyphenols, significantly enhancing their bioavailability and bioactivity. In products such as kombucha, tempeh, and miso, microbial enzymes transform complex polyphenolic structures into smaller and more bioavailable metabolites with improved antioxidant, antimicrobial, and anti-inflammatory effects. These modified polyphenolic metabolites beneficially interact with the gut microbiota, promoting microbial diversity and contributing to metabolic health [25].
2.3. Prebiotic and Synbiotic Properties of Fermented Foods
Fermented foods also function naturally as prebiotic and synbiotic systems. By virtue of their biochemical transformations, fermented foods often contain high concentrations of fermentable substrates such as oligosaccharides and modified polysaccharides, selectively stimulating the growth and activity of beneficial gut microbes such as Bifidobacterium and Lactobacillus species [26]. For instance, fermented soybean products such as tempeh and natto contain increased levels of bioactive oligosaccharides that specifically foster beneficial gut microbial communities, thus enhancing microbial diversity and promoting gut metabolic health [27]. Additionally, the simultaneous presence of probiotics (live beneficial microorganisms) and prebiotic substances (substrates that probiotics selectively utilize) in fermented foods exemplifies their natural synbiotic properties, exerting a synergistic effect that enhances microbiome modulation beyond the individual capabilities of probiotics or prebiotics alone [28].
2.4. Vitamin and Bioactive Peptide Production
Fermentation also markedly increases the levels of certain vitamins (e.g., the vitamin B complex and vitamin K) and bioactive peptides within food matrices. The bioactive peptides generated through microbial proteolytic activities exhibit antihypertensive, antioxidant, immunomodulatory, and antimicrobial properties. Fermented dairy products such as kefir and yogurt are particularly rich in bioactive peptides derived from milk proteins, contributing to their health-promoting potential [29].
Vitamin synthesis is another valuable aspect of fermentation, as certain LAB and yeasts can synthesize essential vitamins, thereby enhancing the nutritional value of the final product. For example, fermentation processes in products such as kefir, yogurt, and fermented vegetables significantly increase the bioavailability of vitamins such as B12, folate, and vitamin K2, which are essential for metabolic, neurological, and cardiovascular health [30].
A wide range of fermented foods contain diverse microbial strains and bioactive metabolites that possess multiple health benefits. Table 1 summarizes the representative fermented foods, their dominant microbial constituents, key fermentation-derived metabolites, and health-related effects based on clinical and preclinical study data.

Table 1.
Summary of fermented foods, dominant microbial taxa, major fermentation metabolites, and associated health-related effects.
3. Fermented Food Microbiomes and Their Dynamics
Fermented foods harbor complex microbial ecosystems comprising diverse populations of beneficial microbes such as LAB, acetic acid bacteria, yeasts, and molds. These microbial communities are not static; rather, they continuously evolve during the fermentation process and respond dynamically to intrinsic factors (e.g., substrate composition, pH changes, and nutrient availability) and extrinsic factors (e.g., temperature, oxygen levels, and fermentation duration) [45,46]. An in-depth understanding of fermented food microbiomes and their ecological dynamics is essential for optimizing their quality, consistency, and safety as well as for accurately predicting and maximizing their health-promoting effects upon consumption.
3.1. Diversity and Composition of Fermented Food Microbiomes
Advancements in molecular techniques, particularly next-generation sequencing and metagenomics, have considerably improved our understanding of the microbial diversity of fermented foods. Different fermentation types are characterized by distinct microbial consortia influenced primarily by the initial substrates, environmental conditions, and traditional or introduced starter cultures.
Traditional fermented vegetables, such as Korean kimchi and European sauerkraut, are primarily dominated by LAB, including species within the genera Lactobacillus, Leuconostoc, Weissella, and Pediococcus [14,47]. In kimchi fermentation, an initial dominance of Leuconostoc mesenteroides is typically observed, establishing an environment conducive to the subsequent growth of acid-tolerant species such as Lactobacillus plantarum and Lactobacillus brevis [48]. This structured microbial succession is critical for developing the distinct sensory and nutritional characteristics of kimchi and for enriching it with vitamins, lactic acid, and various bioactive peptides [20].
Recent studies have highlighted that subtle changes in ingredient formulations markedly influence microbial community structures and metabolite profiles. For example, adding fresh seafood, particularly gizzard shad, to kimchi fermentation can selectively promote the growth of beneficial microbes such as Leuconostoc rapi, a bacterium associated with improved flavor and antioxidant production and reduced undesirable acidity caused by Lactobacillus sakei during later fermentation stages [49].
Similarly, in Korean fermented soybean products, such as doenjang (fermented soybean paste) and gangjang (soy sauce), ingredient modifications considerably reshape the microbiome. The addition of coriander during gangjang fermentation reduces the presence of halophilic Chromohalobacter beijerinckii, a bacterium known to produce biogenic amines. This reduction corresponds to a substantial decrease in the levels of biogenic amines, notably histamine and tyramine, which enhances product safety and quality [50].
In doenjang, supplementation of herbs such as peppermint and Korean mint promotes beneficial microbial shifts by suppressing the growth of potentially harmful bacteria, such as Sphingomonas and Pantoea, while enriching beneficial microbes, such as Saccharopolyspora and Buttiauxella, which are known for their beneficial enzymatic and antimicrobial activities [51]. Such strategic changes in ingredients underscore the potential of targeted microbial modulation for optimizing both product safety and health-promoting functionalities.
Fermented dairy products, including yogurt, kefir, and artisanal cheeses, harbor complex and diverse microbial consortia. Yogurt represents a synergistic partnership of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. In contrast, kefir is characterized by greater microbial complexity involving multiple bacterial species (Lactobacillus kefiranofaciens, Lactococcus lactis, and Leuconostoc mesenteroides) co-existing symbiotically with yeast species such as Saccharomyces cerevisiae and Kluyveromyces marxianus [36,52]. These microbial communities markedly influence the product characteristics, including texture, flavor, and probiotic potential.
Kombucha, a popular fermented tea beverage, represents another fascinating example of complex microbial interactions. Its characteristic microbial community comprises acetic acid bacteria, primarily Komagataeibacter xylinus (previously classified within the genus Gluconacetobacter), and diverse yeasts, such as Zygosaccharomyces, collectively forming a cellulose-based biofilm known as a symbiotic culture of bacteria and yeast. The unique metabolic interactions within kombucha microbiomes produce distinct bioactive metabolites such as acetic, gluconic, and glucuronic acids, contributing to the sensory attributes and potential health benefits of kombucha [53].
3.2. Ecological and Metabolic Dynamics During Fermentation
The fermentation process involves distinct and dynamic ecological successions driven by microbial competition and cooperation, substrate availability, oxygen depletion, and progressive acidification. Initially, a diverse range of microorganisms colonize the fermentation substrate; however, as conditions become more acidic and anaerobic, selective pressures lead to dominance by specific microbial groups adapted to these conditions [54].
During vegetable fermentation, the aerobic microbes present in the initial stages consume residual oxygen, facilitating the subsequent establishment of anaerobic LAB. LAB-driven lactic acid production lowers pH, suppresses the growth of pathogenic organisms, and promotes the selective growth of desirable acid-tolerant microbes [55]. Such ecological shifts directly influence the sensory characteristics, shelf life, and nutritional value of the final product, emphasizing the importance of controlled ecological dynamics to achieve consistent fermented food quality.
4. Effect of Fermented Food Microbiomes on Human Gut Microbiota
The consumption of fermented foods transiently introduces complex microbial communities into the gastrointestinal tract, dynamically interacting with the resident gut microbiota. Although microbes from fermented foods generally do not exhibit long-term colonization, their temporary presence substantially influences microbial diversity, metabolic activity, and ecological resilience within the gut ecosystem [56,57].
Regular intake of fermented products, including yogurt, kefir, kimchi, and sauerkraut, has consistently been associated with beneficial alterations in the gut microbiota. Notably, increased microbial diversity and enrichment of health-promoting taxa such as Bifidobacterium, Lactobacillus, and Akkermansia species have been frequently reported [58,59,60]. These beneficial microbes enhance the gut barrier function, modulate immune responses, and support anti-inflammatory processes.
Recent meta-omics and clinical studies have significantly advanced our understanding of how fermented food consumption modulates the gut microbiota composition. Amplicon sequencing and shotgun metagenomic analyses have identified beneficial taxa such as Komagataeibacter (formerly Gluconacetobacter) and Zygosaccharomyces species as dominant microbes in kombucha fermentations, highlighting their potential functional roles and probable contributions to gut health upon consumption [61]. Complementary evidence from animal studies has demonstrated beneficial microbial shifts following kombucha administration. For instance, Jung et al. [62] reported a significant reduction in the abundance of pro-inflammatory taxa including Erysipelotrichia, Turicibacter, and Clostridium, accompanied by an increase in the abundance of beneficial genera such as Lactobacillus and Mucispirillum in mice with nonalcoholic fatty liver disease (NAFLD). These microbial changes correlated directly with improved liver health and decreased liver fat accumulation, underscoring the role of kombucha in metabolic homeostasis via gut microbiome modulation [62]. More recently, a controlled clinical study confirmed that short-term kombucha consumption in humans enriched the gut microbiota with SCFA-producing taxa, notably Weizmannia coagulans, a probiotic strain typically associated with kombucha fermentation. Although the observed microbiome shifts were modest owing to a short intervention duration and high interindividual variability, this study highlights the potential of kombucha to enhance microbial metabolism and support a healthy gut microbiome through increased SCFA production [63].
Fermented foods also contribute to gut microbial stability and resilience by suppressing the growth of pathogenic microorganisms. Specifically, bacteriocins produced by LAB, such as nisin by Lactococcus lactis and plantaricin by Lactobacillus plantarum, exert selective growth-inhibitory effects against enteric pathogens, thereby protecting gut microbiome integrity [64]. Furthermore, bioactive metabolites resulting from fermentation, particularly modified dietary fibers and polyphenols (described comprehensively in Section 2), improve substrate availability and bioactivity, further supporting the proliferation of beneficial gut microbiota [44,65].
Advances in the understanding of fermented food microbiomes are fueling innovations in precision fermentation, enabling targeted modulation of microbiota and their metabolites for personalized nutritional interventions. By selecting specific microbial consortia and fermentation conditions, precise fermentation approaches have the potential to optimize fermented products tailored to individual microbiome compositions and health objectives [66,67].
In summary, fermented foods considerably influence the gut microbiota through transient interactions among microbes, promotion of ecological resilience, suppression of pathogen growth, and enhancement of microbial diversity. These multidimensional interactions make fermented foods powerful dietary components for personalized microbiome management, extending their health-promoting effects beyond those of conventional probiotic supplements. Leveraging insights from meta-omics and targeted fermentation strategies can further enhance these benefits and foster tailored microbiome-targeted nutritional approaches.
5. Interindividual Variability in Response to and Precision Nutrition Opportunities with Fermented Foods
Fermented food intake represents a promising approach for modulating the gut microbiota and enhancing systemic health, delivering not only live microbes but also diverse metabolites within a complex food matrix. While these benefits are being increasingly recognized, individual responses to fermented food consumption vary substantially owing to the complex interactions among host genetics, baseline microbiota composition, dietary patterns, and lifestyle factors. Such variability influences microbial engraftment, metabolite utilization, and subsequent physiological outcomes.
Recent studies have underscored the individualized effects of fermented foods. Zmora et al. [68] demonstrated that probiotic colonization in the human gut is strongly individualized and is influenced by pre-existing microbiome structures and host characteristics, resulting in distinct colonization patterns. Similarly, Korem et al. [69] highlighted how unique microbiome profiles predict differential glycemic responses among individuals consuming identical foods, further emphasizing the need for tailored nutritional approaches. Zeevi et al. [70] expanded upon this by developing predictive machine learning models that integrate microbiome, dietary, and clinical data to accurately predict individualized metabolic responses, thereby supporting the development of personalized dietary strategies.
The concept of precise nutrition has been increasingly facilitated through advancements in integrative omics technologies including metagenomics, metabolomics, and transcriptomics. Such approaches allow for the detailed characterization of host–microbe interactions, providing a deeper understanding of how fermented foods influence specific biological pathways relevant to health. Machine learning and high-resolution multi-omics datasets are being used to tailor fermented food interventions to individual microbiota compositions, focusing on key health-related pathways such as SCFA synthesis, bile acid metabolism, immune modulation, and metabolic health [71].
Recent advances have facilitated the integration of machine learning and multi-omics technologies to decipher complex microbial dynamics and metabolic pathways in fermented food systems. For instance, the Omics Database of Fermentative Microbes is a curated genomic and metagenomic resource that encompasses microorganisms commonly associated with traditional fermented foods, including LAB, yeasts, and molds. This database enables precise taxonomic assignment, genomic comparisons, and the evaluation of potential starter strains based on omics data, thus supporting targeted strain selection and improved fermentation control [72]. Complementing this, Li et al. [73] applied machine learning algorithms, including logistic regression, random forest, and K-nearest neighbors, along with metagenomic and flavoromic data, to classify abnormal versus optimal fermentation outcomes in sauce-flavor Baijiu. In their study, SHapley Additive exPlanations values helped unveil key microbial and metabolic markers distinguishing fermentation states, while ecological modeling provided insights into community assembly mechanisms under different environmental conditions. These integrative approaches serve as powerful tools for predicting fermentation performance, diagnosing microbial imbalances, and tailoring fermented food production to improve quality and reproducibility.
Innovative precision fermentation techniques enable targeted microbiome modulation through tailored microbial consortia or optimized fermentation conditions, allowing for the design of fermented products that directly address specific health conditions, including metabolic syndrome, inflammatory bowel diseases, and neurological disorders [74,75]. Qian and Ho [76] emphasize that beyond identifying beneficial microbial taxa, understanding the ecological interactions among microbes and their metabolites is critical for developing next-generation precision nutritional interventions.
Future research must include robust clinical trials specifically designed to assess the microbial viability, safety profiles, and dose–response relationships of fermented food interventions while accounting for interindividual variability. Longitudinal studies integrating multi-omics analyses to monitor microbiota dynamics, metabolite shifts, and clinical biomarkers are essential for validating personalized approaches. Additionally, the development of comprehensive databases linking microbial taxa and metabolites to specific health outcomes is critical for advancing regulatory standards and ensuring effective therapeutic applications [77,78].
By addressing interindividual variability through advanced omics and precision fermentation technologies, fermented foods can be strategically used as personalized dietary interventions to maximize their potential in microbiome-driven health promotion.
6. Clinical and Preclinical Evidence Supporting the Health Benefits of Fermented Foods
Substantial preclinical and human study data have confirmed the health-promoting effects of fermented foods across a wide range of physiological systems. Notably, Wastyk et al. [6] conducted a landmark 10-week randomized controlled trial in healthy adults and showed that daily intake of fermented foods significantly increased microbiota diversity and reduced the levels of 19 inflammatory markers, including interleukin (IL)-6 and IL-12b, highlighting their immunomodulatory and anti-inflammatory properties.
Other randomized clinical trials have demonstrated diverse health benefits in different populations. Han et al. [79] reported that fermented kimchi improved the levels of metabolic markers and shifted the gut microbiota in overweight women, whereas a kefir-based intervention led to a reduction in inflammatory symptoms in patients with inflammatory bowel disease [80]. In a study of NAFLD and metabolic syndrome, Chen et al. [81] found that yogurt intake significantly improved insulin sensitivity and reduced liver fat accumulation in obese women.
Emerging evidence suggests that fermented foods support cognitive function. In Korea and Japan, fermented milk enriched with Lactobacillus helveticus (IDCC3801 or CM4) improved memory and cognitive test scores in elderly and middle-aged participants, respectively, as determined using neuropsychological and biomarker analyses [82]. In patients with Alzheimer’s disease, fermented dairy products containing Bifidobacterium bifidum, Lactobacillus casei, and L. acidophilus have shown potential to mitigate cognitive decline [82].
In addition to brain health, several studies have validated the metabolic benefits of fermented foods. For example, a randomized controlled trial conducted in a high-cardiovascular-risk Mediterranean population revealed that dairy product consumption was associated with a decreased risk of developing type 2 diabetes mellitus over time [83]. Another study by Ecklu-Mensah et al. [63] demonstrated that kombucha consumption in healthy adults modulated the gut microbiota composition and improved inflammatory and metabolic markers, including a reduction in blood pressure and fasting blood glucose level.
Preclinical studies further support the physiological benefits of kombucha, particularly through gut–liver and metabolic axis modulation. In a mouse model of diet-induced obesity and NAFLD, kombucha supplementation significantly improved glucose tolerance, improved hyperinsulinemia, and alleviated hepatic steatosis [84]. These effects were linked to the downregulated expression of pro-inflammatory genes such as TNF-α and SREBP-1, decreased collagen deposition in liver tissue, and restoration of insulin signaling via AKT phosphorylation, suggesting strong anti-inflammatory and hepatoprotective effects [84].
The above findings were reinforced in a methionine/choline-deficient mouse model of nonalcoholic steatohepatitis (NASH), in which kombucha administration led to a significant reduction in hepatic triglyceride level, inflammation, and fibrosis [85]. Mechanistically, kombucha promoted hepatocyte survival by reducing apoptosis and enhancing cell proliferation, suppressed lipid accumulation through downregulated expression of genes such as Cd36, Pparγ, Fas, and Srebp1c, and stimulated β-oxidation via upregulated expression of Ppargc1α, Cpt1, and Acox1. Additionally, kombucha attenuated Hedgehog signaling, a key driver of fibrosis in NASH, by suppressing Shh, Smo, and Gli2 expression. Taken together, these hepatoprotective effects highlight the ability of kombucha to modulate both the metabolic and inflammatory pathways. Furthermore, the interaction of kombucha with bile acid receptors such as TGR5 and FXR extends its benefits to lipid regulation and immune modulation.
The consumption of amazake, a traditional Japanese fermented rice beverage, has been shown to reduce serum TNF-α levels and improve symptoms such as muscle cramps and depression in patients with NAFLD and periodontal disease, highlighting the potential of amazake as an anti-inflammatory and quality-of-life-enhancing dietary intervention [86].
The diversity of fermented food types (e.g., kimchi, sauerkraut, miso, sourdough, tempeh, and kombucha) and the broad spectrum of bioactive compounds contribute to their versatile therapeutic applications. According to recent reviews [3,87,88], bioactive metabolites, such as gamma-aminobutyric acid (GABA), EPS, and peptides, produced during fermentation exert direct effects on immune modulation, oxidative stress, and inflammatory pathways. The clinical and preclinical study findings confirm that fermented foods are not only safe but also offer measurable health benefits across the metabolic, cognitive, and immune domains. They highlight the role of fermented foods in preventive and therapeutic nutritional strategies, particularly when guided by microbiome-informed precision tools.
7. Conclusions
Fermented foods represent nutritional systems whose health effects extend beyond providing beneficial live microorganisms. Through intricate biochemical transformations, fermentation enhances dietary fiber accessibility and nutrient bioavailability and generates various bioactive metabolites, including organic acids (e.g., acetic and lactic acids), bacteriocins, EPS, vitamins, and bioactive peptides, indirectly supporting gut microbiota-derived SCFA production. The ecological dynamics of fermented food microbiomes influence gut microbiota composition, resilience, and metabolic health via transient microbial and metabolite interactions.
Clinical and preclinical studies substantiate fermented foods’ roles in immune regulation, metabolic health, inflammation control, and cognitive function. However, interindividual variability underscores the importance of personalized nutritional approaches informed by microbiome profiling and precision fermentation technologies.
Future research should focus on standardized clinical trials, mechanistic insights into microbial and metabolite interactions, and innovative fermentation techniques targeting specific health outcomes. Strengthening regulatory frameworks and consumer education will further optimize fermented foods as precise dietary interventions for improved gut and systemic health.
Author Contributions
Conceptualization, M.M. and I.P.; data curation, M.M. and I.P.; writing—original draft preparation, M.M. and I.P.; writing—review and editing, M.M. and I.P.; project administration, M.M.; funding acquisition, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Youngsan University research fund of 2024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We appreciate Young-Su Seo (Pusan National University) for critical discussions for the construction of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of fermented foods in food guides around the world. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef]
- Caffrey, E.B.; Perelman, D.; Ward, C.P.; Sonnenburg, E.D.; Gardner, C.D.; Sonnenburg, J.L. Unpacking food fermentation: Clinically relevant tools for fermented food identification and consumption. Adv. Nutr. 2025, 16, 100412. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153. [Google Scholar] [CrossRef]
- Kim, N.; Lee, J.; Song, H.S.; Oh, Y.J.; Kwon, M.-S.; Yun, M.; Lim, S.K.; Park, H.K.; Jang, Y.S.; Lee, S.; et al. Kimchi intake alleviates obesity-induced neuroinflammation by modulating the gut-brain axis. Food Res. Int. 2022, 158, 111533. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’toole, P.W.; Beresford, T.P. Fermented foods, health and the gut microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, S.; Seki, N.; Sugiura, Y.; Akiyama, M.; Uchiyama, J.; Yamaguchi, G.; Yakabe, K.; Ejima, R.; Hattori, K.; Kimizuka, T.; et al. Cooperative action of gut-microbiota-accessible carbohydrates improves host metabolic function. Cell Rep. 2022, 40, 3. [Google Scholar] [CrossRef] [PubMed]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented foods, their microbiome and its potential in boosting human health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Jeon, C.O. Kimchi microflora: History, current status, and perspectives for industrial kimchi production. Appl. Microbiol. Biotechnol. 2014, 98, 2385–2393. [Google Scholar] [CrossRef]
- Gaudioso, G.; Weil, T.; Marzorati, G.; Solovyev, P.; Bontempo, L.; Franciosi, E.; Bertoldi, L.; Pedrolli, C.; Tuohy, K.M.; Fava, F. Microbial and metabolic characterization of organic artisanal sauerkraut fermentation and study of gut health-promoting properties of sauerkraut brine. Front. Microbiol. 2022, 13, 929738. [Google Scholar] [CrossRef]
- Fang, L.; Wang, W.; Dou, Z.; Chen, J.; Meng, Y.; Cai, L.; Li, Y. Effects of mixed fermentation of different lactic acid bacteria and yeast on phytic acid degradation and flavor compounds in sourdough. LWT 2023, 174, 114438. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Overby, H.B.; Ferguson, J.F. Gut microbiota-derived short-chain fatty acids facilitate microbiota: Host cross talk and modulate obesity and hypertension. Curr. Hypertens. Rep. 2021, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, S.A.; da Conceição, L.L.; e Dias, M.M.; Siqueira, N.P.; Rosa, D.D.; de Oliveira, L.L.; da Matta, S.L.P.; Peluzio, M.D.C.G. Kefir reduces the incidence of pre-neoplastic lesions in an animal model for colorectal cancer. J. Funct. Foods 2019, 53, 1–6. [Google Scholar] [CrossRef]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W., III. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. Bacteriocins from lactic acid bacteria: Purification, properties and use as biopreservatives. Braz. Arch. Biol. Technol. 2007, 50, 512–542. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Srinivash, M.; Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B.; Keerthivasan, M. Probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from homemade fermented food products. J. Agric. Food Res. 2023, 11, 100517. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic acid bacteria exopolysaccharides in foods and beverages: Isolation, properties, characterization, and health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of fermentation on bioactivity and the composition of polyphenols contained in polyphenol-rich foods: A review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Dong, Y.; Han, M.; Fei, T.; Liu, H.; Gai, Z. Utilization of diverse oligosaccharides for growth by Bifidobacterium and Lactobacillus species and their in vitro co-cultivation characteristics. Int. Microbiol. 2024, 27, 941–952. [Google Scholar] [CrossRef]
- Dafne, V.J.; Kassandra, D.F.S.; Nicolle, M.Z.E.; Rocio, C.V. Tempeh-type fermentation: Impact on food composition, bioaccessibility, and health benefits. In Improving Health and Nutrition Through Functional Foods; Woodhead Publishing: Sawston, UK, 2025; pp. 17–41. [Google Scholar]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Di Cagno, R.; De Angelis, M. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 2010, 50, 716–727. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Yılmaz, B.; Şahin, T.Ö.; Güneşliol, B.E.; Ayten, Ş.; Russo, P.; Spano, G.; Rocha, J.M.; Bartkiene, E.; Özogul, F. Dairy lactic acid bacteria and their potential function in dietetics: The food–gut–health axis. Foods 2021, 10, 3099. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wei, X.; Xu, C.; Cavender, G.; Lin, W.; Sun, S. Invited review: Advances in yogurt development: Microbiological safety, quality, functionality, sensory evaluation, and consumer perceptions across different dairy and plant-based alternative sources. J. Dairy Sci. 2025, 108, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, H.-J. Revisiting the potential anti-obesity effects of kimchi and lactic acid bacteria isolated from kimchi: A lustrum of evidence. J. Ethn. Foods 2024, 11, 36. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, J.-E.; Lee, N.-K.; Paik, H.-D. Antioxidant and anti-inflammatory effect of probiotic Lactobacillus plantarum KU15149 derived from Korean homemade diced-radish kimchi. J. Microbiol. Biotechnol. 2020, 30, 591–598. [Google Scholar] [CrossRef]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibanez, F.C. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016, 7, 196946. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Shah, Y.A.; Khan, M.H.; Hussain, M.; Ikram, A.; Ateeq, H.; Noman, M.; Saewan, S.A.; Khashroum, A.O. Miso: A traditional nutritious & health-endorsing fermented product. Food Sci. Nutr. 2022, 10, 4103–4111. [Google Scholar] [PubMed]
- Rizzo, G. Soy-based tempeh as a functional food: Evidence for human health and future perspective. Front. Biosci. 2024, 16, 3. [Google Scholar] [CrossRef]
- Chen, H.; McGowan, E.M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Qu, X.; Lin, Y. Nattokinase: A promising alternative in prevention and treatment of cardiovascular diseases. Biomark. Insights 2018, 13, 1177271918785130. [Google Scholar] [CrossRef]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Niamah, A.K.; Sahi, A.A.; Al-Sharifi, A.S.N. Effect of feeding soy milk fermented by probiotic bacteria on some blood criteria and weight of experimental animals. Probiotics Antimicrob. Proteins 2017, 9, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.; Curtin, C. Microbial composition of SCOBY starter cultures used by commercial kombucha brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Mannaa, M.; Han, G.; Seo, Y.S.; Park, I. Evolution of food fermentation processes and the use of multi-omics in deciphering the roles of the microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- Zabat, M.A.; Sano, W.H.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Whon, T.W.; Roh, S.W.; Jeon, C.O. Unraveling microbial fermentation features in kimchi: From classical to meta-omics approaches. Appl. Microbiol. Biotechnol. 2020, 104, 7731–7744. [Google Scholar] [CrossRef]
- Mannaa, M.; Seo, Y.-S.; Park, I. Effect of seafood (gizzard shad) supplementation on the chemical composition and microbial dynamics of radish kimchi during fermentation. Sci. Rep. 2019, 9, 17693. [Google Scholar] [CrossRef]
- Mannaa, M.; Seo, Y.-S.; Park, I. Addition of coriander during fermentation of Korean soy sauce (Gangjang) causes significant shift in microbial composition and reduction in biogenic amine levels. Foods 2020, 9, 1346. [Google Scholar] [CrossRef]
- Mannaa, M.; Cho, S.-S.; Seo, Y.-S.; Park, I. Microbial composition of fermented Korean soy paste (Doenjang) prepared by adding different herbs during fermentation. Fermentation 2021, 7, 93. [Google Scholar] [CrossRef]
- Liu, E.; Zheng, H.; Shi, T.; Ye, L.; Konno, T.; Oda, M.; Shen, H.; Ji, Z.-S. Relationship between Lactobacillus bulgaricus and Streptococcus thermophilus under whey conditions: Focus on amino acid formation. Int. Dairy J. 2016, 56, 141–150. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on kombucha tea—Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Dutton, R.J. Fermented foods as experimentally tractable microbial ecosystems. Cell 2015, 161, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Pasolli, E.; De Filippis, F.; Mauriello, I.E.; Cumbo, F.; Walsh, A.M.; Leech, J.; Cotter, P.D.; Segata, N.; Ercolini, D. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nat. Commun. 2020, 11, 2610. [Google Scholar] [CrossRef]
- Derrien, M.; van Hylckama Vlieg, J.E. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does consumption of fermented foods modify the human gut microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, C.I.; Kurilshikov, A.; Leeming, E.R.; Visconti, A.; Bowyer, R.C.E.; Menni, C.; Falchi, M.; Koutnikova, H.; Veiga, P.; Zhernakova, A.; et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 2022, 22, 39. [Google Scholar]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Arıkan, M.; Mitchell, A.L.; Finn, R.D.; Gürel, F. Microbial composition of kombucha determined using amplicon sequencing and shotgun metagenomics. J. Food Sci. 2020, 85, 455–464. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, I.; Mannaa, M.; Kim, J.; Wang, S.; Park, I.; Kim, J.; Seo, Y.S. Effect of kombucha on gut-microbiota in mouse having non-alcoholic fatty liver disease. Food Sci. Biotechnol. 2019, 28, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ecklu-Mensah, G.; Miller, R.; Maseng, M.G.; Hawes, V.; Hinz, D.; Kim, C.; Gilbert, J.A. Modulating the human gut microbiome and health markers through kombucha consumption: A controlled clinical study. Sci. Rep. 2024, 14, 31647. [Google Scholar] [CrossRef] [PubMed]
- Grosu-Tudor, S.S.; Stancu, M.M.; Pelinescu, D.; Zamfir, M. Characterization of some bacteriocins produced by lactic acid bacteria isolated from fermented foods. World J. Microbiol. Biotechnol. 2014, 30, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Boucard, A.S.; Mohseni, A.H.; Taghinezhad-S, S.; Cortes-Perez, N.G.; Bermúdez-Humarán, L.G. Role of gut microbiota and probiotics in colorectal cancer: Onset and progression. Microorganisms 2021, 9, 1021. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405. [Google Scholar] [CrossRef]
- Korem, T.; Zeevi, D.; Zmora, N.; Weissbrod, O.; Bar, N.; Lotan-Pompan, M.; Avnit-Sagi, T.; Kosower, N.; Malka, G.; Rein, M.; et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab. 2017, 25, 1243–1253. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D’Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Rossetto Marcelino, V.; Bryant, R.V.; Costello, S.P.; et al. The future of microbiome-based therapeutics. Aliment. Pharmacol. Ther. 2022, 56, 192–208. [Google Scholar] [CrossRef]
- Whon, T.W.; Ahn, S.W.; Yang, S.; Kim, J.Y.; Kim, Y.B.; Kim, Y.; Hong, J.M.; Jung, H.; Choi, Y.E.; Lee, S.H.; et al. ODFM, an Omics Data Resource from Microorganisms Associated with Fermented Foods. Sci. Data 2021, 8, 113. [Google Scholar] [CrossRef]
- Li, S.; Han, Y.; Yan, M.; Qiu, S.; Lu, J. Machine Learning and Multi-Omics Integration to Reveal Biomarkers and Microbial Community Assembly Differences in Abnormal Stacking Fermentation of Sauce-Flavor Baijiu. Foods 2025, 14, 245. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef]
- Berding, K.; Donovan, S.M. Diet can impact microbiota composition in children with autism spectrum disorder. Front. Neurosci. 2018, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Ho, J.W.K. Challenges and emerging systems biology approaches to discover how the human gut microbiome impact host physiology. Biophys. Rev. 2020, 12, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Louca, P.; Gibson, R.; Menni, C.; Spector, T.D.; Le Roy, C.I. The complexities of the diet–microbiome relationship: Advances and perspectives. Genome Med. 2021, 13, 10. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision nutrition and the microbiome, part I: Current state of the science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Bose, S.; Wang, J.H.; Kim, B.S.; Kim, M.J.; Kim, E.J.; Kim, H. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, İ.; Dolar, M.E.; Özpınar, H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk. J. Gastroenterol. 2019, 30, 242–253. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, R.; Yang, X.; Dai, J.; Huang, M.; Ji, X.; Li, Y.; Okekunle, A.P.; Gao, G.; Onwuka, J.U.; et al. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1611–1619. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Impact of fermented foods on human cognitive function—A review of outcome of clinical trials. Sci. Pharm. 2018, 86, 22. [Google Scholar] [CrossRef] [PubMed]
- Díaz-López, A.; Bulló, M.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Fitó, M.; Gómez-Gracia, E.; Fiol, M.; Garcia de la Corte, F.J.; Ros, E.; et al. Dairy product consumption and risk of type 2 diabetes in an elderly Spanish Mediterranean population at high cardiovascular risk. Eur. J. Nutr. 2016, 55, 349–360. [Google Scholar] [CrossRef]
- Moreira, G.V.; Araujo, L.C.; Murata, G.M.; Matos, S.L.; Carvalho, C.R. Kombucha tea improves glucose tolerance and reduces hepatic steatosis in obese mice. Biomed. Pharmacother. 2022, 155, 113660. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, J.; Wang, S.; Sung, S.; Kim, N.; Lee, H.H.; Seo, Y.S.; Jung, Y. Hepatoprotective effect of kombucha tea in rodent model of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2019, 20, 2369. [Google Scholar] [CrossRef]
- Nagao, Y.; Takahashi, H.; Kawaguchi, A.; Kitagaki, H. Effect of fermented rice drink “Amazake” on patients with nonalcoholic fatty liver disease and periodontal disease: A pilot study. Reports 2021, 4, 36. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One health, fermented foods, and gut microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).