Millettia speciosa and By-Products: A Comprehensive Review of Chemical Composition, Bioactivities, Safety, and Industrial Applications
Abstract
1. Introduction
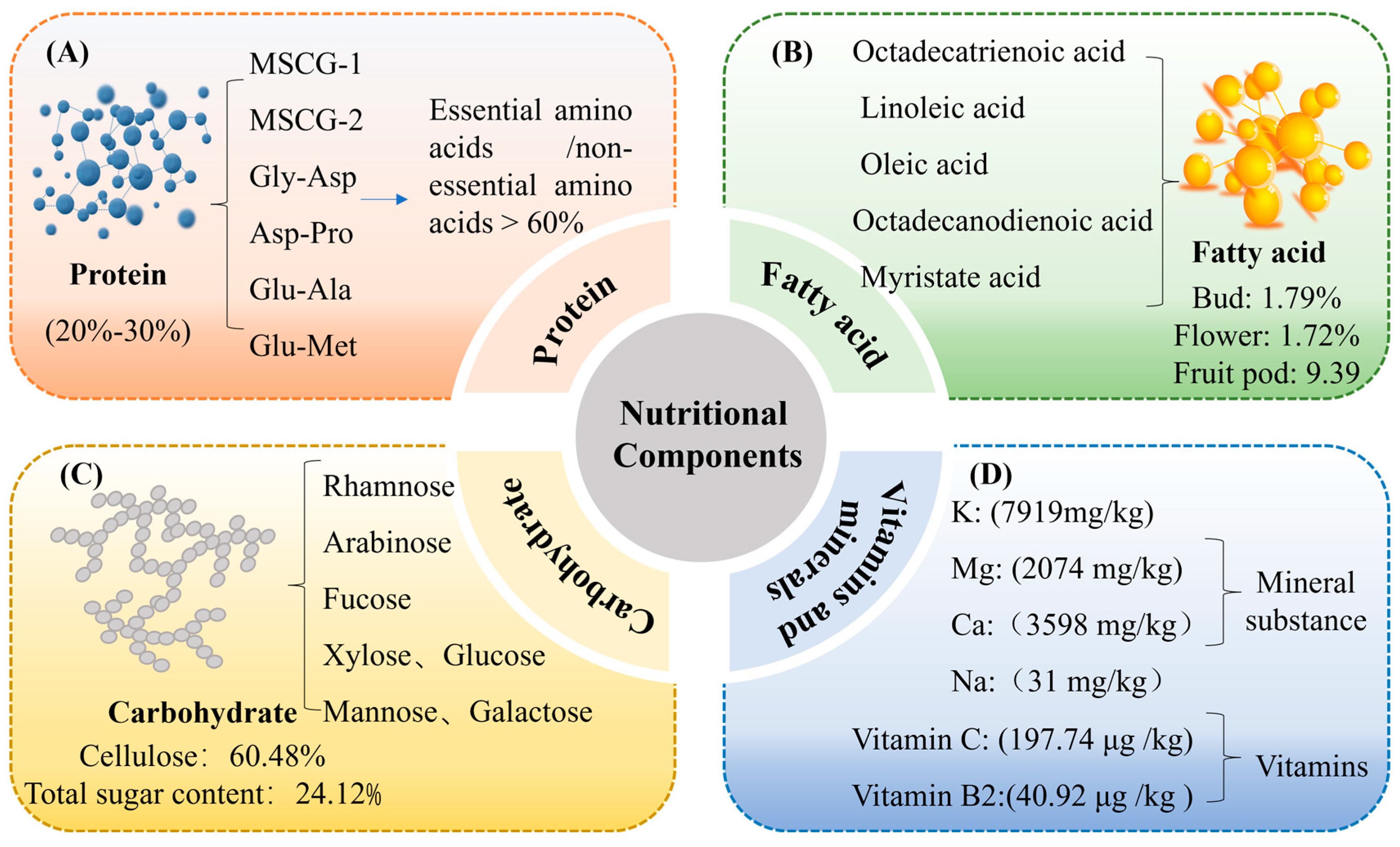
2. Nutritional Components of MS
2.1. Protein
| Classifications | Extraction Method | Nutrition Compositions | Parts | Contents | References |
|---|---|---|---|---|---|
| Amino acid | Weighed 2 g of sample powder was mixed with 10 mL of 6 M hydrochloric acid, sealed, and hydrolyzed at 110 °C for 22 h | Aspartic acid | Seeds | 1.21 g/100 g | [1] |
| Leucine | Seeds | 0.83 g/100 g | |||
| Lysine | Seeds | 0.82 g/100 g | |||
| Serine | Seeds | 0.71 g/100 g | |||
| Glutamic acid | Seeds | 1.76 g/100 g | |||
| Proline | Seeds | 0.58 g/100 g | |||
| Alanine | Seeds | 0.62 g/100 g | |||
| Isoleucine | Seeds | 0.38 g/100 g | |||
| Histidine | Seeds | 0.30 g/100 g | |||
| Tyrosine | Seeds | 0.23 g/100 g | |||
| Methionine | Seeds | 0.05 g/100 g | |||
| Phenylalanine | Seeds | 0.61 g/100 g | |||
| Threonine | Seeds | 0.64 g/100 g | |||
| Valine | Seeds | 0.57 g/100 g | |||
| Arginine | Seeds | 0.48 g/100 g | |||
| Glycine | Seeds | 0.52 g/100 g | |||
| Weighed 2 g of sample powder was mixed with 10 mL of 6 M hydrochloric acid, sealed, and hydrolyzed at 110 °C for 22 h | Aspartic acid | Leaves | 1.57 g/100 g | [1] | |
| Glutamic acid | Leaves | 1.73 g/100 g | |||
| Proline | Leaves | 1.57 g/100 g | |||
| Alanine | Leaves | 0.99 g/100 g | |||
| Leucine | Leaves | 1.20 g/100 g | |||
| Lysine | Leaves | 1.10 g/100 g | |||
| Serine | Leaves | 0.70 g/100 g | |||
| Threonine | Leaves | 0.76 g/100 g | |||
| Arginine | Leaves | 0.77 g/100 g | |||
| Glycine | Leaves | 0.81 g/100 g | |||
| Isoleucine | Leaves | 0.65 g/100 g | |||
| Phenylalanine | Leaves | 0.71 g/100 g | |||
| Histidine | Leaves | 0.36 g/100 g | |||
| Tyrosine | Leaves | 0.28 g/100 g | |||
| Methionine | Leaves | 0.02 g/100 g | |||
| Valine | Leaves | 0.85 g/100 g | |||
| Weighed 2 g of sample powder was mixed with 10 mL of 6 M hydrochloric acid, sealed, and hydrolyzed at 110 °C for 22 h | Aspartic acid | Flower | 1.79 g/100 g | [1] | |
| Glutamic acid | Flower | 1.01 g/100 g | |||
| Proline | Flower | 0.79 g/100 g | |||
| Alanine | Flower | 0.69 g/100 g | |||
| Leucine | Flower | 0.63 g/100 g | |||
| Lysine | Flower | 0.60 g/100 g | |||
| Serine | Flower | 0.55 g/100 g | |||
| Threonine | Flower | 0.52 g/100 g | |||
| Valine | Flower | 0.52 g/100 g | |||
| Arginine | Flower | 0.49 g/100 g | |||
| Glycine | Flower | 0.41 g/100 g | |||
| Isoleucine | Flower | 0.37 g/100 g | |||
| Histidine | Flower | 0.27 g/100 g | |||
| Tyrosine | Flower | 0.14 g/100 g | |||
| Phenylalanine | Flower | 0.35 g/100 g | |||
| Two grams of sample powder were weighed and mixed with 10 mL of deionized water, then ground in a mortar for 10 min. Subsequently, 20 mL of 10% sulfosalicylic acid was added, and the mixture was transferred to a 50 mL centrifuge tube. The tube was stored at 4 °C for 17 h. | Aspartic acid | Champ | 0.0033 g/100 g | [12] | |
| Glutamic acid | Champ | 0.0071 g/100 g | |||
| Proline | Champ | 0.0472 g/100 g | |||
| Alanine | Champ | 0.0064 g/100 g | |||
| Leucine | Champ | 0.0026 g/100 g | |||
| Lysine | Champ | 0.0013 g/100 g | |||
| Serine | Champ | 0.1141 g/100 g | |||
| Threonine | Champ | 0.0089 g/100 g | |||
| Glycine | Champ | 0.0071 g/100 g | |||
| Valine | Champ | 0.0026 g/100 g | |||
| Arginine | Champ | 0.0201 g/100 g | |||
| Tyrosine | Champ | 0.1660 g/100 g | |||
| Phenylalanine | Champ | 0.0011 g/100 g | |||
| Histidine | Champ | 0.0054 g/100 g | |||
| Fatty acid | Weighed 10.0 g of seeds, mixed with 100 mg of gallic acid, and added 95% ethanol and HCl for hydrolysis at 40 min. After cooling, extracted with ether-petroleum ether, and concentrated under reduced pressure to obtain the fat extract. Refluxed with NaOH-methanol, followed by BF3-methanol reflux. Extracted and processed with n-heptane, and finally dried with anhydrous sodium sulfate. | Lauric acid | Seeds | 0.0156% | [1] |
| Myristic acid | Seeds | 0.130% | |||
| Pentadecanoic acid | Seeds | 0.0121% | |||
| Docosanoic acid | Seeds | 1.16% | |||
| Palmitic acid | Seeds | 21.3% | |||
| Palmitoleic acid | Seeds | 0.338% | |||
| Heptadecanoic acid | Seeds | 0.127% | |||
| Erucic acid | Seeds | 0.0811% | |||
| Stearic acid | Seeds | 4.99% | |||
| Oleic acid | Seeds | 27.3% | |||
| Linoleic acid | Seeds | 41.9% | |||
| Linolenic acid | Seeds | 0.807% | |||
| Eicosanoenoic acid | Seeds | 0.305% | |||
| Heneicosanoic acid | Seeds | 0.0559% | |||
| Eicosadienoic acid | Seeds | 0.0230% | |||
| Arachidic acid | Seeds | 0.580% | |||
| Monosaccharide | Hydrolyzed the polysaccharides using TFA (2 M) at 105 °C, followed by methanol distillation to remove excess TFA. Finally, diluted the hydrolysate with distilled water and measured it by ion chromatography. | Rhamnose | Champ | - | [14] |
| Arabinose | Champ | ||||
| Fucose | Champ | ||||
| Glucose | Champ | ||||
| Mannose | Champ | ||||
| Fructose | Champ | ||||
| Galactose | Champ | ||||
| Mineral substance | Weighed 0.20 g of the sample and performed digestion with nitric acid (HNO3). The sample was ventilated at 160 °C for 60 min, and the residue was then diluted with water to a final volume of 50 mL. | Ni | Seeds | 3.0 mg/kg | [1] |
| Cu | Seeds | 7.1 mg/kg | |||
| Na | Seeds | <3 mg/kg | |||
| Zn | Seeds | 38 mg/kg | |||
| Mg | Seeds | 2074 mg/kg | |||
| Sb | Seeds | <0.1 mg/kg | |||
| Pb | Seeds | <0.1 mg/kg | |||
| Al | Seeds | <2 mg/kg | |||
| Se | Seeds | 0.59 mg/kg | |||
| K | Seeds | 7919 mg/kg | |||
| Rb | Seeds | 18 mg/kg | |||
| Ca | Seeds | 925 mg/kg | |||
| Sr | Seeds | 0.24 mg/kg | |||
| Ti | Seeds | <0.1 mg/kg | |||
| Ag | Seeds | <0.1 mg/kg | |||
| V | Seeds | <0.1 mg/kg | |||
| Cd | Seeds | <0.05 mg/kg | |||
| Cr | Seeds | <0.1 mg/kg | |||
| Sn | Seeds | <0.1 mg/kg | |||
| Mn | Seeds | 37 mg/kg | |||
| Fe | Seeds | 34 mg/kg | |||
| Hg | Seeds | <0.05 mg/kg | |||
| Ba | Seeds | 0.42 mg/kg | |||
| Mo | Seeds | 0.26 mg/kg | |||
| Li | Seeds | <0.1 mg/kg | |||
| B | Seeds | 7.8 mg/kg | |||
| Weighed 0.20 g of the sample and performed digestion with nitric acid (HNO3). The sample was ventilated at 160 °C for 60 min, and the residue was then diluted with water to a final volume of 50 mL. | Ni | Leaves | 0.91 mg/kg | [1] | |
| Cu | Leaves | 5.7 mg/kg | |||
| Na | Leaves | 31 mg/kg | |||
| Zn | Leaves | 26 mg/kg | |||
| Mg | Leaves | 1417 mg/kg | |||
| Al | Leaves | 54 mg/kg | |||
| Se | Leaves | 0.24 mg/kg | |||
| K | Leaves | 9547 mg/kg | |||
| Rb | Leaves | 20 mg/kg | |||
| Ca | Leaves | 3598 mg/kg | |||
| Sr | Leaves | 6.2 mg/kg | |||
| Ti | Leaves | 0.44 mg/kg | |||
| Ag | Leaves | <0.1 mg/kg | |||
| V | Leaves | <0.1 mg/kg | |||
| Cd | Leaves | <0.05 mg/kg | |||
| Cr | Leaves | 0.52 mg/kg | |||
| Sn | Leaves | <0.1 mg/kg | |||
| Mn | Leaves | 137 mg/kg | |||
| Sb | Leaves | <0.1 mg/kg | |||
| Fe | Leaves | 106 mg/kg | |||
| Hg | Leaves | <0.05 mg/kg | |||
| Ba | Leaves | 8.4 mg/kg | |||
| Mo | Leaves | <0.1 mg/kg | |||
| Li | Leaves | <0.1 mg/kg | |||
| B | Leaves | 16 mg/kg | |||
| Pb | Leaves | 0.47 mg/kg | |||
| Ni | Flower | 2.2 mg/kg | [1] | ||
| Cu | Flower | 5.3 mg/kg | |||
| Na | Flower | 22 mg/kg | |||
| Zn | Flower | 19 mg/kg | |||
| Mg | Flower | 1101 mg/kg | |||
| Al | Flower | 31 mg/kg | |||
| Se | Flower | <0.1 mg/kg | |||
| K | Flower | 10,642 mg/kg | |||
| Rb | Flower | 24 mg/kg | |||
| Ca | Flower | 1436 mg/kg | |||
| Sr | Flower | 2.2 mg/kg | |||
| Ti | Flower | 0.44 mg/kg | |||
| Ag | Flower | <0.1 mg/kg | |||
| V | Flower | <0.1 mg/kg | |||
| Cd | Flower | <0.05 mg/kg | |||
| Cr | Flower | 0.42 mg/kg | |||
| Sn | Flower | <0.1 mg/kg | |||
| Mn | Flower | 86 mg/kg | |||
| Sb | Flower | <0.1 mg/kg | |||
| Fe | Flower | 78 mg/kg | |||
| Mo | Flower | 0.37 mg/kg | |||
| Li | Flower | <0.1 mg/kg | |||
| B | Flower | 8.3 mg/kg | |||
| Pb | Flower | 0.48 mg/kg | |||
| Ba | Flower | 4.3 mg/kg | |||
| Hg | Flower | <0.05 mg/kg | |||
| Weighed 0.5 g of MS powder into a 50 mL PTFE digestion vessel, added 8 mL of concentrated nitric acid and 2 mL of 30% hydrogen peroxide solution, and performed heating digestion for 30 min | B | Champ | 5.2 mg/kg | [12] | |
| Mg | Champ | 2206.40 mg/kg | |||
| Al | Champ | 42.73 mg/kg | |||
| Ca | Champ | 2878.20 mg/kg | |||
| V | Champ | 0.14 mg/kg | |||
| Mn | Champ | 18.72 mg/kg | |||
| Cr | Champ | 0.16 mg/kg | |||
| Rh | Champ | 0.01 mg/kg | |||
| Cu | Champ | 5.97 mg/kg | |||
| Fe | Champ | 118.85 mg/kg | |||
| Co | Champ | 0.17 mg/kg | |||
| Ni | Champ | 3.02 mg/kg | |||
| Sr | Champ | 56.93 mg/kg | |||
| Zn | Champ | 13.27 mg/kg | |||
| Se | Champ | 0.03 mg/kg | |||
| Mo | Champ | 0.86 mg/kg | |||
| Re | Champ | 0.01 mg/kg | |||
| Cd | Champ | 0.06 mg/kg | |||
| As | Champ | 0.03 mg/kg | |||
| Hg | Champ | 0.11 mg/kg | |||
| Pb | Champ | 1.29 mg/kg |
2.2. Fatty Acids
2.3. Carbohydrates
2.4. Vitamins and Minerals
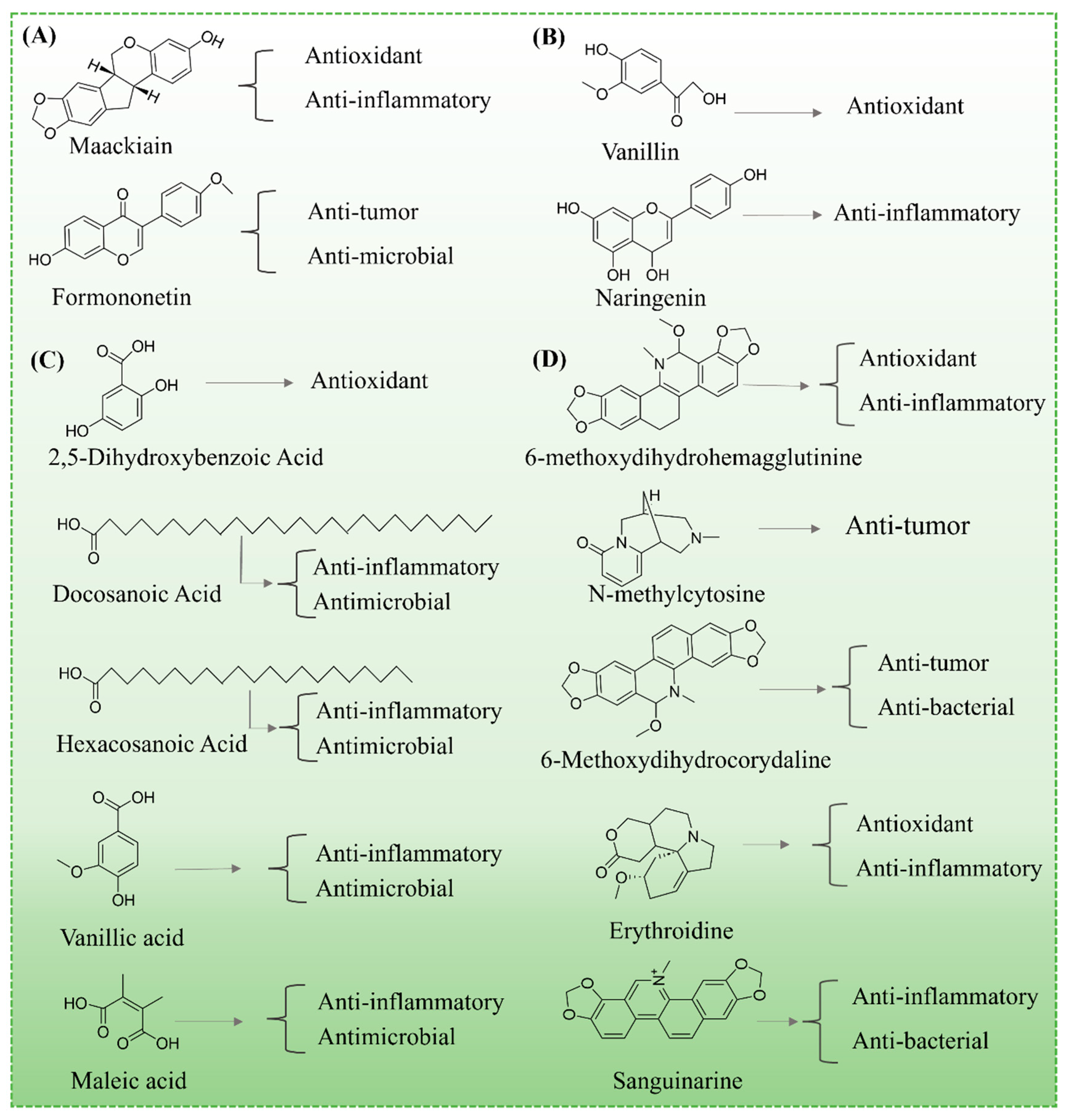
3. Chemical Compositions of MS and By-Products
3.1. Flavonoid Compounds
3.2. Phenolic Acids and Derivatives
3.3. Triterpenoid Compounds
3.4. Organic Acids
3.5. Phytosterols
3.6. Alkaloids
3.7. Esters
3.8. Other Compounds
| Classify | Plant Parts | Names | Extraction Solvents | Analysis Methods | References |
|---|---|---|---|---|---|
| Flavonoids | Champ | Naringenin | 95% ethanol | Silica gel column chromatography Sephadex LH-20 chromatography ODS column chromatography | [6] |
| Champ | Garbanzo | ||||
| Champ | 7-hydroxy-6,4- dimethoxy isoflavone | ||||
| Champ | 2,5,7-trihydroxy-4-methoxy isoflavone | ||||
| Champ | 2-hydroxy biochanin A | ||||
| Champ | 6-methoxycalopogonium isoflavone A | ||||
| Champ | 4,4, -dihydroxy-2′-methoxy chalcone | ||||
| Champ | 2,4-dihydroxy-4-methoxy chalcone | ||||
| Champ | 2,4,4, α-tetrahydroxy dihydrogen chalcone | ||||
| Champ | 3,4-dihydroxy-7-methoxy isoflavones | ||||
| Champ | 4-hydroxy-2,4-dimethoxy chalcone | ||||
| Champ | 2,4, a-trihydroxy-4-methoxy-dihydrogen chalcone | ||||
| Champ | 3,4,7-trihydroxy isoflavones | ||||
| Champ | Formononetin | 95% ethanol | Silica gel column chromatography Sephadex LH-20 chromatography ODS column chromatography | [6] | |
| Champ | 3,4,2′,4′-tetrahydroxychalcone | ||||
| Champ | Homopterocarpin | Ethanol Ethyl acetate Petroleum ether | HPLC-MS | [32] | |
| Champ | Isoliquiritigenin | ||||
| Champ | Maackiain | ||||
| Champ | Pterocarpan | ||||
| Champ | Medicarpin | 95% ethanol | Silica gel column chromatography Sephadex LH-20 chromatography ODS column chromatography | [44] | |
| Champ | 3,7-dihydroxy-2,4′-dimethoxy isoflavone | ||||
| Champ | 4,2,4-trihydroxy chalcone | ||||
| Champ | 4-hydroxy-7-methoxy flavanone | ||||
| Champ | Bisdemethoxycurcumin | 95% ethanol | Silica gel column chromatography Sephadex LH-20 chromatography ODS column chromatography | [45] | |
| Champ | Corylifolin | ||||
| Champ | Quercetin | ||||
| Champ | Isoquercitrin | ||||
| Champ | Licochalcone A | ||||
| Champ | Tectorigenin | ||||
| Champ | Liquiritigenin | ||||
| Champ | Sulfuretin | ||||
| Champ | Amentoflavone | ||||
| Phenolic acid and derivatives | Champ | 1,4-butanediol | 70% methanol, | UHPLC-MS/MS | [8] |
| Champ | Isotrifoliol | ||||
| Champ | 2-pentadecanone | ||||
| Champ | 3-hydroxy-4-methoxy benzoic acid | ||||
| Champ | Vanillic acid | ||||
| Champ | Dicoumarol | ||||
| Champ | Syringin | ||||
| Champ | (+/−)-Gingerol | ||||
| Champ | Nanillic acid | ||||
| Champ | Secoisolariciresinol | ||||
| Champ | Leiocarposide | ||||
| Triterenoids | Champ | 3β-olean-12-en-28,29-dioic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-d-galactopyranoside | 70% methanol, | UHPLC-MS/MS | [21] |
| Champ | 3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucuronopyranoyl-22-O-β-d-glucopyranoside | ||||
| Champ | Betulinic acid 3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl-28-O-β-d-glucopyranoside | ||||
| Champ | Saikogenin G 3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside | ||||
| Champ | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucuronopyranoside | ||||
| Champ | Betulinic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-D-galactopyranoside | ||||
| Champ | Soyasapogrnol B 3-O-α-l-arabinopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)- glucuronopyranosyl-22- O-β-d-glucopyranoside | ||||
| Champ | 3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucuronopyranoyl-22-O-β-d-glucopyranoside | ||||
| Champ | Saikogenin G 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-d-galactopyranoside | ||||
| Champ | 3β-olean-12-en-28,29-dioic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranoside | ||||
| Champ | Betulinic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranosyl-28-O-β-d-glucopyranoside | ||||
| Champ | 3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranosyl-22-O-β-d-glucopyranoside | ||||
| Champ | 3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucuronopyranoyl-22-O-β-d-glucopyranoside | ||||
| Champ | Oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranosyl-28- O-β-d-glucopyranoside | ||||
| Champ | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranoside | ||||
| Champ | Soyasapogrnol B 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranoside | ||||
| Champ | 23-hydroxyl pomalic acid 3-o-α-l-rhamnopyranosyl-(1 → 4)-β-d-glucopyranosyl-(1 → 6)-β-d-galactopyranosyl-28-o-β-d-glucopyranoside | ||||
| Champ | 23-hydroxyl pomalic acid 3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl-28-O-β-d-glucopyranoside | ||||
| Champ | Betulinic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranosyl-28-O-β-d-glucopyranoside | ||||
| Champ | 22β-acetyloxy-3β,24-dihydroxyolean-12-en-29-oic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-d-galactopyranoside | ||||
| Champ | Oleanolic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranoside | ||||
| Champ | 23-hydroxyl pomalic acid 3-o-α-l-rhamnopyranosyl-(1 → 4)-β-d-glucopyranosyl-(1 → 6)-β-d-galactopyranosyl-28-o-β-d-glucopyranoside | ||||
| Champ | Oleanolic acid 3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl-28-O-β-d-glucopyranoside | ||||
| Champ | 3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucopyranosyl-(1 → 2)-β-d-glucopyranosyl-22- O-β-d-glucopyranoside | ||||
| Champ | 22β-acetyloxy-3β,24-dihydroxyolean-12-en-29-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranoside | ||||
| Champ | Betulinic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranoside | ||||
| Champ | Betulinic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside | ||||
| Champ | Soyasapogrnol B 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranosyl-(1 → 2)-glucuronopyranosyl-22- O-β-d-glucopyranoside | ||||
| Champ | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 4)-β-d-glucopyranosyl-(1 → 6)-β-d-galactopyranoside | ||||
| Champ | Saikogenin G 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranoside | ||||
| Champ | Oleanolic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-d-galactopyranoside | ||||
| Champ | 3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucuronopyranoyl-22-O-β-d-glucopyranoside | ||||
| Champ | Saikogenin G 3-O-α-l-arabinopyranosyl-(1 → 2)-[β-d-galactopyranosyl-(1 → 3)]-β-d-galactopyranosyl-(1 → 2)-glucuronopyranoside | ||||
| Champ | Oleanolic acid 3-O-α-l-arabinopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-d-galactopyranoside | ||||
| Champ | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-arabinopyranosyl-(1 → 4)-β-d-glucopyranosyl-(1 → 6)-β-D glucopyranoside | ||||
| Champ | Saikogenin G 3-O-α-l-rhamnopyranosyl-(1 → 4)-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside | ||||
| Champ | Betulinic acid | ||||
| Organic acids | Champ | 2,5-Dihydroxybenzoic acid | 95% ethanol | Silica gel column chromatography Sephadex LH-20 chromatography ODS column chromatography | [46] |
| Champ | Hexacosanoic acid | Ethanol | Silica gel column chromatography Sephadex LH-20 chromatography ODS column chromatography HPLC-MS | [47] | |
| Champ | Dodecanoic acid | ||||
| Champ | Nonanoic acid | 95% ethanol | Silica gel column chromatography Sephadex LH-20 chromatography ODS column chromatography HPLC-MS | [48] | |
| Champ | Pentadecanoic acid | ||||
| Champ | Tetradecanoic acid | ||||
| Champ | Maleic acid | ||||
| Champ | Vanillic acid | ||||
| Champ | Syringic acid | ||||
| Champ | Linoleic acid | 95% ethanol | GC-MS | [49] | |
| Esters | Champ | Caffeic acid lupeol ester | 95% ethanol | GC-MS | [31] |
| Champ | Capsanthin ethyl ester | ||||
| Champ | Methyl 9,12-octadecadienoate | Ethanol | Silica gel column chromatography GC-MS | [36] | |
| Champ | Methyl palmitate | ||||
| Champ | Dibutyl phthalate | ||||
| Champ | Methyl stearate | ||||
| Champ | Methyl oleate | ||||
| Champ | Ethyl palmitate | ||||
| Champ | Ethyl heptanoate | ||||
| Champ | Methyl linoleate | ||||
| Champ | Ethyl linoleate | ||||
| Champ | Myristyl ethyl ester | Ethanol | GC-MS | [49] | |
| Champ | Avenalumic lactone | ||||
| Champ | Linolenic acid lactone | ||||
| Champ | Ethyl heptadecanoate | ||||
| Champ | Ethyl stearate | Water | GC-MS | [50] | |
| Champ | Methyl salicylate | ||||
| Champ | Dihydroactinidiolide |
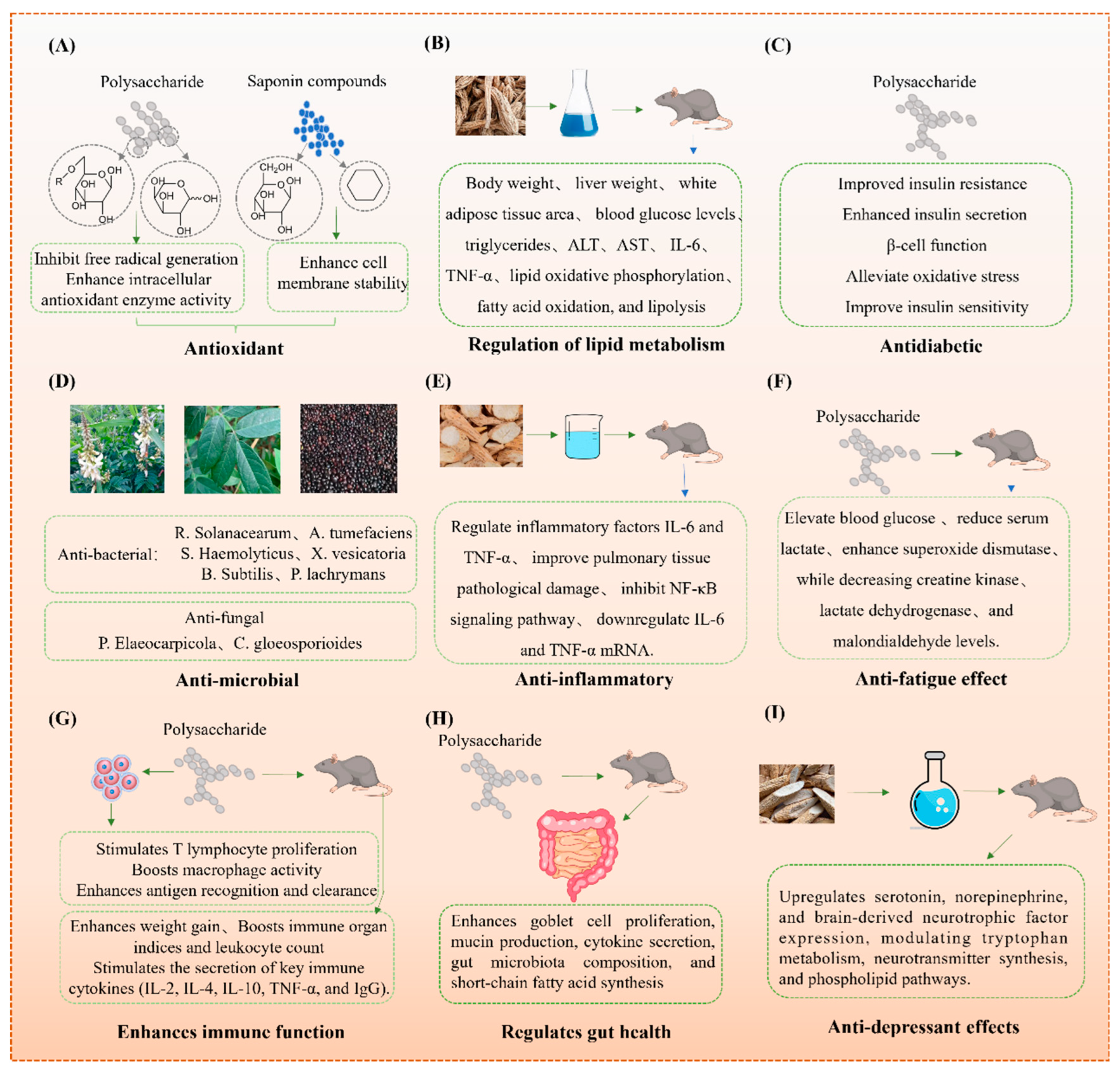
4. Health-Promoting Effects of MS and By-Products
4.1. Antioxidant Activity
4.2. Lipid Metabolism Regulation
4.3. Antidiabetic
4.4. Antibacterial and Antiviral
4.5. Anti-Inflammatory
4.6. Antifatigue
4.7. Immune Enhancement
4.8. Gut Health Regulation
4.9. Antidepressant
5. Safety and Toxicological Assessment of MS
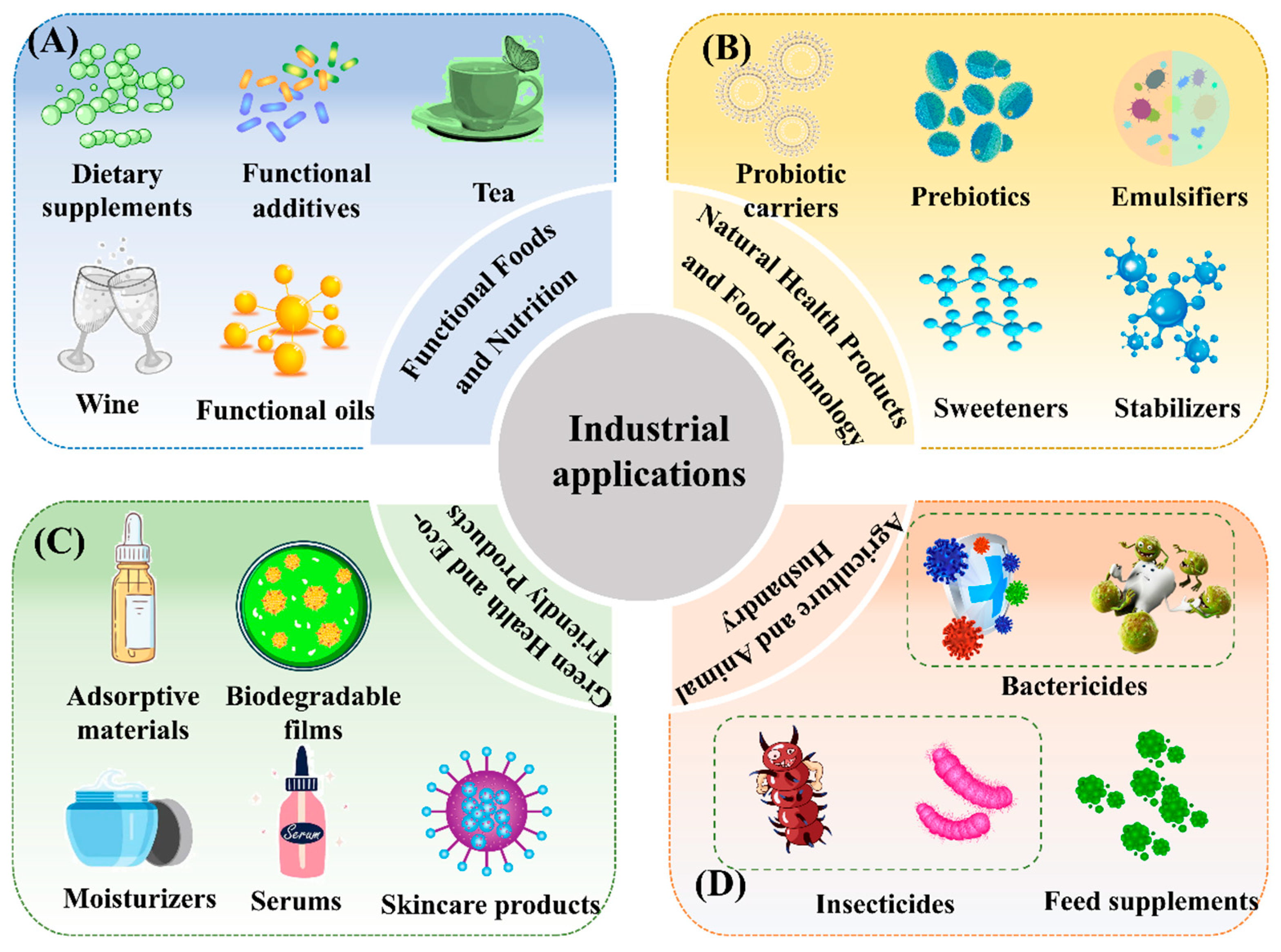
6. Industrial Applications of MS and Its Products
6.1. Functional Beverages
6.2. Natural Food Emulsifiers and Stabilizers
6.3. Food and Functional Additives
6.4. Prebiotics and Probiotic Carriers
6.5. Functional Lipids
6.6. Potential Applications in Health Foods and Dietary Supplements
6.7. Potential Applications in Skincare and Cosmetics
6.8. MS in Agricultural and Ecological Applications
6.8.1. Fungicides and Insecticides
6.8.2. Feed Additives
6.9. MS in Environmental Protection Applications
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Yan, Y.; Li, Y.; Huang, Y.; Zhang, Y.; Yang, L.; Xu, X.; Wu, F.; Du, B.; Mao, Z.; et al. Nutritional value, volatile components, functional metabolites, and antibacterial and cytotoxic activities of different parts of Millettia speciosa Champ., a medicinal and edible plant with potential for development. Plants 2023, 12, 3900. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wei, T.; Li, M.; Liu, S.; Wu, J.; Xu, G.; Zou, J. Effect of aqueous extract of Millettia speciosa Champ on intestinal health maintenance and immune enhancement of Cyprinus carpio. Fish Shellfish. Immunol. 2024, 144, 109227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, X.; Yang, J.; Huang, Z.; Li, C.; Yao, L.; Tan, Z.; Wu, X.; Huang, S.; Yuan, Y.; et al. Effects of climate change on the geographical distribution and potential distribution areas of 35 Millettia species in China. Environ. Sci. Pollut. Res. 2023, 30, 18535–18545. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Lyu, X.F.; Zhai, D.H.; Cai, J.Y. Advances in chemical constituents and pharmacological effects of Millettia speciosa Champ. J. Holist. Integr. Pharm. 2021, 2, 287–300. [Google Scholar] [CrossRef]
- Li, M.F.; Cui, H.L.; Lou, W.Y. Millettia speciosa Champ cellulose-based hydrogel as a novel delivery system for Lactobacillus paracasei: Its relationship to structure, encapsulation and controlled release. Carbohydr. Polym. 2023, 316, 121034. [Google Scholar] [CrossRef]
- Fu, M.Q.; Xiao, G.S.; Xu, Y.J.; Wu, J.J.; Chen, Y.L.; Qiu, S.X. Chemical constituents from roots of Millettia speciosa. Chin. Tradit. Herb Drugs 2016, 8, 385–389. [Google Scholar] [CrossRef]
- Huang, Z.; Zong, M.H.; Lou, W.Y. Effect of acetylation modification on the emulsifying and antioxidant properties of polysaccharide from Millettia speciosa Champ. Food Hydrocoll. 2022, 124, 107217. [Google Scholar] [CrossRef]
- Wang, M.Y.; Ma, W.Y.; Wang, Q.L.; Yang, Q.; Yan, X.X.; Tang, H.; Li, Z.Y.; Li, Y.Y.; Feng, S.X.; Wang, Z.N. Flavonoid-enriched extract from Millettia speciosa Champ. prevents obesity by regulating thermogenesis and lipid metabolism in high-fat diet–induced obese C57BL/6 mice. Food Sci. Nutr. 2022, 10, 445–459. [Google Scholar] [CrossRef]
- Zhang, C.; Mo, Y.Y.; Feng, S.S.; Meng, M.W.; Chen, S.Y.; Haung, H.M.; Ling, X.; Song, H.; Liang, Y.H.; Qu, S.F.; et al. Urinary metabonomics study of anti-depressive mechanisms of Millettia speciosa Champ on rats with chronic unpredictable mild stress-induced depression. J. Pharm. Biomed. Anal. 2021, 205, 114338. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, C.; Lin, Y.; Cai, J. Ameliorating effect on glycolipid metabolism and chemical profile of Millettia speciosa Champ. extract. J. Ethnopharmacol. 2021, 279, 114360. [Google Scholar] [CrossRef]
- Luo, S. Enzymatic Preparation, Characterization, and Application of Millettia speciosa Leaf Peptides. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2021. [Google Scholar]
- Chen, C.; Liu, J.; Luo, N.; Li, A.; Si, W.; Liu, P. Nutritional composition analysis of Millettia speciosa from Hainan. Food Ind. 2016, 37, 287–290. [Google Scholar]
- Zhao, X.N.; Liang, J.L.; Chen, H.B.; Liang, Y.E.; Guo, H.Z.; Su, Z.R.; Li, Y.C.; Zeng, H.F.; Zhang, X.J. Antifatigue and antioxidant activity of the polysaccharides isolated from Millettia speciosa Champ. Leguminosae. Nutrients 2015, 7, 8657–8669. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zong, M.H.; Wu, X.L.; Li, M.F.; Zong, M.H.; Lou, W.Y. Development of Millettia speciosa Champ polysaccharide conjugate stabilized oil-in-water emulsion for oral delivery of β-carotene: Protection effect and in vitro digestion fate. Food Chem. 2022, 397, 133764. [Google Scholar] [CrossRef]
- Chen, J.X.; Liu, Y.F.; Huang, Q.R.; Liu, M.T.; Zeng, Y.J.; Deng, J.Y. Extraction and Antioxidant Activity of Water-Soluble Protein from Millettia speciosa Champ. Mod. Food 2022, 28, 97–102. [Google Scholar]
- Wang, Q.; Liu, R.; Chang, M.; Zhang, H.; Jin, Q.; Wang, X. Dietary oleic acid supplementation and blood inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 2508–2525. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, Y.J.; Chen, X.; Luo, S.Y.; Pu, L.; Li, F.Z.; Zong, M.H.; Lou, W.Y. A novel polysaccharide from the roots of Millettia speciosa Champ: Preparation, structural characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2020, 145, 547–557. [Google Scholar] [CrossRef]
- Chen, X.; Sun, W.; Xu, B.; Wu, E.; Cui, Y.; Hao, K.; Zhang, G.; Zhou, C.; Xu, Y.; Li, J.; et al. Polysaccharides from the roots of Millettia speciosa Champ. modulate gut health and ameliorate cyclophosphamide-induced intestinal injury and immunosuppression. Front. Immunol. 2021, 12, 766296. [Google Scholar] [CrossRef]
- Fang, C.; Wang, D.; Chen, D.; Hui, H. Nutritional and medicinal quality analysis of Millettia speciosa roots from different regions. Chin. J. Mod. Tradit. Chin. Med. 2015, 17, 808–811. [Google Scholar]
- Salehi, M.; Rayatpisheh, N.; Salehi, M.; Mardanparvar, H. Phytochemicals with sodium-glucose co-transporter inhibitory effects for the management of diabetes: A narrative review on recent findings. J. Ren. Endocrinol. 2023, 9, e25095. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Yang, Q.; Wang, Q.; Ma, B.; Li, Z.; Cheng, W.; Tang, H.; Feng, S.; Wang, Z. Metabolomic profiling of Millettia speciosa Champ. at different growth stages. Food Chem. 2022, 376, 131941. [Google Scholar] [CrossRef]
- Oke, I.M.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Makhafola, T.J.; Eze, K.C.; Noreljaleel, A.E.M.; Chukwuma, C.I. Vanillic acid–Zn (II) complex: A novel complex with antihyperglycaemic and anti-oxidative activity. J. Pharm. Pharmacol. 2021, 73, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, J.; Zhang, C.; Cao, L.; Zou, F.; Zhang, Z. Dihydroquercetin (DHQ) ameliorates LPS-induced acute lung injury by regulating macrophage M2 polarization through IRF4/mir-132-3p/FBXW7 axis. Pulm. Pharmacol. Ther. 2023, 83, 102249. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chai, Y.; Lin, H.; Chen, C.; Zhao, M.; Xiong, W.; Zhuang, J.; Fan, X. Dihydroquercetin activates AMPK/Nrf2/HO-1 signaling in macrophages and attenuates inflammation in LPS-induced endotoxemic mice. Front. Pharmacol. 2020, 11, 662. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Liu, Z. A review of plant antipathogenic constituents: Source, activity and mechanism. Pestic. Biochem. Physiol. 2022, 188, 105225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Xu, Y.; Luan, M.; Zhao, F.; Meng, Q. Advances on the anti-inflammatory activity of oleanolic acid and derivatives. Mini Rev. Med. Chem. 2021, 21, 2020–2038. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic acid-based derivatives as potential anti-cancer agents: An update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail; Shahid, M.A.; Babar, A. Role of sugars, amino acids, and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Kalinowska, M.; Mazur, L.; Jabłońska-Trypuć, A.; Lewandowski, W. A new calcium 2,5-dihydroxybenzoate: Synthesis, characterization and antioxidant studies and stress-mediated cytotoxicity in MCF-7 cells. J. Saudi Chem. Soc. 2018, 22, 742–756. [Google Scholar] [CrossRef]
- Greish, K.; Taha, S.; Jasim, A.; Elghany, S.A.; Sultan, A.; AlKhateeb, A.; Othman, M.; Jun, F.; Taurin, S.; Bakhiet, M. Styrene maleic acid encapsulated raloxifene micelles for management of inflammatory bowel disease. Clin. Transl. Med. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Wang, C.W.; Chen, G.Y.; Song, X.P.; Cheng, W.; Shu, H.; Han, C.; Ji, M. Flavonoids from the roots of Millettia speciosa Champ. Chin. Tradit. Pat. Med. 2014, 36, 2111–2114. [Google Scholar]
- Lai, F.L.; Wang, Z.N.; Wang, J.R.; Yan, X.X. Analysis of liposoluble constituents of the leaves from Millettia speciosa by GC-MS. Chin. J. Trop. Crops. 2009, 30, 714–717. [Google Scholar]
- Chen, S.; Wang, R.; Cheng, M.; Wei, G.; Du, Y.; Fan, Y.; Li, J.; Li, H.; Deng, Z. Serum cholesterol-lowering activity of β-sitosterol laurate is attributed to the reduction of both cholesterol absorption and bile acids reabsorption in hamsters. J. Agric. Food Chem. 2020, 68, 10003–10014. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Stalin, A.; Ignacimuthu, S. Molecular docking of γ-sitosterol with some targets related to diabetes. Eur. J. Med. Chem. 2012, 47, 38–43. [Google Scholar] [CrossRef]
- Fu, C.; Guan, G.; Wang, H. The anticancer effect of sanguinarine: A review. Curr. Pharm. Des. 2018, 24, 2760–2764. [Google Scholar] [CrossRef]
- Chen, D.; Gong, B.; Liu, Y.; Fang, C.; Wang, D.; Zheng, X. GC-MS analysis of fat-soluble components in Millettia speciosa roots. Shanxi J. Tradit. Chin. Med. 2015, 36, 1248–1250. [Google Scholar]
- Yuan, S.; Li, W.; Li, Q.; Wang, L.; Cao, J.; Jiang, W. Defense responses, induced by p-coumaric acid and methyl p-coumarate, of jujube (Ziziphus jujuba Mill.) fruit against black spot rot caused by Alternaria alternata. J. Agric. Food Chem. 2019, 67, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Teoh, H.L.; Aminudin, N.; Abdullah, N. In vitro antioxidation, antihepatic steatosis, and anti-inflammatory effects of ethyl acetate fraction from Auricularia nigricans (Agaricomycetes). Int. J. Med. Mushrooms 2021, 23, 43–56. [Google Scholar] [CrossRef]
- Lim, P.T.; Goh, B.H.; Lee, W.L. Taxol: Mechanisms of action against cancer, an update with current research. In Paclitaxel; Academic Press: Cambridge, MA, USA, 2022; pp. 47–71. [Google Scholar]
- Bulbul, I.J.; Uddin, M.E.; Nahar, N.; Kuddus, M.R.; Haque, M.R. Antidiarrheal activity of four different species of Litsea available in Bangladesh. Biomed. Pharmacol. J. 2021, 14, 1259–1266. [Google Scholar] [CrossRef]
- Galiatsatos, P.; Maydan, D.D.; Macalpine, E.; Schleupner, B.; Aitchison, A.H.; Lerner, A.D.; Levy, B.; Halthore, A.; Eward, W. Psoralen: A narrative review of current and future therapeutic uses. J. Cancer Res. Clin. Oncol. 2024, 150, 130. [Google Scholar] [CrossRef]
- Wang, C.; Chen, G.; Song, X.; Chen, W.; Shu, H.; Han, C.; Ji, M. Chemical composition study of Millettia specios. Chin. Herb. Med. 2014, 45, 1515–1520. [Google Scholar]
- Meng, D.; Dong, Y.; Shang, Q.; Sun, Z. Anti-tumor effect and hepatotoxicity mechanisms of psoralen. Front. Pharmacol. 2024, 15, 1442700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.N.; Lai, F.L.; Wang, M.Y.; Wang, J.R. Chemical constituents of the roots of Millettia speciosa. Chin. J. Trop. Crops 2011, 32, 2378–2380. [Google Scholar]
- Wang, M.Y.; Lai, F.L.; Wang, J.R.; Wang, J.R.; Yan, X.X.; Wang, Z.N. Chemical constituents from the vinestems of Millettia speciosa. Nat. Prod. Res. Dev. 2013, 25, 53–55. [Google Scholar]
- Wang, C.H.; Wang, Y.; Wang, G.C.; Zha, J.; Zhang, X.Q.; Ye, W.C. Chemical constituents from roots of Millettia speciosa. Chin. Tradit. Herb Drugs. 2008, 39, 972–975. [Google Scholar]
- Ding, P.; Qiu, J.Y.; Ge, Y.; Dai, L. Chemical constituents of Millettia speciosa. Chin. Tradit. Herb Drugs. 2014, 6, 332–334. [Google Scholar] [CrossRef]
- Wang, C.W.; Chen, G.Y.; Song, X.P.; Chen, W.H.; Shu, H.M.; Han, C.R.; Ji, M.H. Chemical constituents from roots of Millettia speciosa. Chin. Tradit. Herb Drugs. 2014, 45, 1515–1520. [Google Scholar]
- Lai, F.; Wang, Z.; Wang, J.; Yan, X. GC-MS analysis of fat-soluble components in Millettia speciosa vine leaves. Trop. Crops 2009, 30, 714–717. [Google Scholar]
- Li, C.; Li, A.; Xu, X.; Zheng, K. Determination of essential oil components in Millettia speciosa and prediction of their mechanism of action. Jiangsu Agric. Sci. 2020, 48, 217–223. [Google Scholar]
- Bai, L.; Xu, D.; Zhou, Y.M.; Zhang, Y.B.; Zhang, H.; Chen, Y.B.; Cui, Y.L. Antioxidant activities of natural polysaccharides and their derivatives for biomedical and medicinal applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wang, Y.; Chen, M.; Shi, X.; Zhou, X.; Zhang, Z. Phytochemistry and antioxidant activities of the rhizome and radix of Millettia speciosa based on UHPLC-Q-Exactive Orbitrap-MS. Molecules 2022, 27, 7398. [Google Scholar] [CrossRef]
- Huang, Z.; Zong, M.H.; Lou, W.Y. Preparation, structural elucidation, and immunomodulatory activity of a polysaccharide from Millettia speciosa Champ. Ind. Crops Prod. 2022, 182, 114889. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, M.; Lin, L.; Zhao, M. Isolation, identification, and antioxidant activity of Moringa glycoproteins. J. South China Univ. Technol. (Nat. Sci. Ed.) 2015, 43, 8–15+151. [Google Scholar]
- Mo, H.H.; Deng, G.W.; Li, S. Study on the contents of total polysaccharides, total flavonoids, total saponins and the antioxidant activity in the extracts of leaves of Millettia speciosa Champ. Hubei Agric Sci. 2021, 60, 88. [Google Scholar]
- Wu, X.; Wang, T.; Nie, X.; Wu, Y.; Wang, J.; Wang, H.; Dai, R.; Liu, R.; Cui, Y.; Su, M.; et al. Pasteurization and the potential anti-obesity function of fermented beverages: A significant increase in nitrogen-containing aromatic heterocyclic compound content. Fermentation 2024, 10, 646. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, R.; Liu, J.; Huang, J.; Liu, Q.; Yao, M.; Wu, J.; Ye, J.; Li, D.; Gan, L.; et al. Protective role of polysaccharide from the roots of Millettia speciosa Champ in mice with ulcerative colitis induced by dextran sulphate sodium. Nat. Prod. Res. 2024, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhong, Z.; Yu, L.; Yang, Q.; Zhang, J.; Wang, W.; Chen, P.; Li, J.; Zheng, G. Millettia speciosa Champ. root extracts relieve exhaustive fatigue by regulating gut microbiota in mice. Food Chem. Toxicol. 2025, 203, 115557. [Google Scholar] [CrossRef]
- Yu, W.; Han, M.; Duo, S.; Chen, Y.; Yang, Y. The Millettia speciosa Champ polysaccharide attenuates pyroptosis by suppressing the signaling pathway of caspase-1/gasdermin D/Interleukin-1β. Authorea Prepr. 2023, 1–13. [Google Scholar]
- Luo, X.; Lin, C.W.; Chen, J.J.; Wen, Y.R.; Zhu, Y.P.; Mo, J.; Li, D.P. In vivo antifatigue effect of polysaccharides from Millettia speciosa Champ. Nat. Prod. Res. Dev. 2014, 26, 324. [Google Scholar]
- Zhao, X.N.; Wang, X.F.; Liao, J.B.; Guo, H.Z.; Yu, X.D.; Liang, J.L.; Zhang, X.; Su, Z.R.; Zhang, X.J.; Zeng, H.F. Antifatigue effect of Millettia speciosa Champ (Leguminosae) extract in mice. Trop J Pharm Res. 2015, 14, 479–485. [Google Scholar] [CrossRef]
- Qu, P.R.; Jiang, Z.L.; Song, P.P.; Liu, L.C.; Xiang, M.; Wang, J. Saponins and their derivatives: Potential candidates to alleviate anthracycline-induced cardiotoxicity and multidrug resistance. Pharmacol. Res. 2022, 182, 106352. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Su, M.; Wang, T.; Wang, H.; Wu, Y. Fermented Millettia speciosa Champ. (FH03) functional beverage inhibits weight gain in high-fat diet rats: Activation of AMPK pathway suppresses lipid synthesis and promotes lipolysis. In Proceedings of the Conference on Precision R&D and Industrial Development of Nutrition and Health Foods, Fangchenggang, China, 16–17 November 2024; pp. 15–16. [Google Scholar]
- Chen, X.; Wang, Y.; Shen, M.; Yu, Q.; Chen, Y.; Huang, L.; Xie, J. The water-soluble non-starch polysaccharides from natural resources against excessive oxidative stress: A potential health-promoting effect and its mechanisms. Int. J. Biol. Macromol. 2021, 171, 320–330. [Google Scholar] [CrossRef]
- Su, F.L.; Qiu, Z.W.; Sun, X. Hypoglycemic effect of polysaccharide from Millettia speciosa Champ on diabetic mice. Cent. South Pharm. 2019, 17, 1856–1859. [Google Scholar]
- Tuan, N.N.; Thi, H.N.; My, C.L.T.; Hai, T.X.; Trung, H.T.; Kim, A.N.T.; Tan, T.N.; Van, T.L.; Nguyen, C.Q.; Tran, Q.D.; et al. Inhibition of α-glucosidase, acetylcholinesterase, and nitric oxide production by phytochemicals isolated from Millettia speciosa—In vitro and molecular docking studies. Plants 2022, 11, 388. [Google Scholar] [CrossRef]
- Liu, D.D.; Wei, Z.X. Experimental studies on the anti-inflammatory and analgesic effects of the water extract from Millettiae Speciosae Radix. Mod. Chin. Med. 2014, 16, 538–541. [Google Scholar]
- Tang, Z.H.; Luo, Q.L.; Mo, J.; Zhang, X.F.; Zhu, Y.P. Study on mice’s normobaric hypoxia tolerance of polysaccharides from Millettia speciosa Champ. J. Guangxi Univ. (Nat. Sci. Ed.) 2017, 42, 1203–1208. [Google Scholar]
- Wang, L.P.; Shen, M.J.; Yang, B.; Mo, K.; Li, L.; Zhong, X.; Huang, R. Effects of polysaccharides from Millettia speciosa Champ. on proliferation of spleen lymphocyte and secretion of cytokine in mice. Herb. Med. 2017, 36, 480–484. [Google Scholar]
- Zhang, J.; Zhang, W.; Yu, L.; Yang, Q.; Wang, W.; Hu, X.; Li, J.; Zheng, G. Polysaccharides from Millettia speciosa Champ. ameliorate excessive exercise-induced fatigue by regulating gut microbiota. Foodborne Pathog. Dis. 2025, 12, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef]
- Huang, Z. Structural Characterization, Biological Activities and Emulsifying Properties of Millettia speciosa Champ Polysaccharide. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2022. [Google Scholar]
- Su, Z.; Ruan, J.; Liu, X.; Zheng, H.; Ruan, J.; Lu, Y.; Cheng, B.; Wu, F.; Wu, J.; Liu, X.; et al. Combining H-1-NMR-based metabonomics and network pharmacology to dissect the mechanism of antidepressant effect of Millettia speciosa Champ on mice with chronic unpredictable mild stress-induced depression. J. Pharm. Pharmacol. 2021, 73, 881–892. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, P.; Wang, S.; Ma, S. Optimization of ultrasound, microwave and Soxhlet extraction of flavonoids from Millettia speciosa Champ. and evaluation of antioxidant activities in vitro. J. Food Meas. Charact. 2017, 11, 1947–1958. [Google Scholar] [CrossRef]
- Tang, L.; Hu, S.; Chen, M.; Zhang, Z.; Li, W.; Li, D. Subchronic toxicity study of Millettia speciosa. J. Med. Inf. 2024, 37, 62–68. [Google Scholar]
- Nasiruddin Ji, M.; Yu, Z.; Chen, T.M.; Ma, F. Chemical constituents and biological functions of different extracts of Millettia speciosa leaves. J. Food Nutr. Res. 2020, 8, 506–515. [Google Scholar] [CrossRef]
- Mo, H.; Zhang, H. Research progress on the main pharmacological and toxicological effects of Zhuang medicine Millettia speciosa Champ. China Pharm. 2023, 32, 128–131. [Google Scholar]
- Elekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in cancer treatment: Current progress and future prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zong, M.H.; Wang, J.; Peng, S.Y.; Yu, M.; Lou, W.Y. Structural and interfacial properties of acetylated Millettia speciosa Champ polysaccharide and stability evaluation of the resultant O/W emulsion containing β-carotene. Int. J. Biol. Macromol. 2024, 264, 130556. [Google Scholar] [CrossRef]
- Yin, S.; Xu, H.; Xia, J.; Lu, Y.; Xu, D.; Sun, J.; Wang, Y.; Liao, W.; Sun, G. Effect of alpha-linolenic acid supplementation on cardiovascular disease risk profile in individuals with obesity or overweight: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2023, 14, 1644–1655. [Google Scholar] [CrossRef]
- Annevelink, C.E.; Sapp, P.A.; Petersen, K.S.; Shearer, G.C.; Kris-Etherton, P.M. Diet-derived and diet-related endogenously produced palmitic acid: Effects on metabolic regulation and cardiovascular disease risk. J. Clin. Lipidol. 2023, 14, 1644–1655. [Google Scholar] [CrossRef]
- Si, T.; Wang, A.; Yan, H.; Kong, L.; Guan, L.; He, C.; Ma, Y.; Zhang, H.; Ma, H. Progress in the study of natural antimicrobial active substances in Pseudomonas aeruginosa. Molecules 2024, 29, 4400. [Google Scholar] [CrossRef]
- Subhash, S.; Raghavendra, K.V.; Balodi, R.; Deepika; Dubey, N.K. Use of green chemicals in pest and disease management. In Sustainable Management of Potato Pests and Diseases; Springer: Singapore, 2022; pp. 495–524. [Google Scholar]
- Chen, X.; Huang, Z.; Luo, S.Y.; Zong, M.H.; Lou, W.Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Liang, H.; Zhou, J.; Zong, M.; Cao, Y.; Lou, W. A review of advancements in chitosan-essential oil composite films: Better and sustainable food preservation with biodegradable packaging. Int. J. Biol. Macromol. 2024, 274, 133242. [Google Scholar] [CrossRef] [PubMed]
| Part | Assays | Biological Activity | Experimental Model | Main Findings | References |
|---|---|---|---|---|---|
| Rhizomes and roots | In vitro | Antioxidant effects | In vitro antioxidant assays | The extracts of the rhizomes and roots of MS exhibited significant antioxidant activities in vitro, including DPPH radical scavenging, ABTS radical scavenging, and ferric reducing antioxidant power (FRAP) assays. The antioxidant activity of the rhizome extracts was significantly higher than that of the root extracts | [52] |
| Champ | In vitro | Antioxidant effects | In vitro antioxidant assays | The modified polysaccharide from MS Champ exhibits significant antioxidant activity. The modified polysaccharide shows strong free radical scavenging ability in both DPPH and ABTS free radical scavenging assays. | [53] |
| Champ | In vitro | Antioxidant effects | In vitro antioxidant assays | Flavonoid extracts obtained using different extraction methods (ultrasound, microwave, and Soxhlet extraction) all exhibited significant antioxidant activity. In the DPPH radical scavenging assay, the microwave extract showed the highest scavenging capacity (IC50 = 0.34 mg/mL), followed by the ultrasound extract and Soxhlet extract. Additionally, in the ABTS radical scavenging assay and the total antioxidant capacity (FRAP) test, the microwave extract also demonstrated the strongest activity. | [54] |
| Leaves | In vitro | Antioxidant effects | In vitro antioxidant assays | The leaf extract of MS exhibits significant antioxidant activity. The DPPH radical scavenging assay results show that the extract has good scavenging ability. The ABTS radical scavenging assay and reducing power test (FRAP) also demonstrate that the extract shows strong antioxidant effects. Additionally, the study found that the contents of polysaccharides, flavonoids, and saponins in the extract are positively correlated with its antioxidant activity. | [55] |
| Champ | In vivo | Regulation of lipid metabolism | C57BL/6 Mice | The flavonoid extract MS Champ combats obesity by promoting thermogenesis in brown adipose tissue, increasing fatty acid oxidation, inhibiting fat accumulation, and regulating the expression of genes related to lipid metabolism. This results in a reduction in body weight and body fat content in mice, as well as an improvement in their lipid metabolic indicators. | [8] |
| Champ | In vivo | Regulation of lipid metabolism | Mice | The fermented beverage of MS Champ directly activates PKA/PKG-mediated kinase-sensitive hormone-sensitive lipase (p-HSL) through the cAMP signaling pathway, promoting fat breakdown and thus combating obesity. | [56] |
| Seed | In vitro | Anti-bacterial effect | In vitro Anti-bacterial assays | The MS seed extract can significantly inhibit the growth of five Gram-negative strains (Ralstonia solanacearum, Agrobacterium tumefaciens, Xanthomonas vesicatoria, Pseudomonas lachrymans, and Escherichia coli) and two Gram-positive strains (Staphylococcus haemolyticus and Bacillus subtilis). | [1] |
| Leaves | In vitro | Anti-bacterial effect | In vitro Anti-bacterial assays | The MS Leaves extract can significantly inhibit the growth of five Gram-negative strains (Ralstonia solanacearum, Agrobacterium tumefaciens, Xanthomonas vesicatoria, Pseudomonas lachrymans, and Escherichia coli) and two Gram-positive strains (Staphylococcus haemolyticus and Bacillus subtilis). | [1] |
| Flower | In vitro | Anti-bacterial effect | In vitro Anti-bacterial assays | The MS Flower extract can significantly inhibit the growth of five Gram-negative strains (Ralstonia solanacearum, Agrobacterium tumefaciens, Xanthomonas vesicatoria, Pseudomonas lachrymans, and Escherichia coli) and two Gram-positive strains (Staphylococcus haemolyticus and Bacillus subtilis). | [1] |
| Champ | In vivo | Anti-bacterial effect | Mice | The extract of MS regulates glycolipid metabolism, lowers blood glucose levels, improves insulin resistance, and alleviates the metabolic disorders induced by diabetes through the modulation of related metabolic pathways. | [10] |
| Champ | In vivo | Anti-inflammatory activity | Mice | The polysaccharides from MS Champ can alleviate the symptoms of ulcerative colitis induced by dextran sulfate sodium (DSS), significantly reduce the level of intestinal inflammation, decrease pathological damage to the colon tissue, and inhibit the expression of inflammation-related factors. | [57] |
| Champ | In vivo | Anti-inflammatory activity | Mice | MS champ polysaccharides improve gut microbiota and inhibit inflammatory responses. | [58] |
| Champ | In vivo | Anti-inflammatory activity | Mice | MS champ polysaccharides inhibit the expression of pyroptosis-related factors by suppressing the caspase-1/gasdermin D/interleukin-1β signaling pathway, reducing the inflammatory response, and further alleviating tissue damage caused by inflammation. | [59] |
| Champ | In vivo | Antifatigue | Mice | MS extract improves endurance, extends swimming time, and alleviates fatigue symptoms in mice by reducing blood lactate levels and increasing liver glycogen reserves. | [2] |
| Champ | In vivo | Antifatigue | Mice | The polysaccharides from MS champ can enhance the exercise endurance of mice, prolong swimming time, and significantly reduce blood lactate levels. | [60] |
| Champ | In vivo | Antifatigue | Mice | MS champ polysaccharides effectively alleviated fatigue symptoms caused by excessive exercise by enhancing the endurance of mice, prolonging swimming time, reducing blood lactate levels, and increasing liver glycogen reserves. | [61] |
| Champ | In vitro | Regulation of immune function | Cyprinus carpio | The extract of MS champ regulates the expression of immune-related factors, improves the immune response in cyprinus carpio, and enhances resistance to pathogenic microorganisms. | [2] |
| Champ | In vitro | Regulation of immune function | RAW.264 cells | The polysaccharide can enhance the phagocytic function of macrophages, promote the proliferation of leukocytes, regulate the secretion of cytokines by immune cells, and improve the overall function of the immune system. | [17] |
| Champ | In vivo | Improves of intestinal homeostasis | Mice | The polysaccharides from MS can enhance intestinal barrier function, promote the growth of beneficial microbiota, and inhibit the proliferation of harmful bacteria, thereby improving gut health. | [18] |
| Champ | In vitro | Regulation of immune function | RAW.264 cells | MS polysaccharides can significantly enhance the phagocytic ability of macrophages, increase the proliferation rate of leukocytes, and promote immune system activity by regulating the secretion of cytokines. | [53] |
| Champ | In vivo | Regulation of immune function | Mice | MS champ polysaccharides can alleviate cyclophosphamide-induced immunosuppression by improving gut microbiota balance and enhancing immune system function, demonstrating significant immunoregulatory effects. | [60] |
| Champ | In vivo | Improves of intestinal homeostasis | Mice | The polysaccharides from MS champ alleviate intestinal inflammation induced by dextran sulfate sodium (DSS), significantly reduce the level of inflammation in the gut, decrease pathological damage in colon tissue, and inhibit the expression of inflammatory factors, thereby providing protective effects on intestinal health. | [57] |
| Champ | In vivo | Anti-depression | Mice | The extract of MS champ significantly improved the metabolic levels in depressed rats, reducing the changes in metabolites associated with depression, possibly by regulating the synthesis of neurotransmitters and energy metabolism. | [61] |
| Research Type | Research Object | Visual Object | Intervention Time | Dose | Safety Evaluation Description | References |
|---|---|---|---|---|---|---|
| Cytotoxic effect | L929 cells | CCK-8 | 24 h | 50 μg/mL 10 μg/mL 50 μg/mL 200 μg/mL | The extract of MS seeds exhibits good selective cytotoxicity within a certain dosage range and demonstrates high safety for normal cell | [1] |
| Cytotoxic effect | L929 cells | CCK-8 | 24 h | 50 μg/mL 10 μg/mL 50 μg/mL 200 μg/mL | The extract of MS Leaves exhibits good selective cytotoxicity within a certain dosage range and demonstrates high safety for normal cell | [1] |
| Cytotoxic effect | L929 cells | CCK-8 | 24 h | 50 μg/mL 10 μg/mL 50 μg/mL 200 μg/mL | The extract of MS flower exhibits good selective cytotoxicity within a certain dosage range and demonstrates high safety for normal cell | [1] |
| Animal study | Mice | Weight changes, Clinical symptoms | - | - | The given dose of MS Radix extract, no significant acute toxic reactions were observed in the clinical symptoms of mice. | [4] |
| Animal study | Mice | Blood biochemical indicators Histopathology | 14 days | 2 g/(kg·bw) 5 g/(kg·bw) | After a single high-dose administration of MS champ extract, no animal deaths were observed, and the LD50 (median lethal dose) was found to be greater than 5 g/kg, indicating low acute toxicity. | [75] |
| Animal study | Mice | Blood biochemical indicators, Histopathology | 90 days | 0.5 g/(kg·bw) 1 g/(kg·bw) 2 g/(kg·bw) | MS champ extract did not cause significant effects on body weight gain, hematological indicators, blood biochemical markers, or the histopathology of major organs. | [75] |
| Animal study | Mice | Body weight, Hematological parameters, Biochemical indicators, Histopathological changes of major organs | 90 days | 0.86 g/(kg·bw) 2.58 g/(kg·bw) 7.73 g/(kg·bw) | After administration of MS extract, no obvious toxic reactions were observed in the mice’s body weight, hematological parameters, and biochemical indicators, and no significant abnormalities were found in any of the indicators. | [76] |
| Animal study | Mice | Body weight, Hematological parameters, Biochemical indicators, Histopathological changes of major organs | - | - | No significant acute toxic reactions were observed with the given dose of MS leaf extract. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Lou, W. Millettia speciosa and By-Products: A Comprehensive Review of Chemical Composition, Bioactivities, Safety, and Industrial Applications. Foods 2025, 14, 2035. https://doi.org/10.3390/foods14122035
Chen J, Lou W. Millettia speciosa and By-Products: A Comprehensive Review of Chemical Composition, Bioactivities, Safety, and Industrial Applications. Foods. 2025; 14(12):2035. https://doi.org/10.3390/foods14122035
Chicago/Turabian StyleChen, Juntai, and Wenyong Lou. 2025. "Millettia speciosa and By-Products: A Comprehensive Review of Chemical Composition, Bioactivities, Safety, and Industrial Applications" Foods 14, no. 12: 2035. https://doi.org/10.3390/foods14122035
APA StyleChen, J., & Lou, W. (2025). Millettia speciosa and By-Products: A Comprehensive Review of Chemical Composition, Bioactivities, Safety, and Industrial Applications. Foods, 14(12), 2035. https://doi.org/10.3390/foods14122035





