The Role of Hydroxyl Modification of Peptidoglycan to Reduce the TTX Toxicity via Superior Absorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydroxyl-Modified PG
2.3. Determination of Carboxyl Group Content of PGs and HM-PGs
2.4. Structural and Property Characterization of HM-PGs
2.5. Fluorescence Imaging of HM-PGs Treated with TTX Samples
2.6. Quantification and Toxicity Analysis of TTX Samples
2.7. Determination of Intracellular Na+ Concentration Based on SH-SY5Y Cell Model
2.8. Prediction of Action Mode Between HM-PG and TTX
2.9. Analysis of Hydrophobicity and Toxicity Changes of HM-PGs
2.10. Statistical Analysis
3. Results and Discussion
3.1. Condition Optimization of HM-PG Preparation
3.2. Effect of Hydroxyl Modification on Structure of Different PGs
3.3. Effect of Hydroxyl Modification on Physicochemical Properties of PGs
3.4. Effect of HM-PG Treatment on the Adsorption Efficient of TTX
3.4.1. The Influence of HM-PGs on the Removal Effect of TTX
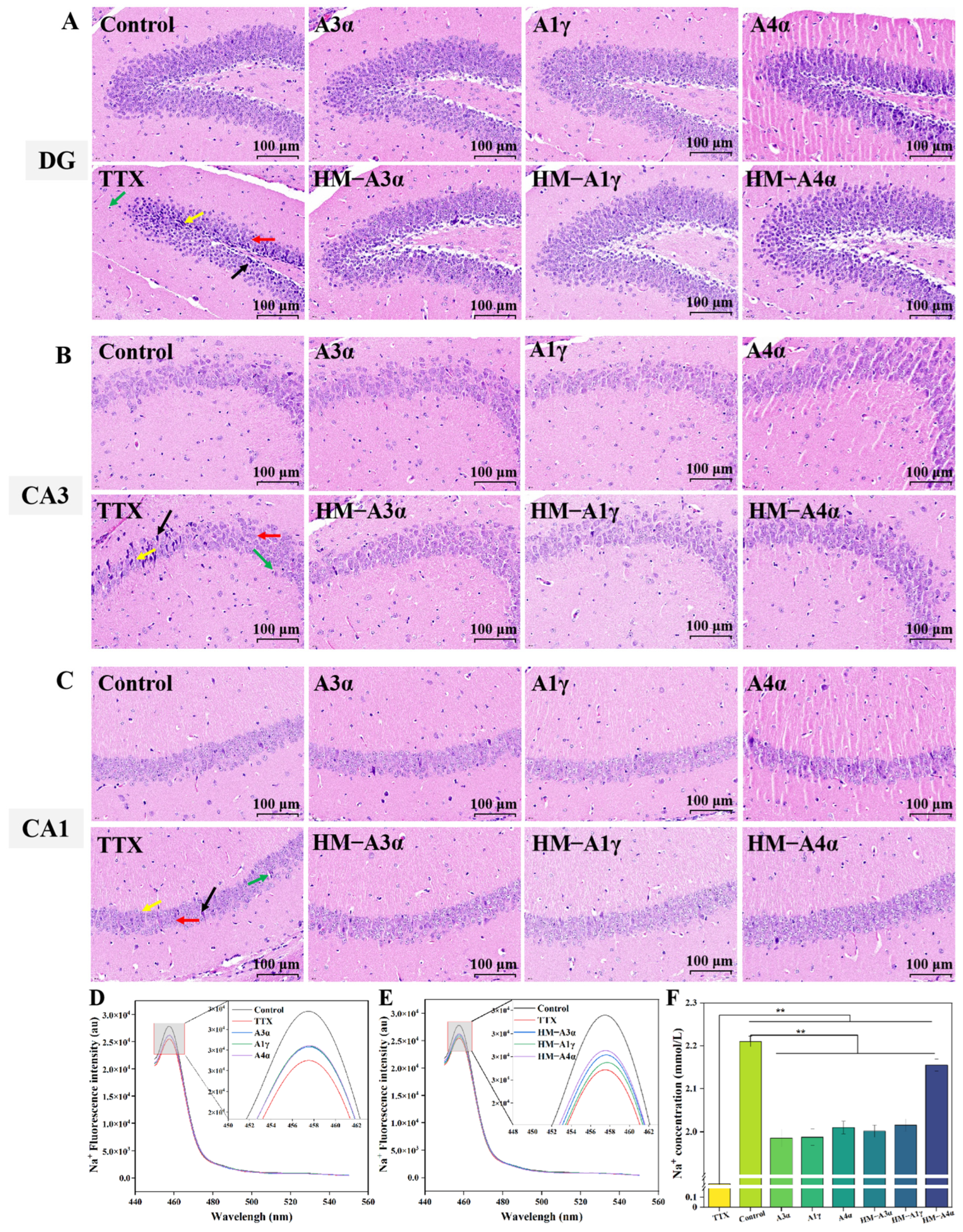
3.4.2. The Reduction Effect of HM-PGs on the Neurotoxicity of TTX
3.5. Effect of Food Matrix on the Adsorption Capacity of TTX by HM-PGs
3.6. Analysis of Binding Mechanism Between HM-PGs and TTX
3.6.1. Prediction of Binding Interaction and Mode Between HM-PG and TTX Based on Molecular Docking
3.6.2. Validation of Binding Forces Between HM-PGs and TTX
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TTX | Tetrodotoxin |
| PG | Peptidoglycan |
| LAB | Lactic acid bacteria |
| VGSCs | Voltage-gated sodium channels |
| HM-PGs | Hydroxyl-modified PGs |
| SEM-EDS | Scanning electron microscopy energy dispersive spectroscopy |
| AFM | Atomic force microscopy |
References
- Zhou, H.; Zhuang, Z.-X.; Sun, Y.-Q.; Chen, Q.; Zheng, X.-Y.; Liang, Y.-T.; Mahboob, S.; Wang, Q.; Zhang, R.; Al-Ghanim, K.A.; et al. Changes in DNA Methylation during Epigenetic-Associated Sex Reversal under Low Temperature in Takifugu rubripes. PLoS ONE 2019, 14, e0221641. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shi, Z.; Zhang, T.; Wang, L.; Dong, R.; Zhang, Y.; Sun, X. Highly Sensitive and Quantitative Fluorescent Strip Immunosensor Based on an Independent Control System for Rapid Detection of Tetrodotoxin in Shellfish. Food Control. 2023, 145, 109403. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, Y.; Gong, Q.-L.; Zhou, L.; Wang, Z.-P. Toxicity of Cultured Puffer Fish and Seasonal Variations in China. Aquac. Res. 2011, 42, 1186–1195. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebesu, J.S.M. Puffer Poisoning: Epidemiology and Treatment. J. Toxicol. Toxin Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- Notice on the Conditional Liberalization of Processing and Operation of Farmed Tiger Puffer and Farmed Obscure Puffer. Available online: https://www.moa.gov.cn/nybgb/2016/dishiqi/201711/t20171126_5919593.htm (accessed on 29 April 2025).
- Fuchi, Y.; Noguchi, T.; Saito, T.; Morisaki, S.; Nakama, S.; Shimazaki, K.; Hayashi, K.; Ohtomo, N.; Hashimoto, K. Mechanisms Involved in the Detoxification of Pufferfish Liver during the Traditional Cooking. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1988, 29, 320–324_1. [Google Scholar] [CrossRef]
- Tu, N.H.K.; Dat, N.V.; Canh, L.V.; Vinh, D.T.T. Detection of the Potential Inactivation of Tetrodotoxin by Lactic Acid Bacterial Exopolysaccharide. Toxins 2018, 10, 288. [Google Scholar] [CrossRef]
- Anraku, K.; Nonaka, K.; Yamaga, T.; Yamamoto, T.; Shin, M.-C.; Wakita, M.; Hamamoto, A.; Akaike, N. Removal of Toxin (Tetrodotoxin) from Puffer Ovary by Traditional Fermentation. Toxins 2013, 5, 193–202. [Google Scholar] [CrossRef]
- Alkassar, M.; Reverté, J.; Fragoso, A.; Torréns, M.; Klijnstra, M.; Gerssen, A.; Diogène, J.; Campàs, M. β-Cyclodextrin Polymer as Tetrodotoxins Scavenger in Oyster Extracts. Microchem. J. 2024, 201, 110585. [Google Scholar] [CrossRef]
- Wang, D.; Sun, L.; Shen, W.-T.; Haggard, A.; Yu, Y.; Zhang, J.A.; Fang, R.H.; Gao, W.; Zhang, L. Neuronal Membrane-Derived Nanodiscs for Broad-Spectrum Neurotoxin Detoxification. ACS Nano 2024, 18, 25069–25080. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, Cellulose, Pectin, Gum, Alginate, Chitin and Chitosan Derived (Nano)Materials for Sustainable Water Treatment: A Review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, R.; Yue, T.; Yuan, Y.; Gao, Z.; Wang, Z. Assessment of Traditional Clarifiers on the Adsorption of Ochratoxin A in Cabernet Sauvignon Red Wine and Their Kinetics. Food Chem. 2022, 373, 131592. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Nan, M.; Bi, Y.; Xue, H.; Lyu, L.; Jiang, D.; Chen, H.; Luo, Q. Removal Capacity and Mechanism of Modified Chitosan for Ochratoxin A Based on Rapid Magnetic Separation Technology. Foods 2025, 14, 666. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Tian, L.; Li, Y.; He, G.; Yu, H.; Qi, H.; Sun, W. Removal Effect and Mechanism of Amphiphilic Chitosan Modified Microbubbles on Microcystis Aeruginosa. J. Water Process Eng. 2022, 46, 102585. [Google Scholar] [CrossRef]
- Liang, J.; He, Q.; Zhao, Y.; Yuan, Y.; Wang, Z.; Gao, Z.; Hu, Z.; Zhao, X.; Yue, T. Synthesis of Sulfhydryl Modified Bacterial Cellulose Gel Membrane and Its Application in Adsorption of Patulin from Apple Juice. LWT 2022, 158, 113159. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Duan, C.; Sun, J.; Xu, Y. Preparation and Characterization of Cellulose-Based Adsorbent and Its Application in Heavy Metal Ions Removal. Carbohydr. Polym. 2019, 206, 837–843. [Google Scholar] [CrossRef]
- Yang, H.; Singh, M.; Kim, S.J.; Schaefer, J. Characterization of the Tertiary Structure of the Peptidoglycan of Enterococcus faecalis. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 2171–2180. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, G.; Ding, S.; Jiang, Y.; Jiang, B.; Ren, D.; Chen, P. Lactobacillus plantarum T3 as an Adsorbent of Aflatoxin B1 Effectively Mitigates the Toxic Effects on Mice. Food Biosci. 2022, 49, 101984. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Fan, Y.; Yang, F.; Li, M.; Sun, X.; Sun, E.; Jin, Y.; Zhao, L. The Adsorption of 2-Amino-1-Methyl-6-Phenyl-Imidazolium [4, 5-B] Pyridine (PhIP) by Lactic Acid Bacteria 37X-15 and Its Peptidoglycan. Food Chem. 2024, 440, 138193. [Google Scholar] [CrossRef]
- Liu, C.; Ye, J.; Wang, C.; Wang, H.; Lu, Y. Lactic Acid Bacteria Reduce the Toxicity of Tetrodotoxin through Peptidoglycan Mediated Binding. Aquac. Fish. 2025, 10, 421–428. [Google Scholar] [CrossRef]
- Gomez-Maldonado, D.; Filpponen, I.; Vega Erramuspe, I.B.; Johansson, L.-S.; Mori, M.F.; Babu, R.J.; Waters, M.N.; Peresin, M.S. Development of a β-Cyclodextrin-Chitosan Polymer as Active Coating for Cellulosic Surfaces and Capturing of Microcystin-LR. Surf. Interfaces 2022, 33, 102192. [Google Scholar] [CrossRef]
- Porfírio, S.; Carlson, R.W.; Azadi, P. Elucidating Peptidoglycan Structure: An Analytical Toolset. Trends Microbiol. 2019, 27, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ren, H.; Yan, Z.; Ma, J.; Feng, X.; Liu, D.; Long, F. Reusable Thiol-Modification Lactobacillus Plantarum Embedded in Cellulose Nanocrystals Composite Aerogel for Efficient Removal of Ochratoxin A in Grape Juice. Food Chem. X 2024, 22, 101336. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, J.; Xu, H.; Zhang, Y.; Mao, X.; Huang, W.-C. Characterization and Comparison of Carboxymethylation and TEMPO-Mediated Oxidation for Polysaccharides Modification. Int. J. Biol. Macromol. 2024, 256, 128322. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Zhang, Y.-N.; Guo, J.-Z.; Lv, J.-Q.; Huan, W.-W.; Li, B. Preparation of Hydrochar with High Adsorption Performance for Methylene Blue by Co-Hydrothermal Carbonization of Polyvinyl Chloride and Bamboo. Bioresour. Technol. 2021, 337, 125442. [Google Scholar] [CrossRef]
- Lu, J.; Luan, H.; Wang, C.; Zhang, L.; Shi, W.; Xu, S.; Jin, Y.; Lu, Y. Molecular and Allergenic Properties of Natural Hemocyanin from Chinese Mitten Crab (Eriocheir sinensis). Food Chem. 2023, 424, 136422. [Google Scholar] [CrossRef]
- GB 5009.206-2016; National Food Safety Standard Determination of Tetrodotoxin in Aquatic Products. China Food and Drug Administration: Beijing, China, 2016.
- Geffeney, S.L.; Cordingley, J.A.; Mitchell, K.; Hanifin, C.T. In Silico Analysis of Tetrodotoxin Binding in Voltage-Gated Sodium Ion Channels from Toxin-Resistant Animal Lineages. Mar. Drugs 2022, 20, 723. [Google Scholar] [CrossRef]
- Ansari, M.A.; Asiri, S.M.M. Green Synthesis, Antimicrobial, Antibiofilm and Antitumor Activities of Superparamagnetic γ-Fe2O3 NPs and Their Molecular Docking Study with Cell Wall Mannoproteins and Peptidoglycan. Int. J. Biol. Macromol. 2021, 171, 44–58. [Google Scholar] [CrossRef]
- Santos, E.S.; Silva, P.C.; Sousa, P.S.A.; Aquino, C.C.; Pacheco, G.; Teixeira, L.F.L.S.; Araujo, A.R.; Sousa, F.B.M.; Barros, R.O.; Ramos, R.M.; et al. Antiviral Potential of Diminazene Aceturate against SARS-CoV-2 Proteases Using Computational and in Vitro Approaches. Chem.-Biol. Interact. 2022, 367, 110161. [Google Scholar] [CrossRef]
- Bursch, M.; Neugebauer, H.; Grimme, S. Structure Optimisation of Large Transition-Metal Complexes with Extended Tight-Binding Methods. Angew. Chem. Int. Ed. 2019, 58, 11078–11087. [Google Scholar] [CrossRef]
- Wróbel, P.; Eilmes, A. Effects of Me–Solvent Interactions on the Structure and Infrared Spectra of MeTFSI (Me = Li, Na) Solutions in Carbonate Solvents—A Test of the GFN2-xTB Approach in Molecular Dynamics Simulations. Molecules 2023, 28, 6736. [Google Scholar] [CrossRef]
- McPherson, J.N.; Elton, T.E.; Colbran, S.B. A Strain-Deformation Nexus within Pincer Ligands: Application to the Spin States of Iron(II) Complexes. Inorg. Chem. 2018, 57, 12312–12322. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H.; Kandler, O. Peptidoglycan Types of Bacterial Cell Walls and Their Taxonomic Implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Asong, J.; Wolfert, M.A.; Maiti, K.K.; Miller, D.; Boons, G.-J. Binding and Cellular Activation Studies Reveal That Toll-like Receptor 2 Can Differentially Recognize Peptidoglycan from Gram-Positive and Gram-Negative Bacteria. J. Biol. Chem. 2009, 284, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Qin, C.; Tong, Z.; Kong, S.; Jia, Z.; Wan, G.; Liu, X. Mechanism and Kinetics Studies of Carboxyl Group Formation on the Surface of Cellulose Fiber in a TEMPO-Mediated System. Cellulose 2017, 24, 2415–2425. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Bhat, A.H.; Ahmad, F. Polymer Blend of PLA/PHBV Based Bionanocomposites Reinforced with Nanocrystalline Cellulose for Potential Application as Packaging Material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef]
- Jia, Y.; Zhai, X.; Fu, W.; Liu, Y.; Li, F.; Zhong, C. Surfactant-Free Emulsions Stabilized by Tempo-Oxidized Bacterial Cellulose. Carbohydr. Polym. 2016, 151, 907–915. [Google Scholar] [CrossRef]
- Jia, X.; Ma, P.; Taylor, K.S.-Y.; Tarwa, K.; Mao, Y.; Wang, Q. Development of Stable Pickering Emulsions with TEMPO-Oxidized Chitin Nanocrystals for Encapsulation of Quercetin. Foods 2023, 12, 367. [Google Scholar] [CrossRef]
- Mota, L.O.; Almeida, Y.A.; de Bispo, D.F.; Souza, M.F.F.; Santos, D.C.; Sobrinho, R.A.L.; Gimenez, I.F. Preparation of TEMPO-Oxidized Cellulose Hydrogels Modified with β-Cyclodextrin and κ-Carrageenan for Potential Adsorption Applications. ACS Omega 2025, 10, 972–984. [Google Scholar] [CrossRef]
- Ahrari, A.; Meseke, M.; Förster, E. Tetrodotoxin Prevents Heat-Shock Induced Granule Cell Dispersion in Hippocampal Slice Cultures. Front. Cell Dev. Biol. 2022, 10, 906262. [Google Scholar] [CrossRef]
- Buckmaster, P.S. Prolonged Infusion of Tetrodotoxin Does Not Block Mossy Fiber Sprouting in Pilocarpine-Treated Rats. Epilepsia 2004, 45, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Aranda, L. Expression of C-Fos Protein in Medial Septum/Diagonal Band of Broca and CA3 Region, Associated with the Temporary Inactivation of the Supramammillary Area. J. Chem. Neuroanat. 2016, 74, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Chiba, Y.; Wakamori, M.; Yamada, T.; Tsunogae, S.; Cho, Y.; Sakakibara, R.; Imazu, T.; Tokoro, S.; Satake, Y.; et al. Differential Binding of Tetrodotoxin and Its Derivatives to Voltage-Sensitive Sodium Channel Subtypes (Nav1.1 to Nav1.7). Br. J. Pharmacol. 2017, 174, 3881–3892. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Mozar, C.A.; Durek, T.; Wingerd, J.S.; Alewood, P.F.; Christie, M.J.; Lewis, R.J. Characterisation of Nav Types Endogenously Expressed in Human SH-SY5Y Neuroblastoma Cells. Biochem. Pharmacol. 2012, 83, 1562–1571. [Google Scholar] [CrossRef]
- Ngum, N.M.; Aziz, M.Y.A.; Mohammed Latif, L.; Wall, R.J.; Duce, I.R.; Mellor, I.R. Non-Canonical Endogenous Expression of Voltage-Gated Sodium Channel NaV1.7 Subtype by the TE671 Rhabdomyosarcoma Cell Line. J. Physiol. 2022, 600, 2499–2513. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, L.; Zhang, T.; Ren, J.; Cheng, W.; Zhang, L.; Meng, P. Adsorption Behavior of Cd (II) on TEMPO-Oxidized Cellulose in Inorganic/Organic Complex Systems. Environ. Res. 2021, 195, 110848. [Google Scholar] [CrossRef]
- Chen, J.; Han, K.; Su, L.; Zhang, L.; Wang, L.; Lian, J.; Xing, L.; Majumder, A.; Lu, J. Enhanced Removal of Methylene Blue by 2-(2,4,5-Tricarboxybenzyloxy)-1,2,3-Propanetricarboxylic Acid Modified Cellulose. Int. J. Biol. Macromol. 2024, 282, 136909. [Google Scholar] [CrossRef]
- Bridgeman, A.J.; Cavigliasso, G.; Ireland, L.R.; Rothery, J. The Mayer Bond Order as a Tool in Inorganic Chemistry. J. Chem. Soc. Dalton Trans. 2001, 14, 2095–2108. [Google Scholar] [CrossRef]
- Amen, R.; Elsayed, I.; Schueneman, G.T.; Hassan, E.B. Self-Assembled Aminated and TEMPO Cellulose Nanofibers (Am/TEMPO-CNF) Aerogel for Adsorptive Removal of Oxytetracycline and Chloramphenicol Antibiotics from Water. Gels 2024, 10, 77. [Google Scholar] [CrossRef]
- Liu, P.; Oksman, K.; Mathew, A.P. Surface Adsorption and Self-Assembly of Cu(II) Ions on TEMPO-Oxidized Cellulose Nanofibers in Aqueous Media. J. Colloid Interface Sci. 2016, 464, 175–182. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Zhao, X.; Sun, H.; Shao, M.; Xu, H. In Vitro Adsorption Mechanism of Acrylamide by Lactic Acid Bacteria. LWT 2019, 100, 119–125. [Google Scholar] [CrossRef]
- Goyal, S.P.; Agarwal, T.; Mishra, V.; Kumar, A.; Saravanan, C. Adsorption Characterization of Lactobacillus sp. for Di-(2-Ethylhexyl) Phthalate. Probiotics Antimicrob Proteins 2024, 16, 519–530. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Guo, Y.; Zhang, L.; Miao, J.; Lu, Y. The Role of Hydroxyl Modification of Peptidoglycan to Reduce the TTX Toxicity via Superior Absorption. Foods 2025, 14, 2145. https://doi.org/10.3390/foods14122145
Wang C, Guo Y, Zhang L, Miao J, Lu Y. The Role of Hydroxyl Modification of Peptidoglycan to Reduce the TTX Toxicity via Superior Absorption. Foods. 2025; 14(12):2145. https://doi.org/10.3390/foods14122145
Chicago/Turabian StyleWang, Chang’e, Yi Guo, Lili Zhang, Junjian Miao, and Ying Lu. 2025. "The Role of Hydroxyl Modification of Peptidoglycan to Reduce the TTX Toxicity via Superior Absorption" Foods 14, no. 12: 2145. https://doi.org/10.3390/foods14122145
APA StyleWang, C., Guo, Y., Zhang, L., Miao, J., & Lu, Y. (2025). The Role of Hydroxyl Modification of Peptidoglycan to Reduce the TTX Toxicity via Superior Absorption. Foods, 14(12), 2145. https://doi.org/10.3390/foods14122145






