Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus Isolated from Guangxi Dairy Farms
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Milk Samples
2.2. Identification and Isolation of S. aureus
2.3. Identification of mecA and Enterotoxin Genes
2.4. Antimicrobial Susceptibility Testing
2.5. Biofilm Formation Assay
2.6. spa Typing
2.7. Statistical Analysis
3. Results
3.1. Isolation and Identification of S. aureus
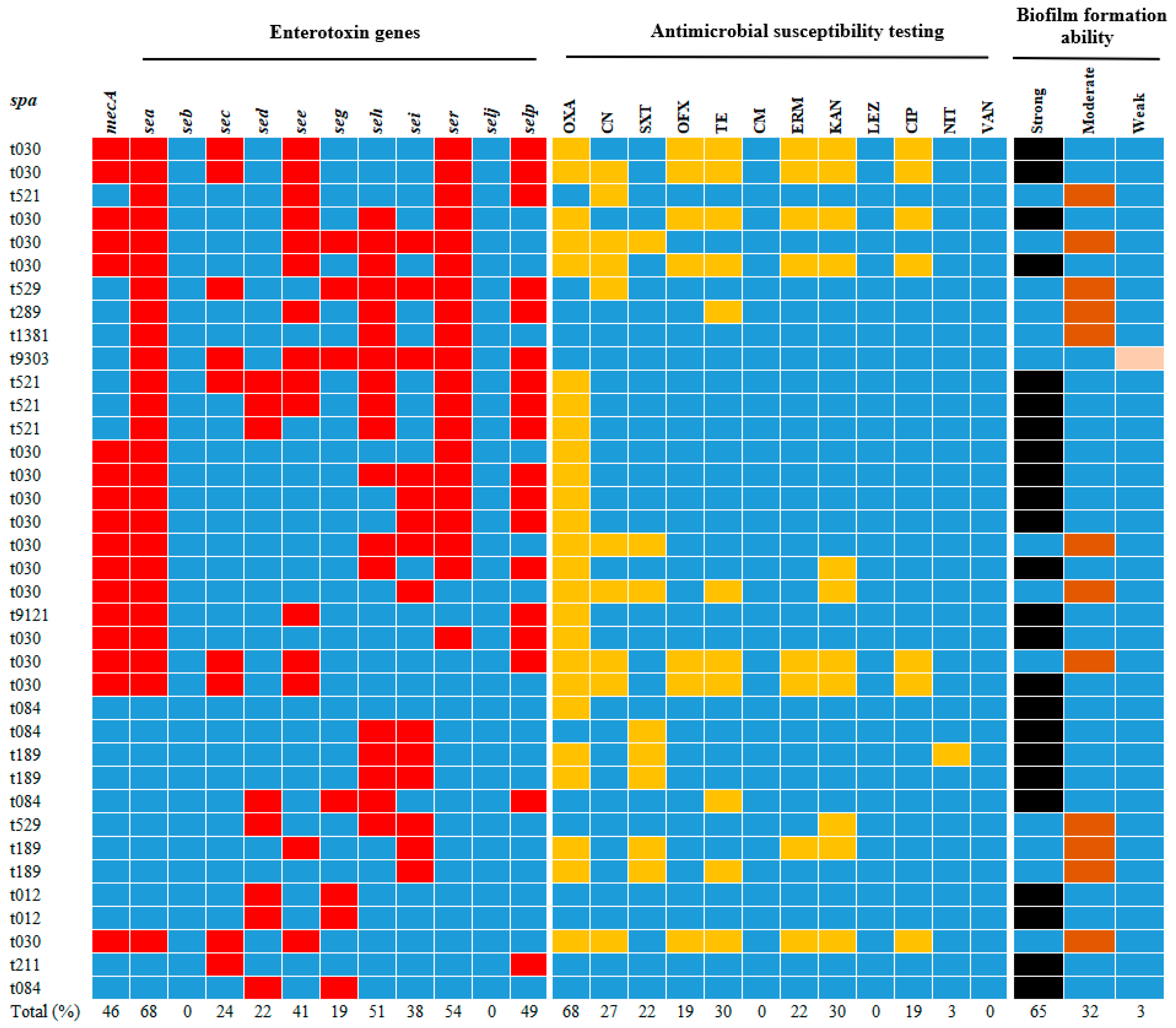
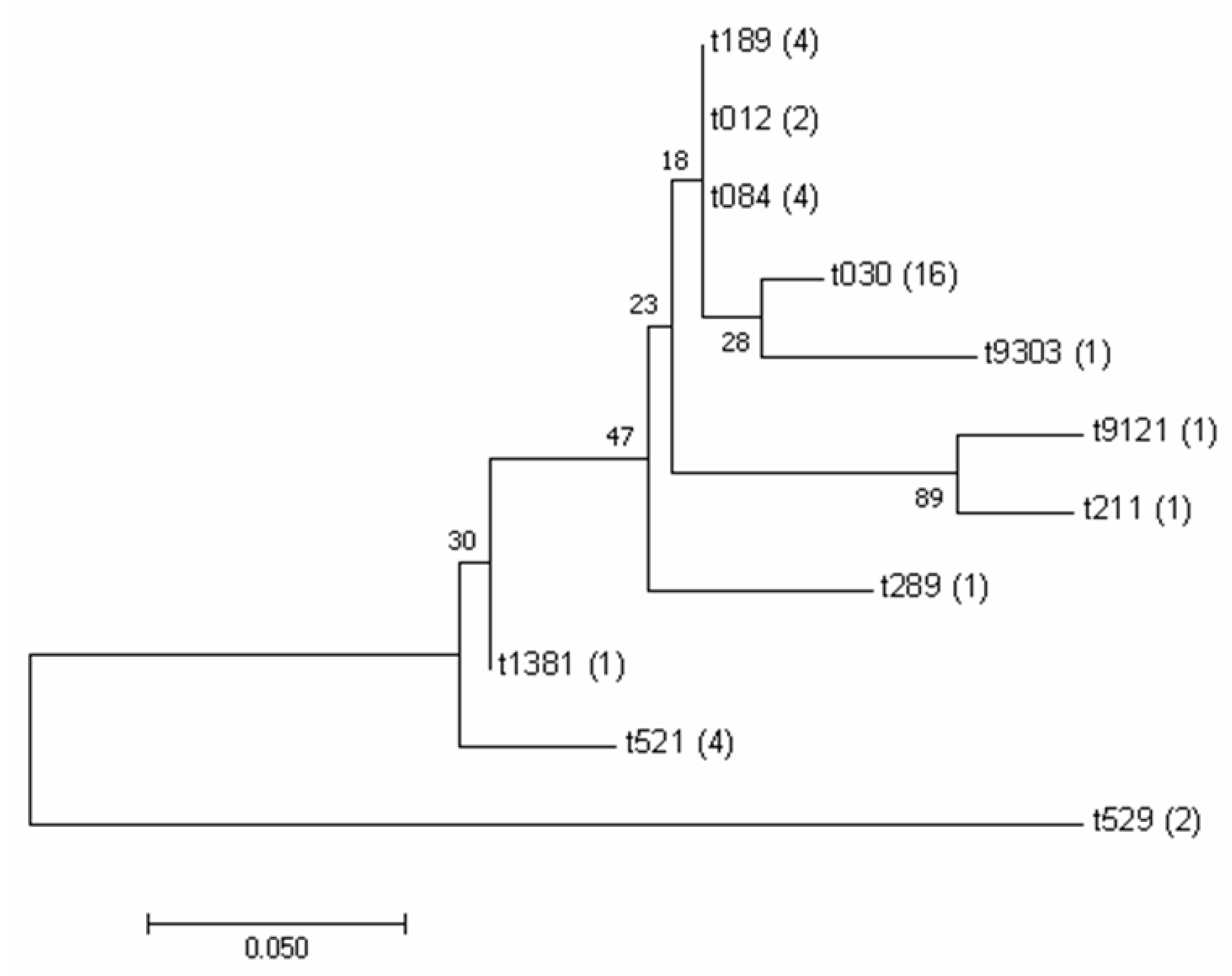
3.2. Analysis and Identification of spa Typing of Isolates
3.3. Enterotoxin Gene Frequencies in Isolates
3.4. Antimicrobial Resistance Analysis of Isolates
3.5. Biofilm Formation Ability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIC | minimum inhibitory concentration |
| DNA | deoxyribonucleic acid |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MDR | multidrug resistance |
| OXA | oxacillin |
| ERM | erythromycin |
| OFX | ofloxacin |
| VAN | vancomycin |
| CN | gentamicin |
| KAN | kanamycin |
| TE | tetracycline |
| CM | chloramphenicol |
| CIP | ciprofloxacin |
| NIT | nitrofurantoin |
| SXT | sulfamethoxazole-trimethoprim |
| LEZ | linezolid |
References
- Xue, T.; You, Y.B.; Hong, D.; Sun, H.P.; Sun, B.L. The KdpDE Two-Component System Couples Extracellular K Sensing and Agr Signaling to Infection Programming. Infect. Immun. 2011, 79, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Haran, K.P.; Godden, S.M.; Boxrud, D.; Jawahir, S.; Bender, J.B.; Sreevatsan, S. Prevalence and Characterization of, Including Methicillin-Resistant, Isolated from Bulk Tank Milk from Minnesota Dairy Farms. J. Clin. Microbiol. 2012, 50, 688–695. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States-Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Johler, S.; Macori, G.; Bellio, A.; Acutis, P.L.; Gallina, S.; Decastelli, L. Characterization of isolated along the raw milk cheese production process in artisan dairies in Italy. J. Dairy. Sci. 2018, 101, 2915–2920. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Hata, E.; Katsuda, K.; Kobayashi, H.; Uchida, I.; Tanaka, K.; Eguchi, M. Genetic Variation among Strains from Bovine Milk and Their Relevance to Methicillin-Resistant Isolates from Humans. J. Clin. Microbiol. 2010, 48, 2130–2139. [Google Scholar] [CrossRef]
- Felipe, V.; Morgante, C.A.; Somale, P.S.; Varroni, F.; Zingaretti, M.L.; Bachetti, R.A.; Correa, S.G.; Porporatto, C. Evaluation of the biofilm forming ability and its associated genes in species isolates from bovine mastitis in Argentinean dairy farms. Microb. Pathogenesis 2017, 104, 278–286. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.H.; Jiang, T.; Peng, Z.X.; Xu, J.; Yi, L.X.; Li, F.Q.; Fanning, S.; Baloch, Z. Prevalence and Characterization of Cultured From Raw Milk Taken From Dairy Cows With Mastitis in Beijing, China. Front. Microbiol. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. Fems Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef]
- Tarekgne, E.K.; Skjerdal, T.; Skeie, S.; Rudi, K.; Porcellato, D.; Félix, B.; Narvhus, J.A. Enterotoxin Gene Profile and Molecular Characterization of Isolates from Bovine Bulk Milk and Milk Products of Tigray Region, Northern Ethiopia. J. Food Protect 2016, 79, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Staphylococcal enterotoxins and enterotoxin-like toxins with special reference to dairy products: An overview. Crit. Rev. Food Sci. 2018, 58, 1943–1970. [Google Scholar] [CrossRef]
- Uhlén, M.; Guss, B.; Nilsson, B.; Gatenbeck, S.; Philipson, L.; Lindberg, M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J. Biol. Chem. 1984, 259, 1695–1702. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.J.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.C.; Crisóstomo, I.; Santos-Sanches, I.; Major, P.; Alves, C.R.; Aires-de-Sousa, M.; Thege, M.K.; de Lencastre, H. Comparison of DNA sequencing of the protein A gene polymorphic region with other molecular typing techniques for typing two epidemiologically diverse collections of methicillin-resistant. J. Clin. Microbiol. 2001, 39, 574–580. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.W.; Zhu, C.F.; Ma, K.; Fang, M.C.; Li, B.B.; Wang, W.H.; Xue, T. Antibiotics Resistance and Virulence of Isolates Isolated from Raw Milk from Handmade Dairy Retail Stores in Hefei City, China. Foods 2022, 11, 2185. [Google Scholar] [CrossRef]
- Ren, Q.; Liao, G.H.; Wu, Z.H.; Lv, J.F.; Chen, W. Prevalence and characterization of isolates from subclinical bovine mastitis in southern Xinjiang, China. J. Dairy. Sci. 2020, 103, 3368–3380. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, L.P.; Xue, T.; Sun, B.L. autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012, 12, 288. [Google Scholar] [CrossRef]
- Zhao, X.N.; Yuan, X.M.; Hu, M.; Zhang, Y.; Li, L.L.; Zhang, Q.; Yuan, X.X.; Wang, W.B.; Liu, Y.Q. Prevalence and characterization of and methicillin-resistant isolated from bulk tank milk in Shandong dairy farms. Food Control 2021, 125, 107836. [Google Scholar] [CrossRef]
- Ahmed, A.A.H.; Maharik, N.M.S.; Valero, A.; Kamal, S.M. Incidence of enterotoxigenic in milk and Egyptian artisanal dairy products. Food Control 2019, 104, 20–27. [Google Scholar] [CrossRef]
- Fetsch, A.; Contzen, M.; Hartelt, K.; Kleiser, A.; Maassen, S.; Rau, J.; Kraushaar, B.; Layer, F.; Strommenger, B. food-poisoning outbreak associated with the consumption of ice-cream. Int. J. Food Microbiol. 2014, 187, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Asao, T.; Kumeda, Y.; Kawai, T.; Shibata, T.; Oda, H.; Haruki, K.; Nakazawa, H.; Kozaki, S. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: Estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol. Infect. 2003, 130, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jamali, H.; Paydar, M.; Radmehr, B.; Ismail, S.; Dadrasnia, A. Prevalence and antimicrobial resistance of isolated from raw milk and dairy products. Food Control 2015, 54, 383–388. [Google Scholar] [CrossRef]
- André, M.C.D.P.B.; Campos, M.R.H.; Borges, L.J.; Kipnis, A.; Pimenta, F.C.; Serafini, A.B. Comparison of isolates from food handlers, raw bovine milk and Minas Frescal cheese by antibiogram and pulsed-field gel electrophoresis following SmaI digestion. Food Control 2008, 19, 200–207. [Google Scholar] [CrossRef]
- Traversa, A.; Gariano, G.R.; Gallina, S.; Bianchi, D.M.; Orusa, R.; Domenis, L.; Cavallerio, P.; Fossati, L.; Serra, R.; Decastelli, L. Methicillin resistance in strains isolated from food and wild animal carcasses in Italy. Food Microbiol. 2015, 52, 154–158. [Google Scholar] [CrossRef]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-Level Mastitis-Associated Costs on Canadian Dairy Farms. Front. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef]
- Schmidt, T.; Kock, M.M.; Ehlers, M.M. Molecular Characterization of Isolated from Bovine Mastitis and Close Human Contacts in South African Dairy Herds: Genetic Diversity and Inter-Species Host Transmission. Front. Microbiol. 2017, 8, 511. [Google Scholar] [CrossRef]
- Xing, X.N.; Zhang, Y.; Wu, Q.; Wang, X.; Ge, W.P.; Wu, C.M. Prevalence and characterization of isolated from goat milk powder processing plants. Food Control 2016, 59, 644–650. [Google Scholar] [CrossRef]
- Shi, C.P.; Yu, Z.N.; Ho, H.; Wang, J.; Wu, W.; Xing, M.R.; Wang, Y.T.; Rahman, S.M.E.; Han, R.W. Occurrence, Antimicrobial Resistance Patterns, and Genetic Characterization of Isolated from Raw Milk in the Dairy Farms over Two Seasons in China. Microb. Drug Resist. 2021, 27, 99–110. [Google Scholar] [CrossRef]
- Khanna, T.; Friendship, R.; Dewey, C.; Weese, J.S. Methicillin resistant colonization in pigs and pig farmers. Vet. Microbiol. 2008, 128, 298–303. [Google Scholar] [CrossRef]
- Brown, D.F.J.; Edwards, D.I.; Hawkey, P.M.; Morrison, D.; Ridgway, G.L.; Towner, K.J.; Wren, M.W.D.; An, J.W.P.B.S. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant (MRSA). J. Antimicrob. Chemoth 2005, 56, 1000–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.C.; Li, Y.; Tang, Y.Y.; Meng, C.; Ingmer, H.; Jiao, X.A. Prevalence and characterization of and in chicken from retail markets in China. Food Control 2019, 96, 158–164. [Google Scholar] [CrossRef]
- Becker, K.; van Alen, S.; Idelevich, E.A.; Schleimer, N.; Seggewiss, J.; Mellmann, A.; Kaspar, U.; Peters, G. Plasmid-Encoded Transferable -Mediated Methicillin Resistance in. Emerg. Infect. Dis. 2018, 24, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Wolska-Gebarzewska, M.; Miedzobrodzki, J.; Kosecka-Strojek, M. Current types of staphylococcal cassette chromosome (SCC) in clinically relevant coagulase-negative staphylococcal (CoNS) species. Crit. Rev. Microbiol. 2024, 50, 1020–1036. [Google Scholar] [CrossRef]
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal Enterotoxins. Toxins 2010, 2, 2177–2197. [Google Scholar] [CrossRef]
- McMillan, K.; Moore, S.C.; McAuley, C.M.; Fegan, N.; Fox, E.M. Characterization of isolates from raw milk sources in Victoria, Australia. Bmc Microbiol. 2016, 16, 169. [Google Scholar] [CrossRef]
- Sato’o, Y.; Omoe, K.; Naito, I.; Ono, H.K.; Nakane, A.; Sugai, M.; Yamagishi, N.; Hu, D.L. Molecular Epidemiology and Identification of a Clone Causing Food Poisoning Outbreaks in Japan. J. Clin. Microbiol. 2014, 52, 2637–2640. [Google Scholar] [CrossRef]
- Yan, X.M.; Wang, B.; Tao, X.X.; Hu, Q.H.; Cui, Z.G.; Zhang, J.Z.; Lin, Y.M.; You, Y.H.; Shi, X.L.; Grundmann, H. Characterization of Strains Associated with Food Poisoning in Shenzhen, China. Appl. Environ. Microb. 2012, 78, 6637–6642. [Google Scholar] [CrossRef]
- Mehli, L.; Hoel, S.; Thomassen, G.M.B.; Jakobsen, A.N.; Karlsen, H. The prevalence, genetic diversity and antibiotic resistance of in milk, whey, and cheese from artisan farm dairies. Int. Dairy. J. 2017, 65, 20–27. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Angelidis, A.S.; Papadopoulos, T.; Kotzamanidis, C.; Zdragas, A.; Papa, A.; Filioussis, G.; Sergelidis, D. and methicillin-resistant (MRSA) in bulk tank milk, livestock and dairy-farm personnel in north-central and north-eastern Greece: Prevalence, characterization and genetic relatedness. Food Microbiol. 2019, 84, 103249. [Google Scholar] [CrossRef]
- Fessler, A.; Scott, C.; Kadlec, K.; Ehricht, R.; Monecke, S.; Schwarz, S. Characterization of methicillin-resistant ST398 from cases of bovine mastitis. J. Antimicrob. Chemoth 2010, 65, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Thiran, E.; Di Ciccio, P.A.; Graber, H.U.; Zanardi, E.; Ianieri, A.; Hummerjohann, J. Biofilm formation of dairy isolates representing different genotypes. J. Dairy. Sci. 2018, 101, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Strommenger, B.; Braulke, C.; Heuck, D.; Schmidt, C.; Pasemann, B.; Nübel, U.; Witte, W. spa typing of as a frontline tool in epidemiological typing. J. Clin. Microbiol. 2008, 46, 574–581. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer | Primer Sequence (5′-3′) | Reference or Source |
|---|---|---|---|
| 16S | 27F | AGAGTTTGATCCTGGCTCAG | This study |
| 1492R | TACCTTGTTACGACTT | ||
| mecA | mecA-F | GTTGTAGTTGTCGGGTTT | This study |
| mecA-R | CCACATTGTTTCGGTCTA | ||
| spa | spa-1113F | TAAAGACGATCCTTCGGTGAGC | Ridom |
| spa-1514R | CAGCAGTAGTGCCGTTTGCTT | Ridom | |
| sea | sea-F | GGTTATCAATGTGCGGGTGG | [16] |
| sea-R | CGGCACTTTTTTCTCTTCGG | ||
| seb | seb-F | GTATGGTGGTGTAACTGAGC | [16] |
| seb-R | CCAAATAGTGACGAGTTAGG | ||
| sec | sec-F | AGATGAAGTAGTTGATGTGTATGG | [16] |
| sec-R | CACACTTTTAGAATCAACCG | ||
| sed | sed-F | CCAATAATAGGAGAAAATAAAAG | [16] |
| sed-R | ATTGGTATTTTTTTTCGTTC | ||
| see | see-F | TGTATGTATGGAGGTGTAAC | [16] |
| see-R | GCCAAAGCTGTCTGAG | ||
| seg | seg-F | GTTAGAGGAGGTTTTATG | [16] |
| seg-R | TTCCTTCAACAGGTGGAGA | ||
| seh | seh-F | CAACTGCTGATTTAGCTCAG | [16] |
| seh-R | CCCAAACATTAGCACCA | ||
| sei | sei-F | GGCCACTTTATCAGGACA | [16] |
| sei-R | AACTTACAGGCAGTCCA | ||
| ser | ser-F | AGATGTGTTTGGAATACCCTAT | [16] |
| ser-R | CTATCAGCTGTGGAGTGCAT | ||
| selj | selj-F | GTTCTGGTGGTAAACCA | [16] |
| selj-R | GCGGAACAACAGTTCTGA | ||
| selp | selp-F | TCAAAAGACACCGCCAA | [16] |
| selp-R | ATTGTCCTTGAGCACCA |
| Locations | No. of Samples | No. of S. aureus (%) | No. of MRSA * (%) |
|---|---|---|---|
| Liuzhou | 70 | 9 (12.9%) | 9 (12.9%) |
| Laibin | 38 | 5 (13.2%) | 3 (7.8%) |
| Guigang | 66 | 14 (21.2%) | 8 (12.1%) |
| Nanning | 38 | 6 (15.8%) | 3 (7.9%) |
| Chongzuo | 30 | 3 (10%) | 2 (6.6%) |
| Total | 242 | 37(15.3%) | 25 |
| spa Type | spa Repeat Succession | No. and Proportion of Isolates |
|---|---|---|
| t030 | 15-12-16-02-24-24 | 16 (43.2%) |
| t521 | 07-23-12-21-17-34-34-34-34-33-34 | 4 (10.8%) |
| t084 | 07-23-12-34-34-12-12-23-02-12-23 | 4 (10.8%) |
| t189 | 07-23-12-21-17-34 | 4 (10.8%) |
| t529 | 04-34 | 2 (5.4%) |
| t012 | 15-12-16-02-16-02-25-17-24-24 | 2 (5.4%) |
| t211 | 11-19-12-12-21-17-34-24-34-22-25 | 1 (2.7%) |
| t289 | 26-23-21-17-34-12-23-02-12-23 | 1 (2.7%) |
| t1381 | 07-23-21-16-34-33-34 | 1 (2.7%) |
| t9303 | 04-20-24-17 | 1 (2.7%) |
| t9121 | 11 | 1 (2.7%) |
| Enterotoxin Genes | No. of S. aureus | Detection Rate |
|---|---|---|
| sea | 25 | 67.6% |
| seb | 0 | 0 |
| sec | 9 | 24.3% |
| sed | 8 | 21.6% |
| see | 15 | 40.5% |
| seg | 7 | 18.9% |
| seh | 19 | 51.4% |
| sei | 14 | 37.8% |
| ser | 20 | 54.1% |
| selj | 0 | 0 |
| selp | 18 | 48.7% |
| Antibiotic Class | Antibiotic | No. of Resistant S. aureus (%) |
|---|---|---|
| β-Lactams | Oxacillin | 25 (67.6%) |
| Aminoglycosides | Gentamicin | 10 (27%) |
| Kanamycin | 11 (29.7%) | |
| Tetracyclines | Tetracycline | 11 (29.7%) |
| Sulfonamides | Sulfamethoxazole-trimethoprim | 8 (21.6%) |
| Chloramphenicol | Chloramphenicol | 0 (0%) |
| Macrolides | Erythromycin | 8 (21.6%) |
| Quinolones | Ofloxacin | 7 (18.9%) |
| Ciprofloxacin | 7 (18.9%) | |
| Oxazolidinones | linezolid | 0 (0%) |
| Nitrofurans | Nitrofurantoin | 1 (2.7%) |
| Glycopeptide | Vancomycin | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Guo, J.; Hu, J.; Liu, Q.; Wang, H.; Xue, T. Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus Isolated from Guangxi Dairy Farms. Foods 2025, 14, 2221. https://doi.org/10.3390/foods14132221
Ma K, Guo J, Hu J, Liu Q, Wang H, Xue T. Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus Isolated from Guangxi Dairy Farms. Foods. 2025; 14(13):2221. https://doi.org/10.3390/foods14132221
Chicago/Turabian StyleMa, Kai, Jia Guo, Jie Hu, Qiuyuan Liu, Hui Wang, and Ting Xue. 2025. "Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus Isolated from Guangxi Dairy Farms" Foods 14, no. 13: 2221. https://doi.org/10.3390/foods14132221
APA StyleMa, K., Guo, J., Hu, J., Liu, Q., Wang, H., & Xue, T. (2025). Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus Isolated from Guangxi Dairy Farms. Foods, 14(13), 2221. https://doi.org/10.3390/foods14132221





