Germination-Induced Changes in the Nutritional, Bioactive, and Digestive Properties of Lima Bean (Phaseolus lunatus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Germination Process of Lima Bean
2.3. Visual Characteristics of Germinated Lima Bean
2.4. Compositions of Germinated Lima Bean
2.4.1. Protein Concentration (PC)
2.4.2. Total Carbohydrate Content (TCC)
2.4.3. Total Polyphenol Content (TPC)
2.4.4. Total Flavonoid Content (TFC)
2.5. Antinutrient Content
2.5.1. Tannic Content (TC)
2.5.2. Phytic Acid Content (PAC)
2.6. Antioxidant Activities
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS Radical Scavenging Activity
2.6.3. Reducing Power (RP)
2.7. In Vitro Limited Proteolysis Profile
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.8. Statistical Analyses
3. Results and Discussion
3.1. Visual Characteristics of Germinated Lima Bean
3.2. Compositions of Germinated Lima Bean
3.2.1. Protein Concentration (PC)
3.2.2. Total Carbohydrate Content (TCC)
3.2.3. Total Polyphenol Content and Total Flavonoid Content (TPC and TFC)
3.3. Antioxidant Activities
3.4. Anti-Nutritional Components (ANFs)
3.5. Non-Reducing SDS-PAGE
3.6. Digestibility of Germinated Lima Bean
3.7. Heat Map Representing the Pearson Correlation Between All Variables
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| G0-G72 | Germinated lima beans during 0, 12, 24, 36, 48, 60, and 72 h |
| D0-D72 (1h–4h) | In vitro limited proteolysis with 1–4 h |
| PC | Protein concentration |
| TCC | Total carbohydrate content |
| TPC | Total polyphenol content |
| TFC | Total flavonoid content |
| TC | Tannin content |
| PAC | Phytic acid content |
| RP | Reducing power |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| ANFs | Antinutritional factors |
References
- Maphosa, Y.; Jideani, V.A. The Role of Legumes in Human Nutrition. In Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Adebo, J.A. A Review on the Potential Food Application of Lima Beans (Phaseolus lunatus L.), an Underutilized Crop. Appl. Sci. 2023, 13, 1996. [Google Scholar] [CrossRef]
- Popoola, J.O.; Ojuederie, O.; Omonhinmin, C.; Adegbite, A. Neglected and Underutilized Legume Crops: Improvement and Future Prospects. In Recent Advances in Grain Crops Research; IntechOpen: London, UK, 2019. [Google Scholar]
- Ogwu, M.; Osawaru, M.; Aiwansoba, R.; Iroh, R. Status and prospects of vegetables in Africa. Status and prospects of vegetables in Africa. In Proceedings of the Joint Biodiversity Conservation Conference of Nigeria Tropical Biology Association and Nigeria Chapter of Society for Conservation Biology on MDGs to SDGs: Toward Sustainable Biodiversity Conservation in Nigeria; Borokini, I.T., Babalola, F.D., Eds.; University of Ilorin: Ilorin, Nigeria, 2016; pp. 47–57. [Google Scholar]
- Chel-Guerrero, L.; Pérez-Flores, V.; Betancur-Ancona, D.; Dávila-Ortiz, G. Functional Properties of Flours and Protein Isolates from Phaseolus lunatus and Canavalia ensiformis Seeds. J. Agric. Food Chem. 2002, 50, 584–591. [Google Scholar] [CrossRef]
- Holland, B.; McCance, R.A.; Widdowson, E.M.; Unwin, I.; Buss, D. Vegetables, Herbs and Spices: Fifth Supplement to McCance and Widdowson’s The Composition of Foods; Royal Society of Chemistry: London, UK, 1991; Volume 5. [Google Scholar]
- Agostini-Costa, T.d.S.; Teodoro, A.F.P.; Alves, R.d.B.d.N.; Braga, L.R.; Ribeiro, I.F.; Silva, J.P.; Quintana, L.G.; Burle, M.L. Total phenolics, flavonoids, tannins and antioxidant activity of lima beans conserved in a Brazilian Genebank. Cienc. Rural. 2014, 45, 335–341. [Google Scholar] [CrossRef]
- Yellavila, S.; Agbenorhevi, J.K.; Asibuo, J.; Sampson, G. Proximate Composition, Minerals Content and Functional Properties of Five Lima Bean Accessions. 2015. Available online: http://pubs.sciepub.com/jfs/3/3/1 (accessed on 25 January 2025).
- El-Gohery, S.S. Effect of Different Treatments on Nutritional Value of Lima Bean (Phaseolus lunatus) and Its Utilization in Biscuit Manufacture. Food Nutr. Sci. 2021, 12, 372–391. [Google Scholar] [CrossRef]
- Farinde, E.O.; Adeniran, H.A.; Abiose, S.H. Comparative Microbial Assessment of Fermented Lima Bean (Phaseolus lunatus) and Locust Bean (Parkia biglobossa) in Production of Daddawa. Br. Microbiol. Res. J. 2014, 4, 772–784. [Google Scholar] [CrossRef]
- Adebayo, S. Effect of soaking time on the proximate, mineral compositions and anti-nutritional factors of lima bean. Food Sci. Qual. Manag. 2014, 27, 2224–6088. [Google Scholar]
- Adeparusi, E. Effect of processing on the nutrients and anti-nutrients of lima bean (Phaseolus lunatus L.) flour. Food/Nahrung 2001, 45, 94–96. [Google Scholar] [CrossRef]
- Televičiūtė, D.; Tarasevičienė, Ž.; Danilčenko, H.; Barčauskaitė, K.; Kandaraitė, M.; Paulauskienė, A. Changes in chemical composition of germinated leguminous under abiotic stress conditions. Food Sci. Technol. 2020, 40, 415–421. [Google Scholar] [CrossRef]
- Sharma, S.; Sahni, P. Dynamics of Germination Behaviour, Protein Secondary Structure, Technofunctional Properties, Antinutrients, Antioxidant Capacity and Mineral Elements in Germinated Dhaincha. Food Technol. Biotechnol. 2021, 59, 238–250. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, N. Lima Bean, in Vegetable Crop Science; CRC Press: Boca Raton, FL, USA, 2017; pp. 701–714. [Google Scholar]
- Alves, A.U.; Dornelas, C.S.; Alves, E.U.; A Cardoso, E.; de Oliveira, A.N.P.; Cruz, I.d.S. Lima beans production and economic revenue as function of organic and mineral fertilization. Hortic. Bras. 2008, 26, 251–254. [Google Scholar] [CrossRef]
- Saleem, Z.M.; Ahmed, S.; Hasan, M.M. Phaseolus lunatus linn: Botany, medicinal uses, phytochemistry and pharmacology. World J. Pharm. Pharm. Sci. 2016, 5, 87–93. [Google Scholar] [CrossRef]
- Rizvi, Q.U.E.H.; Guiné, R.P.F.; Ahmed, N.; Sheikh, M.A.; Sharma, P.; Sheikh, I.; Yadav, A.N.; Kumar, K. Effects of Soaking and Germination Treatments on the Nutritional, Anti-Nutritional, and Bioactive Characteristics of Adzuki Beans (Vigna angularis L.) and Lima Beans (Phaseolus lunatus L.). Foods 2024, 13, 1422. [Google Scholar] [CrossRef] [PubMed]
- Farinde, E.O.; Olanipekun, O.T.; Olasupo, R.B. Nutritional composition and antinutrients content of raw and processed lima bean (Phaseolus lunatus). Ann. Food Sci. Technol. 2018, 19, 250–264. [Google Scholar] [CrossRef]
- Esquivel-Martínez, G.T.; Andueza-Noh, R.H.; Garruña, R.; Villanueva-Couoh, E.; Martínez-Castillo, J.; Díaz-Mayo, J.; Ruiz-Santiago, R.R.; Camacho-Pérez, E. Morphological differentiation and seed quality of Lima bean (Phaseolus lunatus L.). Genet. Resour. Crop Evol. 2024, 71, 69–81. [Google Scholar] [CrossRef]
- Amadeu, L.T.S.; Queiroz, A.J.d.M.; de Figueirêdo, R.M.F.; Paiva, Y.F.; Silva, E.T.d.V.; Ferreira, J.P.d.L.; dos Santos, F.S.; Moura, H.V.; Carvalho, R.d.O. Physicochemical and bioactive aspects of germination of lima bean seeds. Rev. Bras. Eng. Agric. E Ambient. 2025, 29, e287729. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook. Springer Protocols Handbooks; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Nielsen, S.S. Food Analysis; Springer Nature: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Echeverria-Jaramillo, E.; Shin, W. Effects of concentration methods on the characteristics of spray-dried black soybean cooking water. Int. J. Food Sci. Technol. 2022, 57, 7330–7339. [Google Scholar] [CrossRef]
- Lee, S.; Cho, J.-H.; Park, K.D.; Kim, Y.-D.; Yim, S.-H. Assessment of validation and antioxidant activities of novel 12 Korean strawberry cultivars. Food Sci. Technol. 2022, 42, e76121. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Lahare, R.P.; Yadav, H.S.; Bisen, Y.K.; Dashahre, A.K. Estimation of Total Phenol, Flavonoid, Tannin and Alkaloid Content in Different Extracts of Catharanthus roseus from Durg District, Chhattisgarh, India. Sch. Bull. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Peng, X.; Li, H.; Xu, W.; Yang, Q.; Li, D.; Fan, T.; Li, B.; Ding, J.; Ku, W.; Deng, D.; et al. The AtMINPP Gene, Encoding a Multiple Inositol Polyphosphate Phosphatase, Coordinates a Novel Crosstalk between Phytic Acid Metabolism and Ethylene Signal Transduction in Leaf Senescence. Int. J. Mol. Sci. 2024, 25, 8969. [Google Scholar] [CrossRef]
- EL Shafay, S.; El-Sheekh, M.; Bases, E.; El-Shenody, R. Antioxidant, antidiabetic, anti-inflammatory and anticancer potential of some seaweed extracts. Food Sci. Technol. 2022, 42, e20521. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.d.S.; Junior, H.B.d.S.; Guedes, T.J.F.L.; Sandes, R.D.D.; Rajan, M.; Neta, M.T.S.L.; Narain, N. Comparative analysis of fresh and processed mango (Mangifera indica L, cv. “Maria”) pulps: Influence of processing on the volatiles, bioactive compounds and antioxidant activity. Food Sci. Technol. 2022, 42, e54020. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W.; Zhang, J.; Zhang, H.; Li, J.; He, X.; Ma, H. Subunit, amino acid composition and in vitro digestibility of protein isolates from Chinese kabuli and desi chickpea (Cicer arietinum L.) cultivars. Food Res. Int. 2010, 43, 567–572. [Google Scholar] [CrossRef]
- Chavan, U.; McKenzie, D.; Shahidi, F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 2001, 74, 177–187. [Google Scholar] [CrossRef]
- Kim, M.-J.; Shin, W.-S. Structural and functional modification of proteins from black soybean Aquasoya via ultrasonication. Ultrason. Sonochem. 2022, 91, 106220. [Google Scholar] [CrossRef]
- Aguilera, Y.; Díaz, M.F.; Jiménez, T.; Benítez, V.; Herrera, T.; Cuadrado, C.; Martín-Pedrosa, M.; A Martín-Cabrejas, M. Changes in Nonnutritional Factors and Antioxidant Activity during Germination of Nonconventional Legumes. J. Agric. Food Chem. 2013, 61, 8120–8125. [Google Scholar] [CrossRef]
- Bainto-Ancheta, L.C.; Bunyameen, N.; Ogawa, Y. Young seedlings as potential protein sources: Changes during germination and in vitro digestion. LWT 2025, 223, 117713. [Google Scholar] [CrossRef]
- Admassu, S. Potential health benefits and problems associated with phytochemicals in food legumes. East Afr. J. Sci. 2010, 3, 116–133. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A.; Aguilera, Y.; Pedrosa, M.M.; Cuadrado, C.; Hernández, T.; Díaz, S.; Esteban, R.M. The impact of dehydration process on antinutrients and protein digestibility of some legume flours. Food Chem. 2009, 114, 1063–1068. [Google Scholar] [CrossRef]
- Rehman, Z.-U.; Shah, W. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Lekjing, S.; Venkatachalam, K. Effects of germination time and kilning temperature on the malting characteristics, biochemical and structural properties of HomChaiya rice. RSC Adv. 2020, 10, 16254–16265. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Rozan, P.; Lambein, F.; Frias, J.; Vidal-Valverde, C. Effects of different germination conditions on the contents of free protein and non-protein amino acids of commercial legumes. Food Chem. 2004, 86, 537–545. [Google Scholar] [CrossRef]
- Bautista-Expósito, S.; Peñas, E.; Vanderberg, A.; Frias, J.; Martínez-Villaluenga, C. Effect of Time and Legume Type on Germination-Induced Proteolysis of Lentils and Faba Beans. In Proceedings of the International Electronic Conference on Food Science and Functional Foods, Online, 10–25 November 2020; p. 4. [Google Scholar]
- Atudorei, D.; Stroe, S.-G.; Codină, G.G. Impact of Germination on the Microstructural and Physicochemical Properties of Different Legume Types. Plants 2021, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Agiang, M.; Umoh, I.; Essien, A.; Eteng, M. Nutrient Changes and Antinutrient Contents of Beniseed and Beniseed Soup during Cooking using a Nigerian Traditional Method. Pak. J. Biol. Sci. 2010, 13, 1011–1015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dueñas, M.; Hernández, T.; Estrella, I.; Fernández, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic Profiles and Antioxidant Activity of Germinated Legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Muñoz-Llandes, C.B.; Martínez-Villaluenga, C. Time maters: Exploring the dynamics of bioactive compounds content, bioaccessibility and antioxidant activity during Lupinus angustifolius germination. Food Res. Int. 2024, 187, 114426. [Google Scholar] [CrossRef]
- Wu, N.-N.; Li, R.; Li, Z.-J.; Tan, B. Effect of germination in the form of paddy rice and brown rice on their phytic acid, GABA, γ-oryzanol, phenolics, flavonoids and antioxidant capacity. Food Res. Int. 2022, 159, 111603. [Google Scholar] [CrossRef]
- Popoola, O.O. Phenolic compounds composition and in vitro antioxidant activity of Nigerian Amaranthus viridis seed as affected by autoclaving and germination. Meas. Food 2022, 6, 100028. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, S.; Rani, A.; Gulati, A.; Ahuja, P.S. Phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H) and catechins (flavan-3-ols) accumulation in tea. Funct. Integr. Genom. 2008, 9, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Nderitu, A.M.; Dykes, L.; Awika, J.M.; Minnaar, A.; Duodu, K.G. Phenolic composition and inhibitory effect against oxidative DNA damage of cooked cowpeas as affected by simulated in vitro gastrointestinal digestion. Food Chem. 2013, 141, 1763–1771. [Google Scholar] [CrossRef]
- Walter, M.; Marchesan, E. Phenolic compounds and antioxidant activity of rice. Braz. Arch. Biol. Technol. 2011, 54, 371–377. [Google Scholar] [CrossRef]
- Gamel, T.; Abdel-Aal, E.-S.M. Phenolic acids and antioxidant properties of barley wholegrain and pearling fractions. Agric. Food Sci. 2012, 21, 118–131. [Google Scholar] [CrossRef]
- Uchegbu, N.N.; Ishiwu, C.N. Germinated Pigeon Pea (Cajanus cajan): A novel diet for lowering oxidative stress and hyperglycemia. Food Sci. Nutr. 2016, 4, 772–777. [Google Scholar] [CrossRef]
- Wu, F.; Yang, N.; Touré, A.; Jin, Z.; Xu, X. Germinated Brown Rice and Its Role in Human Health. Crit. Rev. Food Sci. Nutr. 2013, 53, 451–463. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Bora, P. Anti-nutritional factors in foods and their effects. J. Acad. Ind. Res. 2014, 3, 285–290. [Google Scholar]
- Feizollahi, E.; Mirmahdi, R.S.; Zoghi, A.; Zijlstra, R.T.; Roopesh, M.; Vasanthan, T. Review of the beneficial and anti-nutritional qualities of phytic acid, and procedures for removing it from food products. Food Res. Int. 2021, 143, 110284. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Yasmin, A.; Zeb, A.; Khalil, A.W.; Paracha, G.M.-U.; Khattak, A.B. Effect of Processing on Anti-nutritional Factors of Red Kidney Bean (Phaseolus vulgaris) Grains. Food Bioprocess Technol. 2008, 1, 415–419. [Google Scholar] [CrossRef]
- Patterson, C.A.; Curran, J.; Der, T. Effect of Processing on Antinutrient Compounds in Pulses. Cereal Chem. 2016, 94, 2–10. [Google Scholar] [CrossRef]
- Olika, E.; Abera, S.; Fikre, A. Physicochemical Properties and Effect of Processing Methods on Mineral Composition and Antinutritional Factors of Improved Chickpea (Cicer arietinum L.) Varieties Grown in Ethiopia. Int. J. Food Sci. 2019, 2019, 9614570. [Google Scholar] [CrossRef]
- Dong, Q.; Saneoka, H. Physiological Characteristics, Phytase Activity, and Mineral Bioavailability of a Low-Phytate Soybean Line during Germination. Plant Foods Hum. Nutr. 2020, 75, 383–389. [Google Scholar] [CrossRef]
- Khattak, A.B.; Zeb, A.; Bibi, N.; Khalil, S.A.; Khattak, M.S. Influence of germination techniques on phytic acid and polyphenols content of chickpea (Cicer arietinum L.) sprouts. Food Chem. 2007, 104, 1074–1079. [Google Scholar] [CrossRef]
- Shimelis, E.A.; Rakshit, S.K. Effect of processing on antinutrients and in vitro protein digestibility of kidney bean (Phaseolus vulgaris L.) varieties grown in East Africa. Food Chem. 2007, 103, 161–172. [Google Scholar] [CrossRef]
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Jacinto-Hernández, C.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Dávila-Ortiz, G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012, 131, 1157–1164. [Google Scholar] [CrossRef]
- Chirinos, R.; Villasante-Bravo, N.; Aguilar-Galvez, A.; Figueroa-Merma, A.; Carpentier, S.; Pedreschi, R.; Campos, D. Antioxidant, antihypertensive and antidiabetic potential of peptidic fractions obtained from tarwi (Lupinus mutabilis) protein hydrolysate and identification of promising multifunctional bioactive peptides. Int. J. Food Sci. Technol. 2022, 57, 7402–7411. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.K.; Sharma, A.; Roy, R.; Kumar, D.; Bh, G.; Tripathi, A.; Chaudhari, B.P.; Das, M.; Jain, S.; et al. Phaseolin: A 47.5 kDa protein of red kidney bean (Phaseolus vulgaris L.) plays a pivotal role in hypersensitivity induction. Int. Immunopharmacol. 2014, 19, 178–190. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Karabulut, G.; Ozkan, G.; Yılmaz, H.; Gültekin-Subaşı, B.; Capanoglu, E. Allergenicity of Alternative Proteins: Reduction Mechanisms and Processing Strategies. J. Agric. Food Chem. 2025, 73, 7522–7546. [Google Scholar] [CrossRef]
- Montoya, C.A.; Leterme, P.; Victoria, N.F.; Toro, O.; Souffrant, W.B.; Beebe, S.; Lallès, J.-P. Susceptibility of Phaseolin to in Vitro Proteolysis Is Highly Variable across Common Bean Varieties (Phaseolus vulgaris). J. Agric. Food Chem. 2008, 56, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mora, P.; Frias, J.; Peñas, E.; Zieliński, H.; Giménez-Bastida, J.A.; Wiczkowski, W.; Zielińska, D.; Martínez-Villaluenga, C. Simultaneous release of peptides and phenolics with antioxidant, ACE-inhibitory and anti-inflammatory activities from pinto bean (Phaseolus vulgaris L. var. pinto) proteins by subtilisins. J. Funct. Foods 2015, 18, 319–332. [Google Scholar] [CrossRef]
- Zambrano, M.; Vásquez, G.; Morales, D.; Vilcacundo, R.; Carrillo, W. Isolation of baby lima bean (Phaseolus lunatus) proteins fractions and evaluation of their antioxidant activity. Ital. J. Food Sci. 2020, 32, 275–291. [Google Scholar]
- Tello, A.; Poveda, T.; Briceno, J.; Vasquez, G.; Del Pozo, F.; Morales, D. Micronutrients and In Vitro Protein Digestibility of Lima Beans (Phaseolus lunatus L.) Grown in Ecuador (P204-214). In Proceedings of the CICABI 2018, Ambato, Ecuador, 25–29 June 2018. [Google Scholar]
- Palupi, H.T.; Estiasih, T.; Yunianta; Sutrisno, A. Characterization of nutritional and functional properties of Lima bean flour (Phaseolus lunatus L.). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Online, 17–18 November 2021; p. 012033. [Google Scholar]
- Fu, Z.; Akula, S.; Thorpe, M.; Hellman, L. Marked difference in efficiency of the digestive enzymes pepsin, trypsin, chymotrypsin, and pancreatic elastase to cleave tightly folded proteins. Biol. Chem. 2021, 402, 861–867. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Singh, B. Characterization of in vitro antioxidant activity, bioactive components, and nutrient digestibility in pigeon pea (Cajanus cajan) as influenced by germination time and temperature. J. Food Biochem. 2019, 43, e12706. [Google Scholar] [CrossRef]
- Sinha, R.; Kawatra, A.; Sehgal, S. Saponin Content and Trypsin Inhibitor Activity of Cowpea: Varietal Differences and Effects of Processing and Cooking Methods. J. Food Sci. Technol. Mysore 2005, 42, 182–185. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20053120239 (accessed on 25 January 2025).

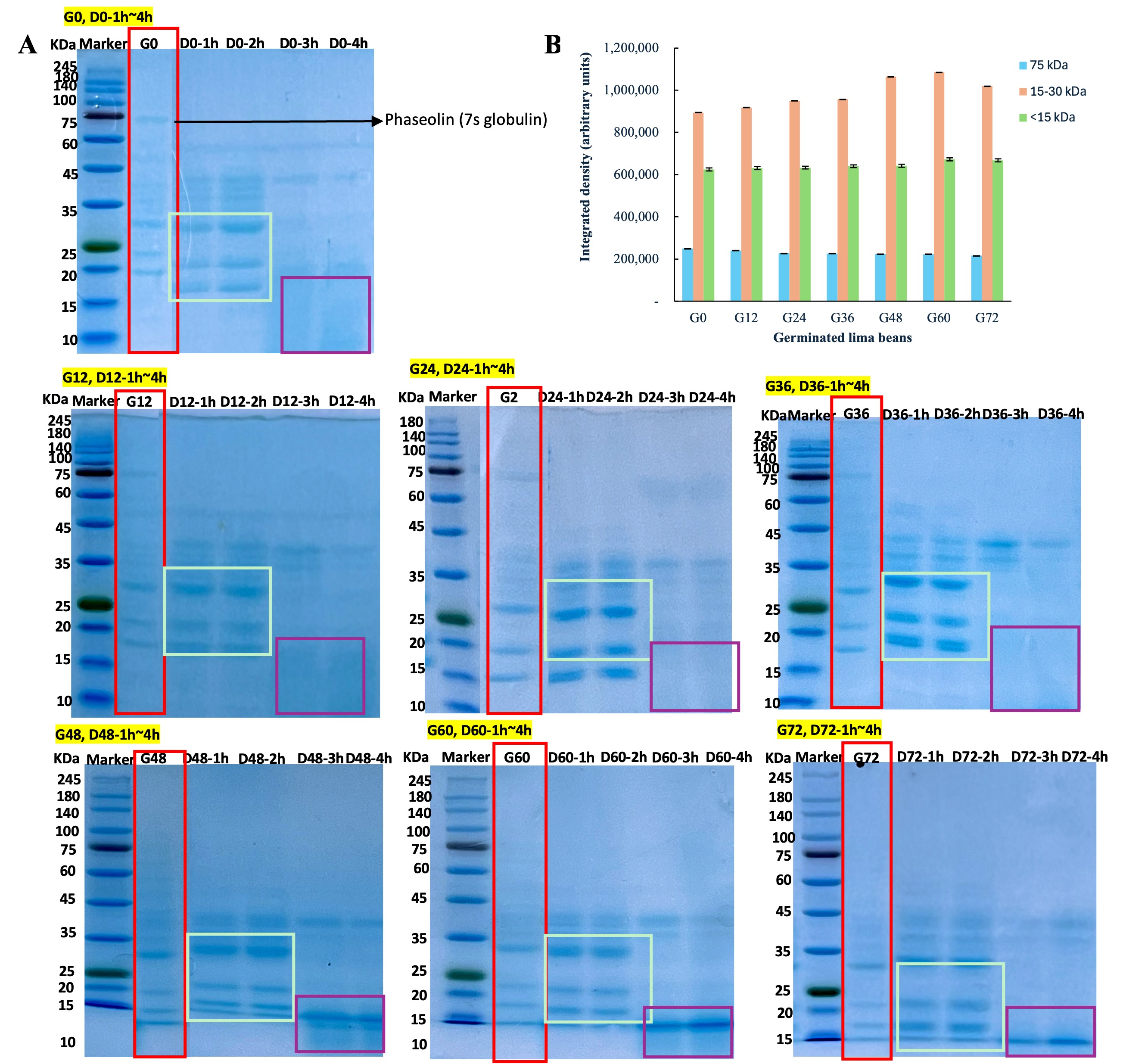
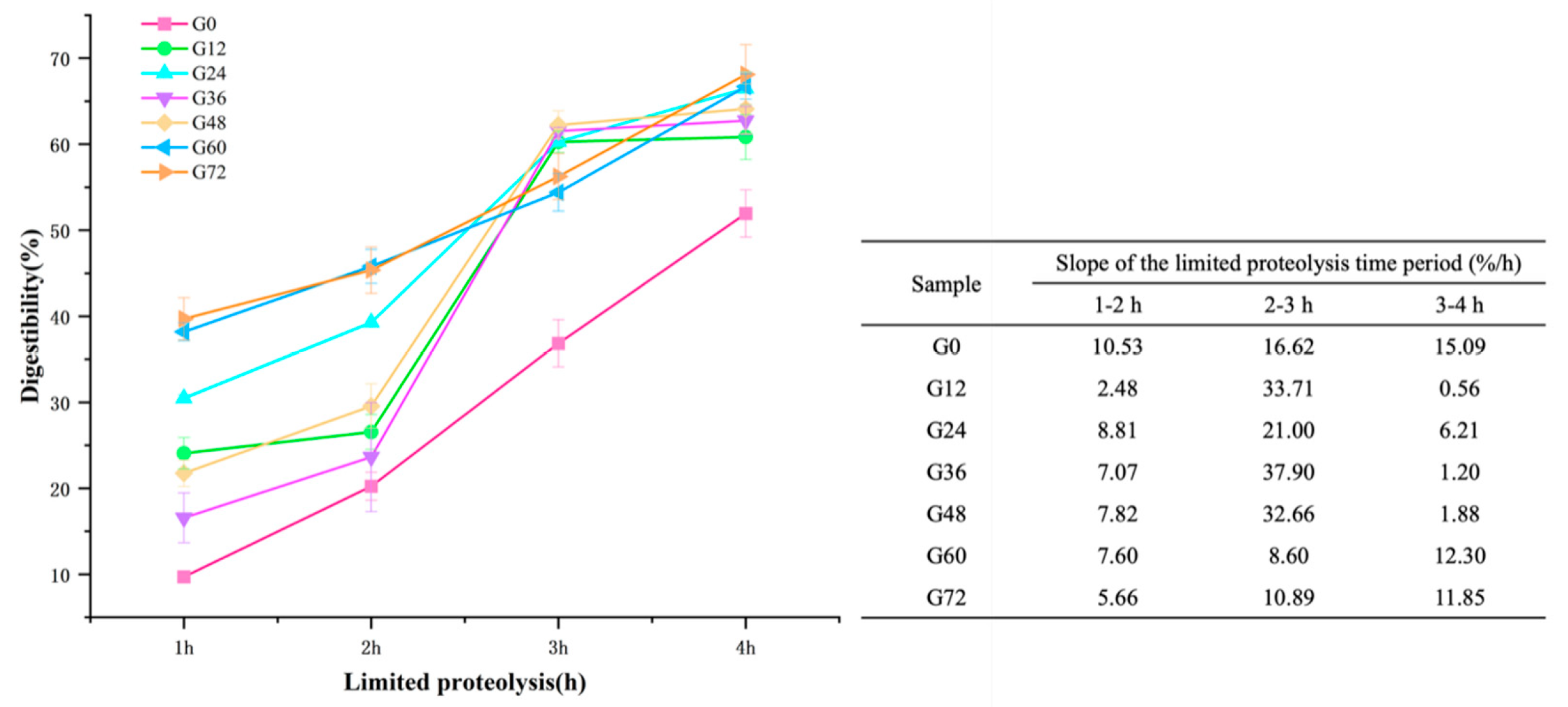
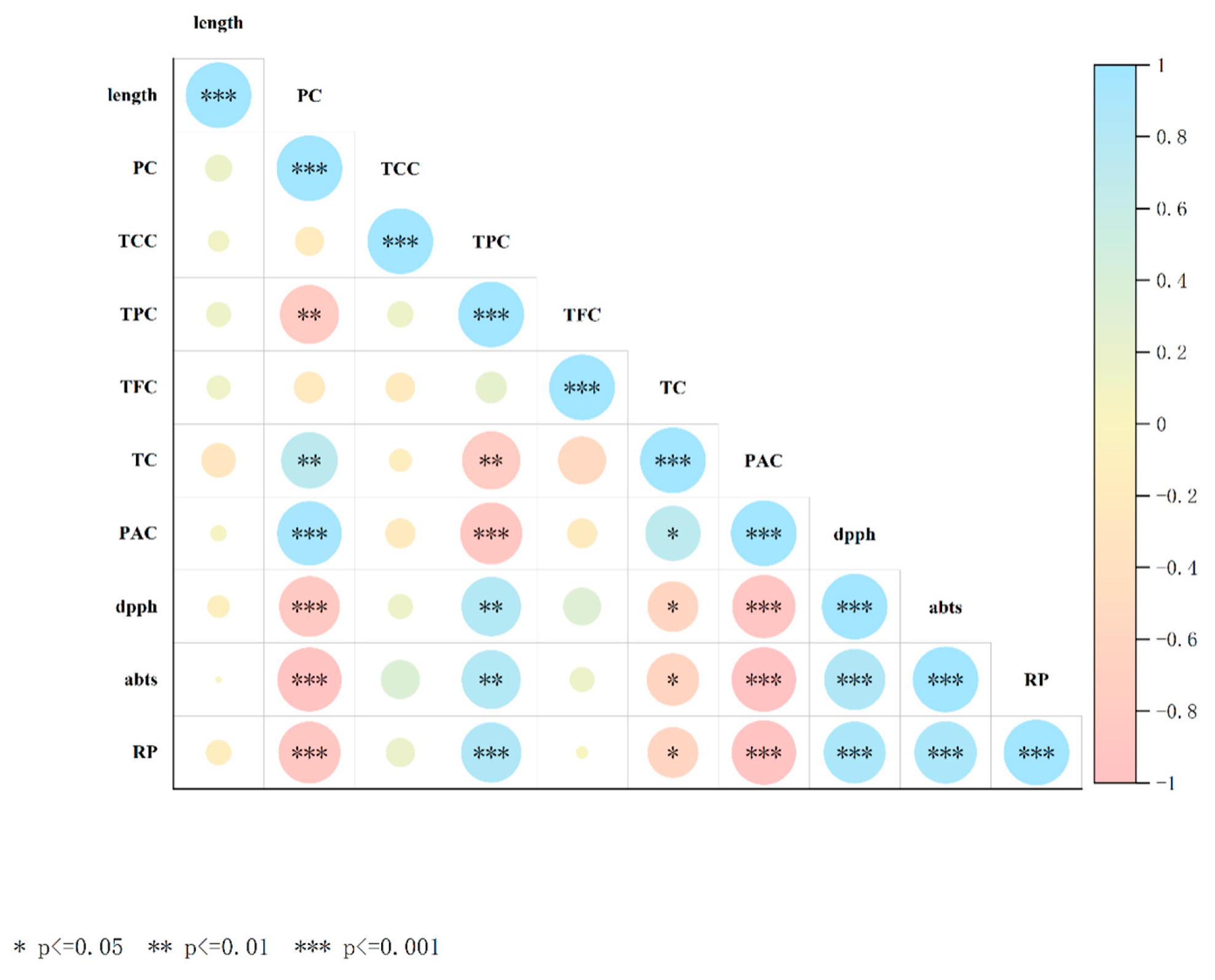
| G0 | G12 | G24 | G36 | G48 | G60 | G72 | |
|---|---|---|---|---|---|---|---|
| Length (cm) | 2.44 ± 0.05 d | 3.06 ± 0.19 c | 3.34 ± 0.154 b | 3.53 ± 0.15 a | 3.55 ± 0.16 a | 3.56 ± 0.16 a | 3.57 ± 0.16 a |
| Germination rate (%) | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 33.05 ± 0.02 b | 51.20 ± 0.01 a | 52.12 ± 0.01 a | 52.15 ± 0.02 a |
| G0 | G12 | G24 | G36 | G48 | G60 | G72 | |
|---|---|---|---|---|---|---|---|
| PC (μg/mL) | 10.08 ± 0.08 a | 9.67 ± 0.16 b | 9.37 ± 0.08 bc | 9.20 ± 0.01 c | 9.08 ± 0.02 c | 8.55 ± 0.08 d | 8.38 ± 0.33 d |
| TCC (mg/g) | 88.4 ± 0.02 a | 88.7 ± 0.01 a | 88.3 ± 0.08 a | 88.6 ± 0.05 a | 88.8 ± 0.12 a | 88.4 ± 0.11 a | 88.8 ± 0.03 a |
| TPC (mg GAE/g) | 124.74 ± 0.17 c | 152.00 ± 24.13 bc | 169.14 ± 5.36 b | 171.52 ± 0.67 ab | 213.67 ± 37.12 a | 194.50 ± 3.87 ab | 215.57 ± 1.34 a |
| TFC (mg RE/g) | 58.16 ± 0.00 c | 58.42 ± 0.37 c | 62.19 ± 1.52 b | 62.50 ± 0.56 b | 62.90 ± 0.00 b | 63.95 ± 0.00 b | 71.84 ± 0.00 a |
| G0 | G12 | G24 | G36 | G48 | G60 | G72 | |
|---|---|---|---|---|---|---|---|
| DPPH radical scavenging activity (%) | 47.47 ± 0.29 e | 48.23 ± 0.61 e | 51.65 ± 2.75 d | 55.58 ± 1.73 c | 59.13 ± 1.98 b | 56.46 ± 2.96 bc | 65.77 ± 0.57 a |
| ABTS radical scavenging activity (%) | 10.50 ± 0.86 d | 12.34 ± 0.50 d | 16.67 ± 4.12 c | 19.92 ± 1.79 c | 24.21 ± 1.28 b | 31.04 ± 0.98 a | 31.75 ± 0.71 a |
| RP (O.D.) | 0.50 ± 0.05 c | 0.53 ± 0.01 c | 0.54 ± 0.00 c | 0.54 ± 0.00 c | 0.63 ± 0.02 b | 0.64 ± 0.02 b | 0.70 ± 0.01 a |
| G0 | G12 | G24 | G36 | G48 | G60 | G72 | |
|---|---|---|---|---|---|---|---|
| TC (mg/g) | 1.58 ± 0.01 a | 0.46 ± 0.01 b | 0.34 ± 0.00 c | 0.32 ± 0.01 c | 0.30 ± 0.02 c | 0.28 ± 0.00 c | 0.26 ± 0.00 c |
| PAC (mg/g) | 34.1 ± 0.07 a | 31.6 ± 0.03 b | 29.2 ± 0.10 c | 26.1 ± 0.03 d | 23.4 ± 0.01 d | 22.5 ± 0.02 e | 18.9 ± 0.05 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Shin, W.-S. Germination-Induced Changes in the Nutritional, Bioactive, and Digestive Properties of Lima Bean (Phaseolus lunatus L.). Foods 2025, 14, 2123. https://doi.org/10.3390/foods14122123
Wu Y, Shin W-S. Germination-Induced Changes in the Nutritional, Bioactive, and Digestive Properties of Lima Bean (Phaseolus lunatus L.). Foods. 2025; 14(12):2123. https://doi.org/10.3390/foods14122123
Chicago/Turabian StyleWu, Yingjinzhu, and Weon-Sun Shin. 2025. "Germination-Induced Changes in the Nutritional, Bioactive, and Digestive Properties of Lima Bean (Phaseolus lunatus L.)" Foods 14, no. 12: 2123. https://doi.org/10.3390/foods14122123
APA StyleWu, Y., & Shin, W.-S. (2025). Germination-Induced Changes in the Nutritional, Bioactive, and Digestive Properties of Lima Bean (Phaseolus lunatus L.). Foods, 14(12), 2123. https://doi.org/10.3390/foods14122123






