Synergistic Probiotic–Postbiotic Therapy Ameliorates Hyperuricemia via Multi-Target Regulation of Purine Metabolism and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Pretreatment
2.3. Animal Treatment
2.4. Serum Biochemical Analysis
2.5. Histopathological Analysis
2.6. Intestinal Analysis by 16S rRNA Gene Sequencing
2.7. Quantification of Short-Chain Fatty Acids via Gas Chromatographic Analysis
2.8. Statistical Analysis
3. Results and Discussion
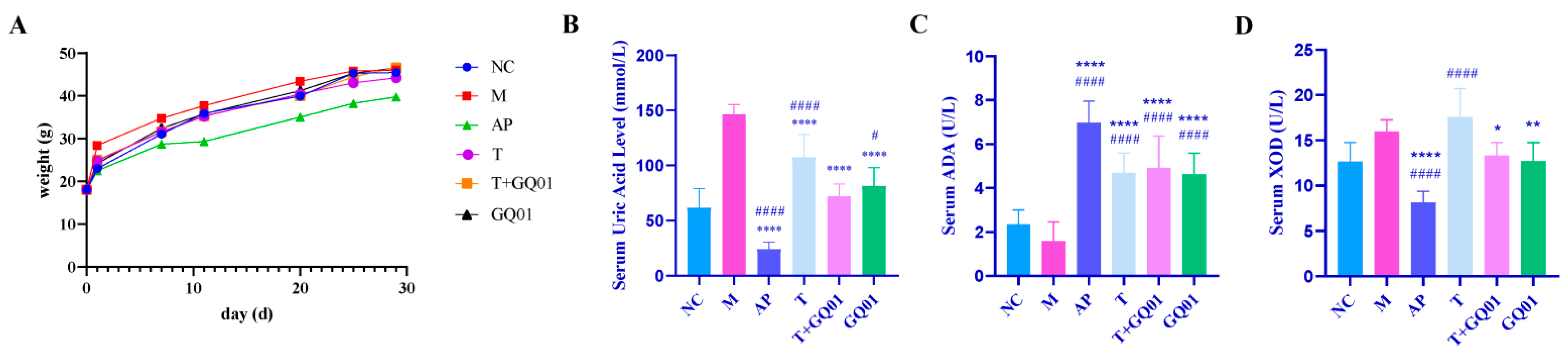
3.1. P. acidilactici GQ01, Buckwheat-Fermented Postbiotics, Collagen Peptide and Multiple Medicinal Food Blends Can Synergistically Improve HUA
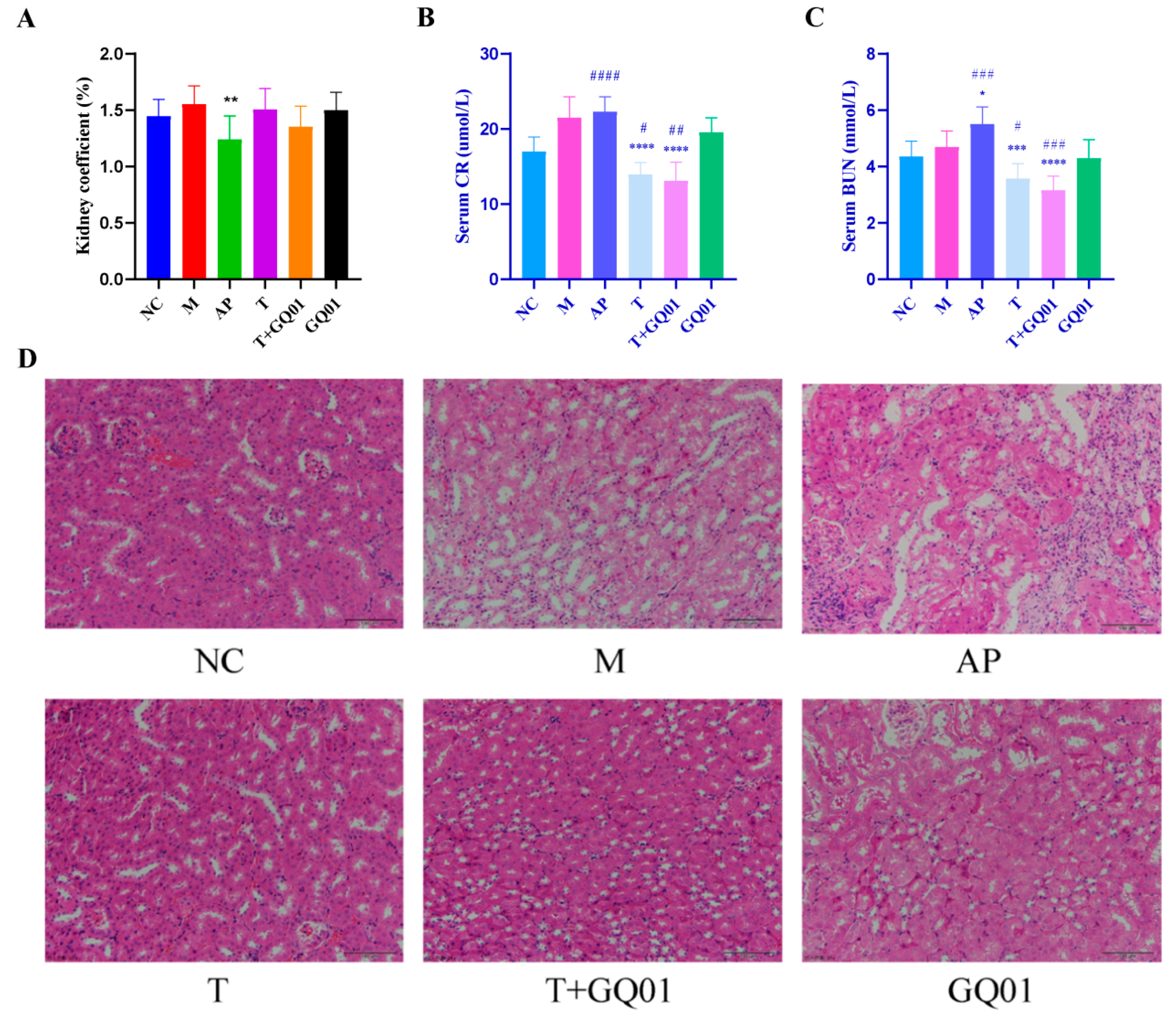
3.2. P. acidilactici GQ01, Buckwheat-Fermented Postbiotics, Collagen Peptide and Multiple Medicinal Food Blends Synergistically Alleviate Kidney Injury in HUA Mice
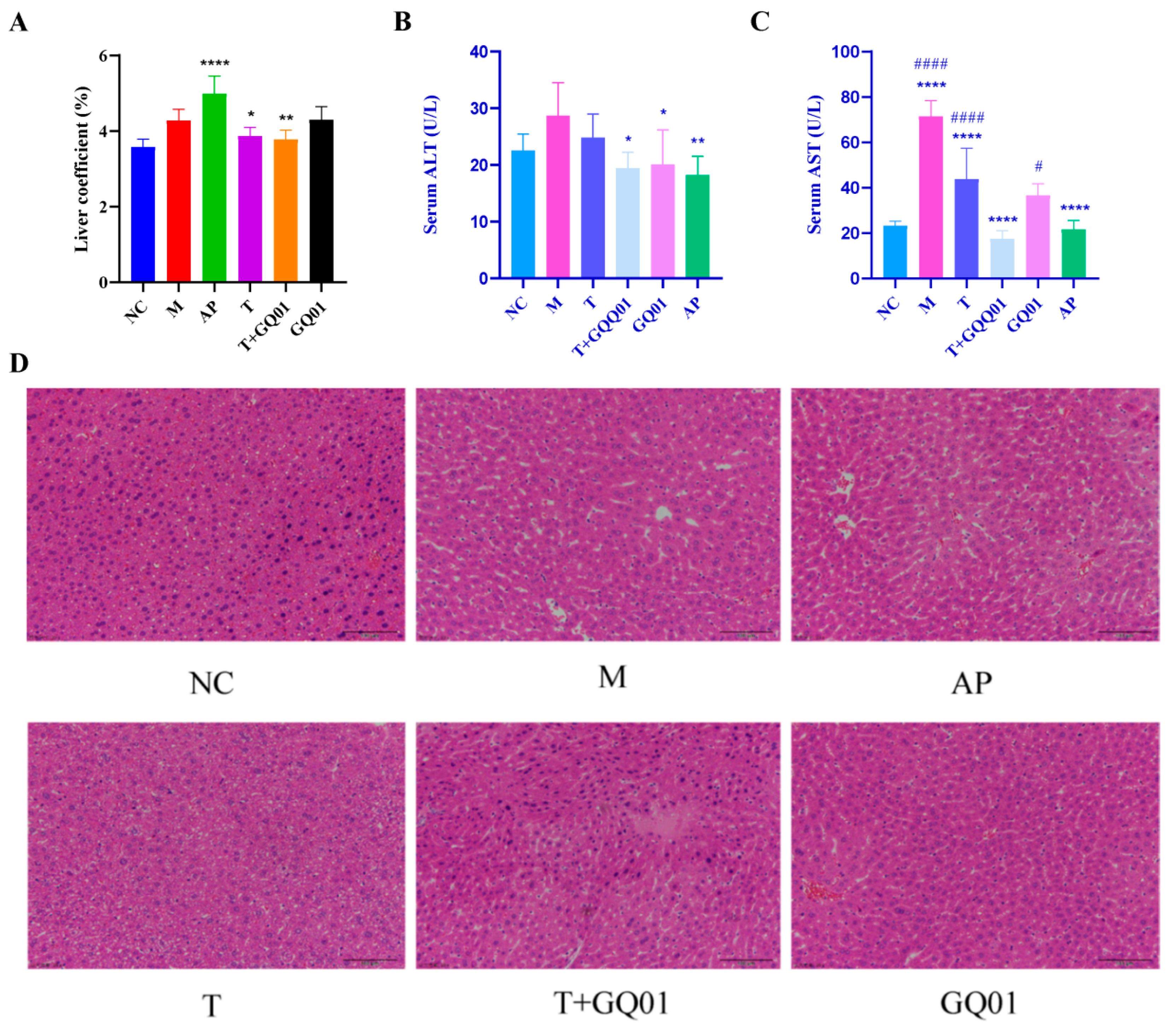
3.3. P. acidilactici GQ01, Buckwheat-Fermented Postbiotics, Collagen Peptide and Multiple Medicinal Food Blends Synergistically Alleviate Liver Injury in HUA Mice
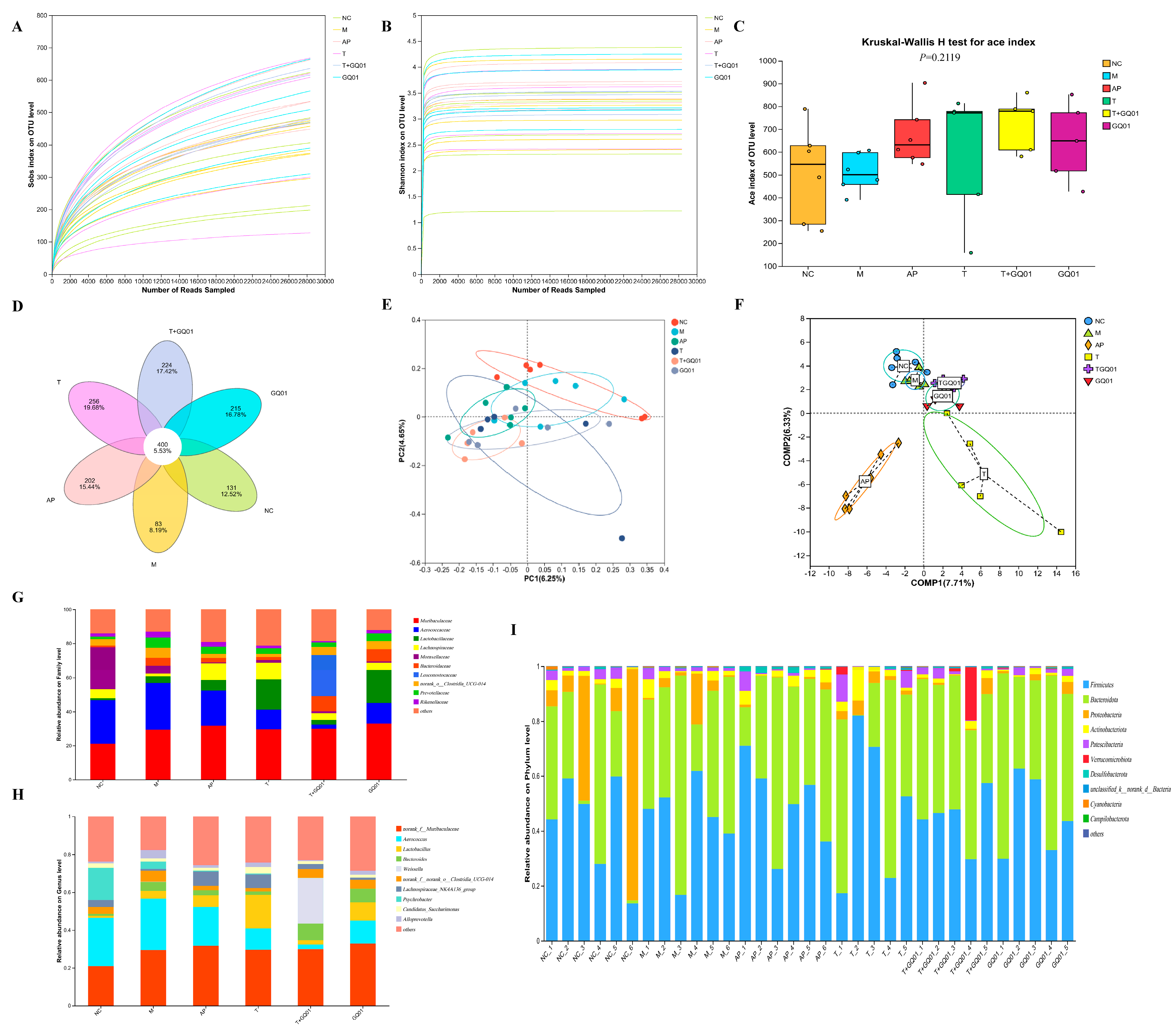
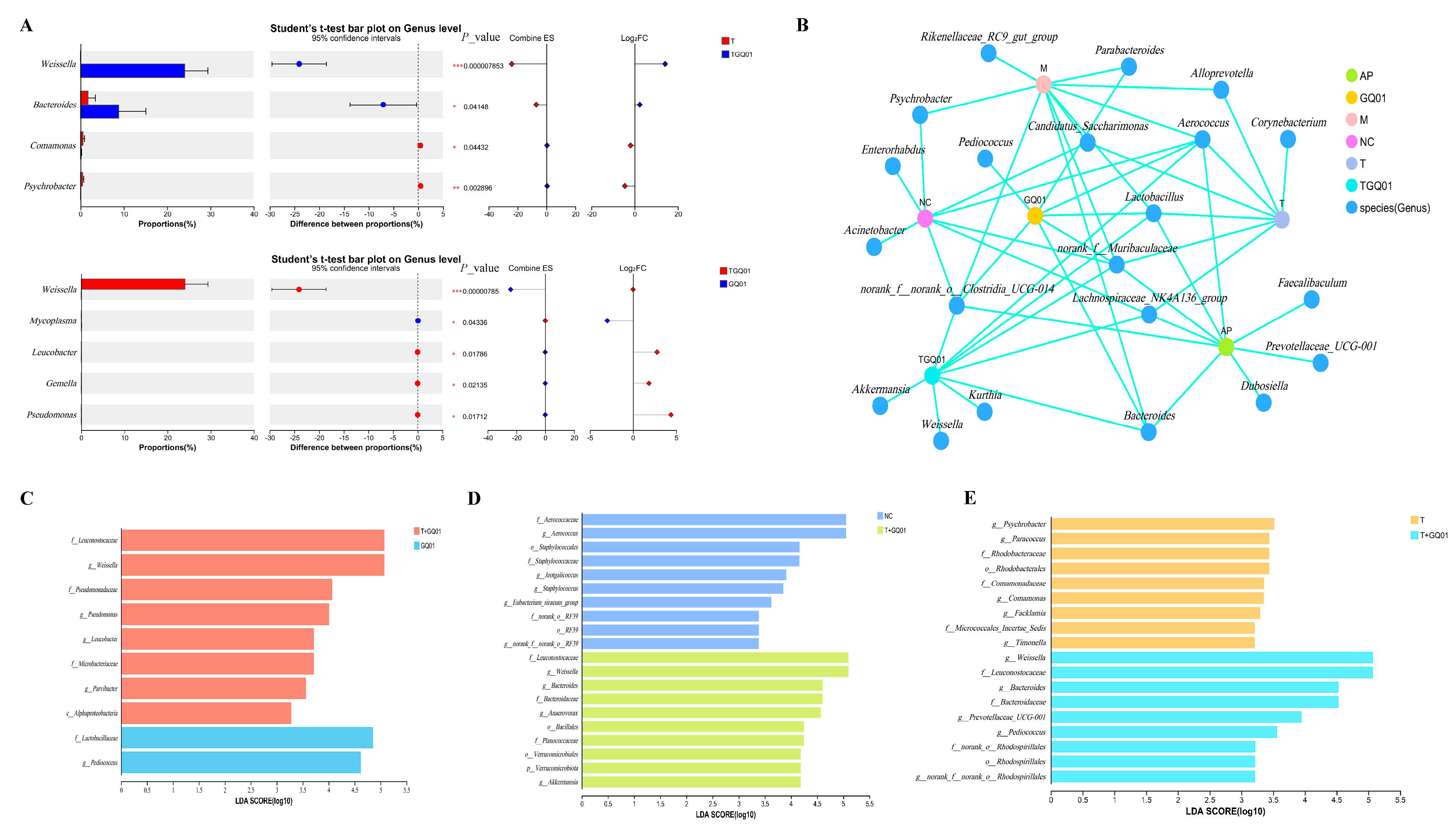
3.4. P. acidilactici GQ01, Buckwheat-Fermented Postbiotics, Collagen Peptide and Multiple Medicinal Food Blends Synergistically Improve the Intestinal Flora Structure in HUA Mice
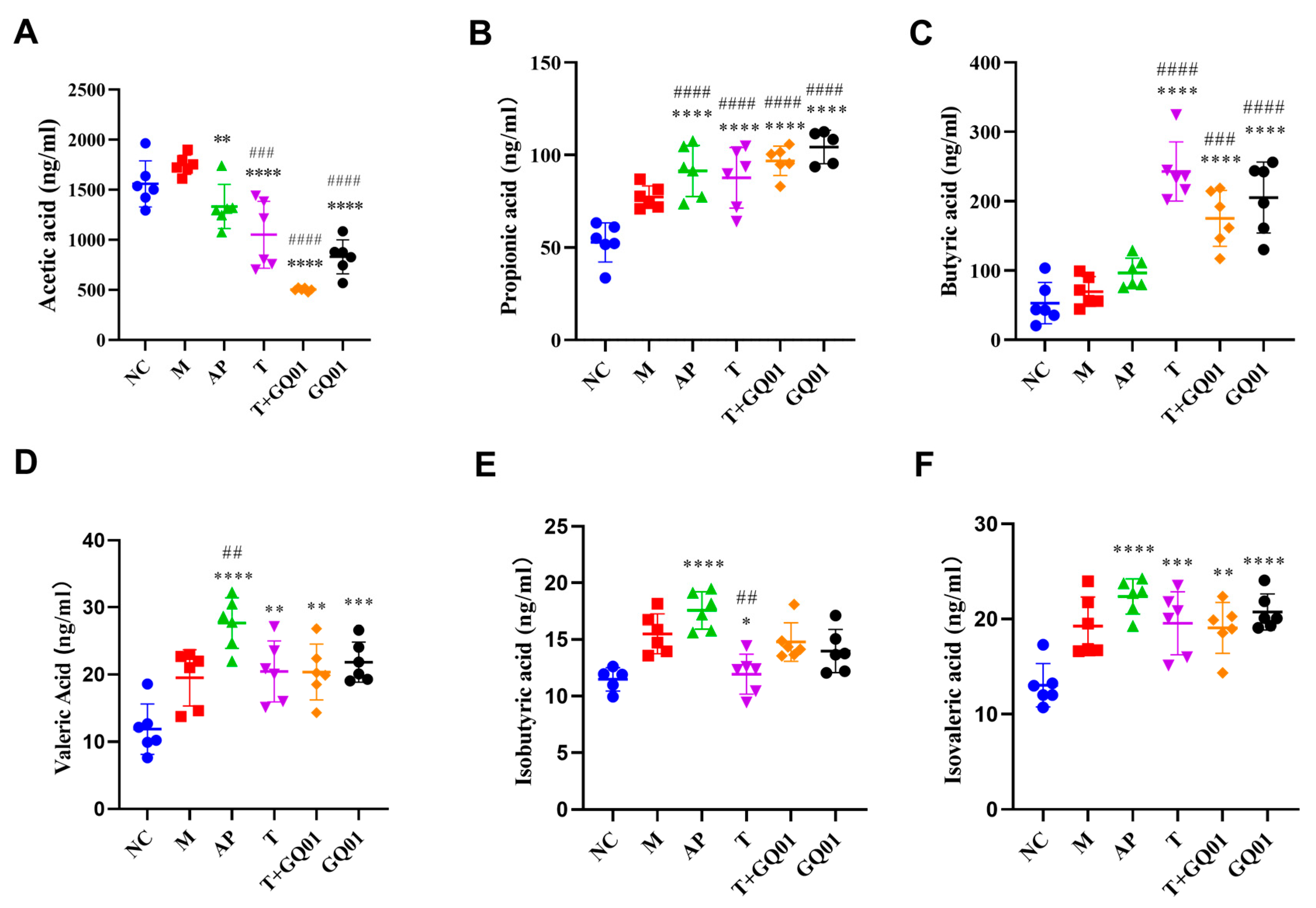
3.5. P. acidilactici GQ01, Buckwheat-Fermented Postbiotics, Collagen Peptide, and Multiple Medicinal Food Blends Can Synergistically Improve Intestinal SCFAs Content
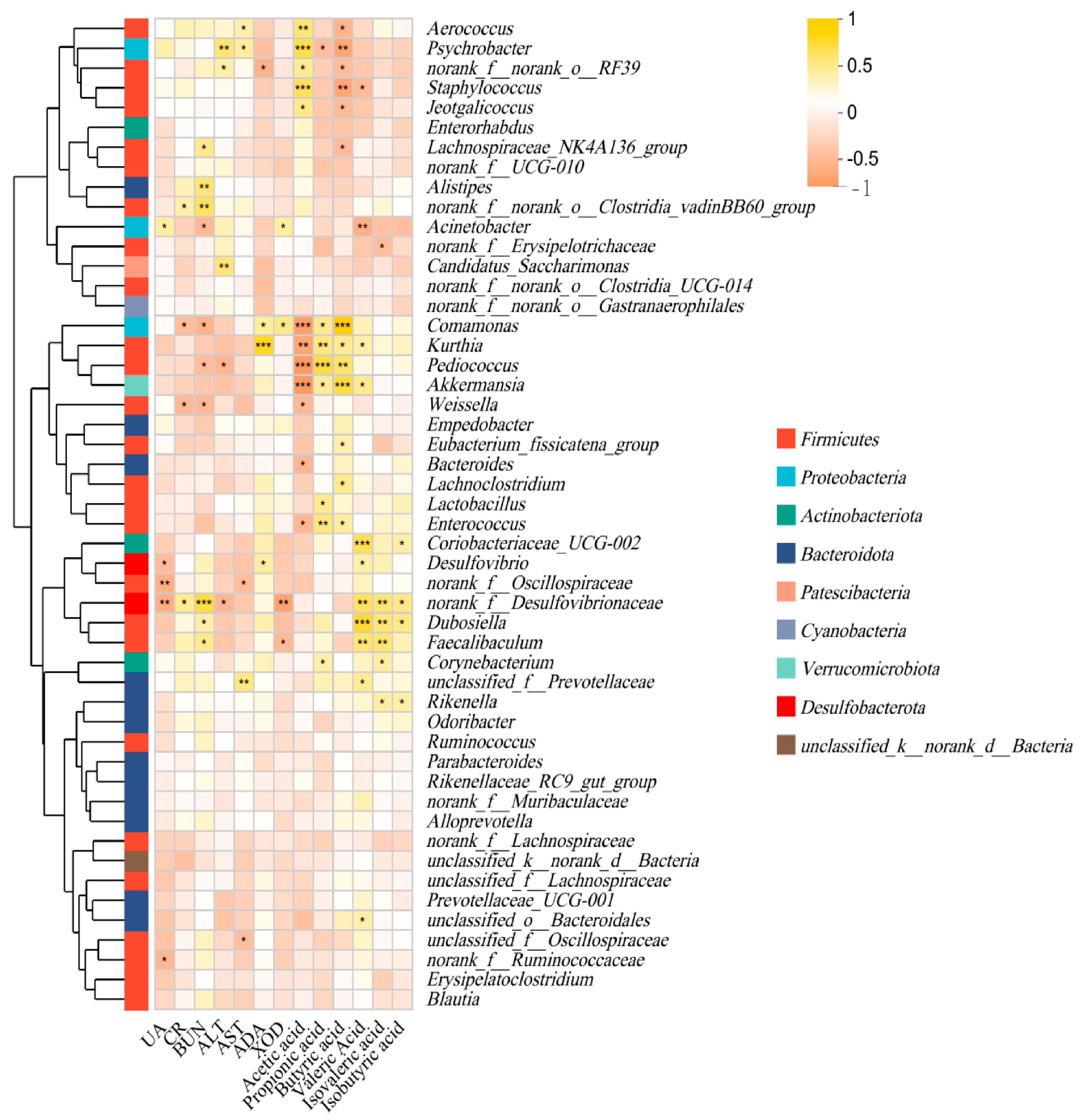
3.6. Effect of P. acidilactici GQ01 Combined with Buckwheat-Fermented Postbiotics, Collagen Peptide and Multiple Medicinal Food Blends on Spearman Correlation Analysis of Intestinal Flora in HUA Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, S.; Arakawa, H.; Tamai, I. Uric acid in health and disease: From physiological functions to pathogenic mechanisms. Pharmacol. Ther. 2024, 256, 108615. [Google Scholar] [CrossRef] [PubMed]
- Gherghina, M.-E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress—Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Vargas-Santos, A.B.; Neogi, T. Management of Gout and Hyperuricemia in CKD. Am. J. Kidney Dis. 2017, 70, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Afinogenova, Y.; Danve, A.; Neogi, T. Update on gout management: What is old and what is new. Curr. Opin. Rheumatol. 2022, 34, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012, 64, 1431–1446. [Google Scholar] [CrossRef]
- Zhu, F. Buckwheat proteins and peptides: Biological functions and food applications. Trends Food Sci. Technol. 2021, 110, 155–167. [Google Scholar] [CrossRef]
- Wu, J.; Alhamoud, Y.; Lv, S.; Feng, F.; Wang, J. Beneficial properties and mechanisms of natural phytochemicals to combat and prevent hyperuricemia and gout. Trends Food Sci. Technol. 2023, 138, 355–369. [Google Scholar] [CrossRef]
- Yang, B.; Xin, M.; Liang, S.; Xu, X.; Cai, T.; Dong, L.; Wang, C.; Wang, M.; Cui, Y.; Song, X.; et al. New insight into the management of renal excretion and hyperuricemia: Potential therapeutic strategies with natural bioactive compounds. Front. Pharmacol. 2022, 13, 1026246. [Google Scholar] [CrossRef]
- Sun, L.; Ni, C.; Zhao, J.; Wang, G.; Chen, W. Probiotics, bioactive compounds and dietary patterns for the effective management of hyperuricemia: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 2016–2031. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2021, 62, 3979–3989. [Google Scholar] [CrossRef]
- Evgenovich Kondratiuk, V.; Mykhailivna Tarasenko, O.; Myroslavivna Karmazina, O.; Valentinovich Taranchuk, V. Impact of the Synbiotics and Urate-Lowering Therapy on Gut Microbiota and Cytokine Profile in Patients with Chronic Gouty Arthritis. J. Med. Life 2020, 13, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, S.; Liu, S.; Prasanthi, H.A.C.; Li, Y.; Cao, J.; Zhong, F.; Guo, L.; Lu, F.; Luo, X. Postbiotic of Pediococcus acidilactici GQ01, a Novel Probiotic Strain Isolated from Natural Fermented Wolfberry, Attenuates Hyperuricaemia in Mice through Modulating Uric Acid Metabolism and Gut Microbiota. Foods 2024, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Xu, X.; Li, W.; Fu, X.; Han, W.; Li, G. Investigating the Vital Role of the Identified Abietic Acid from Helianthus annuus L. Calathide Extract against Hyperuricemia via Human Embryonic Kidney 293T Cell Model. Molecules 2023, 28, 5141. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Ji, X.; Yu, L.; Han, C.; Gao, H.; Cai, Y.; Li, J.; He, Y.; Lu, H.; Song, G.; Xue, P. Investigating the effects of rare ginsenosides on hyperuricemia and associated sperm damage via nontargeted metabolomics and gut microbiota. J. Ethnopharmacol. 2024, 332, 118362. [Google Scholar] [CrossRef]
- Feng, S.; Wu, S.; Xie, F.; Yang, C.S.; Shao, P. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci. Technol. 2022, 123, 87–102. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, S.; Wu, L.; Wang, H.; Xue, H.; Wang, R. Flavonoid-rich extract of Paederia scandens (Lour.) Merrill improves hyperuricemia by regulating uric acid metabolism and gut microbiota. Food Chem. 2025, 471, 142857. [Google Scholar] [CrossRef]
- Ren, B.; Yue, K.; Zhang, Y.; Fu, Y. Collagen-derived peptides as prebiotics to improve gut health. Curr. Opin. Food Sci. 2024, 55, 101123. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, Z.; Feng, P.; Li, R.; Chen, X.; Tian, X.; Han, R.; Kakade, A.; Liu, P.; Li, X. Limosilactobacillus fermentum JL-3 isolated from “Jiangshui” ameliorates hyperuricemia by degrading uric acid. Gut Microbes 2021, 13, 1897211. [Google Scholar] [CrossRef]
- Li, J.; Gong, Y.; Li, J.; Fan, L. In vitro inhibitory effects of polyphenols from Tartary buckwheat on xanthine oxidase: Identification, inhibitory activity, and action mechanism. Food Chem. 2022, 379, 132100. [Google Scholar] [CrossRef]
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de novo purine biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2020, 56, 1832438. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Zhao, X.; Zhang, P.; Guo, J.; Zhuang, Y.; Wang, S. Coffee Leaf Tea Extracts Improve Hyperuricemia Nephropathy and Its Associated Negative Effect in Gut Microbiota and Amino Acid Metabolism in Rats. J. Agric. Food Chem. 2023, 71, 17775–17787. [Google Scholar] [CrossRef]
- Ben Salem, C.; Slim, R.; Fathallah, N.; Hmouda, H. Drug-induced hyperuricaemia and gout. Rheumatology 2017, 56, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, L.; Li, X.; Xiao, Y.; Huang, Y.; Zhang, Y.; Li, J.; Li, M.; Ren, Z. Lactiplantibacillus pentosus P2020 protects the hyperuricemia and renal inflammation in mice. Front. Nutr. 2023, 10, 1094483. [Google Scholar] [CrossRef] [PubMed]
- Dikhit, M.R.; Li, F.; Dong, Y.-Z.; Zhang, D.; Zhang, X.-M.; Lin, Z.-J.; Zhang, B. Molecular mechanisms involved in drug-induced liver injury caused by urate-lowering Chinese herbs: A network pharmacology study and biology experiments. PLoS ONE 2019, 14, e0216948. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Zhang, Q.; Pradhan, M.; Mehrabian, M.; Lusis, A.J.; Bergström, G.; Bäckhed, F.; Rey, F.E. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053.e1010. [Google Scholar] [CrossRef]
- Yin, H.; Liu, N.; Chen, J. The Role of the Intestine in the Development of Hyperuricemia. Front. Immunol. 2022, 13, 845684. [Google Scholar] [CrossRef]
- Mugula, J.K.; Nnko, S.A.M.; Narvhus, J.A.; Sørhaug, T. Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol. 2003, 80, 187–199. [Google Scholar] [CrossRef]
- Xu, X.; Yan, Y.; Huang, J.; Zhang, Z.; Wang, Z.; Wu, M.; Liang, H. Regulation of uric acid and glyoxylate metabolism by UgmR protein in Pseudomonas aeruginosa. Environ. Microbiol. 2022, 24, 3242–3255. [Google Scholar] [CrossRef]
- Chabbi, N.; Chafiki, S.; Telmoudi, M.; Labbassi, S.; Bouharroud, R.; Tahiri, A.; Mentag, R.; El Amri, M.; Bendiab, K.; Hsissou, D.; et al. Plant-Growth-Promoting Rhizobacteria Improve Seeds Germination and Growth of Argania spinosa. Plants 2024, 13, 2025. [Google Scholar] [CrossRef]
- Bakhsh, A.; Joseph, S.; Mannocci, F.; Proctor, G.; Moyes, D.; Niazi, S.A. Apical periodontitis microbiome association with salivary and serum inflammatory burden. Int. Endod. J. 2025, 58, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Sarkar, P. Isolation of a novel uric-acid-degrading microbe Comamonas sp. BT UA and rapid biosensing of uric acid from extracted uricase enzyme. J. Biosci. 2014, 39, 805–819. [Google Scholar] [CrossRef]
- Grzesiak, J.; Kaczyńska, A.; Gawor, J.; Żuchniewicz, K.; Aleksandrzak-Piekarczyk, T.; Gromadka, R.; Zdanowski, M.K. A smelly business: Microbiology of Adélie penguin guano (Point Thomas rookery, Antarctica). Sci. Total Environ. 2020, 714, 136714. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, R.; Jia, W.; Li, P.; Song, C. Clinical Characteristics of Lung Consolidation with Mycoplasma pneumoniae Pneumonia and Risk Factors for Mycoplasma pneumoniae Necrotizing Pneumonia in Children. Infect. Dis. Ther. 2024, 13, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, C.; Zeng, X.; Yuan, Z. Microecological treatment of hyperuricemia using Lactobacillus from pickles. BMC Microbiol. 2020, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ihrie, V.; Shi, P.; Ihrie, M.; Womble, J.; Meares, A.; Granek, J.; Gunsch, C.; Ingram, J. Glucagon-Like Peptide 1 Receptor (Glp1r) Deficiency Does Not Appreciably Alter Airway Inflammation or Gut-Lung Microbiome Axis in a Mouse Model of Obese Allergic Airways Disease and Bariatric Surgery. J. Asthma Allergy 2025, 18, 285–305. [Google Scholar] [CrossRef]
- Gu, X.; Fan, M.; Zhou, Y.; Zhang, Y.; Wang, L.; Gao, W.; Li, T.; Wang, H.; Si, N.; Wei, X.; et al. Intestinal endogenous metabolites affect neuroinflammation in 5×FAD mice by mediating “gut-brain” axis and the intervention with Chinese Medicine. Alzheimer’s Res. Ther. 2024, 16, 222. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Wu, W.; Lou, X.; Gao, R.; Ma, T.; Dheen, S.T.; Cheng, J.; Xiong, J.; Chen, X.; et al. Indole derivatives ameliorated the methamphetamine-induced depression and anxiety via aryl hydrocarbon receptor along “microbiota-brain” axis. Gut Microbes 2025, 17, 2470386. [Google Scholar] [CrossRef]
- Zhao, H.; Abbas, S.; Ren, J.; Huang, H.; Song, Y.; Su, X.; Wu, Q.; Ma, Y.; Tang, H.; Gao, Y.-Z.; et al. Dextran from human feces-derived Weissella cibaria facilitates intestinal mucosal barrier function by modulating gut bacteria and propionate levels. Carbohydr. Polym. 2025, 354, 123300. [Google Scholar] [CrossRef]
- Zhou, Z.; Tran, P.Q.; Kieft, K.; Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 2020, 14, 2060–2077. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Roy, A.; Pal, S.; Mohapatra, B.; Kazy, S.K.; Maiti, M.K.; Sar, P. Enrichment and characterization of hydrocarbon-degrading bacteria from petroleum refinery waste as potent bioaugmentation agent for in situ bioremediation. Bioresour. Technol. 2017, 242, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Teng, T.M.; Han, J.; Kim, H.S. Antibiotic-associated changes in Akkermansia muciniphila alter its effects on host metabolic health. Microbiome 2025, 13, 48. [Google Scholar] [CrossRef]
- Pan, M.; Qian, C.; Huo, S.; Wu, Y.; Zhao, X.; Ying, Y.; Wang, B.; Yang, H.; Yeerken, A.; Wang, T.; et al. Gut-derived lactic acid enhances tryptophan to 5-hydroxytryptamine in regulation of anxiety via Akkermansia muciniphila. Gut Microbes 2025, 17, 2447834. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.-C.; Armougom, F.; Robert, C.; Audoly, G.; Papazian, L.; Raoult, D. High-level colonisation of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int. J. Antimicrob. Agents 2013, 41, 149–155. [Google Scholar] [CrossRef]
- Zhu, H.-Z.; Liang, Y.-D.; Ma, Q.-Y.; Hao, W.-Z.; Li, X.-J.; Wu, M.-S.; Deng, L.-J.; Li, Y.-M.; Chen, J.-X. Xiaoyaosan improves depressive-like behavior in rats with chronic immobilization stress through modulation of the gut microbiota. Biomed. Pharmacother. 2019, 112, 108621. [Google Scholar] [CrossRef]
- Joles, J.A.; García-Arroyo, F.E.; Gonzaga, G.; Muñoz-Jiménez, I.; Blas-Marron, M.G.; Silverio, O.; Tapia, E.; Soto, V.; Ranganathan, N.; Ranganathan, P.; et al. Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS ONE 2018, 13, e0202901. [Google Scholar] [CrossRef]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. Int. J. Biol. Macromol. 2022, 208, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Li, X.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactic acid bacteria strains relieve hyperuricaemia by suppressing xanthine oxidase activityviaa short-chain fatty acid-dependent mechanism. Food Funct. 2021, 12, 7054–7067. [Google Scholar] [CrossRef]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2020, 69, 1487–1495. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Wang, R.; Yu, Y.; Liu, X.; Tian, Z. Protective effect of sodium butyrate on intestinal barrier damage and uric acid reduction in hyperuricemia mice. Biomed. Pharmacother. 2023, 161, 114568. [Google Scholar] [CrossRef] [PubMed]
- Farhadipour, M.; Arnauts, K.; Clarysse, M.; Thijs, T.; Liszt, K.; Van der Schueren, B.; Ceulemans, L.J.; Deleus, E.; Lannoo, M.; Ferrante, M.; et al. SCFAs switch stem cell fate through HDAC inhibition to improve barrier integrity in 3D intestinal organoids from patients with obesity. iScience 2023, 26, 108517. [Google Scholar] [CrossRef] [PubMed]
- Eckenstaler, R.; Benndorf, R.A. The Role of ABCG2 in the Pathogenesis of Primary Hyperuricemia and Gout—An Update. Int. J. Mol. Sci. 2021, 22, 6678. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhao, X.; Wang, F.; Wang, G. A brief review of urate transporter 1 (URAT1) inhibitors for the treatment of hyperuricemia and gout: Current therapeutic options and potential applications. Eur. J. Pharmacol. 2021, 907, 174291. [Google Scholar] [CrossRef]
- Pope, S.D.; Chen, L.-L.; Stewart, V. Purine Utilization byKlebsiella oxytocaM5al: Genes for Ring-Oxidizing and -Opening Enzymes. J. Bacteriol. 2009, 191, 1006–1017. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Liu, S.; Wang, S.; Li, Z.; Lu, F.; Luo, X. Synergistic Probiotic–Postbiotic Therapy Ameliorates Hyperuricemia via Multi-Target Regulation of Purine Metabolism and Gut Microbiota. Foods 2025, 14, 2213. https://doi.org/10.3390/foods14132213
Ren L, Liu S, Wang S, Li Z, Lu F, Luo X. Synergistic Probiotic–Postbiotic Therapy Ameliorates Hyperuricemia via Multi-Target Regulation of Purine Metabolism and Gut Microbiota. Foods. 2025; 14(13):2213. https://doi.org/10.3390/foods14132213
Chicago/Turabian StyleRen, Lu, Shiting Liu, Shangshang Wang, Zhenrui Li, Fuping Lu, and Xuegang Luo. 2025. "Synergistic Probiotic–Postbiotic Therapy Ameliorates Hyperuricemia via Multi-Target Regulation of Purine Metabolism and Gut Microbiota" Foods 14, no. 13: 2213. https://doi.org/10.3390/foods14132213
APA StyleRen, L., Liu, S., Wang, S., Li, Z., Lu, F., & Luo, X. (2025). Synergistic Probiotic–Postbiotic Therapy Ameliorates Hyperuricemia via Multi-Target Regulation of Purine Metabolism and Gut Microbiota. Foods, 14(13), 2213. https://doi.org/10.3390/foods14132213





