Comparative Evaluation of Inulin and High-Ester Pectin for Microencapsulation of Bacillus coagulans TBC-169: Characterization and Probiotic Application in Peanut Butter Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Bacillus coagulans TBC-169 Microcapsules
2.3. Preparation of Prebiotic Peanut Butter
2.4. Determination of the Digestive Stability of Microcapsules in Gastroenteric Fluid
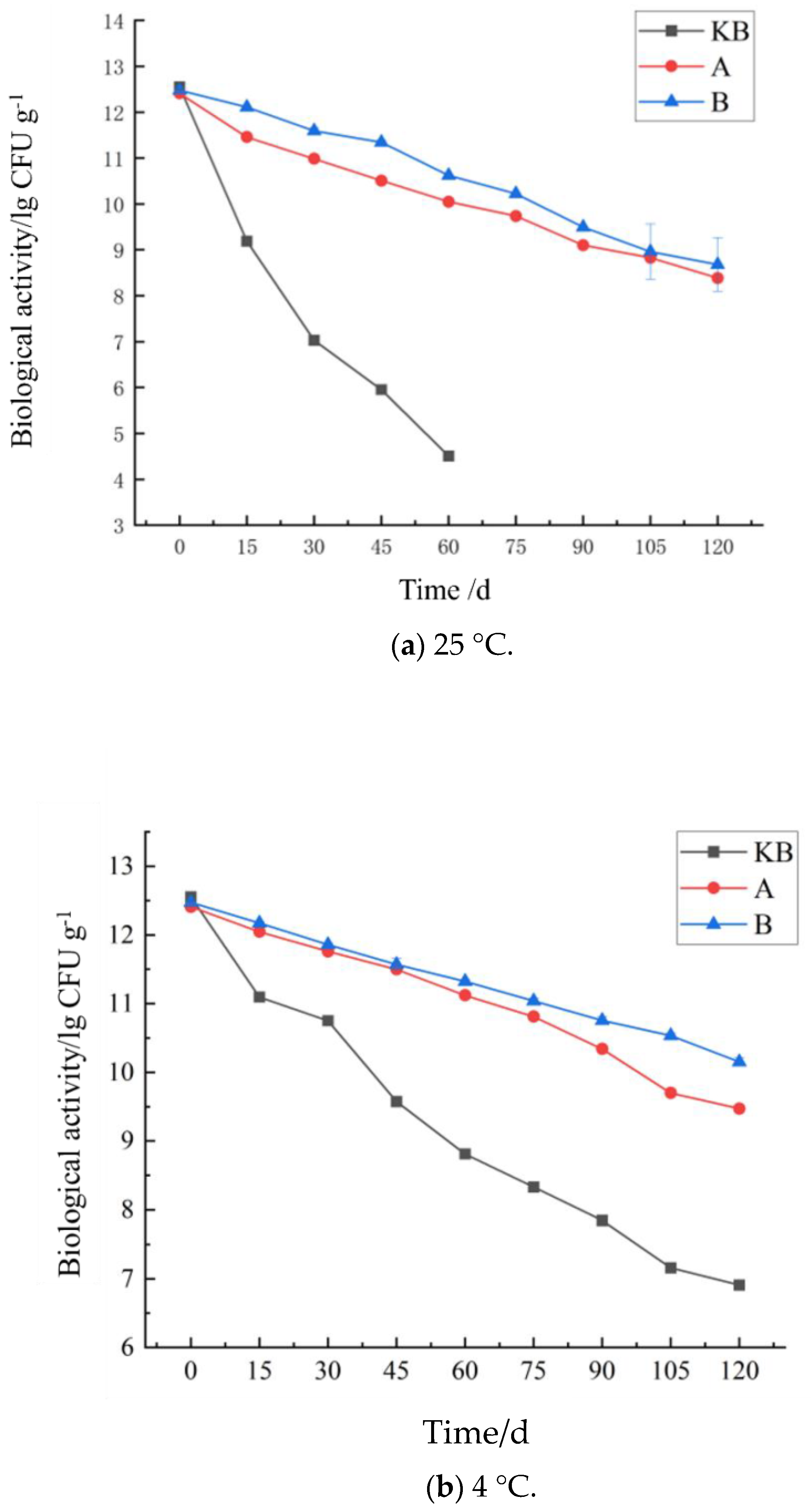
2.5. Storage Stability Test of Microcapsules
2.6. Composition and Bacterial Evaluation
2.7. Examination of Structural and Rheological Properties
2.7.1. SEM
2.7.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.7.3. DSC Analysis
2.7.4. Laser Particle Size Measurement
2.7.5. Analysis of Texture Characteristics
2.7.6. Rheological Properties
2.8. Untargeted Metabolomics Measurement
2.9. Data Processing and Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition and Bacterial Test Results
3.2. Bioactivity of Bacillus coagulans Microcapsules
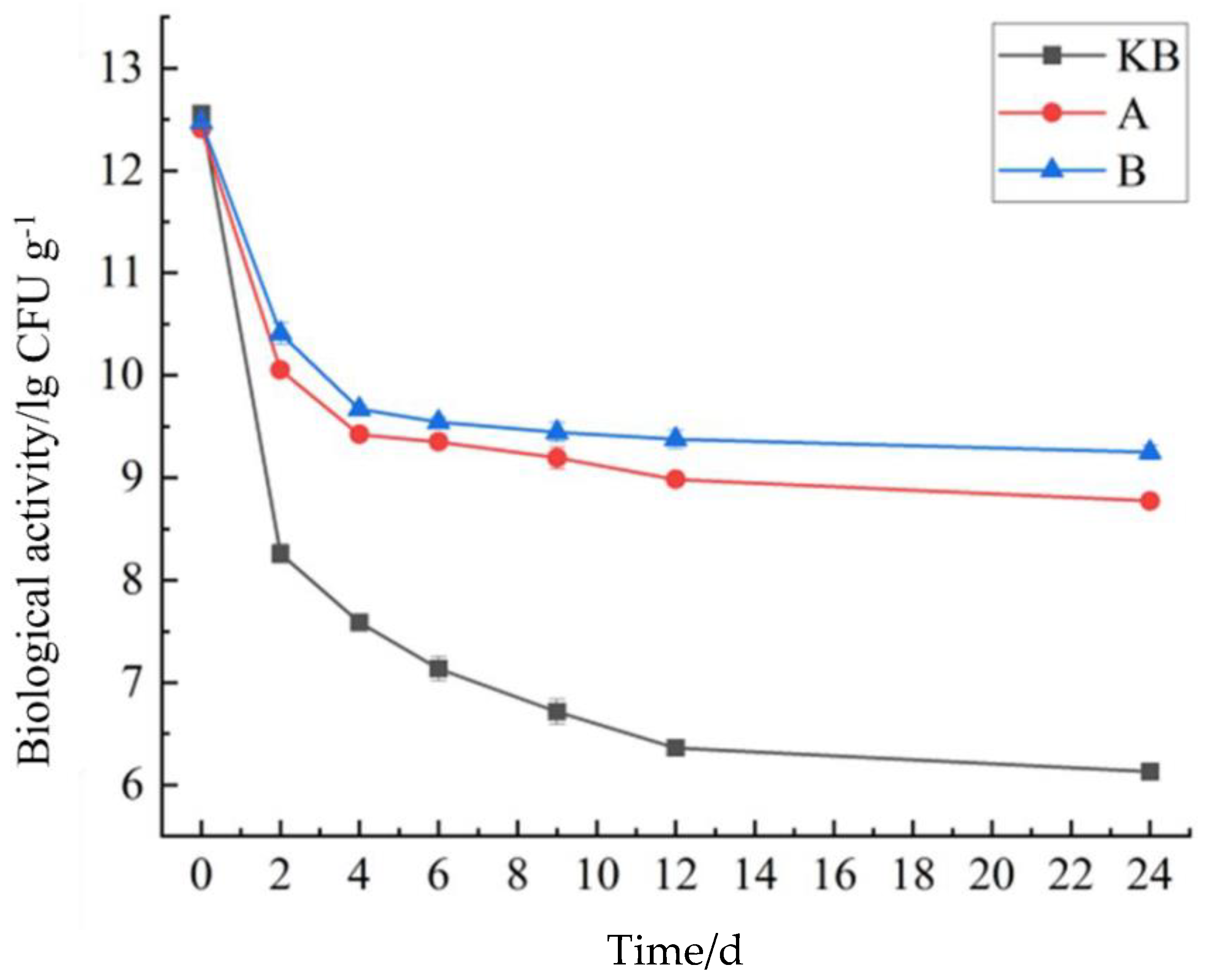
3.3. Influence of Storage Time on the Probiotic Activity of Peanut Butter
3.4. Structural and Rheological Properties
3.4.1. Particle Size
3.4.2. DSC
3.4.3. Texture
3.4.4. Rheological Characteristics
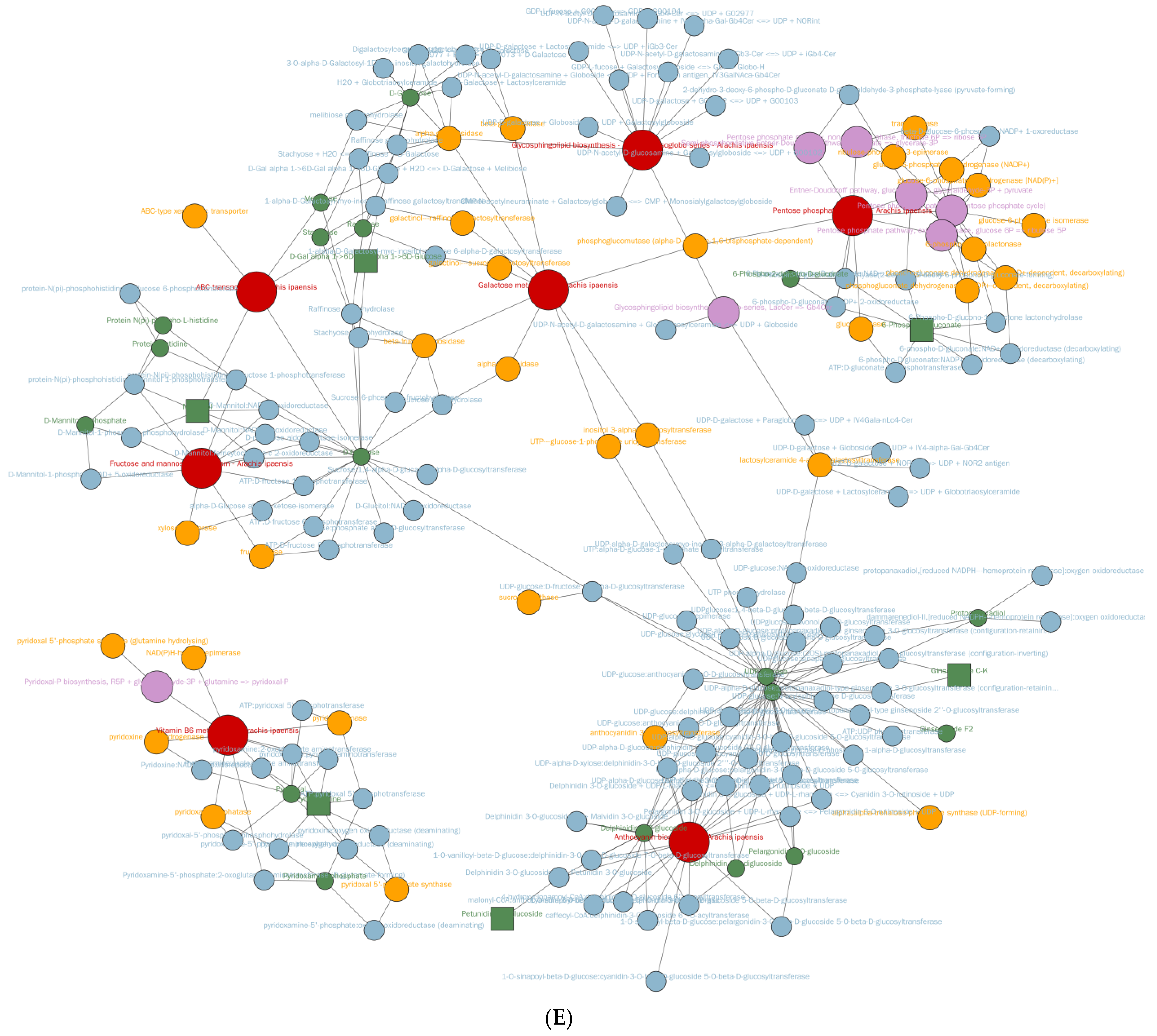
3.5. Metabolomic Differences Between High-Ester Pectin and Inulin Microencapsulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreto, A.G.; Guerra, A.F.; Nogueira, R.I.; Carvalho, C.W.P.; Godoy, R.C.B.; Freitas, S.P. Functional peanut butter stuffed snack development based on Brazilian pine (Araucaria angustifolia) and rice flours. Int. Organ. Sci. Res. 2018, 8, 53–58. [Google Scholar]
- Tanti, R.; Barbut, S.; Marangoni, A.G. Oil stabilization of natural peanut butter using food grade polymers. Food Hydrocoll. Oxf. 2016, 61, 399–408. [Google Scholar] [CrossRef]
- Sudha, M.R.; Yelikar, K.A.; Deshpande, S. Clinical Study of Bacillus coagulans Unique IS-2 (ATCC PTA-11748) in the Treatment of Patients with Bacterial Vaginosis. Indian J. Microbiol. 2012, 52, 396–399. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, E.; Lebbe, L.; Logan, N.A.; Devos, J. Polyphasic characterization of Bacillus coagulans strains, illustrating heterogeneity within this species, and emended description of the species. Syst. Appl. Microbiol. 2004, 27, 50–60. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2019, 64, 103643. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Beale, D.J.; Karpe, A.V.; Shastri, S.; Basheer, W.; Southam, B.; Eri, R. Synbiotic supplementation with prebiotic green banana resistant starch and probiotic Bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases. Eur. J. Nutr. 2020, 59, 3669–3689. [Google Scholar] [CrossRef]
- Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012, 30, 591–600. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gomez-Mascaraque, L.G.; Sarabi-Jamab, M.; Ghorani, B.; Benitez-Paez, A.; Lopez-Rubio, A. Agarose-based freeze-dried capsules prepared by the oil-induced biphasic hydrogel particle formation approach for the protection of sensitive probiotic bacteria. Food Hydrocoll. 2019, 87, 487–496. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Li, D.-Y.; Zhang, Q.; Wang, W. Review of protein and its application in microencapsulation of probiotics. Sci. Technol. Food Ind. 2012, 33, 409–413. [Google Scholar]
- Kiumarsi, M.; Rafe, A.; Yeganehzad, S. Effect of different bulk sweeteners on the dynamic oscillatory and shear rheology of chocolate. Appl. Rheol. 2017, 6, 11–19. [Google Scholar]
- Duque, A.L.R.F.; Demarqui, F.M.; Santoni, M.M.; Zanelli, C.F.; Adorno, M.A.T.; Milenkovic, D.; Mesa, V.; Sivieri, K. Effect of probiotic, prebiotic, and synbiotic on the gut microbiota of autistic children using an in vitro gut microbiome model. Food Res. Int. 2021, 149, 110657. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.M.R.; Iqbal, W.; Javed, S.; Rashid, M.; Iahtisham-UI-Haq, S. Prebiotics and iron bioavailability? Unveiling the hidden association-A review. Trends Food Sci. Technol. 2021, 110, 584–590. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- Wei, B.; Xia, W.; Wang, L.; Jin, X.; Yang, W.; Rao, D.; Chen, S.; Wu, J. Diverse prebiotic effects of isomaltodextrins with different glycosidic linkages and molecular weights on human gut bacteria in vitro. Carbohydr. Polym. 2022, 279, 118986. [Google Scholar] [CrossRef]
- Morshedi, M.; Saghafi-Asl, M.; Hosseinifard, E.S. The potential therapeutic effects of the gut microbiome manipulation by synbiotic containing-Lactobacillus plantarum on neuropsychological performance of diabetic rats. J. Transl. Med. 2020, 18, 1–14. [Google Scholar] [CrossRef]
- Hijova, E.; Strojn, L.; Bertková, I.; Bomba, A. Dietary Lactobacillus plantarum LS/07 and inulin in the management of chronic disease risk factors. Acta Biochim. Pol. 2020, 67, 547–551. [Google Scholar] [CrossRef]
- Rivera-Huerta, M.; Lizárraga-Grimes, V.L.; Castro-Torres, I.G.; Tinoco-Méndez, M.; Macías-Rosales, L.; Sánchez-Bartéz, F.; Tapia-Pérez, G.G.; Romero-Romero, L.; Gracia-Mora, M.I. Functional Effects of Prebiotic Fructans in Colon Cancer and Calcium Metabolism in Animal Models. BioMed Res. Int. 2017, 2017, 9758982. [Google Scholar] [CrossRef]
- Liu, J.R.; Nakamura, A.; Corredig, M. Addition of Pectin and Soy Soluble Polysaccharide Affects the Particle Size Distribution of Casein Suspensions Prepared from Acidified Skim Milk. J. Agric. Food Chem. 2006, 54, 6241–6246. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Poletto, G.; Deus, C.D.; Codevilla, C.F.; Menezes, C.R.D. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res. Int. 2020, 130, 108902. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Erasmus, S.W.; Zhao, S.; Wang, Q.; van Ruth, S.M. An explorative study on the relationships between the quality traits of peanut varieties and their peanut butters. LWT 2021, 151, 112068. [Google Scholar] [CrossRef]
- Sun, R.; Lv, Y.; Zhang, X.; Zhao, J.; Qian, Z.; Lan, Q.; Wang, Z.; He, F.; Liu, T. Silicification-interlayered nanofiber substrates regulated crumpled ultrathin polyamide nanofilms for highly enhanced nanofiltration. J. Membr. Sci. 2023, 672, 121476. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein–polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, K.; Samara, N. The Association Between Peanut and Peanut Butter Consumption and Cognitive Function. Curr. Dev. Nutr. 2021, 5, 26. [Google Scholar] [CrossRef]
- Kozowicz, K.; Góral, M.; Góral, D.; Pankiewicz, U.; Bronowicka-Mielniczuk, U. Effect of ice cream storage on the physicochemical properties and survival of probiotic bacteria supplemented with zinc ions. LWT-Food Sci. Technol. 2019, 116, 108562. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Xu, F. Versatile Functionalization of Polysaccharides via Polymer Grafts: From Design to Biomedical Applications. Acc. Chem. Res. 2017, 50, 281. [Google Scholar] [CrossRef]
- Qin, X.-S.; Gao, Q.-Y.; Luo, Z.-G. Enhancing the storage and gastrointestinal passage viability of probiotic powder (Lactobacillus Plantarum) through encapsulation with pickering high internal phase emulsions stabilized with WPI-EGCG covalent conjugate nanoparticles. Food Hydrocoll. 2021, 116, 106658. [Google Scholar] [CrossRef]
- Weinbreck, F. Evidence and characterization of complex coacervates containing plant proteins: Application to the microencapsulation of oil droplets. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 239–247. [Google Scholar]
- Zhang, D.; Shen, D.; Cao, Y.; Duan, X.; Sun, H. Widely targeted metabolomic approach reveals dynamic changes in non-volatile and volatile metabolites of peanuts during roasting. Food Chem. 2023, 412, 135577. [Google Scholar] [CrossRef]





| Group | Energy J/100 g | Protein g/100 g | Lipid g/100 g | Carbohydrates g/100 g | Sodium mg/100 g |
|---|---|---|---|---|---|
| KB | 2527 ± 125 | 21.5 ± 3.5 | 46.6 ± 7.2 | 25.7 ± 3.1 | 293 ± 21 |
| FB | 2592 ± 137 | 21.6 ± 2.7 | 49.6 ± 6.9 | 22.9 ± 2.7 | 287 ± 27 |
| A | 2584 ± 164 | 21.5 ± 3.2 | 50 ± 5.9 | 21.7 ± 2.8 | 302 ± 32 |
| B | 2583 ± 143 | 21.5 ± 2.9 | 49.5 ± 6.2 | 22.7 ± 2.5 | 291 ± 43 |
| Group | Elastic Index | Hardness/g | Gumminess/g | Viscosity/mJ |
|---|---|---|---|---|
| KB | 0.97 ± 0.01 a | 44.5667 ± 2.54 a | 30.5333 ± 4.02 a | 2.7100 ± 0.12 a |
| FB | 0.97 ± 0.03 a | 40.5667 ± 0.57 b | 30.9 ± 1.47 a | 2.3867 ± 0.21 ab |
| A | 0.97 ± 0.03 a | 38.3333 ±0.65 bc | 36.3333 ± 1.42 a | 2.1500 ± 0.04 b |
| B | 0.9767 ± 0.03 a | 36.3333 ± 0.81 c | 27.3667 ± 2.99 a | 2.1667 ± 0.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Tian, Y.; Zhang, L.; Yu, M. Comparative Evaluation of Inulin and High-Ester Pectin for Microencapsulation of Bacillus coagulans TBC-169: Characterization and Probiotic Application in Peanut Butter Formulation. Foods 2025, 14, 2151. https://doi.org/10.3390/foods14132151
Xie M, Tian Y, Zhang L, Yu M. Comparative Evaluation of Inulin and High-Ester Pectin for Microencapsulation of Bacillus coagulans TBC-169: Characterization and Probiotic Application in Peanut Butter Formulation. Foods. 2025; 14(13):2151. https://doi.org/10.3390/foods14132151
Chicago/Turabian StyleXie, Mengxi, Yuan Tian, Liangchen Zhang, and Miao Yu. 2025. "Comparative Evaluation of Inulin and High-Ester Pectin for Microencapsulation of Bacillus coagulans TBC-169: Characterization and Probiotic Application in Peanut Butter Formulation" Foods 14, no. 13: 2151. https://doi.org/10.3390/foods14132151
APA StyleXie, M., Tian, Y., Zhang, L., & Yu, M. (2025). Comparative Evaluation of Inulin and High-Ester Pectin for Microencapsulation of Bacillus coagulans TBC-169: Characterization and Probiotic Application in Peanut Butter Formulation. Foods, 14(13), 2151. https://doi.org/10.3390/foods14132151






