Multi-Omics Joint Analysis of Molecular Mechanisms of Compound Essential Oils Inhibiting Spoilage Yeast in Paocai
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cells and Culture Conditions
2.3. Inhibitory Activity of Compounded Essential Oils Against P. manshurica
2.4. The Morphological Changes Caused by CEOs
2.5. RNA-Seq Analysis
2.6. LC-MS/MS Analysis
2.7. Bioinformatics Analysis
3. Results
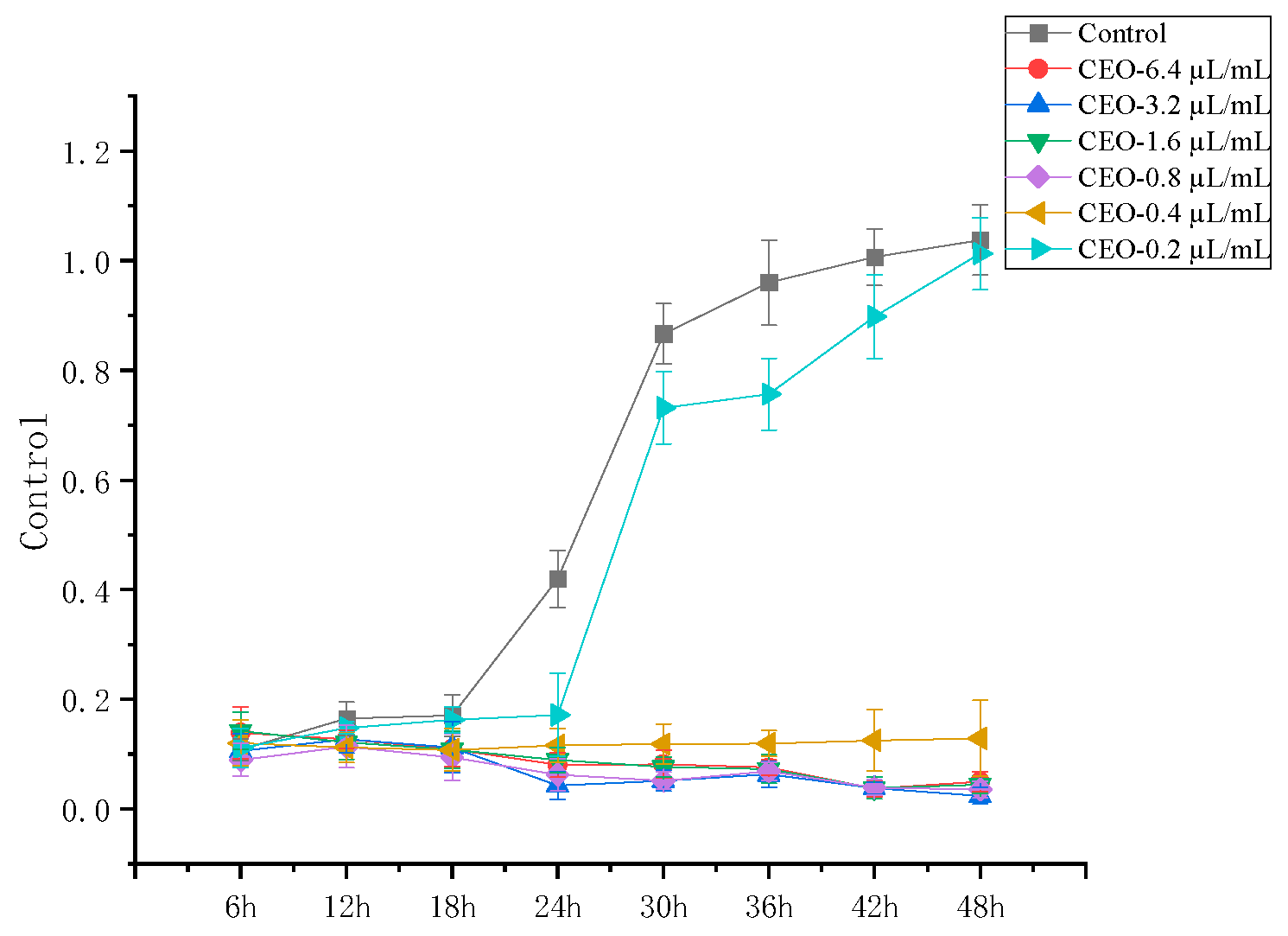
3.1. Effect of CEOs on P. manshurica MICs
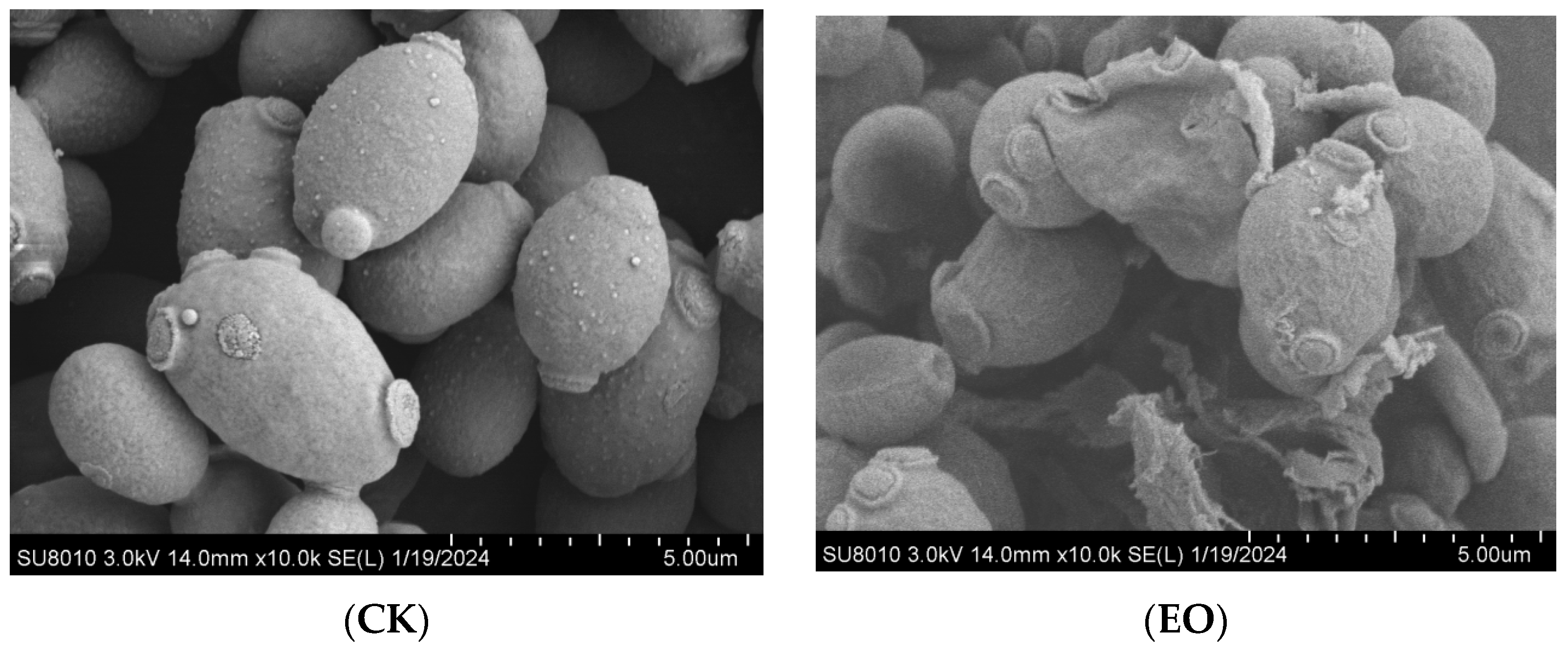
3.2. Effect of CEOs on P. manshurica Cell Morphology
3.3. Transcriptome Sequencing Analysis
3.3.1. Data Processing and Analysis
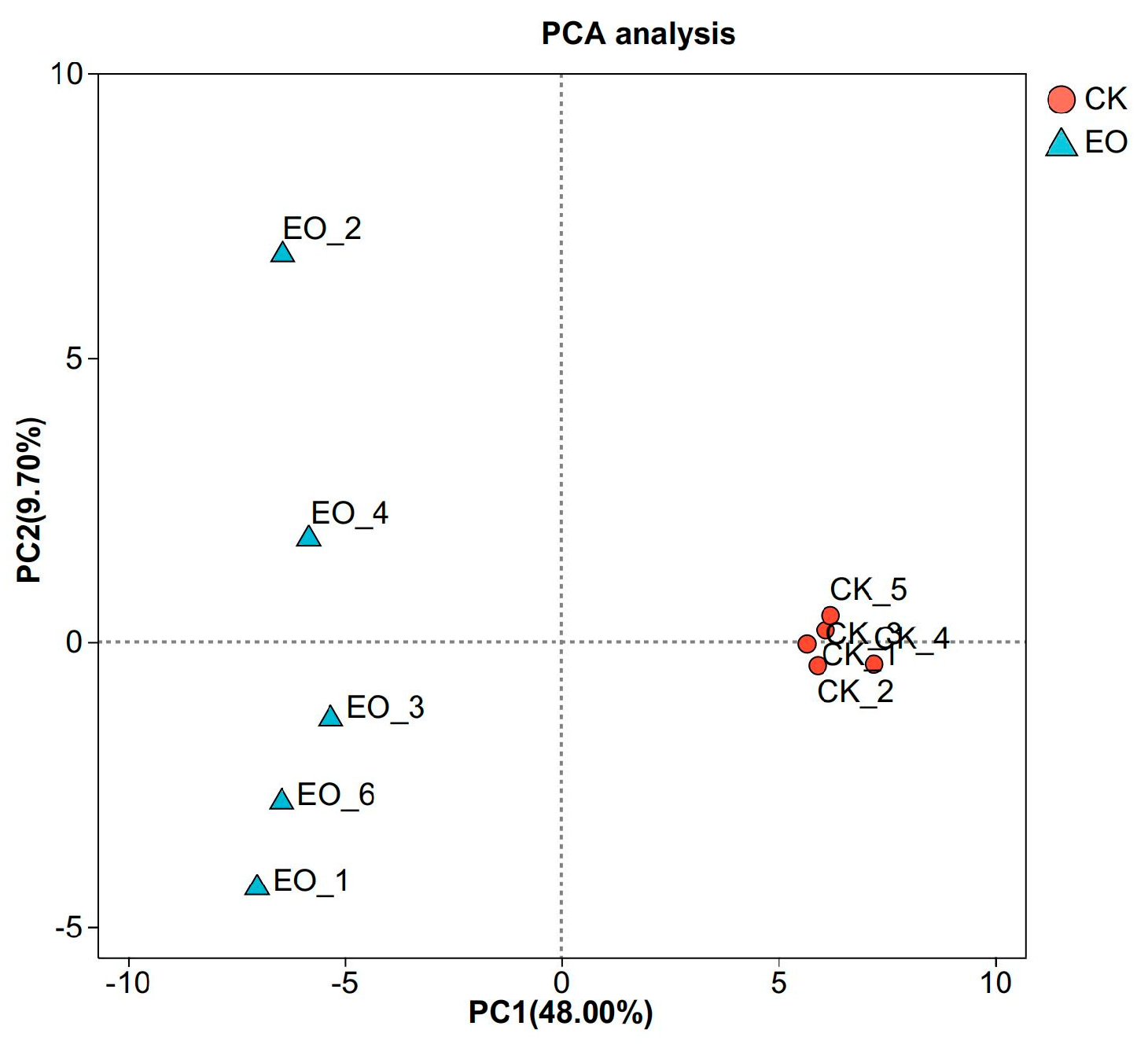
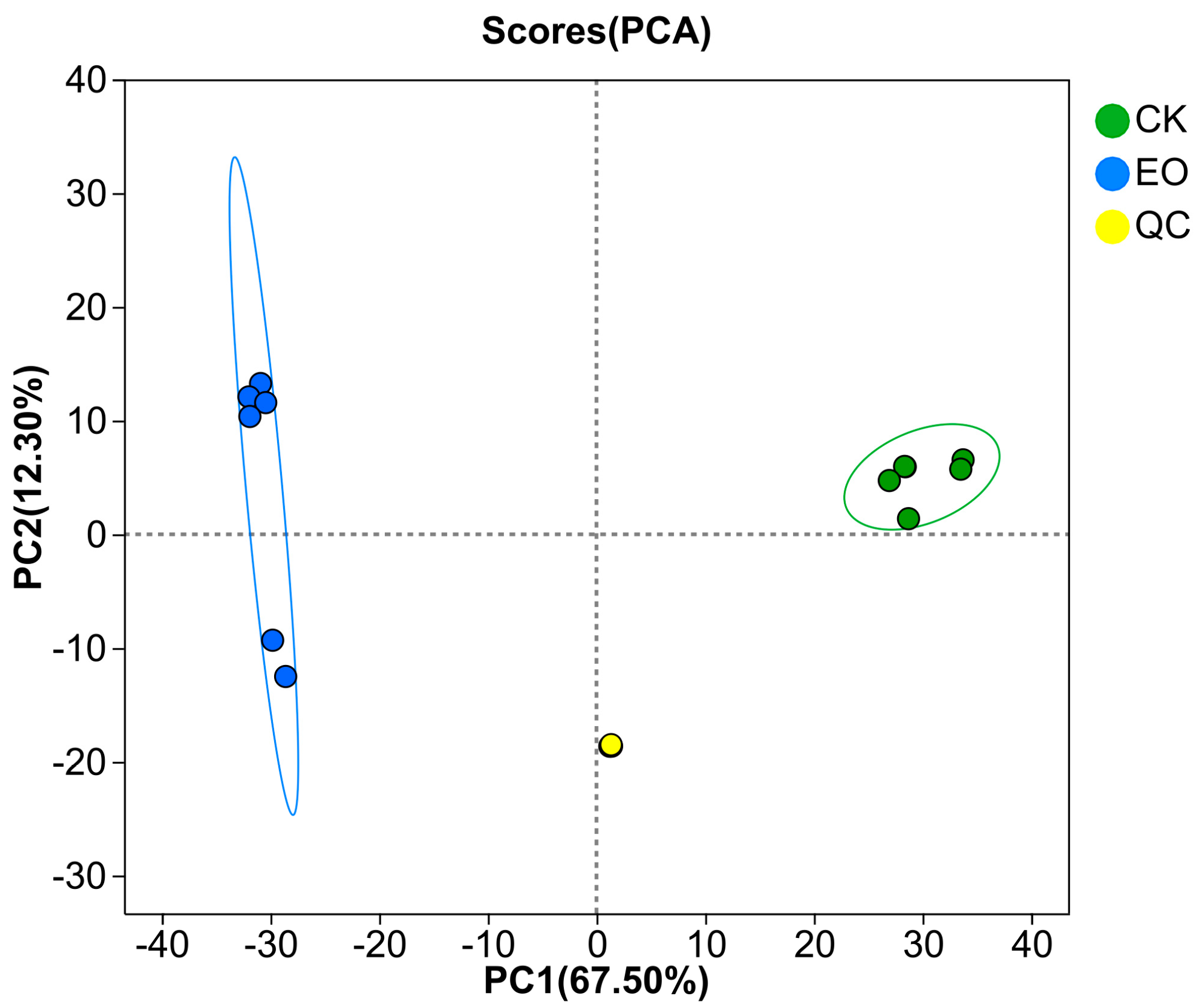
3.3.2. Diversity Analysis
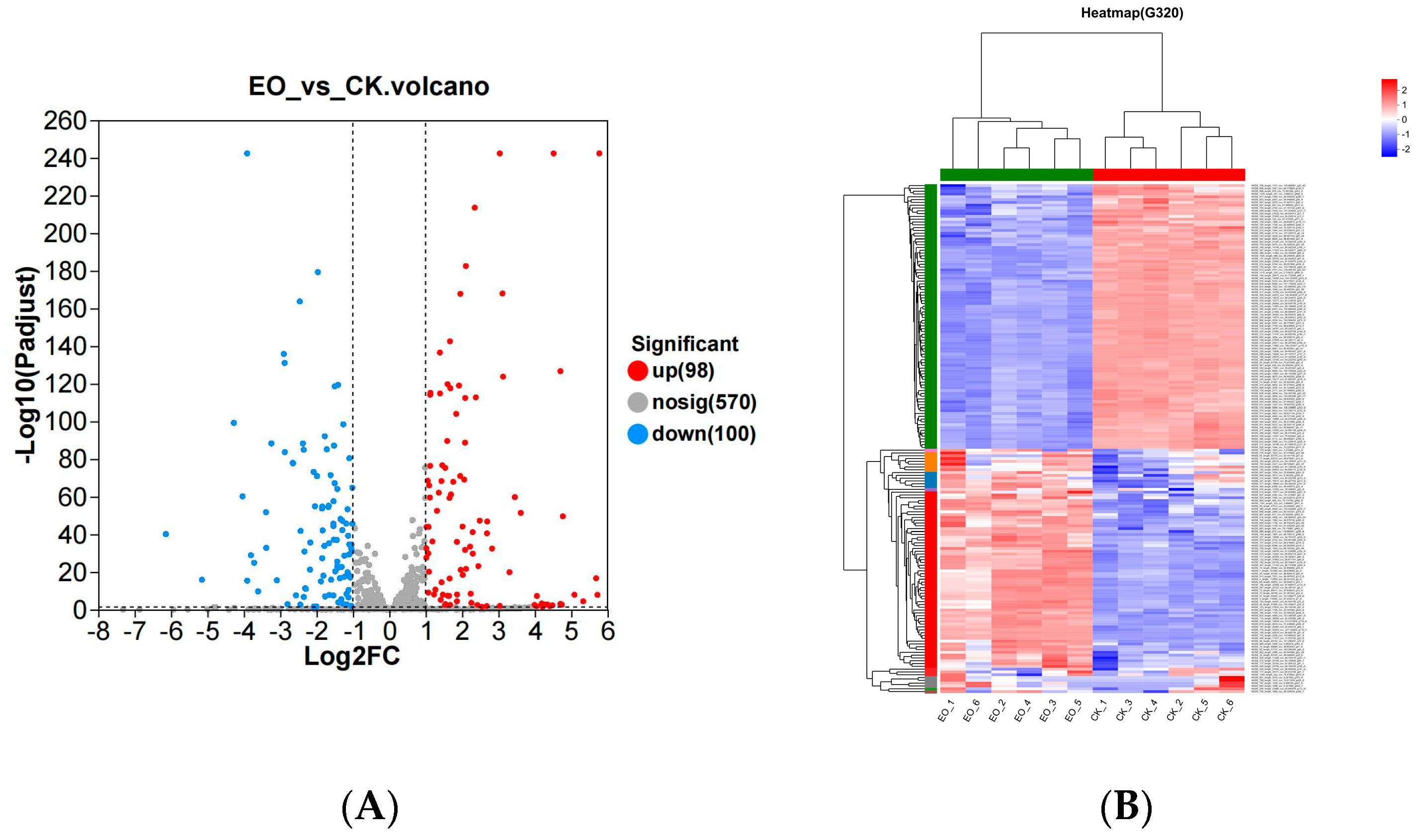
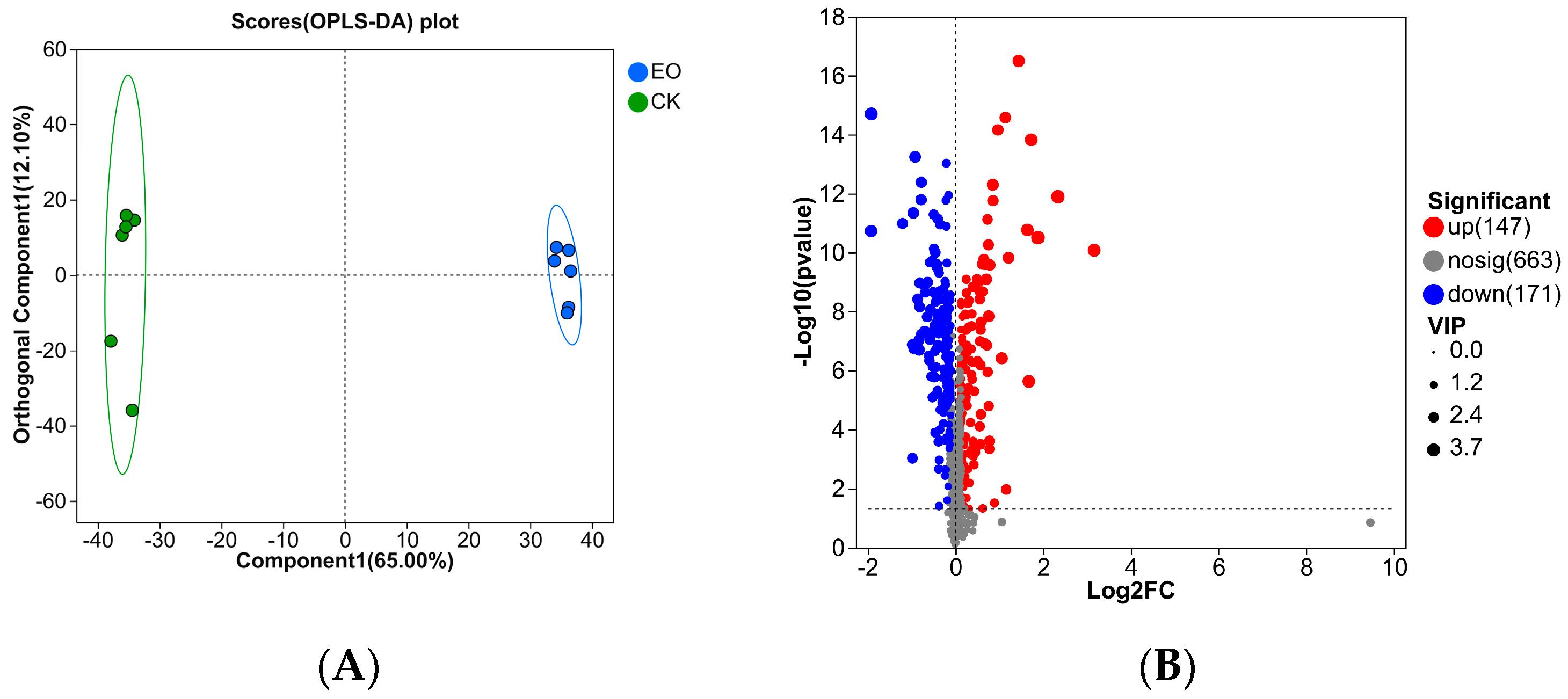
3.3.3. DEGs
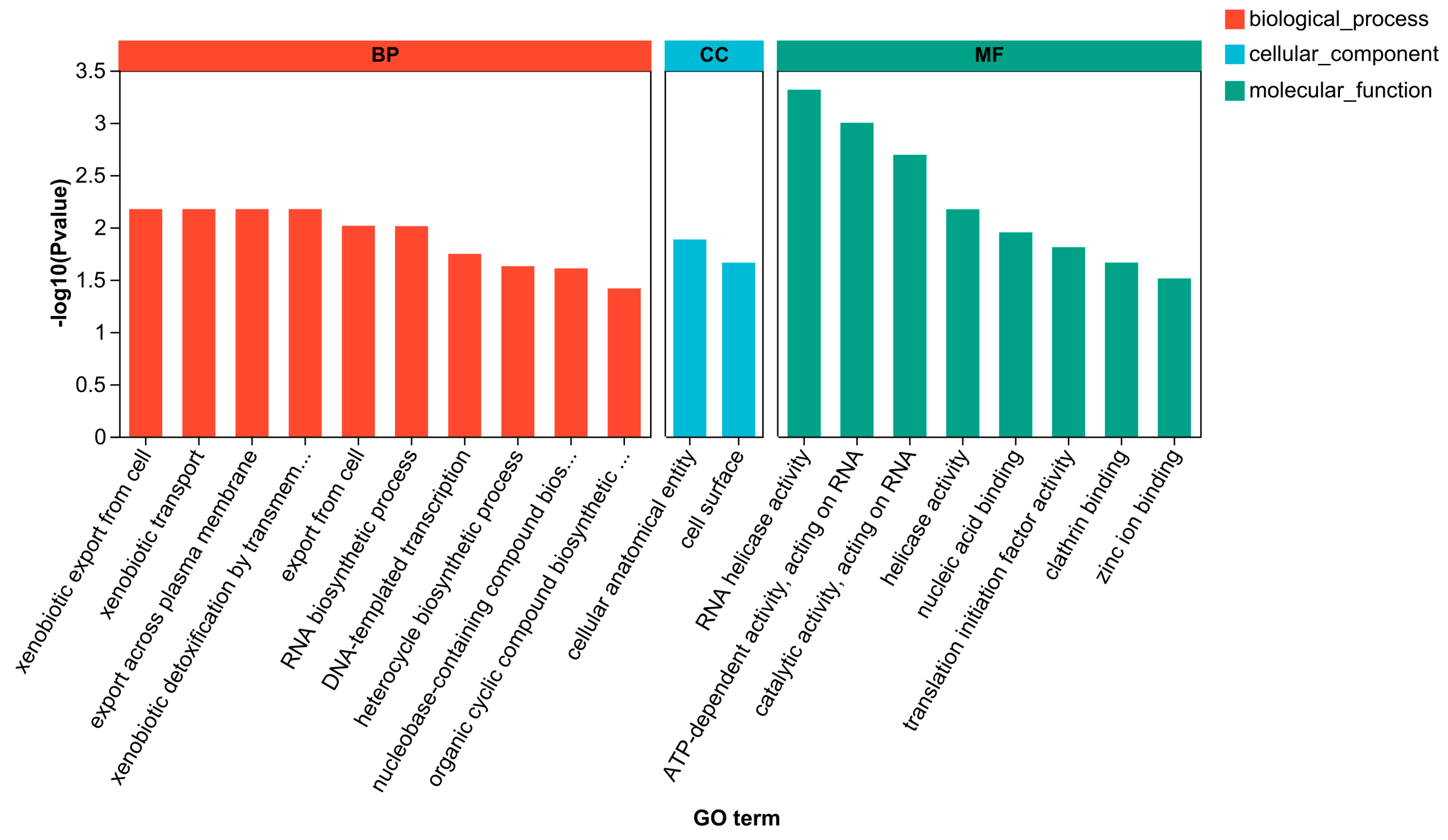
3.3.4. GO Enrichment Analysis
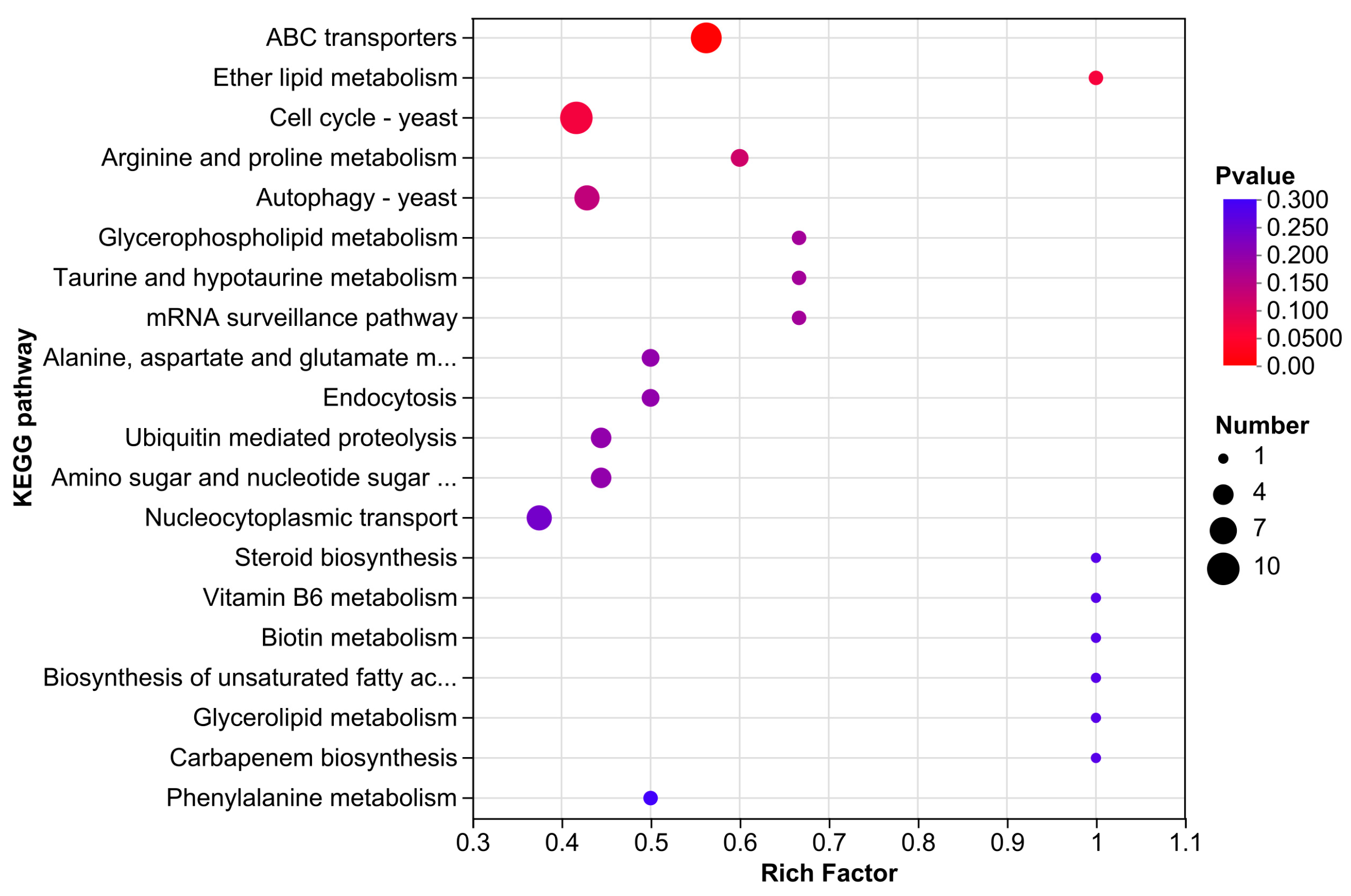
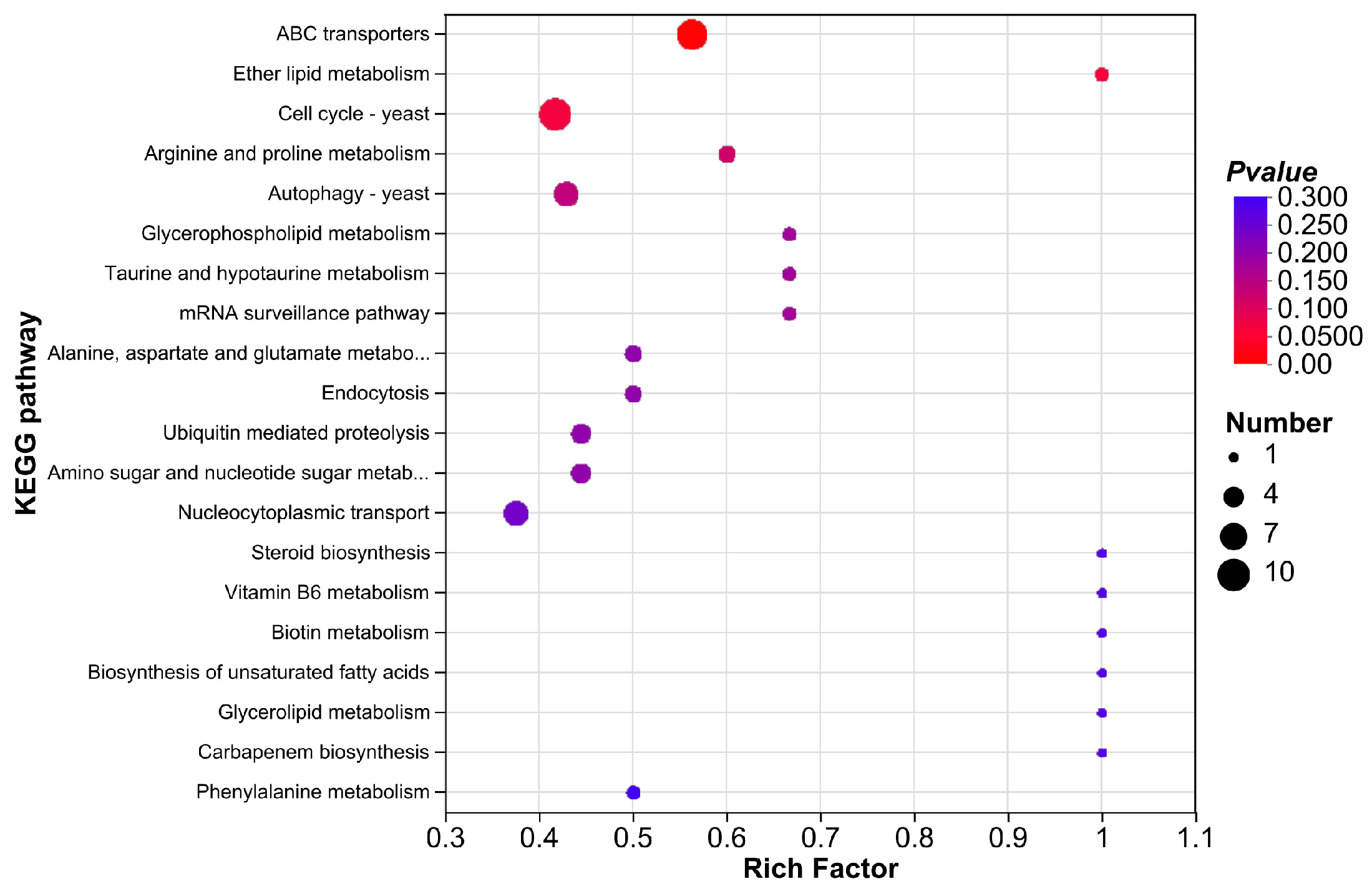
3.3.5. KEGG Enrichment Analysis
3.4. Metabolome Analysis
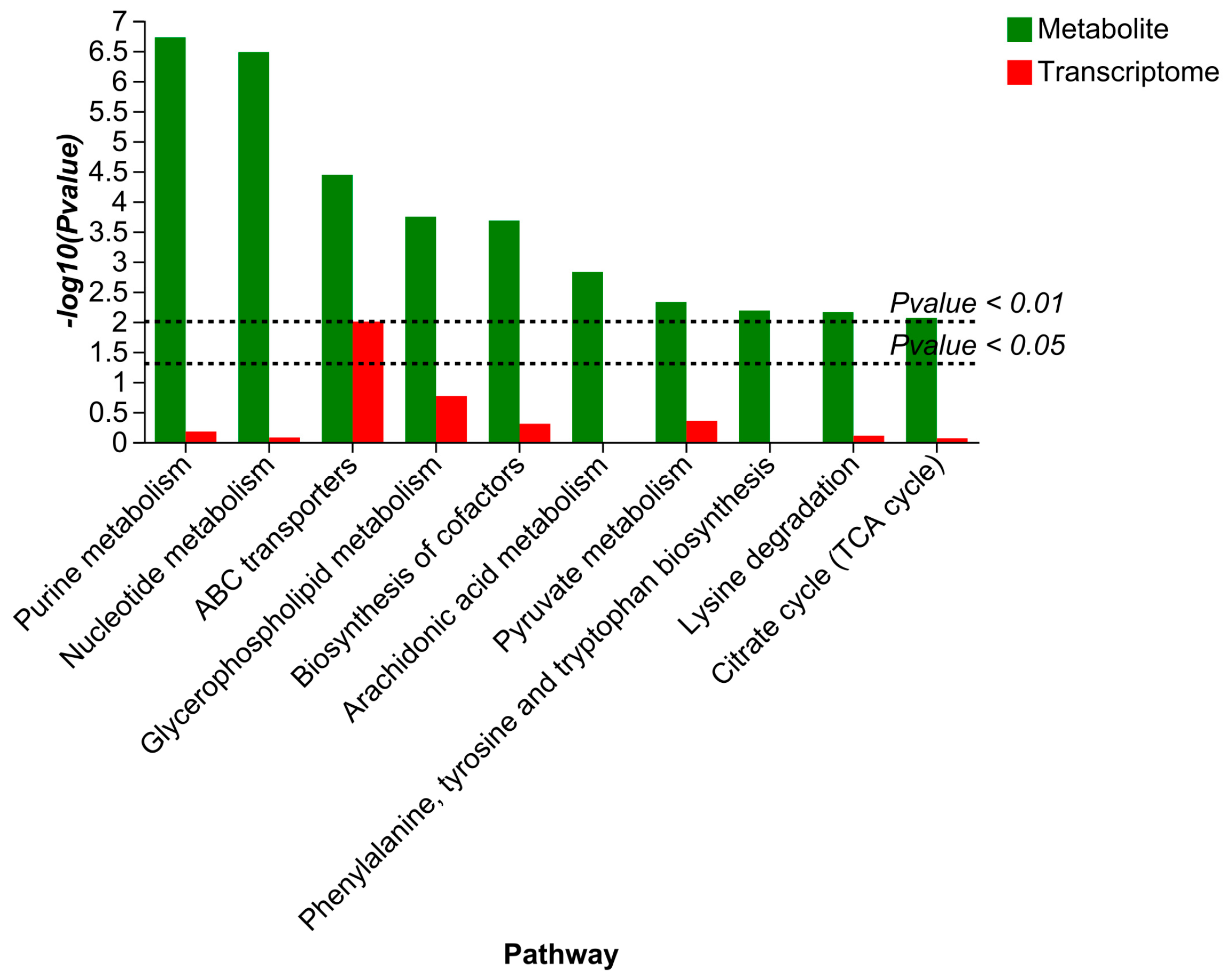
3.5. Multi-Omics Association Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, A.; Li, X.; Pu, B.; Ao, X.; Zhou, K.; He, L.; Chen, S.; Liu, S. Use of Psychrotolerant Lactic Acid Bacteria (Lactobacillus spp. and Leuconostoc spp.) Isolated from Chinese Traditional Paocai for the Quality Improvement of Paocai Products. J. Agric. Food Chem. 2017, 65, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Che, Z.; Li, H.; Liu, L. Influence of oxygen exposure on fermentation process and sensory qualities of Sichuan pickle (paocai). RSC Adv. 2019, 9, 38520–38530. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, G.; Tang, Y.; Li, H.; Shen, W.; Wang, M.; Liu, S.; Qin, W.; Zhang, Q. Effects of temperature on paocai bacterial succession revealed by culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2020, 317, 108463. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Shang, Z.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Effect of ripening and variety on the physiochemical quality and flavor of fermented Chinese chili pepper (Paojiao). Food Chem. 2022, 368, 130797. [Google Scholar] [CrossRef]
- Zheng, X.F.; Yang, Z.Q.; Zhang, H.; Jin, W.X.; Xu, C.W.; Gao, L.; Rao, S.Q.; Jiao, X.A. Isolation of virulent phages infecting dominant mesophilic aerobic bacteria in cucumber pickle fermentation. Food Microbiol. 2020, 86, 103330. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, H.W.; Kim, J.Y.; Kang, S.E.; Roh, S.W.; Hong, S.W.; Yoo, S.R.; Kim, T.W. Impact of fermentation conditions on the diversity of white colony-forming yeast and analysis of metabolite changes by white colony-forming yeast in kimchi. Food Res. Int. 2020, 136, 109315. [Google Scholar] [CrossRef]
- Franco, W.; Pérez-Díaz, I.M. Role of selected oxidative yeasts and bacteria in cucumber secondary fermentation associated with spoilage of the fermented fruit. Food Microbiol. 2012, 32, 338–344. [Google Scholar] [CrossRef]
- Yang, Y.; Lian, Y.; Yin, S.; Suo, H.; Zeng, F.; Wang, H.; Song, J.; Zhang, Y. Inhibition of Lactobacillus fermentum SHY10 on the white membrane production of soaked pickled radish. Food Sci. Nutr. 2022, 10, 2236–2244. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Coimbra, A.; Ferreira, S.; Duarte, A.P. Biological properties of Thymus zygis essential oil with emphasis on antimicrobial activity and food application. Food Chem. 2022, 393, 133370. [Google Scholar] [CrossRef]
- Ashaq, B.; Rasool, K.; Habib, S.; Bashir, I.; Nisar, N.; Mustafa, S.; Ayaz, Q.; Nayik, G.A.; Uddin, J.; Ramniwas, S.; et al. Insights into chemistry, extraction and industrial application of lemon grass essential oil-A review of recent advances. Food Chem. X 2024, 22, 101521. [Google Scholar] [CrossRef] [PubMed]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 2020, 202, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Hlebová, M.; Hleba, L.; Medo, J.; Kováčik, A.; Čuboň, J.; Ivana, C.; Uzsáková, V.; Božik, M.; Klouček, P. Antifungal and synergistic activities of some selected essential oils on the growth of significant indoor fungi of the genus Aspergillus. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2021, 56, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Shi, P.; Zhang, S.; Xiang, W.L.; Liu, J.Y.; Lin, Z.X.; Tang, J. Inhibition of Perilla frutescens Essential Oil on Pellicle Formation of Candida tropicalis and Pichia kluyveri and Its Effect on Volatile Compounds in Sichuan Pickles. Foods 2023, 12, 15. [Google Scholar] [CrossRef]
- Yu, H.; Lin, Z.X.; Xiang, W.L.; Huang, M.; Tang, J.; Lu, Y.; Zhao, Q.H.; Zhang, Q.; Liu, L. Antifungal activity and mechanism of d-limonene against foodborne opportunistic pathogen Candida tropicalis. LWT 2022, 159, 113144. [Google Scholar] [CrossRef]
- Li, Y.; Ren, F.; Chen, D.; Chen, H.; Chen, W. Antibacterial Mechanism of Linalool against Pseudomonas fragi: A Transcriptomic Study. Foods 2022, 11, 2058. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, J.; Miao, Z.; Liang, W.; Wang, G. Metabolome and Transcriptome Profiling Reveal Carbon Metabolic Flux Changes in Yarrowia lipolytica Cells to Rapamycin. J. Fungi 2022, 8, 939. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Chen, H.; Zhong, Q.; Yun, Y.; Chen, W. Metabolomics analysis of the deterioration mechanism and storage time limit of tender coconut water during storage. Foods 2020, 9, 46. [Google Scholar] [CrossRef]
- Sangster, T.; Major, H.; Plumb, R.; Wilson, A.J.; Wilson, I.D. A pragmatic and readily implemented quality control strategy for HPLC-MS and GC-MS-based metabonomic analysis. Analyst 2006, 131, 1075–1078. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. Imeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Shim, J.M.; Kim, D.W.; Yao, Z.; Kim, J.A.; Kim, H.J.; Kim, J.H. Effects of different types of salts on the growth of lactic acid bacteria and yeasts during kimchi fermentation. Food Sci. Biotechnol. 2017, 27, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, N.; Xiao, G.; Bai, W.; Zhang, X.; Zhao, W. Lemon essential oil/vermiculite encapsulated in electrospun konjac glucomannan-grafted-poly (acrylic acid)/polyvinyl alcohol bacteriostatic pad: Sustained control release and its application in food preservation. Food Chem. 2021, 348, 129021. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Simal-Gandara, J.; Murugan, M.; Dhanya, M.K.; Pandian, A. Nutmeg (Myristica fragrans Houtt.) essential oil: A review on its composition, biological, and pharmacological activities. Phytother. Res. 2022, 36, 2839–2851. [Google Scholar] [CrossRef]
- Kang, J.; Sun, Y.; Huang, X.; Ye, L.; Chen, Y.; Chen, X.; Zheng, X.; Han, B.Z. Unraveling the microbial compositions, metabolic functions, and antibacterial properties of Huangshui, a byproduct of Baijiu fermentation. Food Res. Int. 2022, 157, 111320. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants Metabolome Study: Emerging Tools and Techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and Future of Plant Stress Detection: An Overview From Remote Sensing to Positron Emission Tomography. Front. Plant Sci. 2021, 11, 609155. [Google Scholar] [CrossRef]
- Yazgan, H.; Ozogul, Y.; Kuley, E. Antimicrobial influence of nanoemulsified lemon essential oil and pure lemon essential oil on food-borne pathogens and fish spoilage bacteria. Int. J. Food Microbiol. 2019, 306, 108266. [Google Scholar] [CrossRef]
- Rodrigues, L.; Coelho, E.; Madeira, R.; Teixeira, P.; Henriques, I.; Coimbra, M.A. Food Ingredients Derived from Lemongrass Byproduct Hydrodistillation: Essential Oil, Hydrolate, and Decoction. Molecules 2022, 27, 2493. [Google Scholar] [CrossRef]
- Kalli, S.; Vallieres, C.; Violet, J.; Sanders, J.W.; Chapman, J.; Vincken, J.P.; Avery, S.V.; Araya-Cloutier, C. Cellular Responses and Targets in Food Spoilage Yeasts Exposed to Antifungal Prenylated Isoflavonoids. Microbiol. Spectr. 2023, 11, e0132723. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, Z.; Sun, H. Antifungal activities and mechanisms of trans-cinnamaldehyde and thymol against food-spoilage yeast Zygosaccharomyces rouxii. J. Food Sci. 2022, 87, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Meng, Q.; Wang, Y.; Xia, G.; Xia, X.; Shen, J. Comprehensive proteomic and metabolomic profiling of mcr-1-mediated colistin resistance in Escherichia coli. Int. J. Antimicrob. Agents 2019, 53, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Al Naggar, Y.; Wu, Y.; Liu, D.; Hu, Y.; Wang, K.; Jin, X.; Peng, W. Untargeted metabolomics description of propolis’s in vitro antibacterial mechanisms against Clostridium perfringens. Food Chem. 2023, 406, 135061. [Google Scholar] [CrossRef]
- Zhao, L.; Yao, L.; Liu, M.; Qiu, S.; He, J.; Lin, J.; Tao, Z.; Lu, Y.; Deng, S.; Chen, H.; et al. Longistylin A from Cajanus cajan (L.) Millsp. disturbs glycerophospholipid metabolism and cytokinin biosynthesis of Nocardia seriolae. J. Ethnopharmacol. 2024, 330, 118199. [Google Scholar] [CrossRef]
- De Smet, J.; Zimmermann, M.; Kogadeeva, M.; Ceyssens, P.J.; Vermaelen, W.; Blasdel, B.; Bin Jang, H.; Sauer, U.; Lavigne, R. High coverage metabolomics analysis reveals phage-specific alterations to Pseudomonas aeruginosa physiology during infection. ISME J. 2016, 10, 1823–1835. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, G.; Sun, J.; Guo, L.; Jiang, M.; Ou, B.; Zhang, W.; Si, H. Transcriptomic Analysis of Staphylococcus aureus Under the Stress Condition Caused by Litsea cubeba L. Essential Oil via RNA Sequencing. Front. Microbiol. 2020, 11, 1693. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Ning, Y.; Ma, M.; Zhang, Y.; Zhang, D.; Hou, L.; Yang, K.; Fu, Y.; Wang, Z.; Jia, Y. Antibacterial mechanism of sucrose laurate against Bacillus cereus by attacking multiple targets and its application in milk beverage. Food Res. Int. 2022, 154, 111018. [Google Scholar] [CrossRef]
- Liang, S.; Hu, X.; Wang, R.; Fang, M.; Yu, Y.; Xiao, X. The combination of thymol and cinnamaldehyde reduces the survival and virulence of Listeria monocytogenes on autoclaved chicken breast. J. Appl. Microbiol. 2022, 132, 3937–3950. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhu, Z.; Tian, H.; Liu, L.; Li, X.; Pan, J.; Hu, Y.; Niu, Z.; Wang, H.; Liu, X. Multi-Omics Joint Analysis of Molecular Mechanisms of Compound Essential Oils Inhibiting Spoilage Yeast in Paocai. Foods 2025, 14, 1998. https://doi.org/10.3390/foods14111998
Wu X, Zhu Z, Tian H, Liu L, Li X, Pan J, Hu Y, Niu Z, Wang H, Liu X. Multi-Omics Joint Analysis of Molecular Mechanisms of Compound Essential Oils Inhibiting Spoilage Yeast in Paocai. Foods. 2025; 14(11):1998. https://doi.org/10.3390/foods14111998
Chicago/Turabian StyleWu, Xinyi, Zhiyan Zhu, Hao Tian, Li Liu, Xuerui Li, Jun Pan, Yifan Hu, Zhirui Niu, Hanmo Wang, and Xiuwei Liu. 2025. "Multi-Omics Joint Analysis of Molecular Mechanisms of Compound Essential Oils Inhibiting Spoilage Yeast in Paocai" Foods 14, no. 11: 1998. https://doi.org/10.3390/foods14111998
APA StyleWu, X., Zhu, Z., Tian, H., Liu, L., Li, X., Pan, J., Hu, Y., Niu, Z., Wang, H., & Liu, X. (2025). Multi-Omics Joint Analysis of Molecular Mechanisms of Compound Essential Oils Inhibiting Spoilage Yeast in Paocai. Foods, 14(11), 1998. https://doi.org/10.3390/foods14111998



