Evaluating the Microstructure and Bioaccessibility of Bioactive Compounds and Antioxidant Activity After the Dehydration of Red Cabbage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
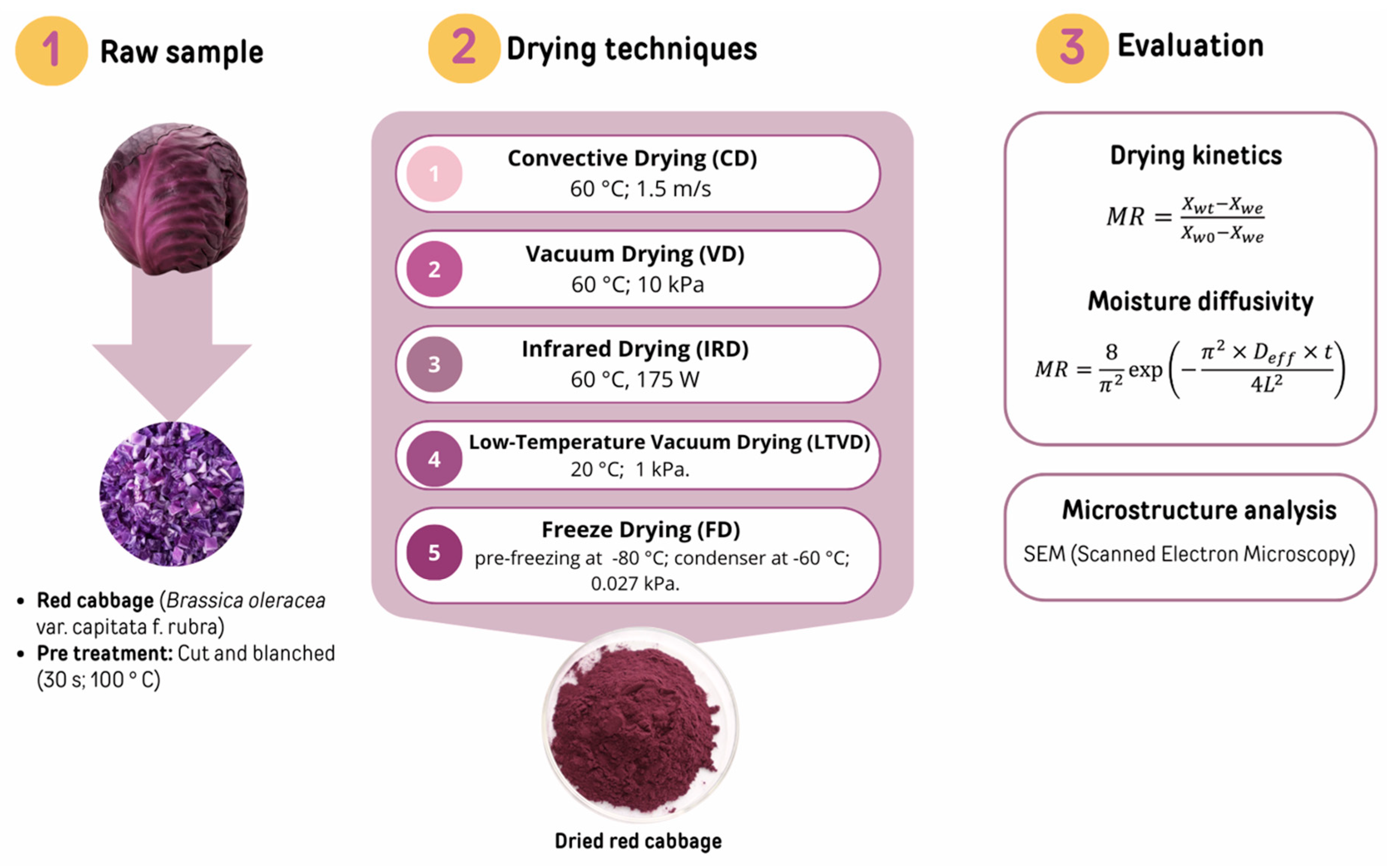
2.2. Preparation of Study Materials, the Drying Procedure, and Microstructure Analysis
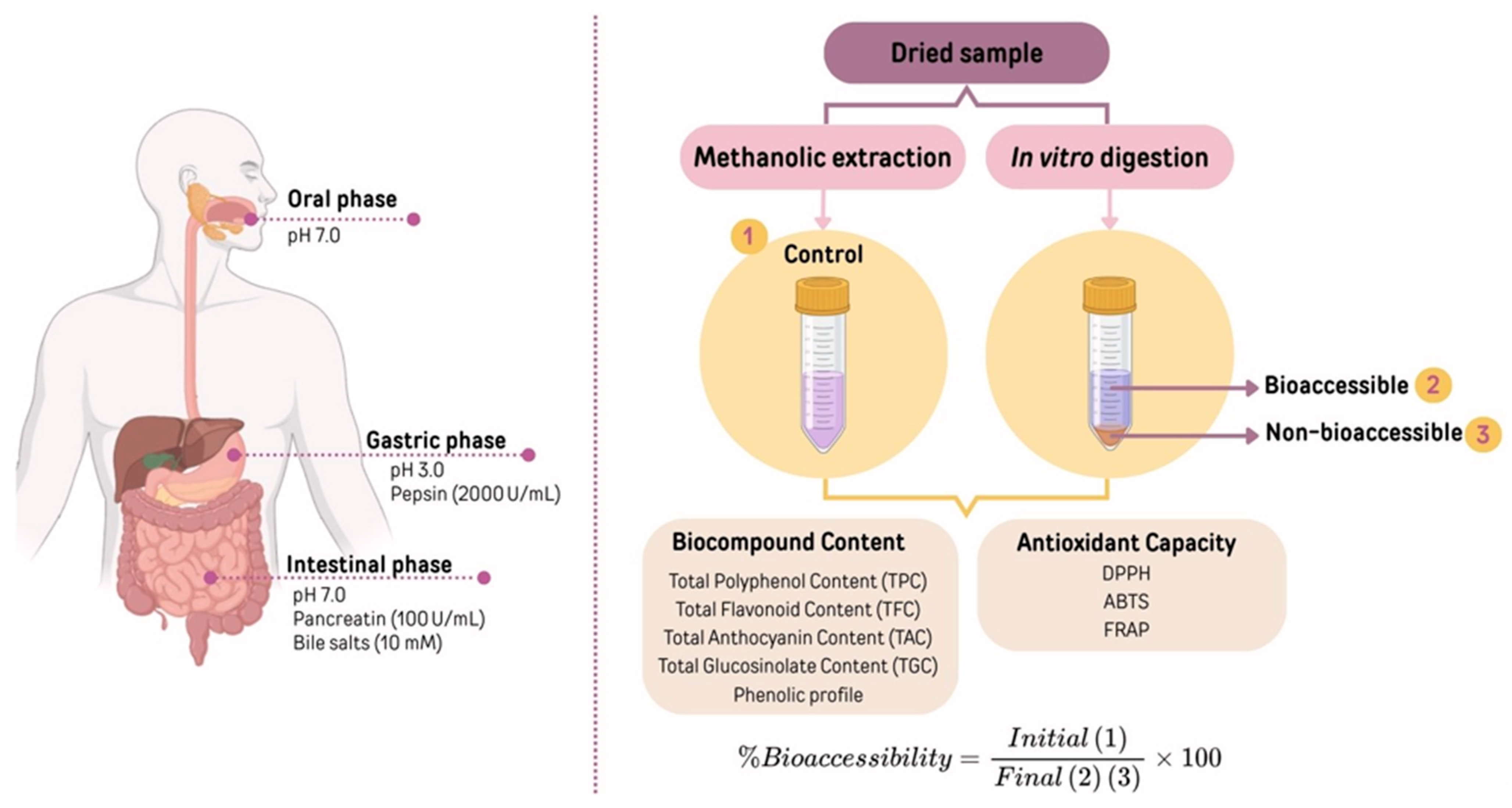
2.3. Procedure for Extracting Bioactive Compounds from Red Cabbage
2.3.1. Extracts Obtained from Dried Red Cabbage
2.3.2. Extracts Obtained During Simulated In Vitro Gastrointestinal Digestion
2.3.3. Extracts Obtained from Digesta
2.4. Bioactive Compound Quantification of Extracts
2.4.1. Quantification of Polyphenol Content
2.4.2. Quantification of Flavonoid Content
2.4.3. Quantification of Anthocyanin Content
2.4.4. Quantification of Glucosinolates Content
2.4.5. Quantification of Individual Phenolic Compounds
2.5. Antioxidant Potential of Extracts
2.5.1. DPPH Radical Scavenging Assay
2.5.2. Ferric-Reducing Antioxidant Power Assay
2.5.3. ABTS Radical Cation Scavenging Capacity Assay
2.6. Statistical Analysis
3. Results and Discussion
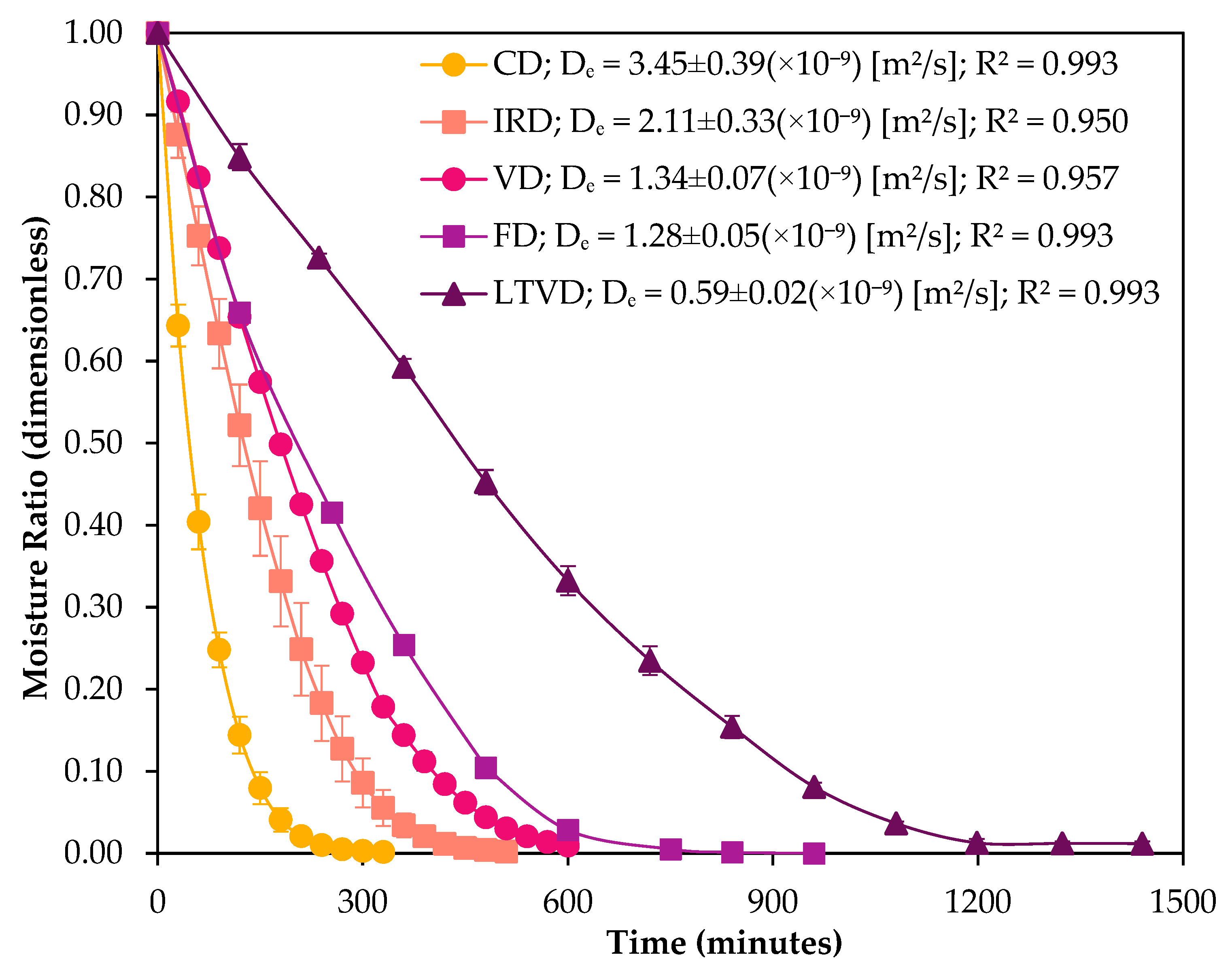
3.1. Drying Kinetics
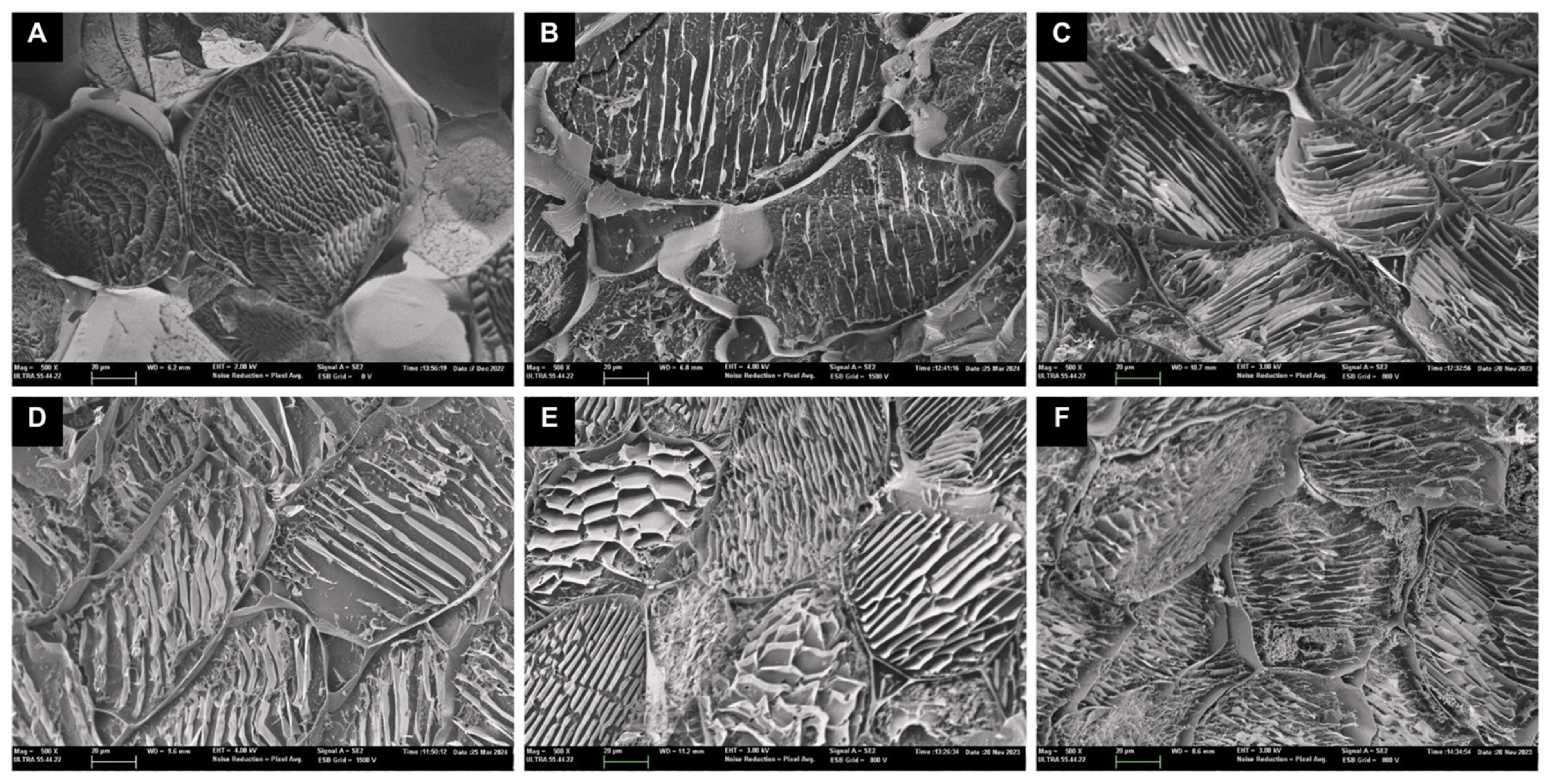
3.2. Microstructure Analysis
3.3. Drying Changes Bioactive Components in Red Cabbage
3.4. Drying Influences the Bioaccessibility of Bioactive Components in Red Cabbage After In Vitro Digestion
3.5. Drying Influences the Bioaccessibility of Individual Phenolic Compounds in Red Cabbage After In Vitro Digestion
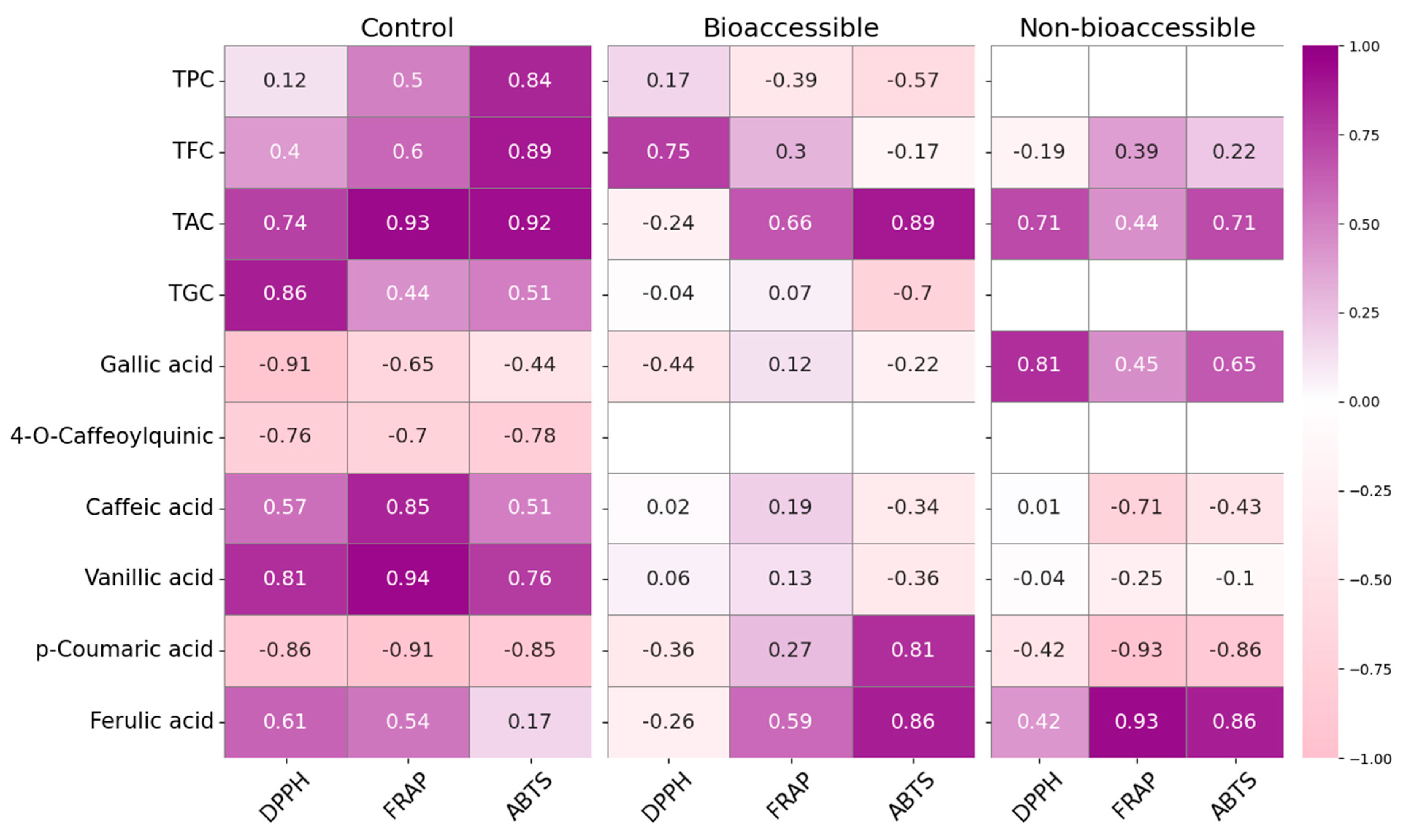
3.6. Drying Influences the Bioaccessibility of Antioxidant Propierties in Red Cabbage After In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, T.; Xing, Y.; Xu, Q.; Yang, S.; Xu, L.; Wang, X.; Yang, P. Physical and chemical properties of purple cabbage as affected by drying conditions. Int. J. Food Prop. 2021, 24, 997–1010. [Google Scholar] [CrossRef]
- Sendri, N.; Singh, S.; Bhatt, S.; Gupta, M.; Bhandari, P. Insight into the influence of oxygen, sunlight and temperature on the stability and color attributes of red cabbage anthocyanins and in vitro gastrointestinal behaviour. Food Chem. Adv. 2023, 3, 100359. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Albuquerque, J.C.; de Andrade, E.W.V.; Gregório, M.G.; Santos, R.M.S.; Rodrigues, T.J.A.; Carvalho, R.O.; Gomes, M.M.A.; Moura, H.V.; et al. Optimization of ultrasound pre-treatment and the effect of different drying techniques on antioxidant capacity, bioaccessibility, structural and thermal properties of purple cabbage. Chem. Eng. Process. 2024, 201, 109801. [Google Scholar] [CrossRef]
- Ávila, S.; Zalamanski, S.; Tanikawa, L.M.; Kruger, C.C.H.; Ferreira, S.M.R. Influence of cooking methods on in vitro bioaccessibility of phenolics, flavonoids, and antioxidant activity of red cabbage. Plant Foods Hum. Nutr. 2023, 78, 124–131. [Google Scholar] [CrossRef]
- Tan, S.; Lan, X.; Chen, S.; Zhong, X.; Li, W. Physical character, total polyphenols, anthocyanin profile and antioxidant activity of red cabbage as affected by five processing methods. Food Res. Int. 2023, 169, 112929. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Obregón-Cano, S.; Mesa-Plata, O.; Cartea-González, M.E.; de Haro-Bailón, A. Quantification and in vitro bioaccessibility of glucosinolates and trace elements in Brassicaceae leafy vegetables. Food Chem. 2021, 339, 127860. [Google Scholar] [CrossRef]
- Luo, S.; An, R.; Zhou, H.; Zhang, Y.; Ling, J.; Hu, H.; Li, P. The glucosinolate profiles of Brassicaceae vegetables responded differently to quick-freezing and drying methods. Food Chem. 2022, 383, 132624. [Google Scholar] [CrossRef]
- Jaiju, D.; Ravichandran, R.; Haridas, P.C.; Delfia, A.; Uchoi, D.; Chatterjee, N.S. Insights from untargeted metabolomics and molecular networking on the effect of infrared drying and electrical oven drying of Turbinaria conoides (J. Agardh) Küzing for nutraceutical application. Food Humanit. 2025, 4, 100630. [Google Scholar] [CrossRef]
- Sakare, P.; Prasad, N.; Thombare, N.; Singh, R.; Sharma, S.C. Infrared drying of food materials: Recent advances. Food Eng. Rev. 2020, 12, 381–398. [Google Scholar] [CrossRef]
- Addo, P.W.; Gariepy, Y.; Shearer, M.; Taylor, N.; MacPherson, S.; Raghavan, V.; Orsat, V.; Lefsrud, M. Microwave-assisted hot air drying of Cannabis sativa: Effect of vacuum and pre-freezing on drying kinetics and quality. Ind. Crops Prod. 2024, 218, 119015. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, K.; Wang, Y.; Dai, B.; Liu, S.; Li, Y. Moisture diffusion and shrinkage characteristics of broad bean during lowtemperature vacuum drying. Int. J. Food Prop. 2020, 23, 2217–2230. [Google Scholar] [CrossRef]
- Mejías, N.; Vega-Gálvez, A.; Gómez-Pérez, L.S.; Pastén, A.; Uribe, E.; Cortés, A.; Valenzuela-Barra, G.; Camus, J.; Delporte, C.; Bernal, G. Health-promoting properties of processed red cabbage (Brassica oleracea var. capitata f. rubra): Effects of drying methods on bio-compound retention. Foods 2024, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- King, V.A.E.; Zall, R.R.; Ludington, D.C. Controlled low-temperature vacuum dehydration–A new approach for low-temperature and low-pressure food drying. J. Food Sci. 1989, 54, 1573–1579. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Lagnika, C.; Li, D.; Liu, C.; Jiang, N.; Song, J.; Zhang, M. A comparative evaluation of nutritional properties, antioxidant capacity and physical characteristics of cabbage (Brassica oleracea var. Capitata var L.) subjected to different drying methods. Food Chem. 2020, 309, 125620. [Google Scholar] [CrossRef]
- Li, X.; Yi, J.; He, J.; Dong, J.; Duan, X. Comparative evaluation of quality characteristics of fermented napa cabbage subjected to hot air drying, vacuum freeze drying, and microwave freeze drying. LWT—Food Sci. Technol. 2024, 192, 115740. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, Y.; Ma, Y.; Zhang, M. Different thermal drying methods affect the phenolic profiles, their bioaccessibility and antioxidant activity in Rhodomyrtus tomentosa (Ait.) Hassk berries. LWT—Food Sci. Technol. 2017, 79, 260–266. [Google Scholar] [CrossRef]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of vegetable fresh-processing residues as functional powdered ingredients. A review on the potential impact of pretreatments and drying methods on bioactive compounds and their bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 654313. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Ozdemir, F.A.; Kołodziej, B. In vitro bioaccessibility and activity of basil (Ocimum basilicum L.) phytochemicals as affected by cultivar and postharvest preservation method—Convection drying, freezing, and freeze-drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Ozkan, K.; Karadag, A.; Sagdic, O. The effects of different drying methods on the in vitro bioaccessibility of phenolics, antioxidant capacity, minerals and morphology of black ‘Isabel’ grape. LWT—Food Sci. Technol. 2022, 158, 113185. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Vega-Gálvez, A.; Gómez-Pérez, L.S.; Ah-Hen, K.S.; Zepeda, F.; García-Segovia, P.; Bilbao-Sainz, C.; Mejías, N.; Pastén, A. Convective hot air drying of red cabbage (Brassica oleracea var. capitata rubra): Mathematical modeling, energy consumption and microstructure. Processes 2024, 12, 509. [Google Scholar] [CrossRef]
- Sánchez-García, J.; Muñoz-Pina, S.; García-Hernández, J.; Heredia, A.; Andrés, A. Fermented quinoa flour: Implications of fungal solid-state bioprocessing and drying on nutritional and antioxidant properties. LWT—Food Sci. Technol. 2023, 182, 114885. [Google Scholar] [CrossRef]
- Muñoz-Pina, S.; Duch-Calabuig, A.; Ruiz de Assín David, E.; Ros-Lis, J.V.; Amorós, P.; Argüelles, Á.; Andrés, A. Bioactive compounds and enzymatic browning inhibition in cloudy apple juice by a new magnetic UVM-7-SH mesoporous material. Food Res. Int. 2022, 162, 112073. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.; Hawkesford, M.J.; De Kok, L.J. The significance of glucosinolates for sulfur storage in Brassicaceae seedlings. Front. Plant Sci. 2014, 5, 704. [Google Scholar] [CrossRef]
- Tanleque-Alberto, F.; Juan-Borrás, M.; Escriche, I. Antioxidant characteristics of honey from Mozambique based on specific flavonoids and phenolic acid compounds. J. Food Compos. Anal. 2020, 86, 103377. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Daneshmandi, M.S.; Sabouri, A.; Kakhki, S.; Emami, S. Assessment of different drying methods with the stability of apocarotenoids and mucilage concentration in saffron (Crocus sativus L.) stigmas based on mathematical modeling. Sci. Hortic. 2024, 338, 113684. [Google Scholar] [CrossRef]
- Luka, B.S.; Vihikwagh, Q.M.; Ngabea, S.A.; Mactony, M.J.; Zakka, R.; Yuguda, T.K.; Adnouni, M. Convective and microwave drying kinetics of white cabbage (Brassica oleracea var. capitata L.): Mathematical modelling, thermodynamic properties, energy consumption and reconstitution kinetics. J. Agric. Food Res. 2023, 12, 100605. [Google Scholar] [CrossRef]
- Wang, D.; Su, D.; Wang, S.; Xu, H.; Song, H. Penetrating microwave vacuum drying: A superior alternative to traditional drying methods for enhancing sensory quality, biological activity and flavor of ‘Akizuki’ pear (Pyrus pyrifolia Nakai). J. Food Compos. Anal. 2025, 142, 107415. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Jiang, R.; Zhang, G.; Dang, Y. Effects of drying methods on the active ingredient content and biological activities of pomegranate peels: An investigation of the potential mechanism of polyphenols as antifungal agents. Food Biosci. 2025, 64, 105947. [Google Scholar] [CrossRef]
- Silva, A.C.C.; Schmidt, F.C. Intensification of freeze-drying rate of coffee extract by vacuum freezing. Innov. Food Sci. Emerg. Technol. 2022, 78, 103022. [Google Scholar] [CrossRef]
- Ozdemir, M.; Pehlivan, S.; Gungor-Bilgicer, V. Ultrasound-assisted vacuum drying of limequat peels and characterization of thermal, morphological and functional properties. Therm. Sci. Eng. Prog. 2024, 54, 102841. [Google Scholar] [CrossRef]
- Pongmalai, P.; Fu, N.; Soponronnarit, S.; Chiewchan, N.; Devahastin, S.; Chen, X.D. Microwave pretreatment enhances the formation of cabbage sulforaphane and its bioaccessibility as shown by a novel dynamic soft rat stomach model. J. Funct. Foods 2018, 43, 186–195. [Google Scholar] [CrossRef]
- Lei, D.; Liu, Y.; Xie, Y.; Sun, W.; Guo, J.; Xu, C. Combined radio frequency heating and pulsed vacuum technology to enhance drying characteristics and heat-mass transfer in peanut pod drying. Innov. Food Sci. Emerg. Technol. 2025, 102, 103976. [Google Scholar] [CrossRef]
- Li, M.X.; Wang, B.; Li, Y.; Nie, X.R.; Mao, J.; Guo, Q.; Xu, N.; Fu, R.; Guo, Z.J.; Zhao, X.L.; et al. Exploration of the impact of different drying methods on the quality of Gastrodia elata: A study based on drying kinetics and multidimensional quality evaluation. Food Chem. 2025, 464, 141628. [Google Scholar] [CrossRef]
- Song, J.; Jin, X.; Han, Y.; Zhai, S.; Zhang, K.; Jia, W.; Chen, J. Infrared drying effects on the quality of jujube and process optimization using response surface methodology. LWT—Food Sci. Technol. 2024, 214, 117089. [Google Scholar] [CrossRef]
- Xie, L.; Mujumdar, A.S.; Fang, X.M.; Wang, J.; Dai, J.W.; Du, Z.L.; Xiao, H.W.; Liu, Y.; Gao, Z.J. Far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) of wolfberry (Lycium barbarum L.): Effects on drying kinetics and quality attributes. Food Bioprod. Process 2017, 102, 320–331. [Google Scholar] [CrossRef]
- Wu, J.; Pan, N.; Chen, X.; Shan, D.; Shi, H.; Qiu, Y.; Liu, Z.; Su, Y.; Weng, J. Comparative evaluation of physical characteristics and volatile flavor components of Bangia fusco-purpurea subjected to hot air drying and vacuum freeze-drying. Curr. Res. Food Sci. 2023, 7, 100624. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, G.; Shanmugam, S.; Galvao, M.S.; Sandes, R.D.D.; Neta, M.T.S.L.; Narain, N.; Mujumdar, A.S. Comparative evaluation of physical properties and volatiles profile of cabbages subjected to hot air and freeze drying. LWT—Food Sci. Technol. 2017, 80, 501–509. [Google Scholar] [CrossRef]
- Morais, R.A.; Polmann, G.; Ferreira, S.R.S.; Block, J.M. In vitro simulated gastrointestinal digestion and its influence on the bioaccessibility and bioactivity of phenolic compounds from Buritirana (Mauritiella armata) pulp, shells, and seeds. Food Res. Int. 2025, 206, 116036. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Pei, Y.; Fan, L.; Wang, C.; Tian, G.; Meng, X.; Li, Z.; Xu, W. Intelligent control of ginger far-infrared radiation and hot-air drying based on multi-sensor fusion technology. Food Bioprod. Process. 2025, 149, 415–427. [Google Scholar] [CrossRef]
- Karabacak, A.O. Effects of different drying methods on drying characteristics, colour and in-vitro bioaccessibility of phenolics and antioxidant capacity of blackthorn pestil (leather). Heat Mass Transf. 2019, 55, 2739–2750. [Google Scholar] [CrossRef]
- Luka, B.S.; Mactony, M.J.; Vihikwagh, Q.M.; Oluwasegun, T.H.; Zakka, R.; Joshua, B.; Muhammed, I.B. Microwave-based and convective drying of cabbage (Brassica oleracea L. var capitata L.): Computational intelligence modeling, thermophysical properties, quality and mid-infrared spectrometry. Meas. Food 2024, 15, 100187. [Google Scholar] [CrossRef]
- Baranzelli, J.; Somacal, S.; Bonini, C.A.A.; Smaniotto, F.A.; Monteiro, C.S.; da Silva, D.T.; de Oliveira Mello, R.; Boldori, J.R.; Denardin, C.C.; Rodrigues, E.; et al. Influence of sprouting on the bioaccessibility and bioactivity of benzoxazinoids, phenolic acids, and flavonoids of soft and hard wheat cultivars. Food Res. Int. 2023, 173, 113338. [Google Scholar] [CrossRef]
- Caicedo-Lopez, L.H.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Campos-Vega, R.; Mendoza, S.; Loarca-Piña, G. Effect of the in vitro gastrointestinal digestion on free-phenolic compounds and mono/oligosaccharides from Moringa oleifera leaves: Bioaccessibility, intestinal permeability and antioxidant capacity. Food Res. Int. 2019, 120, 631–642. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Deng, H.; Yuan, H.; Fan, X.; Yang, H.; Tan, S. In vitro and in vivo bioaccessibility, antioxidant activity, and color of red radish anthocyanins as influenced by different drying methods. Food Chem. X 2023, 18, 100633. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Becerra, C.; Parada, J.; Robert, P. The microencapsulation of maqui (Aristotelia chilensis (Mol.) Stuntz) juice by spray-drying and freeze-drying produces powders with similar anthocyanin stability and bioaccessibility. Molecules 2018, 23, 5127. [Google Scholar] [CrossRef]
- García-Chacón, J.M.; Rodríguez-Pulido, F.J.; Heredia, F.J.; González-Miret, M.L.; Osorio, C. Characterization and bioaccessibility assessment of bioactive compounds from camu-camu (Myrciaria dubia) powders and their food applications. Food Res. Int. 2024, 176, 113820. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Arasu, M.V.; Lee, M.K.; Chun, J.H.; Seo, J.M.; Al-Dhabi, N.A.; Kim, S.J. Analysis and metabolite profiling of glucosinolates, anthocyanins and free amino acids in inbred lines of green and red cabbage (Brassica oleracea L.). LWT 2014, 58, 203–213. [Google Scholar] [CrossRef]
- Martínez-Castro, J.; de Haro-Bailón, A.; Obregón-Cano, S.; García Magdaleno, I.M.; Moreno Ortega, A.; Cámara-Martos, F. Bioaccessibility of glucosinolates, isothiocyanates and inorganic micronutrients in cruciferous vegetables through INFOGEST static in vitro digestion model. Food Res. Int. 2023, 166, 112598. [Google Scholar] [CrossRef]
- Fernández-León, A.M.; Fernández-León, M.F.; González-Gómez, D.; Ayuso, M.C.; Bernalte, M.J. Quantification and bioaccessibility of intact glucosinolates in broccoli ‘Parthenon’ and Savoy cabbage ‘Dama’. J. Food Compos. Anal. 2017, 61, 40–46. [Google Scholar] [CrossRef]
- García-Pérez, P.; Tomas, M.; Rivera-Pérez, A.; Patrone, V.; Giuberti, G.; Capanoglu, E.; Lucini, L. Exploring the bioaccessibility of polyphenols and glucosinolates from Brassicaceae microgreens by combining metabolomics profiling and computational chemometrics. Food Chem. 2024, 452, 139565. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the bioaccessibility of glucosinolates and breakdown products of cruciferous sprouts by simulated in vitro gastrointestinal digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef]
- Vancoillie, F.; Verkempinck, S.H.E.; Sluys, L.; De Mazière, S.; Van Poucke, C.; Hendrickx, M.E.; Van Loey, A.M.; Grauwet, T. Stability and bioaccessibility of micronutrients and phytochemicals present in processed leek and Brussels sprouts during static in vitro digestion. Food Chem. 2024, 445, 138644. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Bolívar, A.; Rabasco-Vílchez, L.; Lafont-Déniz, F.; Luque-Ojeda, J.L.; Pérez-Rodríguez, F. Exploring the bioaccessibility, in vitro colonic fermentation, and the impact on the intestinal microbiota of allyl- and benzyl-isothiocyanate from white and Ethiopian mustard. Food Res. Int. 2025, 203, 115781. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Płatosz, N.; Bartoszek, A. Phytochemical composition and biological activities of differently pigmented cabbage (Brassica oleracea var. capitata) and cauliflower (Brassica oleracea var. botrytis) varieties. J. Sci. Food Agric. 2019, 99, 5499–5507. [Google Scholar] [CrossRef] [PubMed]
- Drozdowska, M.; Leszczyńska, T.; Koronowicz, A.; Piasna-Słupecka, E.; Dziadek, K. Comparative study of young shoots and the mature red headed cabbage as antioxidant food resources with antiproliferative effect on prostate cancer cells. RSC Adv. 2020, 10, 43021–43034. [Google Scholar] [CrossRef] [PubMed]
- Zafar, I.; Hussain, A.I.; Fatima, T.; Alnasser, S.M.A.; Ahmad, A. Inter-varietal variation in phenolic profile, sugar contents, antioxidant, anti-proliferative and antibacterial activities of selected Brassica species. Appl. Sci. 2022, 12, 5811. [Google Scholar] [CrossRef]
- Pashazadeh, H.; Redha, A.A.; Koca, I. Effect of convective drying on phenolic acid, flavonoid and anthocyanin content, texture and microstructure of black rosehip fruit. J. Food Compos. Anal. 2024, 125, 105738. [Google Scholar] [CrossRef]
- Jaouhari, Y.; Bordiga, M.; Travaglia, F.; Coisson, J.D.; Costa-Barbosa, A.; Sampaio, P.; Botelho, C.; Gullón, B.; Ferreira-Santos, P. Microwave-assisted extraction of raspberry pomace phenolic compounds, and their bioaccessibility and bioactivity. Food Chem. 2025, 478, 143641. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brnčić, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef]
- Oancea, S. A review of the current knowledge of thermal stability of anthocyanins and approaches to their stabilization to heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef]
- Colasanto, A.; Disca, V.; Travaglia, F.; Bordiga, M.; Coïsson, J.D.; Arlorio, M.; Locatelli, M. Bioaccessibility of phenolic compounds during simulated gastrointestinal digestion of black rice (Oryza sativa L., cv. Artemide). Food Chem. 2025, 472, 142889. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S. Some recent advances in microstructural modification and monitoring of foods during drying: A review. J. Food Eng. 2014, 123, 148–156. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Lu, P.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. Bioaccessibility and bioactivities of phenolic compounds from roasted coffee beans during in vitro digestion and colonic fermentation. Food Chem. 2022, 386, 132794. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin–Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Noutfia, Y.; Ropelewska, E.; Szwejda-Grzybowska, J.; Mieszczakowska-Frąc, M.; Siarkowski, S.; Rutkowski, K.P.; Konopacka, D. Effects of mild infrared and convective drying on physicochemical properties, polyphenol compounds, and image features of two date palm cultivars: ‘Mejhoul’ and ‘Boufeggous’. LWT–Food Sci. Technol. 2025, 218, 117502. [Google Scholar] [CrossRef]
- Ucar, T.M.; Karadag, A. The effects of vacuum and freeze-drying on the physicochemical properties and in vitro digestibility of phenolics in oyster mushroom (Pleurotus ostreatus). J. Food Meas. Charact. 2019, 13, 2298–2309. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.-N.; Wang, A.-Y.; Zhang, Y.-H.; Guo, Z.; Ye, Y.-Y.; Chen, M.-B. Bioaccessibility and antioxidant activity of fermented chrysanthemum rice wine on in vitro simulated digestion. Food Biosci. 2024, 62, 105144. [Google Scholar] [CrossRef]
- Ozay-Arancioglu, I.; Bekiroglu, H.; Karadag, A.; Saroglu, O.; Tekin-Çakmak, Z.H.; Karasu, S. Effect of different drying methods on the bioactive, microstructural, and in-vitro bioaccessibility of bioactive compounds of the pomegranate arils. Food Sci. Technol. 2022, 42, e06221. [Google Scholar] [CrossRef]
- Domingues, Y.O.; Lemes, G.A.; de Oliveira, F.L.; de Souza, T.R.; Silva, B.; Bento, J.A.C.; Morzelle, M.C. In vitro simulated gastrointestinal digestion and bioaccessibility of phenolic compounds and antioxidants of soursop (Annona muricata L.) peel and pulp. LWT–Food Sci. Technol. 2024, 208, 116694. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]

| Parameter; Extract | Drying Methods | ||||
|---|---|---|---|---|---|
| CD | VD | IRD | LTVD | FD | |
| TPC (mg GAE/g d.m.); Control | 12.64 ± 1.07 aB | 11.22 ± 0.01 bA | 10.53 ± 0.17 bB | 11.29 ± 0.26 bB | 11.39 ± 0.83 abB |
| TPC (mg GAE/g d.m.); Bioaccessible | 21.92 ± 1.72 bA | 13.09 ± 2.38 cA | 31.14 ± 3.28 aA | 21.03 ± 0.65 bA | 15.73 ± 1.83 cA |
| TPC (mg GAE/g d.m.); Non-bioaccessible | n.d. | n.d. | n.d. | n.d. | n.d. |
| TFC (mg CAE/g d.m.); Control | 4.49 ± 0.18 aB | 3.78 ± 0.09 bA | 3.84 ± 0.04 abB | 4.18 ± 0.18 aA | 4.20 ± 0.22 aA |
| TFC (mg CAE/g d.m.); Bioaccessible | 5.09 ± 0.15 aA | 4.02 ± 0.35 cA | 4.74 ± 0.46 abA | 4.24 ± 0.34 bcA | 4.45 ± 0.07 bcA |
| TFC (mg CAE/g d.m.); Non-bioaccessible | 0.49 ± 0.06 aC | 0.38 ± 0.05 abB | 0.37 ± 0.03 bC | 0.39 ± 0.05 abB | 0.42 ± 0.12 abB |
| TAC (mg Cya3glu/g d.m.); Control | 5.03 ± 0.70 abA | 4.30 ± 0.38 bA | 2.62 ± 0.20 cA | 5.17 ± 1.26 aA | 5.65 ± 0.62 aA |
| TAC (mg Cya3glu/g d.m.); Bioaccessible | 3.84 ± 0.23 bcB | 4.01 ± 0.42 bA | 2.94 ± 0.31 dA | 3.57 ± 0.24 cB | 5.72 ± 0.48 aA |
| TAC (mg Cya3glu/g d.m.); Non-bioaccessible | 0.76 ± 0.07 abC | 0.49 ± 0.04 cB | 0.47 ± 0.05 cB | 0.86 ± 0.09 aC | 0.67 ± 0.07 bB |
| TGC (µmol SE/g d.m.); Control | 85.94 ± 4.25 abB | 87.65 ± 1.78 abB | 78.53 ± 2.44 bB | 94.78 ± 9.70 aB | 86.00 ± 2.49 abB |
| TGC (µmol SE/g d.m.); Bioaccessible | 109.50 ± 4.95 bcA | 107.91 ± 9.44 bA | 106.81 ± 11.92 bA | 146.59 ± 13.41 aA | 101.49 ± 4.79 cA |
| TGC (µmol SE/g d.m.); Non-bioaccessible | n.d. | n.d. | n.d. | n.d. | n.d. |
| Phenolic Acids (mg/g d.m.) | Drying Methods | |||||
|---|---|---|---|---|---|---|
| CD | VD | IRD | LTVD | FD | ||
| Gallic acid | Control | 0.18 ± 0.00 aA | 0.18 ± 0.00 aB | 0.18 ± 0.01 aA | n.d. | n.d. |
| Bioaccessible | 0.11 ± 0.01 cB | 0.26 ± 0.01 bA | 0.12 ± 0.00 cA | 0.47 ± 0.09 aA | 0.24 ± 0.01 bA | |
| Non-bioaccessible | 0.02 ± 0.00 bC | 0.02 ± 0.00 bC | 0.03 ± 0.00 bB | 0.04 ± 0.00 aB | 0.01 ± 0.00 cB | |
| 4-O-Caffeoylquinic | Control | 0.10 ± 0.00 bc | 0.10 ± 0.01 b | 0.15 ± 0.01 a | 0.08 ± 0.00 c | 0.10 ± 0.01 bc |
| Bioaccessible | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Non-bioaccessible | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Caffeic acid | Control | 0.06 ± 0.00 cA | 0.06 ± 0.00 cB | 0.06 ± 0.00 dA | 0.09 ± 0.00 bA | 0.18 ± 0.00 aA |
| Bioaccessible | 0.07 ± 0.00 bcA | 0.08 ± 0.01 abA | 0.06 ± 0.00 dA | 0.09 ± 0.01 aA | 0.07 ± 0.01 cdB | |
| Non-bioaccessible | 0.03 ± 0.00 cB | 0.05 ± 0.00 bC | 0.04 ± 0.00 cB | 0.05 ± 0.01 bB | 0.07 ± 0.01 aB | |
| Vanillic acid | Control | 0.40 ± 0.02 cA | 0.29 ± 0.00 dA | 0.09 ± 0.00 eA | 0.60 ± 0.01 bA | 0.87 ± 0.06 aA |
| Bioaccessible | 0.19 ± 0.01 bB | 0.25 ± 0.04 aA | 0.11 ± 0.01 cA | 0.27 ± 0.04 aB | 0.15 ± 0.02 bcB | |
| Non-bioaccessible | 0.01 ± 0.00 dC | 0.01 ± 0.00 cB | n.d. | 0.01 ± 0.00 bC | 0.02 ± 0.00 aC | |
| p-Coumaric acid | Control | 0.08 ± 0.00 cB | 0.10 ± 0.00 bB | 0.12 ± 0.00 aB | 0.06 ± 0.00 dB | 0.05 ± 0.00 dB |
| Bioaccessible | 0.44 ± 0.02 bA | 0.43 ± 0.02 bA | 0.49 ± 0.03 bA | 0.41 ± 0.06 bA | 0.58 ± 0.07 aA | |
| Non-bioaccessible | 0.07 ± 0.00 aB | 0.07 ± 0.01 aB | 0.08 ± 0.00 aB | 0.07 ± 0.01 aB | 0.08 ± 0.01 aB | |
| Ferulic acid | Control | 0.02 ± 0.00 bC | 0.03 ± 0.00 aB | 0.02 ± 0.01 bB | 0.03 ± 0.00 aC | 0.03 ± 0.01 aB |
| Bioaccessible | 0.37 ± 0.02 bA | 0.35 ± 0.00 bA | 0.36 ± 0.03 bA | 0.35 ± 0.01 bA | 0.45 ± 0.01 aA | |
| Non-bioaccessible | 0.06 ± 0.00 aB | 0.06 ± 0.01 aB | 0.05 ± 0.00 aB | 0.06 ± 0.01 aB | 0.05 ± 0.00 aB | |
| Total | Control | 0.84 ± 0.02 | 0.77 ± 0.01 | 0.62 ± 0.04 | 0.86 ± 0.01 | 1.24 ± 0.09 |
| Bioaccessible | 1.19 ± 0.07 | 1.38 ± 0.08 | 1.14 ± 0.07 | 1.59 ± 0.20 | 1.49 ± 0.11 | |
| Non-bioaccessible | 0.19 ± 0.01 | 0.21 ± 0.02 | 0.20 ± 0.01 | 0.24 ± 0.03 | 0.23 ± 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejias, N.; Vega-Galvez, A.; Pasten, A.; Uribe, E.; Andrés, A.; Muñoz-Pina, S.; Khvostenko, K.; García-Segovia, P. Evaluating the Microstructure and Bioaccessibility of Bioactive Compounds and Antioxidant Activity After the Dehydration of Red Cabbage. Foods 2025, 14, 1932. https://doi.org/10.3390/foods14111932
Mejias N, Vega-Galvez A, Pasten A, Uribe E, Andrés A, Muñoz-Pina S, Khvostenko K, García-Segovia P. Evaluating the Microstructure and Bioaccessibility of Bioactive Compounds and Antioxidant Activity After the Dehydration of Red Cabbage. Foods. 2025; 14(11):1932. https://doi.org/10.3390/foods14111932
Chicago/Turabian StyleMejias, Nicol, Antonio Vega-Galvez, Alexis Pasten, Elsa Uribe, Ana Andrés, Sara Muñoz-Pina, Kateryna Khvostenko, and Purificación García-Segovia. 2025. "Evaluating the Microstructure and Bioaccessibility of Bioactive Compounds and Antioxidant Activity After the Dehydration of Red Cabbage" Foods 14, no. 11: 1932. https://doi.org/10.3390/foods14111932
APA StyleMejias, N., Vega-Galvez, A., Pasten, A., Uribe, E., Andrés, A., Muñoz-Pina, S., Khvostenko, K., & García-Segovia, P. (2025). Evaluating the Microstructure and Bioaccessibility of Bioactive Compounds and Antioxidant Activity After the Dehydration of Red Cabbage. Foods, 14(11), 1932. https://doi.org/10.3390/foods14111932







