Texture, Nutrition, and Flavor of Different Freshwater Fish Muscles: Comparative Study and Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sample
2.2. Basic Physicochemical Analysis
2.2.1. Determination of Muscle Texture Characteristics
2.2.2. Determination of Conventional Nutritional Components
2.3. Determination of Fatty Acid and Amino Acid Composition
2.4. Nutritional Evaluation
- AA1: Amino acid content in sample protein
- AA2: Amino acid content in scoring protein
- AA3: Amino acid content in whole egg protein
- n: The number of essential amino acids compared.
- A, B, …H: The content of essential amino acids in muscle protein, expressed in mg/g protein.
- AE, BE, …HE: The corresponding amino acid content in whole egg protein, expressed in mg/g protein.
2.5. Electronic Nose Analysis
2.6. HS-SPME-GC-MS Analysis
2.7. Molecular Docking
2.8. Statistical Analysis
3. Results and Discussion
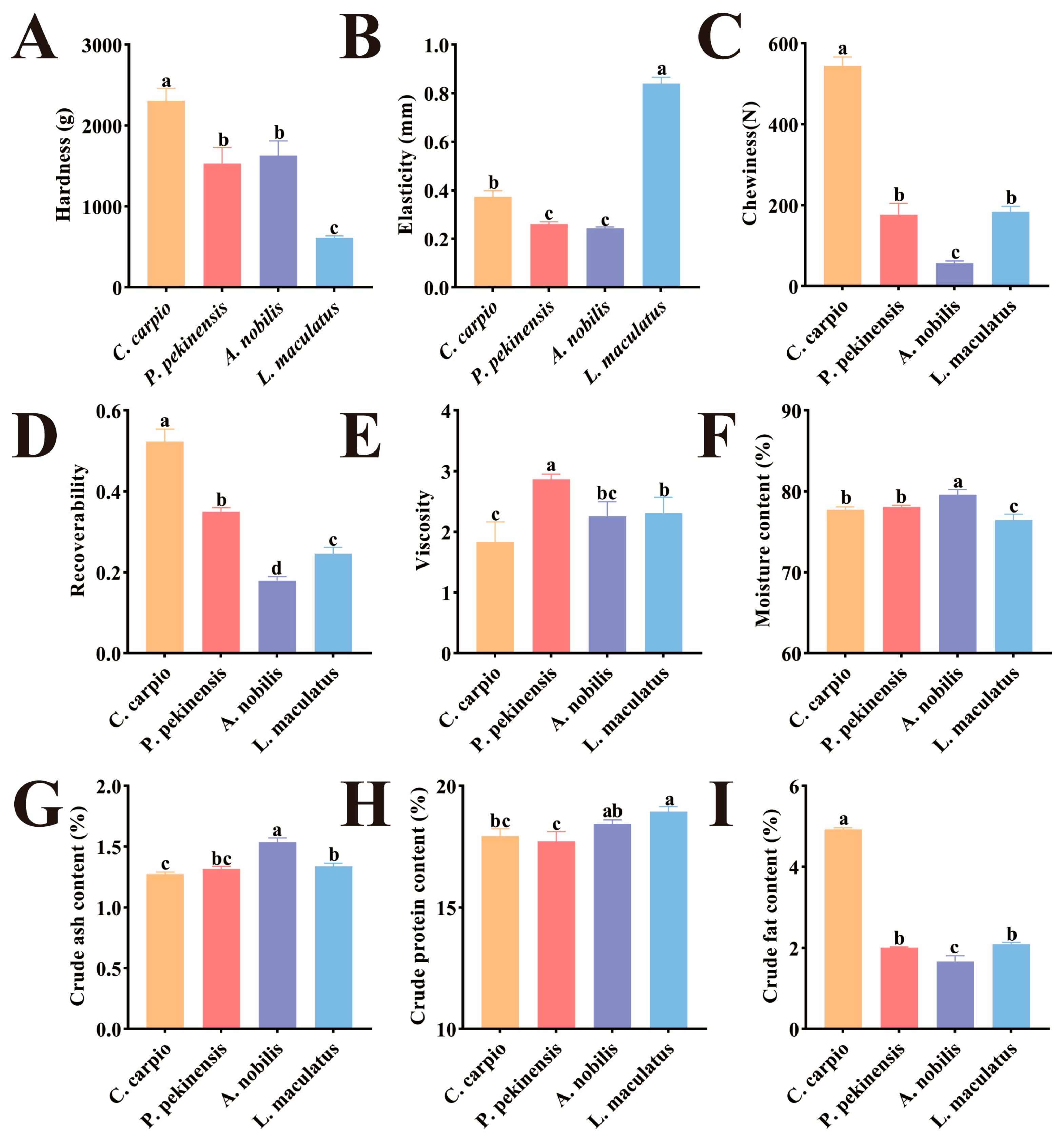
3.1. Muscle Texture and Proximate Composition
3.2. Analysis of Muscle Fatty Acid Composition
3.3. Analysis of Muscle Amino Acid Composition
3.4. Electronic Nose Detection and Volatile Compound Analysis
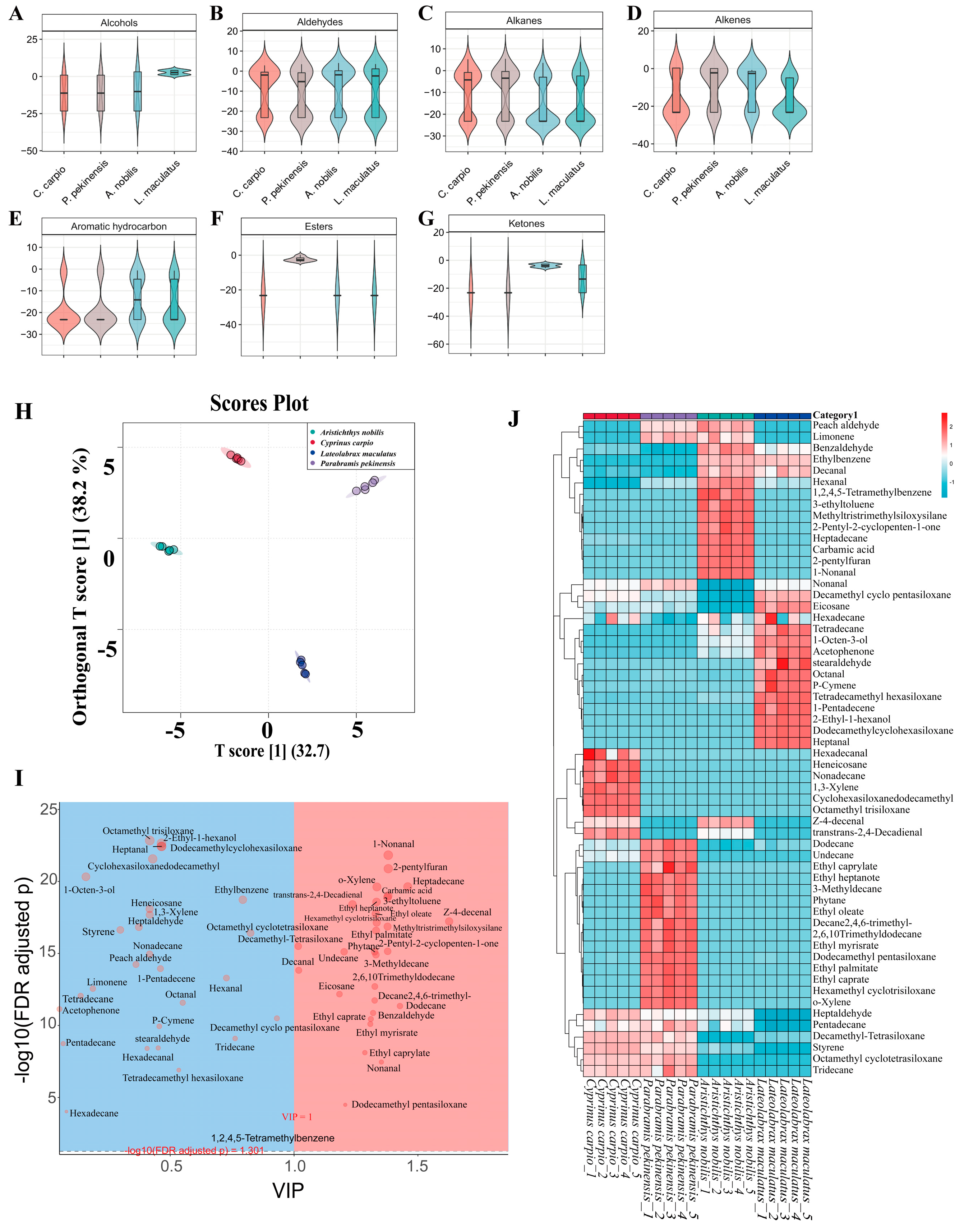
3.5. HS-SPME-GC-MS Analysis and Volatile Compound Profiling
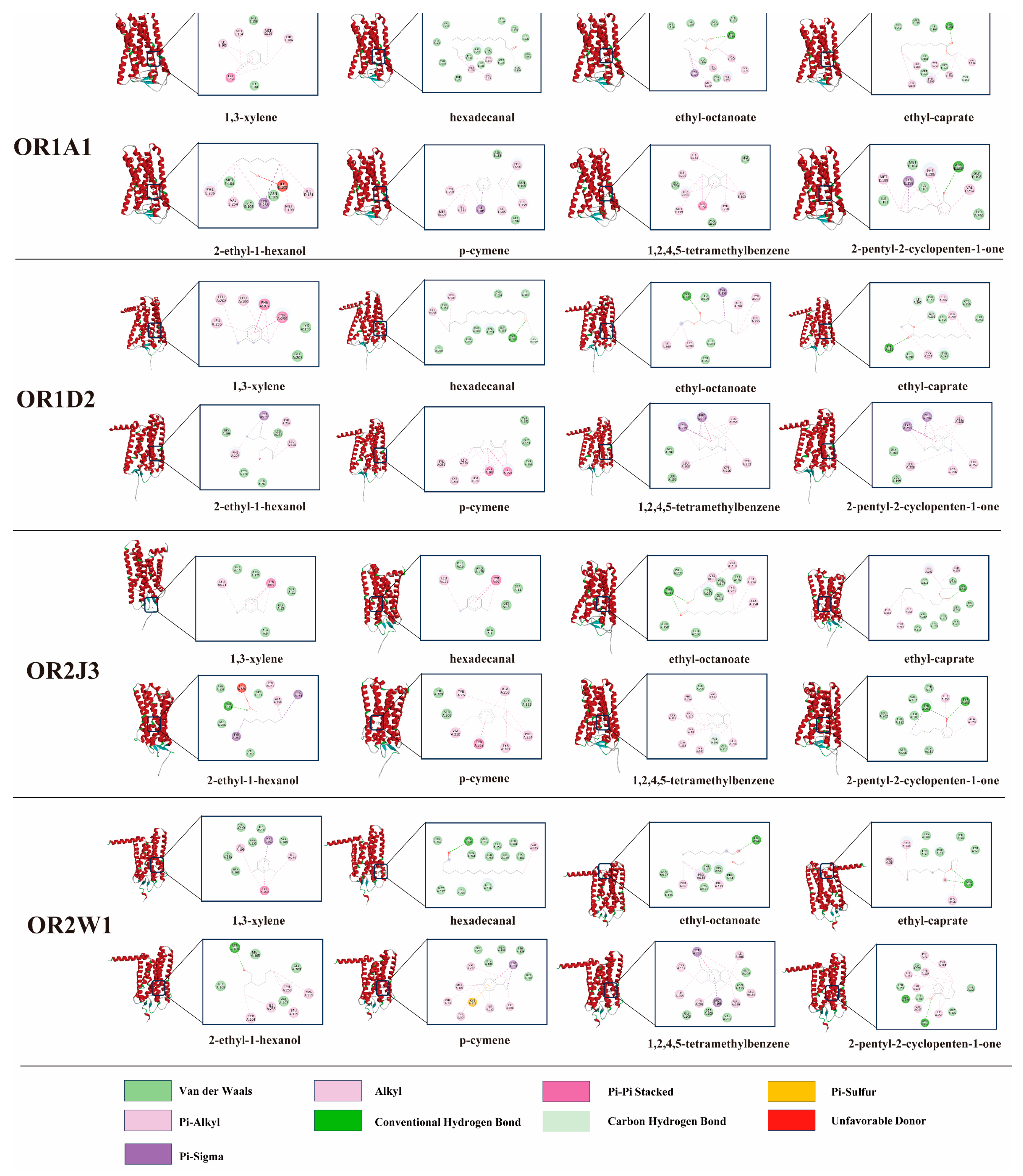
3.6. Molecular Docking Between Key Differential Volatile Flavor Compounds and ORs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Meng, F.; Yu, Z.; Tan, Y. Spatial–temporal characteristics and influencing factors of farmland expansion in different agricultural regions of Heilongjiang Province, China. Land Use Policy 2022, 115, 106007. [Google Scholar] [CrossRef]
- Kang, B.; Deng, J.; Wu, Y.; Chen, L.; Zhang, J.; Qiu, H.; Lu, Y.; He, D. Mapping C hina’s freshwater fishes: Diversity and biogeography. Fish Fish. 2014, 15, 209–230. [Google Scholar] [CrossRef]
- Suomela, J.-P.; Lundén, S.; Kaimainen, M.; Mattila, S.; Kallio, H.; Airaksinen, S. Effects of origin and season on the lipids and sensory quality of European whitefish (Coregonus lavaretus). Food Chem. 2016, 197, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Adámek, Z.; Mössmer, M.; Hauber, M. Current principles and issues affecting organic carp (Cyprinus carpio) pond farming. Aquaculture 2019, 512, 734261. [Google Scholar] [CrossRef]
- Tian, X.; Gao, P.; Xu, Y.; Xia, W.; Jiang, Q. Reduction of biogenic amines accumulation with improved flavor of low-salt fermented bream (Parabramis pekinensis) by two-stage fermentation with different temperature. Food Biosci. 2021, 44, 101438. [Google Scholar] [CrossRef]
- Chen, J.; Tang, L.; Peng, F.; Huang, Z.; Feng, X.; Liu, X.; Wang, Y.; Xiao, Y.; Liu, W. Study on biological characteristics and excellent characters of Baling Tieshan organic bighead carp. Reprod. Breed. 2023, 3, 219–228. [Google Scholar] [CrossRef]
- Sheng, Z.; Xu, J.; Zhang, Y.; Wang, Z.; Chen, N.; Li, S. Dietary protein hydrolysate effects on growth, digestive enzymes activity, and expression of genes related to amino acid transport and metabolism of larval snakehead (Channa argus). Aquaculture 2023, 563, 738896. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Y.; Shen, W.; Zheng, W.; Liu, T.; Wang, J.; Feng, F. Evaluating dynamic effects of dietary glycerol monolaurate on the productive performance and flesh quality of large yellow croaker (Larimichthys crocea). Food Chem. 2022, 387, 132833. [Google Scholar] [CrossRef]
- Zhu, Z.; Bassey, A.P.; Cao, Y.; Du, X.; Huang, T.; Cheng, Y.; Huang, M. Meat quality and flavor evaluation of Nanjing water boiled salted duck (NWSD) produced by different Muscovy duck (Cairina moschata) ingredients. Food Chem. 2022, 397, 133833. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Ayseli, M.T. A systematic review on nano-delivery systems enriched with aromatic compounds: Flavor, odor, and chemical quality perspectives in fish. Food Chem. Adv. 2024, 5, 100750. [Google Scholar] [CrossRef]
- Han, G.; Li, Q.; Hong, H.; You, J.; Yin, T.; Xiong, S.; Liu, R. Exploring the quality changes of carp roes: Perspective from flavor characteristics and microstructure. Food Res. Int. 2025, 218, 116765. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, P.; Jiang, W.-D.; Liu, Y.; Ma, Y.-B.; Kuang, S.-Y.; Li, S.-W.; Tang, L.; Zhou, X.-Q.; Feng, L. A nutritional strategy for improving sub-adult grass carp (Ctenopharyngodon idella) flesh flavor: The role of α-lipoic acid in regulating muscle nucleotide and fatty acid metabolism. Aquaculture 2025, 603, 742437. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, X.-Q.; Jiang, W.-D.; Wu, P.; Liu, Y.; Ma, Y.-B.; Jin, X.-W.; Ren, H.-M.; Feng, L. Histidine improves flesh quality: An assessment of grass carp (Ctenopharyngodon idella) muscle in terms of texture, nutritional value and flavor. Food Chem. 2025, 474, 143214. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Song, Z.; Wang, F.; Lin, L.; Tao, N. Effect of short-term depuration on the flavor of crucian carp (Carassius auratus) and exploration of prediction model for fishy odor. J. Food Compos. Anal. 2025, 142, 107454. [Google Scholar] [CrossRef]
- Li, X.; Song, J.; Jiao, J.; Geng, Q.; Lin, S. Interaction mechanisms of sea cucumber proteins with flavor compounds: Adsorption behavior, dynamic equilibrium and structural changes. Food Chem. 2025, 487, 144767. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, W.; Wang, X.; Li, Y.; Yang, J. Identification of the deterioration flavor markers based on E-nose, GC-IMS and GC–MS during fresh sea cucumber storage and preservation by thymol. Food Chem. 2025, 488, 144942. [Google Scholar] [CrossRef]
- Fu, C.; Zou, Y.; Zhang, Y.; Liao, M.; Chen, D.; Guo, Z. Comparison of Different Deodorizing Treatments on the Flavor of Paddy Field Carp, Analyzed by the E-Nose, E-Tongue and Gas Chromatography–Ion Mobility Spectrometry. Foods 2024, 13, 2623. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, W.; Tian, H.; Wang, L.; Zhu, J.; Luo, W.; Liang, J.; Xiang, L.; Cai, X.; Wang, S.; et al. Influence of EGCG (Epigallocatechin gallate) on Physicochemical–Rheological Properties of Surimi Gel and Mechanism Based on Molecular Docking. Foods 2024, 13, 2412. [Google Scholar] [CrossRef]
- He, M.; Liu, W.; Zhang, C.; Liu, Y.; Zhuang, H.; O’Hagan, D. Selectively Fluorinated Citronellol Analogues Support a Hydrogen Bonding Donor Interaction with the Human OR1A1 Olfactory Receptor. Org. Lett. 2022, 24, 4415–4420. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Z.; Zhang, K.; Deng, X.; Wang, G.; Ye, Z.; Liu, M.; Chen, J.; Chen, S.; Ye, X.; et al. Revealing the off-flavors in hydro-distilled essential oils of sweet orange (Citrus sinensis) by flavoromics strategy and computational simulation. Food Chem. 2025, 465, 141990. [Google Scholar] [CrossRef]
- Xia, B.; Zhao, D.; Hao, Q.; Yu, J.; Han, Y.; Ling, L.; Zhao, R.; Zhao, J. Effects of fishing stress on fatty acid and amino acid composition and glycolipid metabolism in triploid rainbow trout. Food Chem. 2024, 461, 140904. [Google Scholar] [CrossRef] [PubMed]
- GB-5009.6; Chinese National Standard GB5009.6-2016. National Food Safety Standard: Determination of Lipid in Foods. Standards Press of China: Beijing, China, 2016. (In Chinese)
- GB-5009.5; Chinese National Standard GB5009.5-2016. National Food Safety Standard: Determination of Protein in Foods. Standards Press of China: Beijing, China, 2016. (In Chinese)
- GB-5009.168; Chinese National Standard GB5009.168-2016. National Food Safety Standard: Determination of Fatty Acids in Foods. Standards Press of China: Beijing, China, 2016. (In Chinese)
- GB-5009.124; Chinese National Standard GB 5009.124-2016. National Food Safety Standard: Determination of Amino Acids in Food. Standards Press of China: Beijing, China, 2016. (In Chinese)
- Wang, H.; Sui, Y.; Liu, J.; Kong, B.; Li, H.; Qin, L.; Chen, Q. Analysis and comparison of the quality and flavour of traditional and conventional dry sausages collected from northeast China. Food Chem. X 2023, 20, 100979. [Google Scholar] [CrossRef]
- Johnston, I.A.; Alderson, R.; Sandham, C.; Dingwall, A.; Mitchell, D.; Selkirk, C.; Nickell, D.; Baker, R.; Robertson, B.; Whyte, D. Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L.). Aquaculture 2000, 189, 335–349. [Google Scholar] [CrossRef]
- Spierts, I.L.; Leeuwen, J.L.V. Kinematics and muscle dynamics of C-and S-starts of carp (Cyprinus carpio L.). J. Exp. Biol. 1999, 202, 393–406. [Google Scholar] [CrossRef]
- Franco-López, J.; Abarca-Arenas, L.G.; Peláez-Rodríguez, E.; Viveros-Legorreta, J.L.; González-Acosta, A.F. Biometric relationships of the fat sleeper Dormitator maculatus (Bloch, 1792) (Teleostei: Eleotridae) from Alvarado lagoon, Veracruz, Mexico. Ecosistemas Y Recur. Agropecu. 2021, 8, e2679. [Google Scholar]
- Zhang, T.; Wu, Y.; Li, L.; Wang, Y.; Ren, Z. Correlation analysis of sensory with instrumental texture measurement of salted fish. J. Fish. China 2013, 37, 303–310. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Wang, Y.; Bai, C.; Jiang, Y.; Yuan, M.; Zhao, L.; Chen, L. Characterization of the flavor profile of four major Chinese carps using HS-SPME-GC–MS combined with ultra-fasted gas chromatography-electronic nose. Food Chem. 2025, 463, 141264. [Google Scholar] [CrossRef]
- Erdorul, O.; Erbilir, F. Heavy metal and trace elements in various fish samples from Sir Dam Lake, Kahramanmaras, Turkey. Environ. Monit. Assess. 2007, 130, 373. [Google Scholar] [CrossRef]
- Meyer, G.; Fracalossi, D.M. Protein requirement of jundia fingerlings, Rhamdia quelen, at two dietary energy concentrations. Aquaculture 2004, 240, 331–343. [Google Scholar] [CrossRef]
- Romvári, R.; Hancz, C.; Petrási, Z.; Molnár, T.; Horn, P. Non-invasive measurement of fillet composition of four freshwater fish species by computer tomography. Aquac. Int. 2002, 10, 231–240. [Google Scholar] [CrossRef]
- Aghababaei, F.; McClements, D.J.; Hadidi, M. Ultrasound processing for enhanced digestibility of plant proteins. Food Hydrocoll. 2024, 155, 110188. [Google Scholar] [CrossRef]
- Xia, B.; Zou, H.; Li, L.; Zhang, B.; Xiang, Y.; Zou, Y.; Shen, Z.; Xue, S.; Han, Y. Screening and fermentation medium optimization of a strain favorable to Rice–fish Coculture. Front. Microbiol. 2022, 13, 1054797. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional value of fish: Lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Calder, P.C.; Deckelbaum, R.J. Dietary fatty acids in health and disease: Greater controversy, greater interest. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 111–115. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Monnard, C.R.; Dulloo, A.G. Polyunsaturated fatty acids as modulators of fat mass and lean mass in human body composition regulation and cardiometabolic health. Obes. Rev. 2021, 22, e13197. [Google Scholar] [CrossRef]
- World Health Organization; FAO. Energy and Protein Requirements: Report of a Joint FAO/WHO Ad Hoc Expert Committee, Rome, 22 March–2 April 1971; Food and Agriculture Organization: Rome, Italy, 1973. [Google Scholar]
- Tan, K.; Xu, P.; Huang, L.; Luo, C.; Choong, K.; Li, Z.; Guo, Y.; Cheong, K.-L. Quantitative evaluation of essential amino acids and omega-3 long-chain polyunsaturated fatty acids from global marine bivalve aquaculture. Food Chem. X 2025, 25, 102181. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Nunes, A.J.P.; Sá, M.V.C.; Browdy, C.L.; Vazquez-Anon, M. Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture 2014, 431, 20–27. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Zhang, J.-b.; Li, M.-x.; Zhang, Y.-f.; Qin, Y.-w.; Li, Y.; Su, L.-l.; Li, L.; Bian, Z.-h.; Lu, T.-l. E-eye, flash GC E-nose and HS-GC-MS combined with chemometrics to identify the adulterants and geographical origins of Ziziphi Spinosae Semen. Food Chem. 2023, 424, 136270. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Jiao, X.; Zhang, Z.; Zhang, L.; Song, J.; Wu, H.; Xiao, J. Correlation between volatile oxidation products and inflammatory markers in docosahexaenoic acid: Insights from OPLS-DA and predictive modeling. Food Biosci. 2025, 63, 105756. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace–gas chromatography–ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.; Zhao, X.; Wang, Y.; Li, M.; Wang, Y.; Tian, M.; Zhou, J. Classification and characterization on sorghums based on HS-GC-IMS combined with OPLS-DA and GA-PLS. Curr. Res. Food Sci. 2024, 8, 100692. [Google Scholar] [CrossRef]
- Wan, J.-B.; Bai, X.; Cai, X.-J.; Rao, Y.; Wang, Y.-S.; Wang, Y.-T. Chemical differentiation of Da-Cheng-Qi-Tang, a Chinese medicine formula, prepared by traditional and modern decoction methods using UPLC/Q-TOFMS-based metabolomics approach. J. Pharm. Biomed. Anal. 2013, 83, 34–42. [Google Scholar] [CrossRef]
- Ma, Q.; Hamid, N.; Bekhit, A.; Robertson, J.; Law, T. Evaluation of pre-rigor injection of beef with proteases on cooked meat volatile profile after 1 day and 21 days post-mortem storage. Meat Sci. 2012, 92, 430–439. [Google Scholar] [CrossRef]
- Machiels, D.; Istasse, L.; van Ruth, S.M. Gas chromatography-olfactometry analysis of beef meat originating from differently fed Belgian Blue, Limousin and Aberdeen Angus bulls. Food Chem. 2004, 86, 377–383. [Google Scholar] [CrossRef]
- Fu, B.; Zheng, M.; Yang, H.; Zhang, J.; Li, Y.; Wang, G.; Tian, J.; Zhang, K.; Xia, Y.; Li, Z. The effect of broad bean diet on structure, flavor and taste of fresh grass carp: A comprehensive study using E-nose, E-tongue, TPA, HS-SPME-GC-MS and LC-MS. Food Chem. 2024, 436, 137690. [Google Scholar] [CrossRef]
- Ruiz, J.; Ventanas, J.; Cava, R.; Andrés, A.; García, C. Volatile compounds of dry-cured Iberian ham as affected by the length of the curing process. Meat Sci. 1999, 52, 19–27. [Google Scholar] [CrossRef]
- Muriel, E.; Antequera, T.; Petrón, M.; Andrés, A.; Ruiz, J. Volatile compounds in Iberian dry-cured loin. Meat Sci. 2004, 68, 391–400. [Google Scholar] [CrossRef]
- Chi, X.; Shao, Y.; Pan, M.; Yang, Q.; Yang, Y.; Zhang, X.; Ai, N.; Sun, B. Distinction of volatile flavor profiles in various skim milk products via HS-SPME–GC–MS and E-nose. Eur. Food Res. Technol. 2021, 247, 1539–1551. [Google Scholar] [CrossRef]
- Machiels, D.; Van Ruth, S.M.; Posthumus, M.A.; Istasse, L. Gas chromatography-olfactometry analysis of the volatile compounds of two commercial Irish beef meats. Talanta 2003, 60, 755–764. [Google Scholar] [CrossRef]
- Montanari, C.; Bargossi, E.; Gardini, A.; Lanciotti, R.; Magnani, R.; Gardini, F.; Tabanelli, G. Correlation between volatile profiles of Italian fermented sausages and their size and starter culture. Food Chem. 2016, 192, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Yang, Z.; Liu, M.; Shi, Z.; Li, J.; Chen, W.; Qiao, X. Time-dependent categorization of volatile aroma compound formation in stewed Chinese spicy beef using electron nose profile coupled with thermal desorption GC–MS detection. Food Sci. Hum. Wellness 2017, 6, 137–146. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Zhang, H.; Wang, Y.; Chen, Q.; Kong, B. Physicochemical properties and flavour profile of fermented dry sausages with a reduction of sodium chloride. LWT 2020, 124, 109061. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Li, C.; Li, L.; Yang, X.; Wu, Y.; Chen, S.; Zhao, Y. Novel insight into physicochemical and flavor formation in naturally fermented tilapia sausage based on microbial metabolic network. Food Res. Int. 2021, 141, 110122. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Song, S.; Tan, C.; Jia, C.; Xia, S. Identification of characteristic flavour precursors from enzymatic hydrolysis-mild thermal oxidation tallow by descriptive sensory analysis and gas chromatography–olfactometry and partial least squares regression. J. Chromatogr. B 2013, 913, 69–76. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Sun, Z.; Shen, T.; Kou, X.; Niu, Y.; Xiao, Z. Unraveling the interaction mechanism between enantiomers of lactone compounds (γ-octalactone and γ-undecalactone) in Longjing tea and OR1A1 olfactory receptor using molecular docking and molecular dynamics simulation. Food Biosci. 2025, 66, 106282. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, Y.; Yang, H.; Zhao, R.; Zhu, J.; Wang, F. Characterization of honey-like characteristic aroma compounds in Zunyi black tea and their molecular mechanisms of interaction with olfactory receptors using molecular docking. LWT 2024, 191, 115640. [Google Scholar] [CrossRef]
- Jia, X.; Gao, Y.; Xi, H.; Cui, C.; Yang, X.; He, B.; Xu, C.; Gao, M.; Li, T. A flavor imitation method for Osmanthus aroma based on molecular docking screening and odor activity value analysis. LWT 2025, 223, 117697. [Google Scholar] [CrossRef]
- Haag, F.; Di Pizio, A.; Krautwurst, D. The key food odorant receptive range of broadly tuned receptor OR2W1. Food Chem. 2022, 375, 131680. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, Q.; Niu, Y.; She, Y.; Sun, Z.; Zhang, J.; Wang, Z.; Zhou, R.; Zhu, J. Mechanism of the interaction between olfactory receptors and characteristic aroma compounds in sweet orange juice. LWT 2024, 207, 116660. [Google Scholar] [CrossRef]
- Zhai, C.; Mariscal, A.; Liu, W. Molecular recognition in water by synthetic hydrogen-bonding receptors. Trends Chem. 2025, 7, 70–84. [Google Scholar] [CrossRef]
- Zhao, Y.; Templonuevo, R.M.; Chun, J. Enhancement of polycaprolactone nanofiber film performance by hydrogen bonding interactions with chitosan for food packaging. Int. J. Biol. Macromol. 2025, 300, 139437. [Google Scholar] [CrossRef]
- Yang, J.; Cao, P.; Dou, Q.; Guo, L.; Li, Y.; Zhang, Y.; Yan, J.; Wang, Q.; Xu, Z.; Xie, H.; et al. Synergistic enhancement of low-temperature strength and toughness in poly(boron-urethane) elastomers via π-π stacking and hydrogen bonding. Chem. Eng. J. 2025, 503, 158459. [Google Scholar] [CrossRef]
- Yang, C.; Ge, X.; Ge, C.; Zhao, P.; Liang, S.; Xiao, Z. Taste characterization and molecular docking study of novel umami flavor peptides in Yanjin black bone Chicken meat. Food Chem. 2025, 464, 141695. [Google Scholar] [CrossRef]
- Katada, S.; Hirokawa, T.; Oka, Y.; Suwa, M.; Touhara, K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: Mapping the odorant-binding site. J. Neurosci. 2005, 25, 1806–1815. [Google Scholar] [CrossRef]
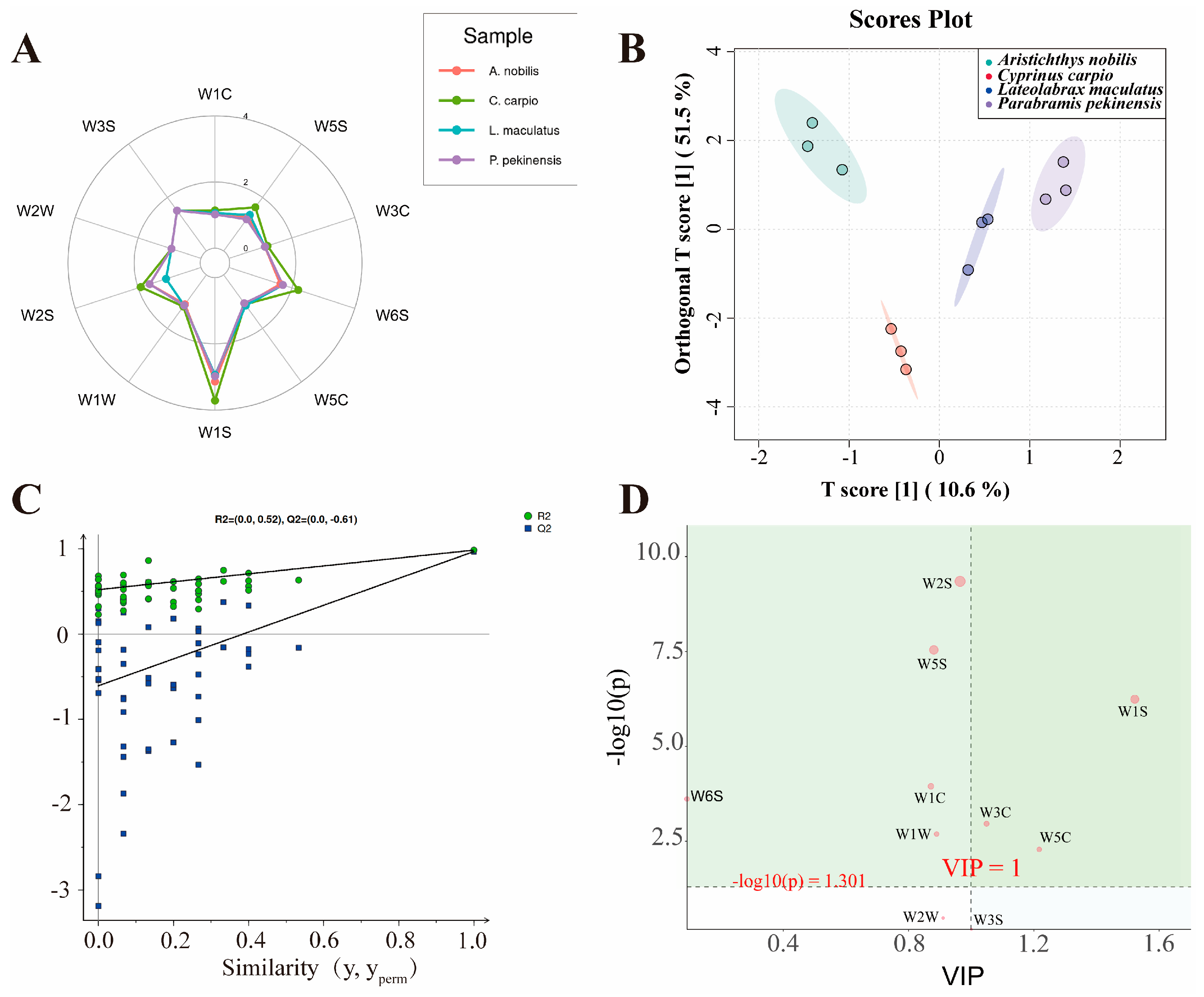
| Sensor | Substances for Sensing |
|---|---|
| W1C | Aromatic benzene |
| W5S | Nitrogen oxides |
| W3C | Aromatic ammonia |
| W6S | Hydrogen |
| W5C | Alkane aromatic compounds |
| W1S | Short-chain alkanes |
| W1W | Sulfides and terpenes |
| W2S | Alcohols, aldehydes, and ketones |
| W2W | Organic sulfides and aromatic components |
| W3S | Long-chain alkanes |
| Fatty Acids (g/100 g Total Fatty Acid) | C. carpio | P. pekinensis | A. nobilis | L. maculatus |
|---|---|---|---|---|
| Lauric acid (C12:0) | 0.03 ± 0.01 b | - | 0.15 ± 0.03 a | - |
| Tridecanoic acid (C13:0) | - | - | - | - |
| Myristic acid (C14:0) | 1.47 ± 0.12 b | 1.30 ± 0.12 c | 5.69 ± 0.02 a | 0.68 ± 0.03 d |
| Pentadecanoic acid (C15:0) | 0.50 ± 0.01 b | 0.08 ± 0.02 c | 1.01 ± 0.04 a | 0.11 ± 0.01 c |
| Palmitic acid (C16:0) | 20.27 ± 2.06 a | 19.05 ± 1.13 a | 18.99 ± 0.83 a | 14.16 ± 0.74 b |
| Heptadecanoic acid (C17:0) | 0.54 ± 0.09 b | 0.45 ± 0.07 b | 1.12 ± 0.07 a | - |
| Stearic acid (C18:0) | 6.30 ± 0.56 a | 5.80 ± 0.10 a | 4.68 ± 0.60 b | 3.58 ± 0.14 c |
| Arachidic acid (C20:0) | 0.20 ± 0.05 b | - | 0.47 ± 0.01 a | 0.23 ± 0.02 b |
| Heneicosanoic acid (C21:0) | 0.21 ± 0.04 b | - | - | 0.56 ± 0.03 a |
| Behenic acid (C22:0) | 0.07 ± 0.02 b | 0.12 ± 0.02 b | 0.24 ± 0.07 a | 0.30 ± 0.04 a |
| Myristoleic acid (C14:1) | - | - | - | - |
| Palmitoleic acid (C16:1) | 7.72 ± 0.23 b | 7.10 ± 0.27 c | 9.20 ± 0.41 a | 3.50 ± 0.11 d |
| Heptadecenoic acid (C17:1) | 0.30 ± 0.07 a | 0.12 ± 0.01 b | - | 0.12 ± 0.01 b |
| Oleic acid (C18:1) | 23.87 ± 0.91 b | 18.64 ± 0.35 c | 13.17 ± 1.16 d | 37.53 ± 1.08 a |
| Eicosenoic acid (C20:1) | 1.38 ± 0.27 b | 0.54 ± 0.01 c | 0.53 ± 0.10 c | 2.13 ± 0.04 a |
| Erucic acid (C22:1n9) | - | - | 0.03 ± 0.01 b | 0.73 ± 0.06 a |
| Nervonic acid (C24:1) | - | - | 0.14 ± 0.04 a | 0.13 ± 0.03 a |
| Linoleic acid (C18:2n6c) | 15.96 ± 1.13 a | 11.45 ± 0.21 b | 6.66 ± 1.03 c | 10.90 ± 0.21 b |
| Gamma-linolenic acid (C18:3n6) | 0.19 ± 0.01 c | 0.06 ± 0.02 d | 0.50 ± 0.05 a | 0.36 ± 0.01 b |
| Alpha-linolenic acid (C18:3n3) | 3.31 ± 0.29 c | 9.92 ± 0.35 a | 7.59 ± 0.73 b | 1.73 ± 0.03 d |
| Eicosadienoic acid (C20:2) | 0.94 ± 0.11 a | 0.52 ± 0.03 c | 0.49 ± 0.06 c | 0.66 ± 0.04b |
| Eicosatrienoic acid (C20:3n3) | 0.22 ± 0.01 c | 1.02 ± 0.06 a | 0.66 ± 0.07 b | 0.14 ± 0.01 d |
| Di-γ-linolenic acid (C20:3n6) | 0.85 ± 0.05 b | 1.47 ± 0.07 a | 0.78 ± 0.21 b | 1.57 ± 0.05 a |
| Arachidonic acid (C20:4n6) | 4.80 ± 0.41 b | 5.98 ± 0.21 a | 5.68 ± 0.82 ab | 2.82 ± 0.13 c |
| EPA (C20:5n3) | 5.61 ± 0.14 b | 5.24 ± 0.02 b | 9.49 ± 1.41 a | 0.23 ± 0.01 c |
| DHA (C22:6n3) | 5.18 ± 0.43 c | 11.19 ± 0.30 b | 13.20 ± 1.28 a | 2.03 ± 0.11 d |
| ∑SFA | 29.57 ± 1.84 b | 26.80 ± 1.04 c | 32.35 ± 1.89 a | 19.62 ± 0.74 d |
| ∑MUFA | 33.27 ± 1.01 b | 26.40 ± 0.40 c | 23.06 ± 1.34 d | 44.16 ± 0.48 a |
| ∑PUFA | 37.06 ± 3.14 a | 46.85 ± 0.36 a | 45.06 ± 10.2 a | 20.44 ± 0.18 b |
| ∑(n − 3) PUFA | 14.32 ± 0.36 c | 27.37 ± 0.62 b | 30.94 ± 0.33 a | 4.13 ± 0.13 d |
| ∑(n − 6) PUFA | 21.79 ± 1.34 a | 18.95 ± 0.21 b | 13.62 ± 0.41 d | 15.65 ± 0.41 c |
| ∑(n − 3) PUFA/∑(n − 6) PUFA | 0.66 ± 0.13 c | 1.44 ± 0.05 b | 2.27 ± 0.01 a | 0.26 ± 0.04 d |
| Amino Acids | Content (mg/g) | Taste Attribute | |||
|---|---|---|---|---|---|
| C. carpio | P. pekinensis | A. nobilis | L. maculatus | ||
| Threonine * | 0.82 ± 0.01 c | 1.13 ± 0.01 a | 0.75 ± 0.01 b | 0.94 ± 0.01 d | Sweet |
| Valine * | 0.97 ± 0.01 c | 1.34 ± 0.01 a | 0.91 ± 0.06 d | 1.10 ± 0.01 b | Bitter |
| Methionine * | 0.58 ± 0.01 c | 0.81 ± 0.01 a | 0.54 ± 0.02 d | 0.68 ± 0.01 b | Bitter |
| Isoleucine * | 0.87 ± 0.04 c | 1.21 ± 0.04 a | 0.82 ± 0.03 c | 1.04 ± 0.04 b | Bitter |
| Leucine * | 1.68 ± 0.06 c | 2.40 ± 0.05 a | 1.57 ± 0.01 d | 1.95 ± 0.01 b | Bitter |
| Phenylalanine * | 0.68 ± 0.01 c | 0.91 ± 0.02 a | 0.58 ± 0.01 d | 0.76 ± 0.04 b | Bitter |
| Lysine * | 1.86 ± 0.01 c | 2.61 ± 0.01 a | 1.75 ± 0.01 d | 2.12 ± 0.02 b | Tasteless |
| Aspartic acid + | 1.81 ± 0.01 c | 2.56 ± 0.01 a | 1.66 ± 0.02 d | 2.11 ± 0.02 b | Umami |
| Glutamic acid + | 2.65 ± 0.01 c | 3.78 ± 0.03 a | 2.53 ± 0.01 d | 3.08 ± 0.01 b | Umami |
| Glycine + | 1.13 ± 0.04 c | 1.44 ± 0.01 a | 0.93 ± 0.01 d | 1.15 ± 0.02 b | Sweet |
| Alanine + | 1.21 ± 0.01 c | 1.64 ± 0.01 a | 1.05 ± 0.04 d | 1.33 ± 0.01 b | Sweet |
| Histidine ^ | 0.51 ± 0.00 a | 0.52 ± 0.01 a | 0.37 ± 0.02 b | 0.34 ± 0.05 b | Bitter |
| Arginine ^ | 1.17 ± 0.04 c | 1.69 ± 0.05 a | 1.09 ± 0.02 d | 1.31 ± 0.03 b | Sweet |
| Serine | 0.74 ± 0.02 c | 1.04 ± 0.02 a | 0.69 ± 0.01 d | 0.86 ± 0.01 b | Sweet |
| Cysteine | 0.12 ± 0.01 d | 0.18 ± 0.01 b | 0.20 ± 0.01 a | 0.14 ± 0.01 c | Tasteless |
| Tyrosine | 0.51 ± 0.03 d | 0.75 ± 0.04 a | 0.51 ± 0.02 c | 0.63 ± 0.02 b | Bitter |
| Proline | 0.51 ± 0.01 b | 0.63 ± 0.01 a | 0.53 ± 0.03 b | 0.53 ± 0.01 b | Tasteless |
| ∑EAA | 7.45 ± 0.14 c | 10.41 ± 0.21 a | 6.92 ± 0.17 b | 8.59 ± 0.27 d | – |
| ∑DAA | 6.8 ± 0.33 c | 9.42 ± 0.12 a | 6.17 ± 0.14 d | 7.67 ± 0.51 b | – |
| ∑NEAA | 10.37 ± 0.22 c | 14.23 ± 0.09 a | 9.56 ± 0.31 d | 11.48 ± 0.29 b | – |
| ∑TAA | 17.82 ± 0.31 c | 24.64 ± 0.33 a | 16.48 ± 0.34 d | 20.07 ± 0.25 b | – |
| ∑EAA/∑TAA | 0.42 ± 0.01 ab | 0.42 ± 0.01 b | 0.42 ± 0.01 ab | 0.43 ± 0.01 a | – |
| ∑DAA/∑TAA | 0.38 ± 0.01 a | 0.38 ± 0.02 a | 0.37 ± 0.01 a | 0.38 ± 0.01 a | – |
| ∑EAA/∑NEAA | 0.72 ± 0.02 b | 0.73 ± 0.01 ab | 0.72 ± 0.02 b | 0.75 ± 0.01 a | – |
| Isoleucine | AAS | 1.22 | 1.23 | 1.24 | 1.3 |
| CS | 1.00 | 1 | 1.01 | 1.06 | |
| Leucine | AAS | 1.34 | 1.39 | 1.36 | 1.39 |
| CS | 1.16 | 1.2 | 1.18 | 1.2 | |
| Lysine | AAS | 1.9 | 1.87 | 1.93 | 1.92 |
| CS | 1.58 | 1.56 | 1.61 | 1.6 | |
| Threonine | AAS | 1.15 | 1.14 | 1.13 | 1.12 |
| CS | 1.02 | 1.02 | 1.01 | 1.04 | |
| Valine | AAS | 1.08 | 1.09 | 0.91 | 1.1 |
| CS | 1.01 | 1 | 0.84 | 1.01 | |
| Methionine + Cysteine | AAS | 1.12 | 1.14 | 1.28 | 1.16 |
| CS | 0.84 | 0.85 | 0.96 | 0.87 | |
| Phenylalanine + Tyrosine | AAS | 1.11 | 1.12 | 1.1 | 1.14 |
| CS | 0.78 | 0.78 | 0.77 | 0.77 | |
| Essential Amino Acid Index (EAAI) | 84.80 | 80.29 | 88.80 | 88.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, B.; Zhang, J.; Li, C.; Wu, S.; Huang, L.; Qin, D.; Hao, Q.; Gao, L. Texture, Nutrition, and Flavor of Different Freshwater Fish Muscles: Comparative Study and Molecular Docking. Foods 2025, 14, 2258. https://doi.org/10.3390/foods14132258
Xia B, Zhang J, Li C, Wu S, Huang L, Qin D, Hao Q, Gao L. Texture, Nutrition, and Flavor of Different Freshwater Fish Muscles: Comparative Study and Molecular Docking. Foods. 2025; 14(13):2258. https://doi.org/10.3390/foods14132258
Chicago/Turabian StyleXia, Banghua, Jiaming Zhang, Chenhui Li, Song Wu, Li Huang, Dongli Qin, Qirui Hao, and Lei Gao. 2025. "Texture, Nutrition, and Flavor of Different Freshwater Fish Muscles: Comparative Study and Molecular Docking" Foods 14, no. 13: 2258. https://doi.org/10.3390/foods14132258
APA StyleXia, B., Zhang, J., Li, C., Wu, S., Huang, L., Qin, D., Hao, Q., & Gao, L. (2025). Texture, Nutrition, and Flavor of Different Freshwater Fish Muscles: Comparative Study and Molecular Docking. Foods, 14(13), 2258. https://doi.org/10.3390/foods14132258






