Valorization of Olive Mill Wastewater by Selective Sequential Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. OMWW Samples
2.2. Bacterial Strains and Culture Conditions
2.3. General Experimental Workflow
2.4. Alcoholic Fermentation
2.5. Screening of AAB Strains
2.6. Acetic Acid Fermentations
2.7. Analytical Determinations
2.8. Total Phenolic Compounds and Antioxidant Activity
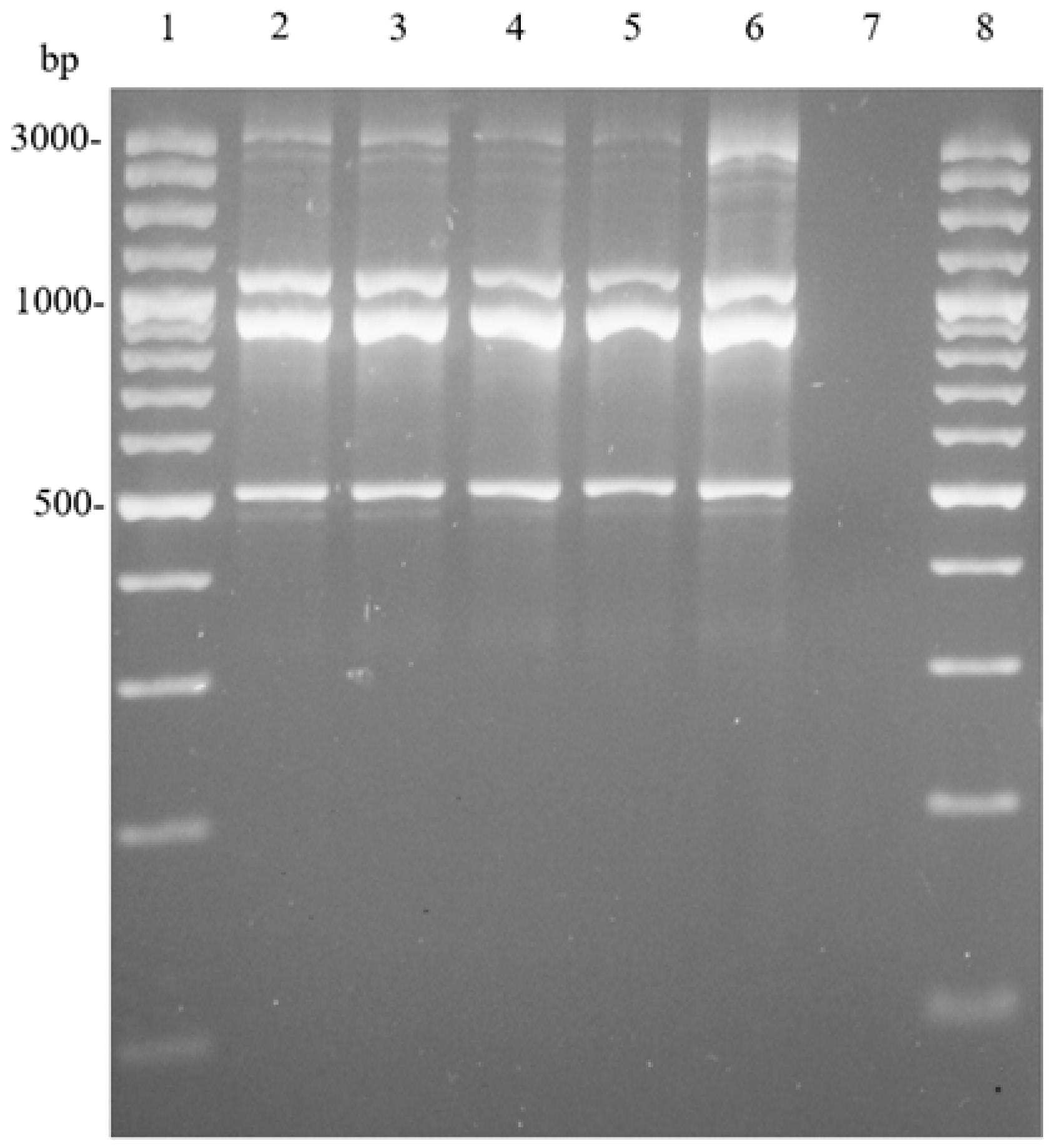
2.9. Genomic DNA Extraction and Amplification of (GTG)5/rep-PCR
2.10. Statistical Analysis
3. Results and Discussion
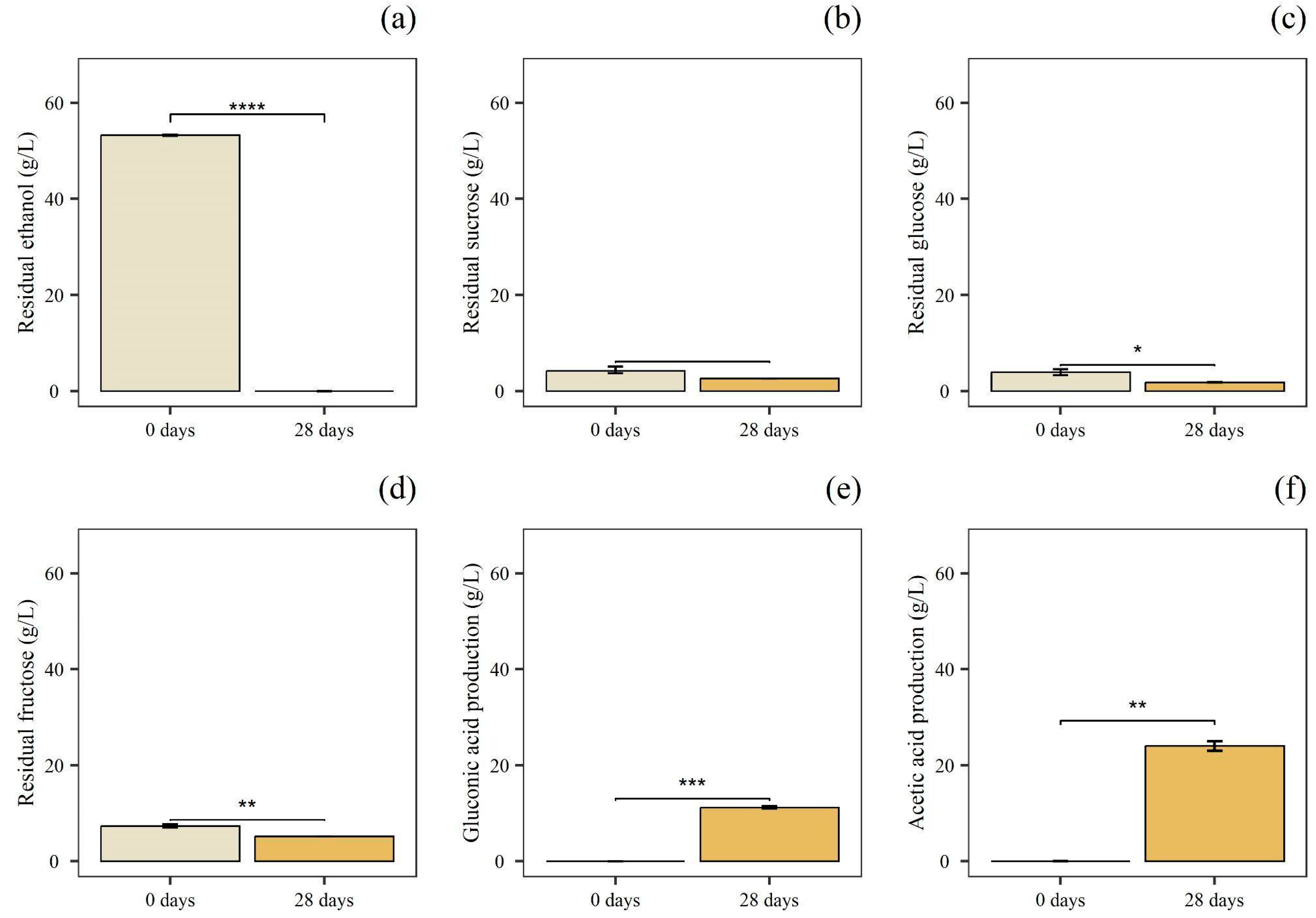
3.1. OMWW Chemical Characterization and Alcoholic Fermentation
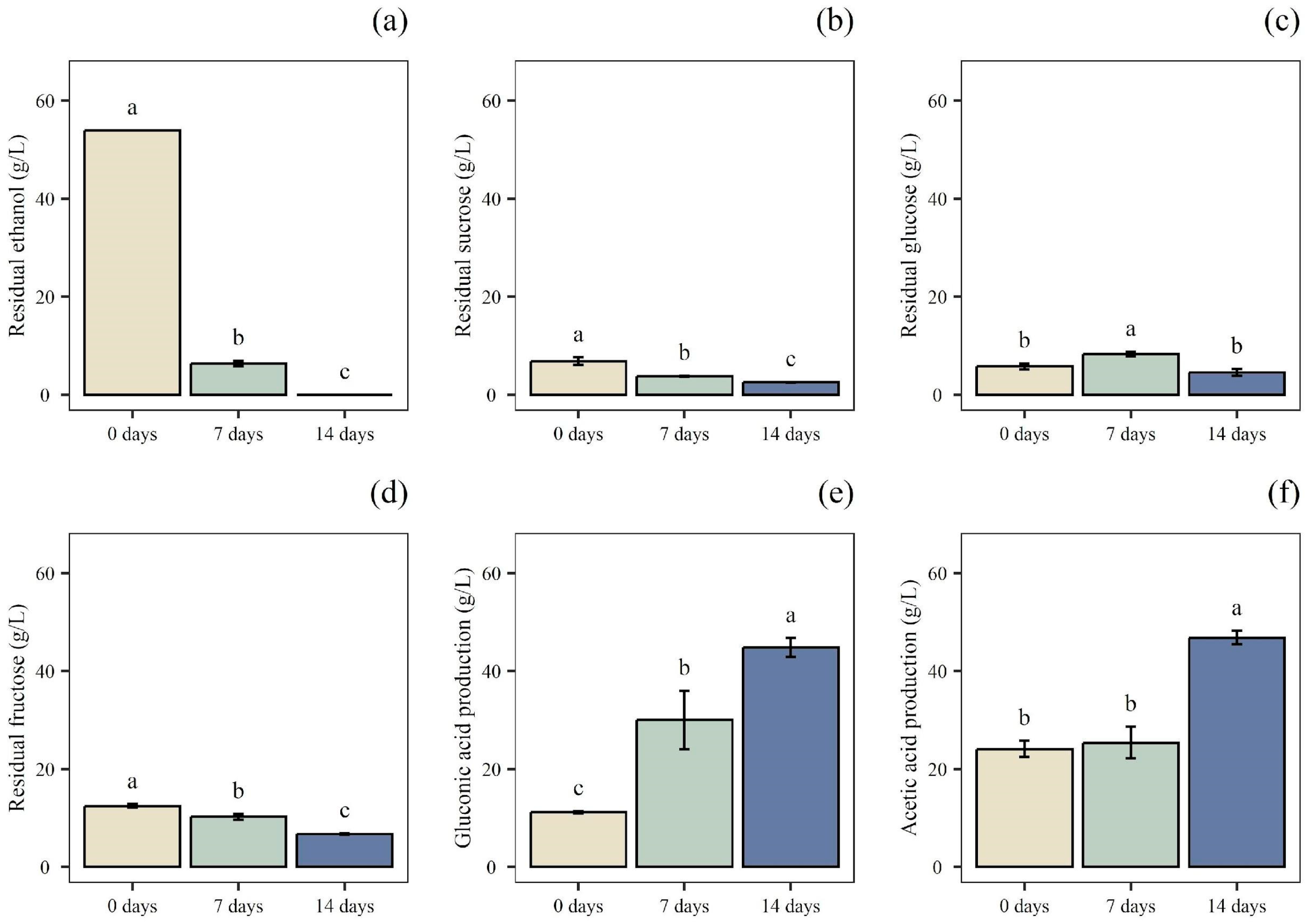
3.2. Screening of AAB for the Acetic Acid Fermentations
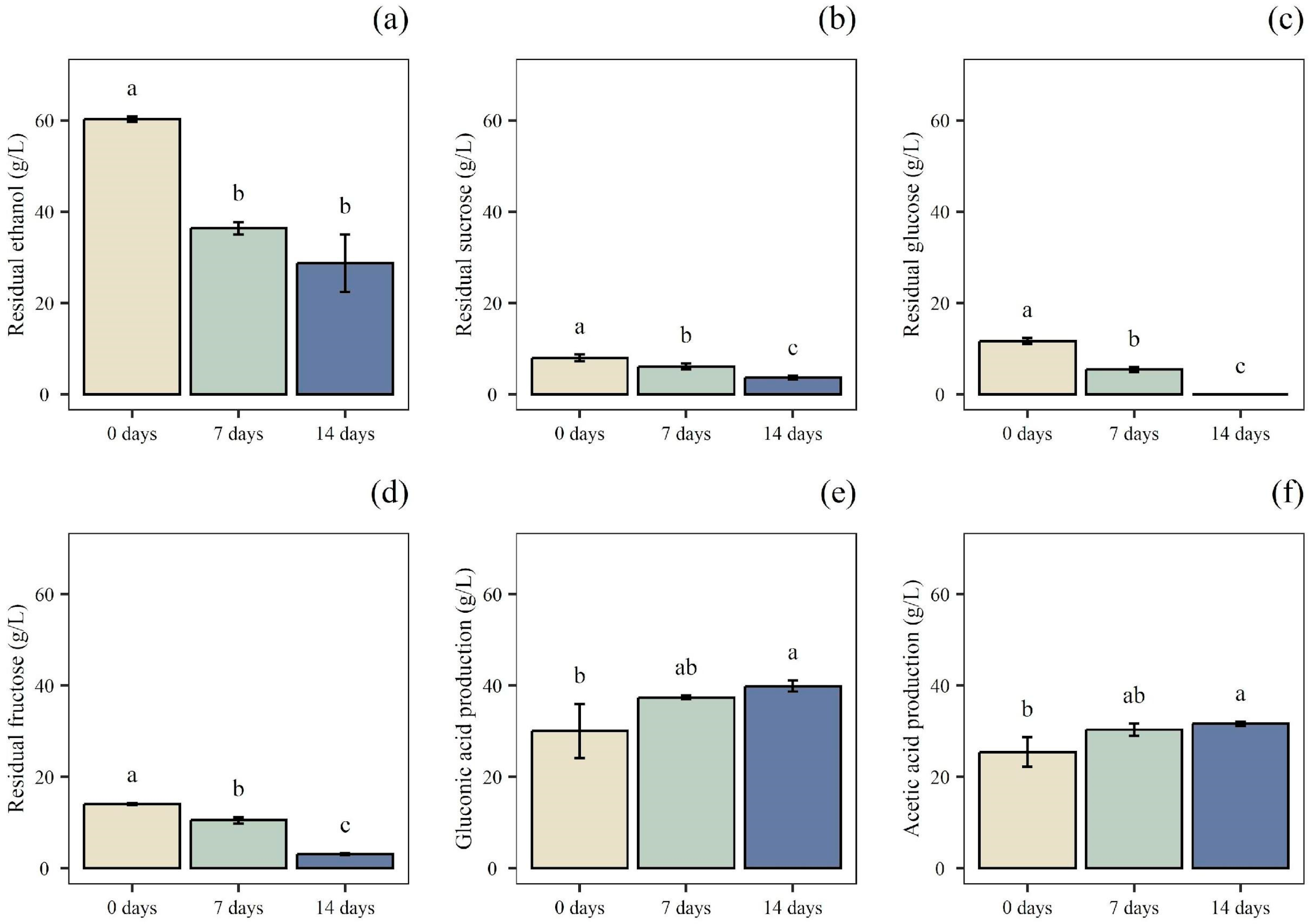
3.3. Acetic Acid Fermentations
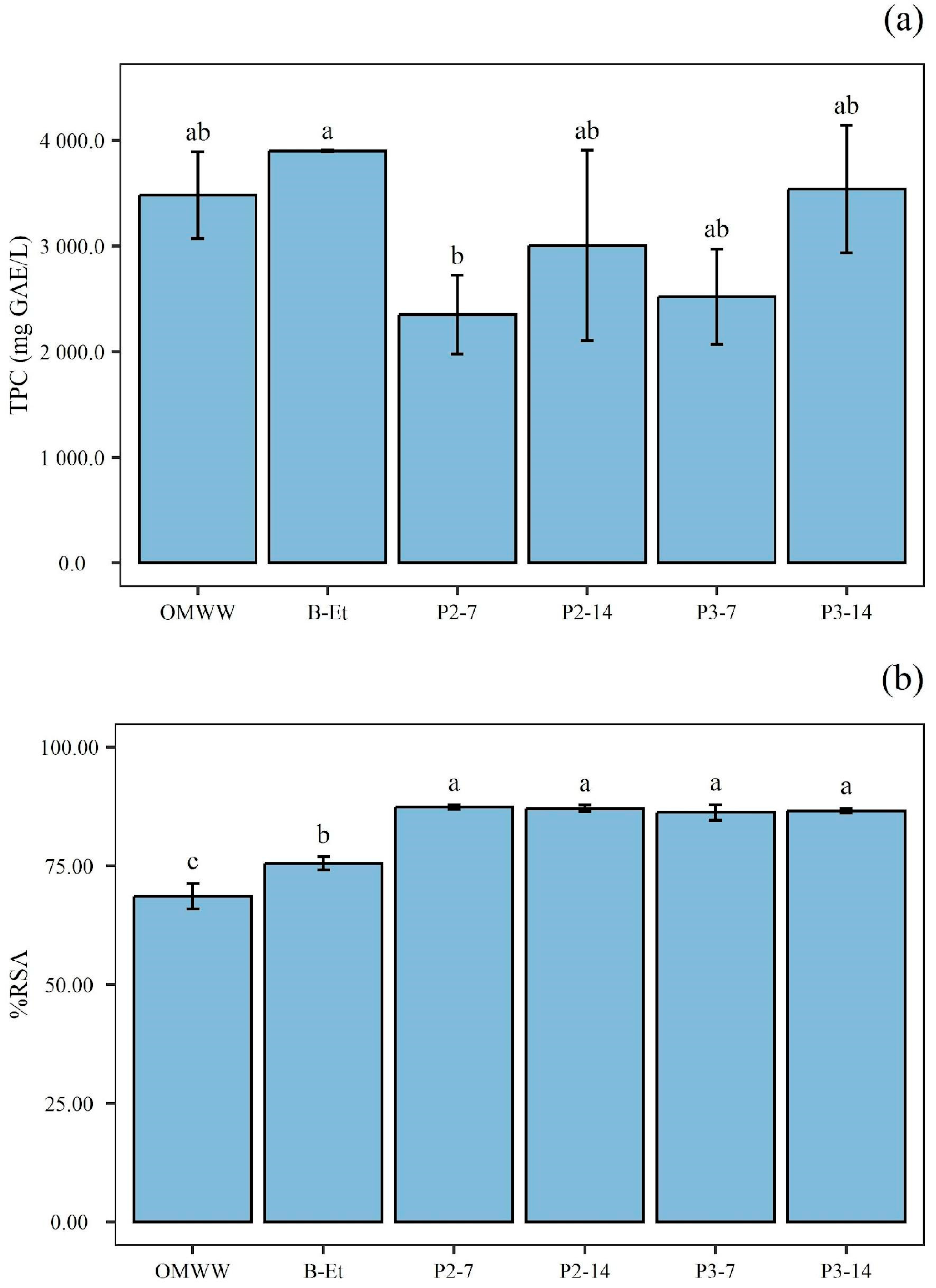
3.4. Total Phenolic Compounds and Antioxidant Activity
3.5. Testing Survival of A. pasteurianus UMCC 1754 During Static and Submerged Fermentation Phases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrakis, C. Boskou, D., Ed.; 9—Olive Oil Extraction. In Olive Oil, 2nd ed.; AOCS Press: Urbana, IL, USA, 2006; pp. 191–223. ISBN 978-1-893997-88-2. [Google Scholar]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management: Literature Review and Patent Survey. In Waste Management Series, 2nd ed.; Elsevier: London, UK, 2006; Volume 5, pp. 23–64. ISBN 978-0-08-044851-0. [Google Scholar]
- McNamara, C.J.; Anastasiou, C.C.; O’Flaherty, V.; Mitchell, R. Bioremediation of Olive Mill Wastewater. Int. Biodeterior. Biodegrad. 2008, 61, 127–134. [Google Scholar] [CrossRef]
- Zahi, M.R.; Zam, W.; El Hattab, M. State of Knowledge on Chemical, Biological and Nutritional Properties of Olive Mill Wastewater. Food Chem. 2022, 381, 132238. [Google Scholar] [CrossRef]
- Benaddi, R.; Osmane, A.; Zidan, K.; Harfi, K.E.; Ouazzani, N. A Review on Processes for Olive Mill Waste Water Treatment. Ecol. Eng. Environ. Technol. 2023, 24, 196–207. [Google Scholar] [CrossRef]
- Vaz, T.; Quina, M.M.J.; Martins, R.C.; Gomes, J. Olive Mill Wastewater Treatment Strategies to Obtain Quality Water for Irrigation: A Review. Sci. Total. Environ. 2024, 931, 172676. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Mameri, N.; Aioueche, F.; Belhocine, D.; Grib, H.; Lounici, H.; Piron, D.; Yahiat, Y. Preparation of activated carbon from olive mill solid residue. J. Chem. Technol. Biotechnol. 2000, 75, 625–631. [Google Scholar] [CrossRef]
- Bouzid, O.; Navarro, D.; Roche, M.; Asther, M.; Haon, M.; Delattre, M.; Lorquin, J.; Labat, M.; Asther, M.; Lesage-Meessen, L. Fungal Enzymes as a Powerful Tool to Release Simple Phenolic Compounds from Olive Oil By-Product. Process. Biochem. 2005, 40, 1855–1862. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Esposito, A.; Vegliò, F. Multi-Metallic Modelling for Biosorption of Binary Systems. Water Res. 2002, 36, 4095–4105. [Google Scholar] [CrossRef]
- Albarrán, A.; Celis, R.; Hermosín, M.C.; López-Piñeiro, A.; Cornejo, J. Behaviour of Simazine in Soil Amended with the Final Residue of the Olive-Oil Extraction Process. Chemosphere 2004, 54, 717–724. [Google Scholar] [CrossRef]
- Siracusa, G.; La Rosa, A.D.; Siracusa, V.; Trovato, M. Eco-Compatible Use of Olive Husk as Filler in Thermoplastic Composites. J. Polym. Environ. 2001, 9, 157–161. [Google Scholar] [CrossRef]
- Khdair, A.; Abu-Rumman, G. Sustainable Environmental Management and Valorization Options for Olive Mill Byproducts in the Middle East and North Africa (MENA) Region. Processes 2020, 8, 671. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Aguiar, N.F.B.; Voss, G.B.; Pintado, M.E. Properties of Fermented Beverages from Food Wastes/By-Products. Beverages 2023, 9, 45. [Google Scholar] [CrossRef]
- Brugnoli, M.; Cantadori, E.; Arena, M.P.; De Vero, L.; Colonello, A.; Gullo, M. Zero- and Low-Alcohol Fermented Beverages: A Perspective for Non-Conventional Healthy and Sustainable Production from Red Fruits. Fermentation 2023, 9, 457. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-Enabled Wellness Foods: A Fresh Perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Mezzetti, F.; Fay, J.C.; Giudici, P.; De Vero, L. Genetic Variation and Expression Changes Associated with Molybdate Resistance from a Glutathione Producing Wine Strain of Saccharomyces cerevisiae. PLoS ONE 2017, 12, e0180814. [Google Scholar] [CrossRef]
- Mezzetti, F.; De Vero, L.; Giudici, P. Evolved Saccharomyces cerevisiae Wine Strains with Enhanced Glutathione Production Obtained by an Evolution-Based Strategy. FEMS Yeast Res. 2014, 14, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Bleve, G.; Lezzi, C.; Chiriatti, M.A.; D’Ostuni, I.; Tristezza, M.; Venere, D.D.; Sergio, L.; Mita, G.; Grieco, F. Selection of Non-Conventional Yeasts and Their Use in Immobilized Form for the Bioremediation of Olive Oil Mill Wastewaters. Bioresour. Technol. 2011, 102, 982–989. [Google Scholar] [CrossRef]
- Ntougias, S.; Bourtzis, K.; Tsiamis, G. The Microbiology of Olive Mill Wastes. BioMed Res. Int. 2013, 2013, 784591. [Google Scholar] [CrossRef]
- Romeo, F.V.; Granuzzo, G.; Foti, P.; Ballistreri, G.; Caggia, C.; Rapisarda, P. Microbial Application to Improve Olive Mill Wastewater Phenolic Extracts. Molecules 2021, 26, 1944. [Google Scholar] [CrossRef]
- Gullo, M.; Zanichelli, G.; Verzelloni, E.; Lemmetti, F.; Giudici, P. Feasible Acetic Acid Fermentations of Alcoholic and Sugary Substrates in Combined Operation Mode. Process Biochem. 2016, 51, 1129–1139. [Google Scholar] [CrossRef][Green Version]
- Sievers, M.; Sellmer, S.; Teuber, M. Acetobacter europaeus sp. Nov., a Main Component of Industrial Vinegar Fermenters in Central Europe. Syst. Appl. Microbiol. 1992, 15, 386–392. [Google Scholar] [CrossRef]
- Wu, J.; Gullo, M.; Chen, F.; Giudici, P. Diversity of Acetobacter pasteurianus Strains Isolated from Solid-State Fermentation of Cereal Vinegars. Curr. Microbiol. 2010, 60, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; Caggia, C.; De Vero, L.; Giudici, P. Characterization of Acetic Acid Bacteria in “Traditional Balsamic Vinegar”. Int. J. Food Microbiol. 2006, 106, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Aiello, E.; Arena, M.P.; De Vero, L.; Montanini, C.; Bianchi, M.; Mescola, A.; Alessandrini, A.; Pulvirenti, A.; Gullo, M. Wine Yeast Strains Under Ethanol-Induced Stress: Morphological and Physiological Responses. Fermentation 2024, 10, 631. [Google Scholar] [CrossRef]
- Foti, P.; Occhipinti, P.S.; Russo, N.; Scilimati, A.; Miciaccia, M.; Caggia, C.; Perrone, M.G.; Randazzo, C.L.; Romeo, F.V. Olive Mill Wastewater Fermented with Microbial Pools as a New Potential Functional Beverage. Molecules 2023, 28, 646. [Google Scholar] [CrossRef]
- Palmeri, R.; Siracusa, L.; Carrubba, M.; Parafati, L.; Proetto, I.; Pesce, F.; Fallico, B. Olive Leaves, a Promising Byproduct of Olive Oil Industry: Assessment of Metabolic Profiles and Antioxidant Capacity as a Function of Cultivar and Seasonal Change. Agronomy 2022, 12, 2007. [Google Scholar] [CrossRef]
- Paredes, C.; Cegarra, J.; Roig, A.; Sánchez-Monedero, M.A.; Bernal, M.P. Characterization of Olive Mill Wastewater (Alpechin) and Its Sludge for Agricultural Purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Iorizzo, M.; Lombardi, S.J.; Lopez, F.; Marconi, E. Effective Assay for Olive Vinegar Production from Olive Oil Mill Wastewaters. Food Chem. 2018, 240, 437–440. [Google Scholar] [CrossRef]
- Zanichelli, D.; Carloni, F.; Hasanaj, E.; D’Andrea, N.; Filippini, A.; Setti, L. Production of Ethanol by an Integrated Valorization of Olive Oil Byproducts. The Role of Phenolic Inhibition (2 Pp). Environ. Sci. Pollut. Res. 2007, 14, 5–6. [Google Scholar] [CrossRef]
- Bambalov, G.; Israilides, C.; Tanchev, S. Alcohol Fermentation in Olive Oil Extraction Effluents. Biol. Wastes 1989, 27, 71–75. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.-E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of Olive Mill Wastewater into High-Added Value Products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol Production from Sweet Sorghum Juice Using Very High Gravity Technology: Effects of Carbon and Nitrogen Supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef]
- Sarris, D.; Giannakis, M.; Philippoussis, A.; Komaitis, M.; Koutinas, A.A.; Papanikolaou, S. Conversions of Olive Mill Wastewater-Based Media by Saccharomyces cerevisiae through Sterile and Non-Sterile Bioprocesses. J. Chem. Technol. Biotechnol. 2013, 88, 958–969. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of Phenolic Compounds on the Growth of Selected Probiotic and Pathogenic Bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Fu, S.; Zhou, M.; Xu, N.; Li, D.; Wang, C.; Hu, Y. Coordination of Characteristic Cytomembrane and Energy Metabolism Contributes to Ethanol-Tolerance of Acetobacter pasteurianus. LWT 2022, 169, 113950. [Google Scholar] [CrossRef]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic Submerged Fermentation by Acetic Acid Bacteria for Vinegar Production: Process and Biotechnological Aspects. Process. Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Mill Wastewater as Renewable Raw Materials to Generate High Added-Value Ingredients for Agro-Food Industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Çelik, G.; Saygın, Ö.; Akmehmet Balcıoğlu, I. Multistage Recovery Process of Phenolic Antioxidants with a Focus on Hydroxytyrosol from Olive Mill Wastewater Concentrates. Sep. Purif. Technol. 2021, 259, 117757. [Google Scholar] [CrossRef]
- Chen, C.; Wu, S.; Li, Y.; Huang, Y.; Yang, X. Effects of Different Acetic Acid Bacteria Strains on the Bioactive Compounds, Volatile Compounds and Antioxidant Activity of Black Tea Vinegar. LWT 2022, 171, 114131. [Google Scholar] [CrossRef]
- Gullo, M.; Mamlouk, D.; De Vero, L.; Giudici, P. Acetobacter pasteurianus Strain AB0220: Cultivability and Phenotypic Stability Over 9 Years of Preservation. Curr. Microbiol. 2012, 64, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; De Vero, L.; Giudici, P. Succession of Selected Strains of Acetobacter pasteurianus and Other Acetic Acid Bacteria in Traditional Balsamic Vinegar. Appl. Environ. Microbiol. 2009, 75, 2585–2589. [Google Scholar] [CrossRef]
- Hidalgo, C.; Vegas, C.; Mateo, E.; Tesfaye, W.; Cerezo, A.B.; Callejón, R.M.; Poblet, M.; Guillamón, J.M.; Mas, A.; Torija, M.J. Effect of Barrel Design and the Inoculation of Acetobacter pasteurianus in Wine Vinegar Production. Int. J. Food Microbiol. 2010, 141, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sainz, F.; Navarro, D.; Mateo, E.; Torija, M.J.; Mas, A. Comparison of D-Gluconic Acid Production in Selected Strains of Acetic Acid Bacteria. Int. J. Food Microbiol. 2016, 222, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Anguluri, K.; La China, S.; Brugnoli, M.; De Vero, L.; Pulvirenti, A.; Cassanelli, S.; Gullo, M. Candidate Acetic Acid Bacteria Strains for Levan Production. Polymers 2022, 14, 2000. [Google Scholar] [CrossRef]
- Brugnoli, M.; Cantadori, E.; Arena, M.P.; Gullo, M. Oxidative Fermentation of Glucose and Ethanol in Designed Media and Cooked Grape Must by Acetic Acid Bacteria. J. Agric. Food Res. 2024, 15, 101028. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377. [Google Scholar] [CrossRef]
- La China, S.; Zanichelli, G.; De Vero, L.; Gullo, M. Oxidative Fermentations and Exopolysaccharides Production by Acetic Acid Bacteria: A Mini Review. Biotechnol. Lett. 2018, 40, 1289–1302. [Google Scholar] [CrossRef]
- Qin, Z.; Yu, S.; Chen, J.; Zhou, J. Dehydrogenases of Acetic Acid Bacteria. Biotechnol. Adv. 2022, 54, 107863. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Jiang, W.; Jia, R.; Zhou, X.; Xu, Y. Directional Enhancement of 2-Keto-Gluconic Acid Production from Enzymatic Hydrolysate by Acetic Acid-Mediated Bio-Oxidation with Gluconobacter oxydans. Bioresour. Technol. 2022, 348, 126811. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, V.K.; Qazi, G.N.; Kumar, A. Gluconobacter oxydans: Its Biotechnological Applications. J. Mol. Microbiol. Biotechnol. 2001, 3, 445–456. [Google Scholar]
- Silberbach, M.; Maier, B.; Zimmermann, M.; Büchs, J. Glucose Oxidation by Gluconobacter Oxydans: Characterization in Shaking-Flasks, Scale-up and Optimization of the pH Profile. Appl. Microbiol. Biotechnol. 2003, 62, 92–98. [Google Scholar] [CrossRef]
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Ehrenreich, A.; Liebl, W.; García-García, I. Gluconic Acid: Properties, Production Methods and Applications—An Excellent Opportunity for Agro-Industrial by-Products and Waste Bio-Valorization. Process. Biochem. 2016, 51, 1891–1903. [Google Scholar] [CrossRef]
- Feki, M.; Allouche, N.; Bouaziz, M.; Gargoubi, A.; Sayadi, S. Effect of Storage of Olive Mill Wastewaters on Hydroxytyrosol Concentration. Eur. J. Lipid Sci. Technol. 2006, 108, 1021–1027. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kallel, L.; Desseaux, V.; Hamdi, M.; Stocker, P.; Ajandouz, E.H. Insights into the Fermentation Biochemistry of Kombucha Teas and Potential Impacts of Kombucha Drinking on Starch Digestion. Food Res. Int. 2012, 49, 226–232. [Google Scholar] [CrossRef]
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in Free-Radical Scavenging Ability of Kombucha Tea during Fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef]
- Erskine, E.; Ozkan, G.; Lu, B.; Capanoglu, E. Effects of Fermentation Process on the Antioxidant Capacity of Fruit Byproducts. ACS Omega 2023, 8, 4543–4553. [Google Scholar] [CrossRef]
- Kachouri, F.; Setti, K.; Ksontini, H.; Mechmeche, M.; Hamdi, M. Improvement of Antioxidant Activity of Olive Mill Wastewater Phenolic Compounds by Lactobacillus plantarum Fermentation. Desalination Water Treat. 2016, 57, 27125–27137. [Google Scholar] [CrossRef]
- Foti, P.; Conti-Nibali, S.; Randazzo, C.L.; Reina, S.; Romeo, F.V.; Caggia, C.; De Pinto, V. Protective Effect of Treated Olive Mill Wastewater on Target Bacteria and Mitochondrial Voltage-Dependent Anion-Selective Channel 1. Antioxidants 2023, 12, 322. [Google Scholar] [CrossRef]
- Fki, I.; Allouche, N.; Sayadi, S. The Use of Polyphenolic Extract, Purified Hydroxytyrosol and 3,4-Dihydroxyphenyl Acetic Acid from Olive Mill Wastewater for the Stabilization of Refined Oils: A Potential Alternative to Synthetic Antioxidants. Food Chem. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Gomes, R.J.; Borges, M.D.F.; Rosa, M.D.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Cleenwerck, I.; De Vos, P.; De Vuyst, L. (GTG)5-PCR Reference Framework for Acetic Acid Bacteria. FEMS Microbiol. Lett. 2009, 301, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M.; Tesfaye, W.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. HPLC Determination of Amino Acids with AQC Derivatization in Vinegars along Submerged and Surface Acetifications and Its Relation to the Microbiota. Eur. Food Res. Technol. 2008, 227, 93–102. [Google Scholar] [CrossRef]
- Trček, J.; Raspor, P.; Teuber, M. Molecular Identification of Acetobacter Isolates from Submerged Vinegar Production, Sequence Analysis of Plasmid pJK2-1 and Application in the Development of a Cloning Vector. Appl. Microbiol. Biotechnol. 2000, 53, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Schüller, G.; Hertel, C.; Hammes, W.P. Gluconacetobacter entanii Sp. Nov., Isolated from Submerged High-Acid Industrial Vinegar Fermentations. IJSEM 2000, 50, 2013–2020. [Google Scholar] [CrossRef]
- Karničnik, B.; Accetto, T.; Fanedl, L.; Jugović, I.; Trček, J. Isolation and Characterization of Komagataeibacter piraceti sp. Nov. and Novacetimonas labruscae sp. Nov.: Two Novel Microaerobic Cellulose-Producing Acetic Acid Bacteria from Vinegars. Microorganisms 2025, 13, 456. [Google Scholar] [CrossRef]
- Brandão, P.R.; Crespo, M.T.B.; Nascimento, F.X. Phylogenomic and Comparative Analyses Support the Reclassification of Several Komagataeibacter Species as Novel Members of the Novacetimonas Gen. Nov. and Bring New Insights into the Evolution of Cellulose Synthase Genes. IJSEM 2022, 72, 005252. [Google Scholar] [CrossRef]
- Holzapfel, W.H. Appropriate Starter Culture Technologies for Small-Scale Fermentation in Developing Countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef] [PubMed]



| Strain | Species | Isolation Source |
|---|---|---|
| UMCC 855 = 21T2 | Saccharomyces cerevisiae | Wine [17] |
| DSM 6160T | Komagataeibacter europaeus | Vinegar [23] |
| UMCC 1806 = ZJ555 | Komagataeibacter europaeus | Cereal vinegar [24] |
| DSM 3509T | Acetobacter pasteurianus | Beer (Leibniz Institute DSMZ) |
| UMCC 1754 = AB0220 | Acetobacter pasteurianus | Wine vinegar [25] |
| UMCC 1786 = DL13 | Acetobacter pasteurianus | Cereal vinegar [24] |
| Process | Time (Days) | Code |
|---|---|---|
| Small scale batch static fermentation, Phase 1 | 28 | P1 |
| Static fermentation, Phase 2 | 7 | P2-7 |
| 14 | P2-14 | |
| Submerged fermentation, Phase 3 | 7 | P3-7 |
| 14 | P3-14 |
| Sample | pH | Glucose (g/L) | Fructose (g/L) | Titratable Acidity (% w/v) | TPC (mg GAE/L) | Dry Matter (% w/v) |
|---|---|---|---|---|---|---|
| OMWW | 4.99 ± 0.07 | 24.91 ± 0.93 | 24.11 ± 0.91 | 0.24 ± 0.01 | 3480.60 ± 409.12 | 5.60 ± 0.02 |
| Time (Days) | pH | Titratable Acidity (% w/v) |
|---|---|---|
| 0 | 3.47 ± 0.16 a | 1.80 ± 0.20 c |
| 7 | 3.69 ± 0.02 a | 3.90 ± 0.13 b |
| 14 | 3.70 ± 0.03 a | 4.41 ± 0.05 a |
| Time (Days) | pH | Titratable Acidity (% w/v) |
|---|---|---|
| 0 | 4.23 ± 0.84 a | 2.29 ± 0.17 c |
| 7 | 4.14 ± 0.10 a | 2.56 ± 0.01 b |
| 14 | 4.08 ± 0.27 a | 2.91 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorello, L.; Arena, M.P.; Brugnoli, M.; Romeo, F.V.; Gullo, M. Valorization of Olive Mill Wastewater by Selective Sequential Fermentation. Foods 2025, 14, 2170. https://doi.org/10.3390/foods14132170
Signorello L, Arena MP, Brugnoli M, Romeo FV, Gullo M. Valorization of Olive Mill Wastewater by Selective Sequential Fermentation. Foods. 2025; 14(13):2170. https://doi.org/10.3390/foods14132170
Chicago/Turabian StyleSignorello, Lara, Mattia Pia Arena, Marcello Brugnoli, Flora V. Romeo, and Maria Gullo. 2025. "Valorization of Olive Mill Wastewater by Selective Sequential Fermentation" Foods 14, no. 13: 2170. https://doi.org/10.3390/foods14132170
APA StyleSignorello, L., Arena, M. P., Brugnoli, M., Romeo, F. V., & Gullo, M. (2025). Valorization of Olive Mill Wastewater by Selective Sequential Fermentation. Foods, 14(13), 2170. https://doi.org/10.3390/foods14132170









