Metal–Phenolic Network-Directed Coating of Lactobacillus plantarum: A Promising Strategy to Increase Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Single-Cell Encapsulation Carriers
2.3. Characterization of Single-Cell Encapsulation Carriers
2.4. Influence of Polyphenols on the Growth of Lactobacillus plantarum
2.5. In Vitro Storage and Gastric Digestion Experiments
2.6. Statistical Analysis
3. Results and Discussion
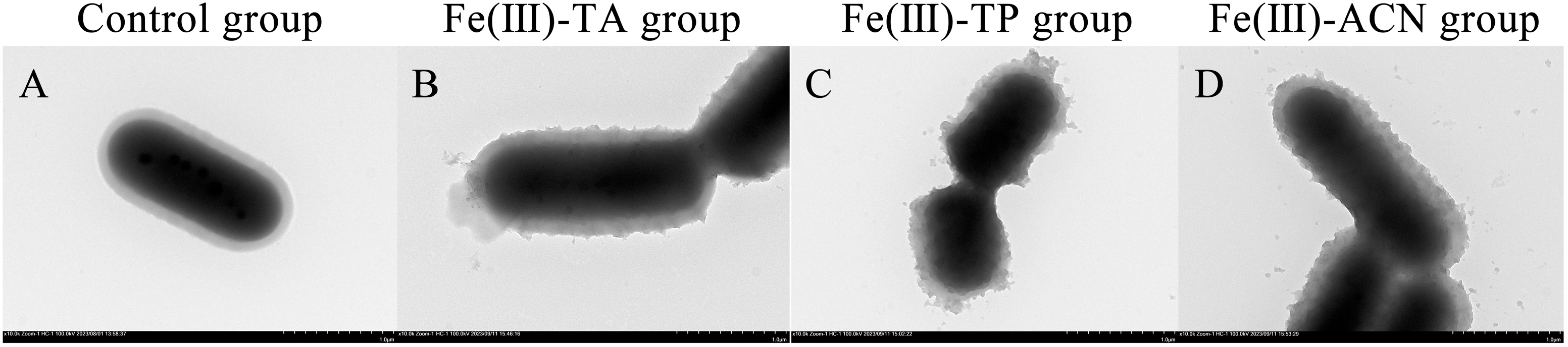
3.1. TEM
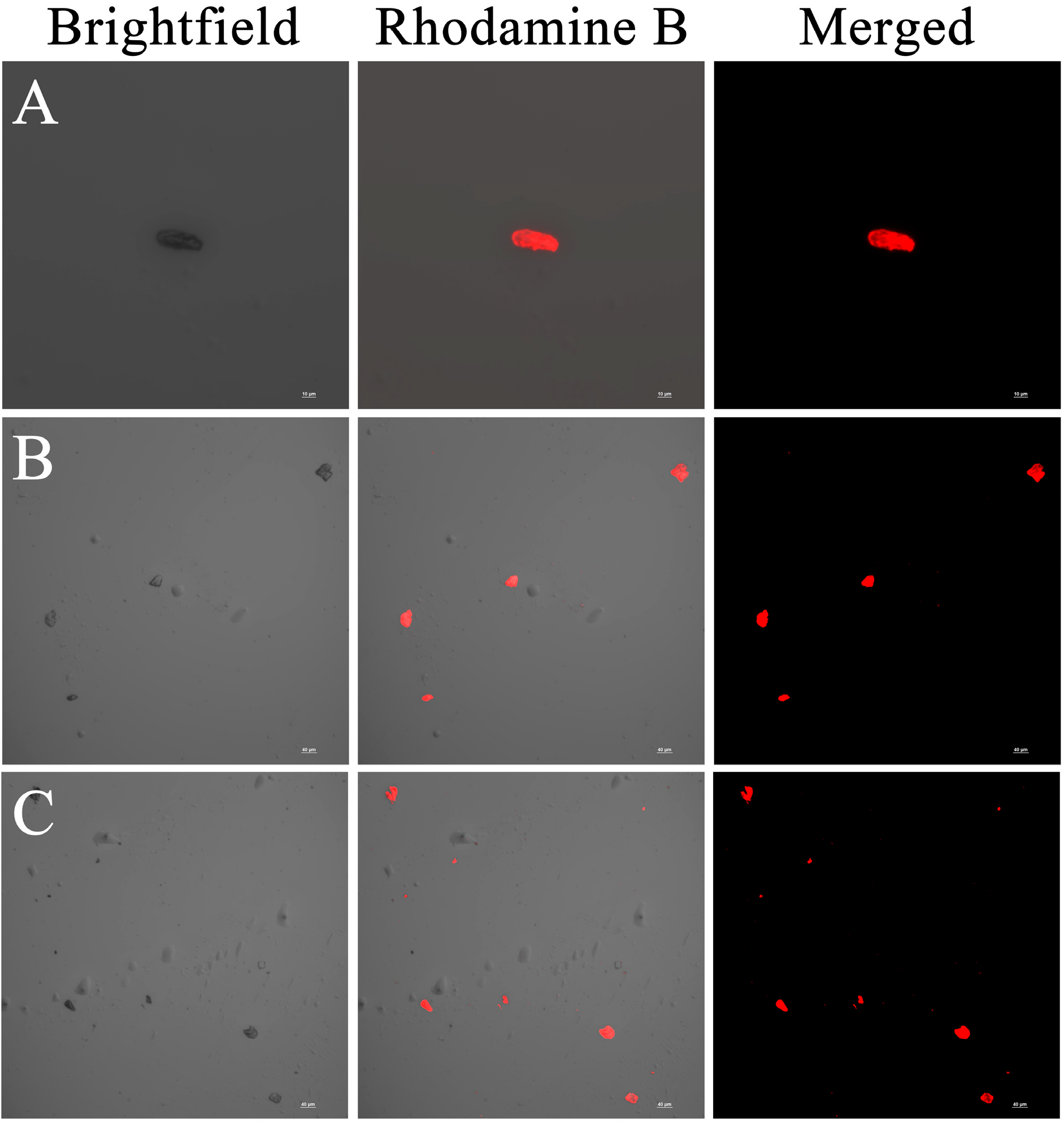
3.2. CLSM
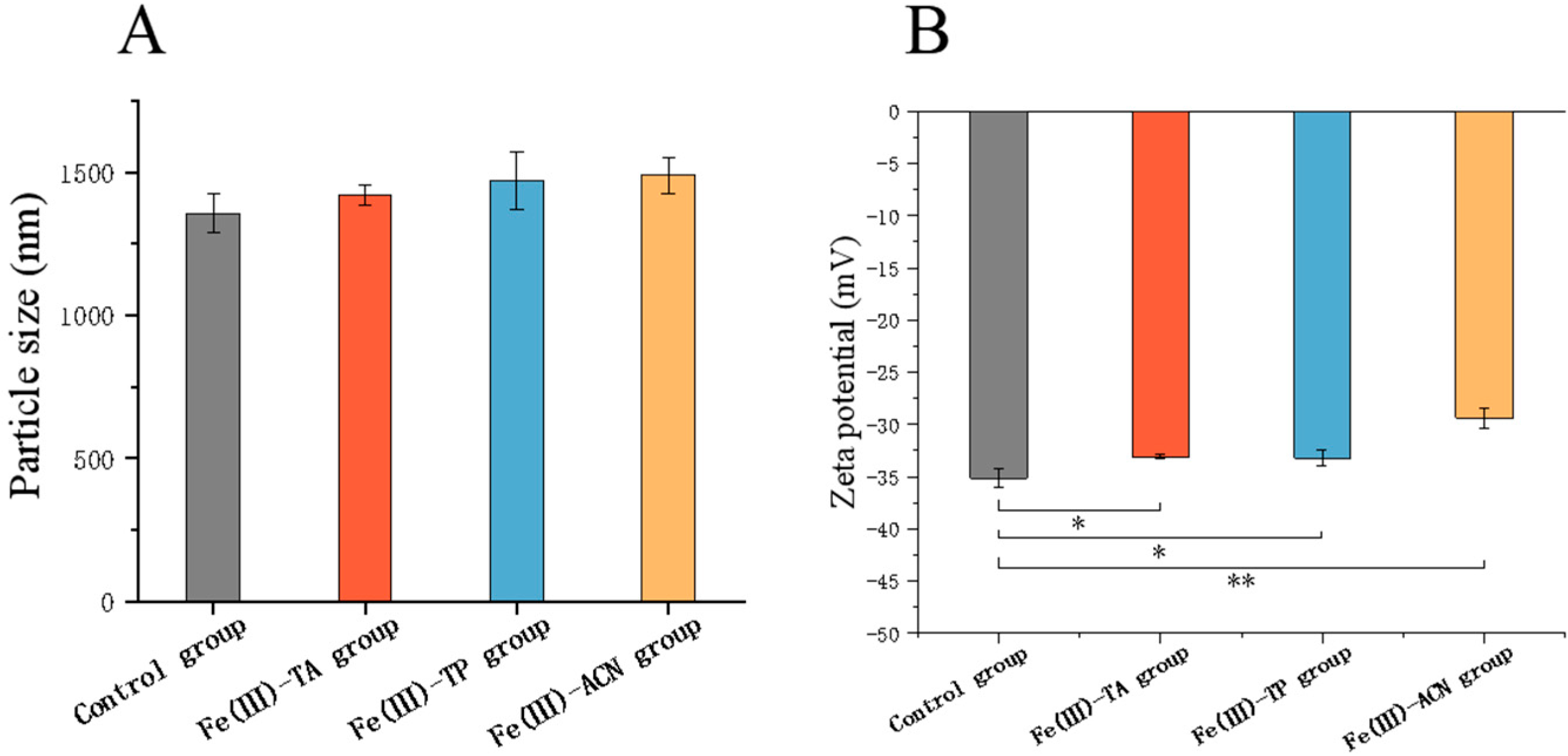
3.3. Particle Size and Zeta Potential
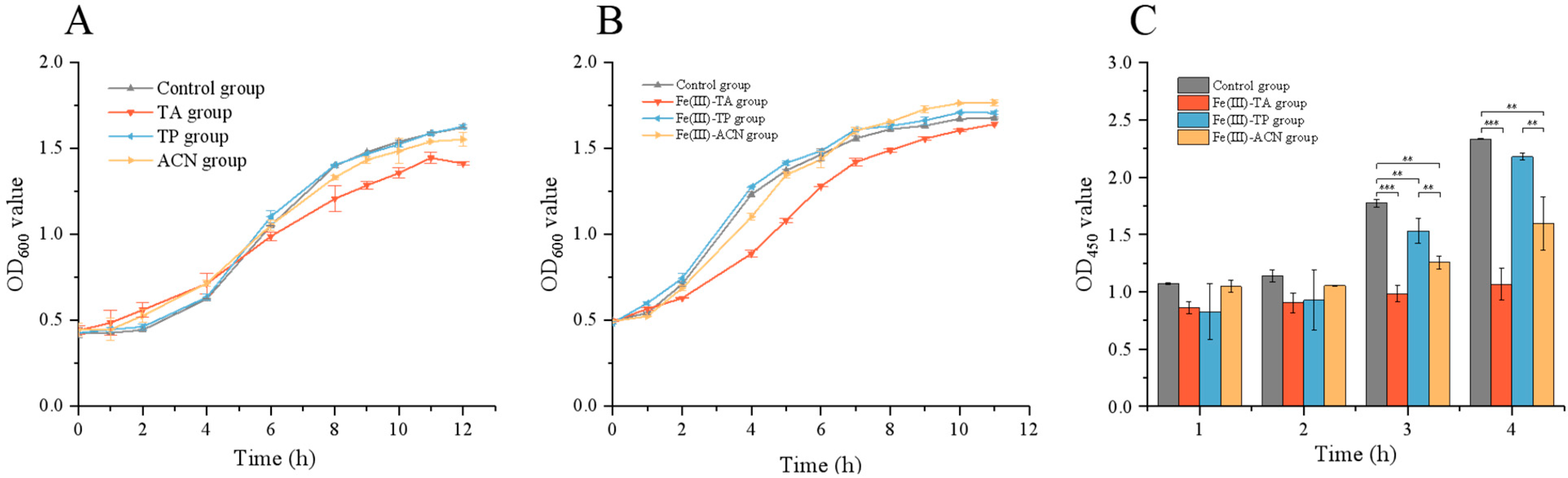
3.4. Effects of Single-Cell Encapsulation on the Growth of Lactobacillus plantarum
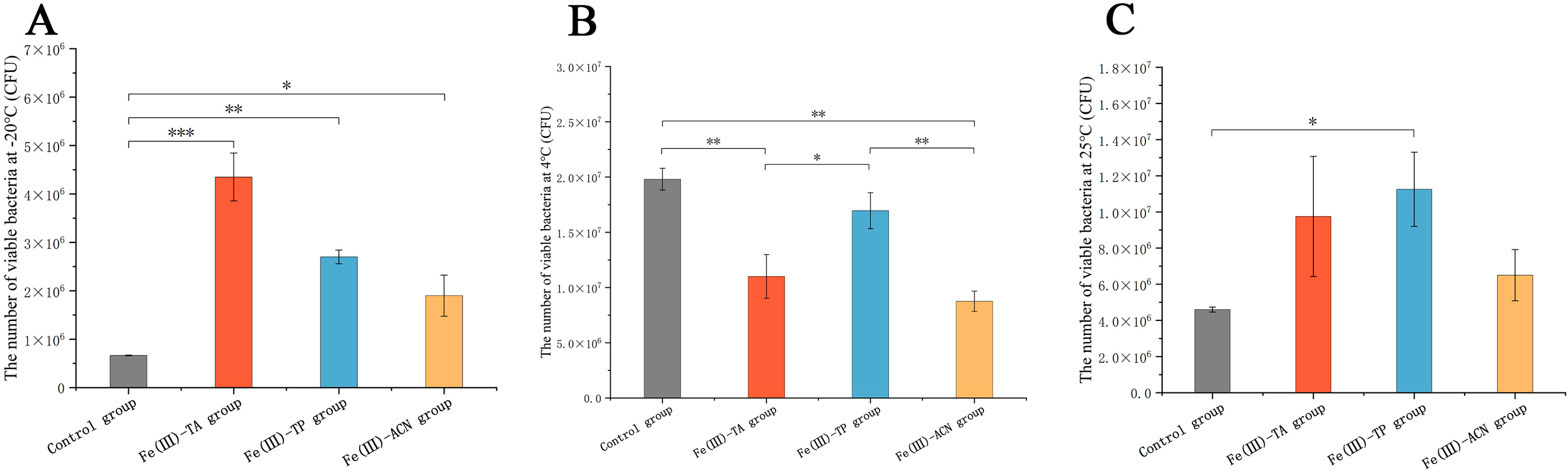
3.5. In Vitro Storage Experiments
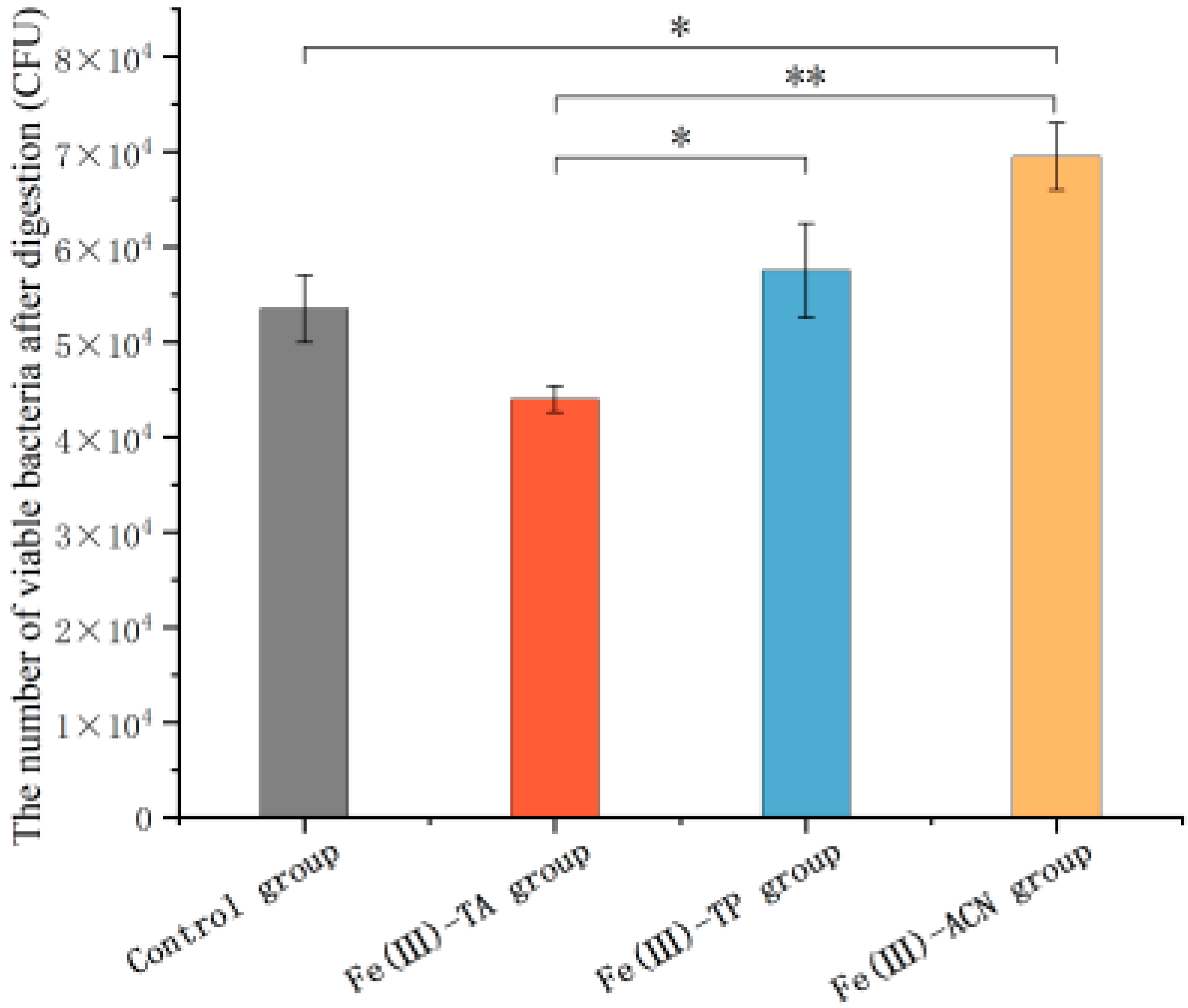
3.6. The Survival Ability for Gastric Assaults
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colin, H.; Francisco, G.; Gregor, R.; Gibson, G.G.; Merenstein, J.D.; Bruno, P.; Lorenzo, M.; Berni, C.R.; Flint, H.J.; Seppo, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z.; Hou, J. Purification and Antimicrobial Mechanism of a Novel Bacteriocin Produced by Lactobacillus rhamnosus 1.0320. LWT 2020, 137, 110338. [Google Scholar] [CrossRef]
- Hou, Q.; Zhao, F.; Liu, W.; Lv, R.; Khine, W.W.T.; Han, J.; Sun, Z.; Lee, Y.K.; Zhang, H. Probiotic-Directed Modulation of Gut Microbiota is Basal Microbiome Dependent. Gut Microbes 2020, 12, 1736974. [Google Scholar] [CrossRef] [PubMed]
- Chong-Su, K.; Lina, C.; Minju, S.; Sungwoong, J.; Young, C.W.; Wook, B.H.; Dong-Mi, S. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Elderly: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 76, 32–40. [Google Scholar]
- Uma, M.M.; XinYee, A.; BoonKiat, L.; Fiona, C.Y.; Afiqah, A.S.N.; Abd, H.I.J.; Abu, B.M.H.; Syazni, R.N.; Xiaojun, L.; Xiaohong, K.; et al. Correction to: Probiotic Bifidobacterium Lactis Probio-M8 Treated and Prevented Acute RTI, Reduced Antibiotic Use and Hospital Stay in Hospitalized Young Children: A Randomized, Double-Blind, Placebo-Controlled Study. Eur. J. Nutr. 2022, 61, 1679–1691. [Google Scholar]
- Yeganegi, M.; Watson, C.S.; Martins, A.; Kim, S.O.; Reid, G.; Challis, J.R.G.; Bocking, A.D. Effect of Lactobacillus rhamnosus GR-1 Supernatant and Fetal Sex on Lipopolysaccharide-Induced Cytokine and Prostaglandin-Regulating Enzymes in Human Placental Trophoblast Cells: Implications for Treatment of Bacterial Vaginosis and Prevention of Preterm Labor. Am. J. Obstet. Gynecol. 2008, 200, e531–e532. [Google Scholar]
- Filannino, P.; De Angelis, M.; Di Cagno, R.; Gozzi, G.; Riciputi, Y.; Gobbetti, M. How Lactobacillus plantarum Shapes Its Transcriptome in Response to Contrasting Habitats. Environ. Microbiol. 2018, 20, 3700–3716. [Google Scholar] [CrossRef]
- Leónides, F.; Nivia, C.; Rebeca, A.; Susana, M.; Esther, J.; Virginia, M.; Miguel, R.J. Prevention of Infectious Mastitis by Oral Administration of Lactobacillus Salivarius PS2 During Late Pregnancy. Clin. Infect. Dis. 2016, 62, civ974. [Google Scholar]
- Shen, F.; Zhu, H.; Bao, Z.; Han, D.; Du, J.; Zhao, M.; Feng, F.; He, G.; Mo, Q. Lactiplantibacillus Plantarum T34 Alleviates Constipation by Enhancing Intestinal Barrier and Modulating Gut Homeostasis. Food Biosci. 2025, 66, 106195. [Google Scholar] [CrossRef]
- Kuziel, G.A.; Rakoff-Nahoum, S. The Gut Microbiome. Curr. Biol. 2022, 32, R257–R264. [Google Scholar] [CrossRef]
- Israr, K.; Junshu, W.; Anping, L.; Zhirong, L.; Pingrong, Y.; Yaping, J.; Xinjun, C.; Tang, Z.; Yanrui, B.; Lajia, Z.; et al. Lactobacillus plantarum Strains Attenuated DSS-Induced Colitis in Mice by Modulating the Gut Microbiota and Immune Response. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2022, 25, 587–603. [Google Scholar]
- Haining, H.; Xinyi, Z.; Lingjun, T.; Qiqi, L.; Xi, L.; Yushan, B.; Pimin, G.; Tongjie, L.; Lanwei, Z.; Yongjun, X.; et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice
. Front. Immunol. 2021, 12, 777147. [Google Scholar]
- Yuting, Z.; Yanning, L.; Xiuquan, W.; Jingshu, H.; Xize, F.; Chunwei, S.; Wentao, Y.; Yanlong, J.; Xin, C.; Jianzhong, W.; et al. Lactobacillus plantarum Nc8 and Its Metabolite Acetate Alleviate Type 1 Diabetes via Inhibiting NLRP3. Microb. Pathog. 2023, 182, 106237. [Google Scholar]
- Feiyu, Y.; Jiyu, W.; Hongxing, Z.; Yuanhong, X.; Junhua, J.; Hui, L.; Xiaona, P.; Hongwei, H. Hypoglycemic Effects of Space-induced Lactobacillus plantarum SS18-5 on Type 2 Diabetes in a Rat Model. J. Food Biochem. 2021, 45, e13899. [Google Scholar]
- Renjie, S.; Jin, Y.; Hua, F.; Chunxia, X.; Danna, W.; Bing, X.; Zhenting, Z.; Beita, Z.; Xiaoshuang, D.; Xuebo, L. Lactobacillus plantarum LLY-606 Supplementation Ameliorates Hyperuricemia via Modulating Intestinal Homeostasis and Relieving Inflammation. Food Funct. 2023, 14, 5663–5677. [Google Scholar]
- Li, S.; Han, X.; Liu, N.; Chang, J.; Liu, G.; Hu, S. Lactobacillus plantarum Attenuates Glucocorticoid-Induced Osteoporosis by Altering the Composition of Rat Gut Microbiota and Serum Metabolic Profile. Front. Immunol. 2024, 14, 1285442. [Google Scholar] [CrossRef]
- Hae-Ji, L.; Jin-Ju, J.; Joo, H.M.; Dong-Hyun, K. Lactobacillus plantarum C29 Alleviates Tnbs-Induced Memory Impairment in Mice. J. Microbiol. Biotechnol. 2018, 28, 175–179. [Google Scholar]
- Cong, X.; Qingfeng, B.; Wan, W.; Juncai, H.; Zhanmei, J. Novel Nano-Encapsulated Probiotic Agents: Encapsulate Materials, Delivery, and Encapsulation Systems. J. Control. Release Off. J. Control. Release Soc. 2022, 349, 184–205. [Google Scholar]
- Pires-Santos, M.; Nadine, S.; Mano, J.F. Unveiling the Potential of Single-Cell Encapsulation in Biomedical Applications: Current Advances and Future Perspectives. Small Sci. 2024, 4, 2300332. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, T.; Li, J.; Niu, R.; Liu, D.; Wang, W. Single-Cell Encapsulation Systems for Probiotic Delivery: Armor Probiotics. Adv. Colloid Interface Sci. 2024, 332, 103270. [Google Scholar] [CrossRef]
- Feng, W.; Jinyao, L. Decorated Bacteria and the Application in Drug Delivery. Adv. Drug Deliv. Rev. 2022, 188, 114443. [Google Scholar]
- Xu, L.Q.; Neoh, K.-G.; Kang, E.-T. Natural Polyphenols as Versatile Platforms for Material Engineering and Surface Functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Zhong, H.; Jiang, J.; Hussain, M.; Zhang, H.; Chen, L.; Guan, R. The Encapsulation Strategies for Targeted Delivery of Probiotics in Preventing and Treating Colorectal Cancer: A Review. Adv. Sci. 2025, 12, 2500304. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Han, S.; Lee, K.B.; Choi, I.S. Biphasic Supramolecular Self-Assembly of Ferric Ions and Tannic Acid across Interfaces for Nanofilm Formation. Adv. Mater. 2017, 29, 1700784. [Google Scholar] [CrossRef]
- Pan, J.; Gong, G.; Wang, Q.; Shang, J.; He, Y.; Catania, C.; Birnbaum, D.; Li, Y.; Jia, Z.; Zhang, Y. A Single-Cell Nanocoating of Probiotics for Enhanced Amelioration of Antibiotic-Associated Diarrhea. Nat. Commun. 2022, 13, 2117. [Google Scholar] [CrossRef]
- Fan, G.; Wasuwanich, P.; Rodriguez-Otero, M.R.; Furst, A.L. Protection of Anaerobic Microbes from Processing Stressors Using Metal-Phenolic Networks. J. Am. Chem. Soc. 2022, 144, 2438–2443. [Google Scholar] [CrossRef]
- Yang, K.; Wang, Q.; Wang, Y.; Li, S.; Gu, Y.; Gao, N.; Zhang, F.; Lei, P.; Wang, R.; Xu, H. Poly(γ-glutamic acid) Nanocoating To Enhance the Viability of Pseudomonas stutzeri NRCB010 through Cell Surface Engineering. ACS Appl. Mater. Interfaces 2021, 13, 39957–39966. [Google Scholar] [CrossRef]
- Yuan, Y.; Kong, Z.-Y.; Sun, Y.-E.; Zeng, Q.-Z.; Yang, X.-Q. Complex Coacervation of Soy Protein with Chitosan: Constructing Antioxidant Microcapsule for Algal Oil Delivery. LWT Food Sci. Technol. 2017, 75, 171–179. [Google Scholar] [CrossRef]
- Zipei, Z.; Min, G.; Xiaomeng, Y.; Sela, A.D.; Hang, X.; Julian, M.D. Encapsulation of Bifidobacterium in Alginate Microgels Improves Viability and Targeted Gut Release. Food Hydrocoll. 2021, 116, 106634. [Google Scholar]
- Yang, X. Study on the Probiotic Delivery System with the Functional Coating to Enhance Intestinal Colonization for the Treatment of Enteritis. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2022. [Google Scholar]
- Wang, H.; Wang, D.; Yu, J.; Zhang, Y.; Zhou, Y. Applications of Metal-Phenolic Networks in Nanomedicine: A review. Biomater Sci. 2022, 10, 5786–5808. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Yu, R.; Chen, Y.; Wang, D.; Zhao, W.; Ge, S.; Liu, H.; Li, J. Metal-Phenolic Network Biointerface-Mediated Cell Regulation for Bone Tissue Regeneration. Mater. Today Bio 2025, 30, 101400. [Google Scholar] [CrossRef]
- Zhao, M.; Mu, L.; Xiang, F.; Lv, W.; Jiang, H.; Tao, G.; Li, B. Oral Delivery of Probiotic Combined Natural Phenolic-Metal Framework with Microencapsulation for Prolonged Intestinal Retention and Alleviation of Inflammatory Bowel Disease. Chem. Eng. J. 2025, 512, 162763. [Google Scholar] [CrossRef]
- Ma, L.; Su, C.; Lin, X.; Wang, H.; Luo, M.; Chen, Z.; Zhang, B.; Zhu, J.; Yuan, Y. Preparation and Characterization of Bilayered Microencapsulation for Co-Delivery Lactobacillus casei and Polyphenols via Zein-Chitosan Complex Coacervation. Food Hydrocoll. 2024, 148, 109410. [Google Scholar] [CrossRef]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of Nanoparticle Size, Shape, and Zeta Potential on Drug Delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pan, S.; Zhou, J.; Lin, Z.; Qu, Y.; Glab, A.; Han, Y.; Richardson, J.J.; Caruso, F. Assembly of Bioactive Nanoparticles via Metal–Phenolic Complexation. Adv. Mater. 2022, 34, 2108624. [Google Scholar] [CrossRef]
- Zhang, L. Modification of Bacterial Surface Based on FeIIITA Multi-Functional Coating and Its Antibacterial Ability. Master’s Thesis, Anhui Medical University, Hefei, China, 2023. [Google Scholar]
- Taisong, F.; Songbai, L. Metal-Phenolic Network Directed Coating of Single Probiotic Cell Followed by Photoinitiated Thiol-Ene Click Fortification to Enhance Oral Therapy. Small Weinh. Bergstr. Ger. 2023, 20, e2308146. [Google Scholar]
- Duñach, M.; Seigneuret, M.; Rigaud, J.L.; Padrós, E. Influence of Cations on the Blue to Purple Transition of Bacteriorhodopsin. Comparison of Ca2+ and Hg2+ Binding and Their Effect on the Surface Potential. J. Biol. Chem. 1988, 263, 17378–17384. [Google Scholar] [CrossRef]
- Trentin, D.S.; Silva, D.B.; Amaral, M.W.; Zimmer, K.R.; Silva, M.V.; Lopes, N.P.; Giordani, R.B.; Macedo, A.J. Tannins Possessing Bacteriostatic Effect Impair Pseudomonas Aeruginosa Adhesion and Biofilm Formation. PLoS ONE 2017, 8, e66257. [Google Scholar] [CrossRef]
- Guofeng, D.; Haiyang, L.; Xiao, Y.; Xiaoxiao, Z.; Hong, L.; Tieli, Z.; Jianming, C. Antimicrobial and Anti-Biofilm Activity of Tannic Acid Against Staphylococcus Aureus. Nat. Prod. Res. 2017, 32, 2225–2228. [Google Scholar]
- Boakye, Y.D.; Agyare, C.; Abotsi, W.K.M.; Ayande, P.G.; Ossei, P.P.S. Anti-Inflammatory Activity of Aqueous Leaf Extract of Phyllanthus muellerianus (Kuntze) Exell. and Its Major Constituent, Geraniin. J. Ethnopharmacol. 2016, 187, 17–27. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Johnson, B.J.; Hickey, T.E.; Pons, T.; Ligler, F.S. Binding and Neutralization of Lipopolysaccharides by Plant Proanthocyanidins. J. Nat. Prod. 2007, 70, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, C.; Zhong, W.; Shu, Y.; Zhang, Y.; Yang, D. Antibacterial Effect and Mechanism of Anthocyanin from Lycium ruthenicum Murr. Front. Microbiol. 2022, 13, 974602. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Li, H.B.; Corke, R.Y. Tannins as an Alternative to Antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Su, S.; Sun, A.; Du, T.; Wang, J.; Liu, J.; Zhang, W. Metal-Phenolic Networks Enhanced the Protection of Excipients for Probiotics During Freeze-Drying. Food Res. Int. 2025, 206, 116097. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Taranto, M.P.; Gerez, C.L.; Agriopoulou, S.; Smaoui, S.; Varzakas, T.; Enshasy, H.A.E. Recent Advances in the Understanding of Stress Resistance Mechanisms in Probiotics: Relevance for the Design of Functional Food Systems. Probiotics Antimicrob. Proteins 2025, 17, 138–158. [Google Scholar] [CrossRef]
- Chu, Y.; Hou, J.; Boyer, C.; Richardson, J.J.; Liang, K.; Xu, J. Biomimetic Synthesis of Coordination Network Materials: Recent advances in MOFs and MPNs. Appl. Mater. Today 2018, 10, 93–105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, H.; Zhong, H. Metal–Phenolic Network-Directed Coating of Lactobacillus plantarum: A Promising Strategy to Increase Stability. Foods 2025, 14, 2277. https://doi.org/10.3390/foods14132277
Zhang H, Zhang H, Zhong H. Metal–Phenolic Network-Directed Coating of Lactobacillus plantarum: A Promising Strategy to Increase Stability. Foods. 2025; 14(13):2277. https://doi.org/10.3390/foods14132277
Chicago/Turabian StyleZhang, Haoxuan, Huange Zhang, and Hao Zhong. 2025. "Metal–Phenolic Network-Directed Coating of Lactobacillus plantarum: A Promising Strategy to Increase Stability" Foods 14, no. 13: 2277. https://doi.org/10.3390/foods14132277
APA StyleZhang, H., Zhang, H., & Zhong, H. (2025). Metal–Phenolic Network-Directed Coating of Lactobacillus plantarum: A Promising Strategy to Increase Stability. Foods, 14(13), 2277. https://doi.org/10.3390/foods14132277



