Abstract
Meat is a vital source of high-quality proteins, amino acids, vitamins, and minerals essential for human health. Growing demand for healthier lifestyles and technological advancements has heightened the focus on meat products, whose quality depends on meat protein properties, such as texture, water holding capacity (WHC), and structural integrity. Non-thermal processing technologies are gaining attention for enhancing the gelation properties of meat protein gels (MPGs) by optimizing solubilization, denaturation, and aggregation while preserving nutritional and sensory qualities and avoiding the drawbacks of thermal treatments. This review focuses on advanced non-thermal processing techniques, including high-pressure processing (HPP), pulsed electric fields (PEFs), ultrasound, and cold plasma, and their impact on MPGs. It also examines vibrational spectroscopy methods, such as Fourier Transform Infrared (FTIR) and Raman spectroscopy, for non-invasive analysis of MPGs. The integration of these approaches with hyperspectral imaging and machine learning is also explored as a tool to improve quality control and assessment.
1. Introduction
The growing global population and rising consumer expectations have intensified demand for high-quality meat products, which remain a key source of essential proteins, vitamins, and minerals [1,2]. According to the Organization for Economic Cooperation and Development, global meat consumption reached 35 kg per capita in 2024, highlighting the need to improve meat quality while ensuring safety and nutritional value.
The fundamental quality attributes of meat texture, WHC, and sensory traits are closely linked to the functional behavior of myofibrillar proteins such as myosin and actin [3] (Figure 1A). These proteins play essential roles in forming three-dimensional gel networks during thermal processing (Figure 1B). However, excessive heating can compromise their functionality [4], which has prompted increasing interest in non-thermal techniques that preserve protein structure while enhancing product quality [5,6].
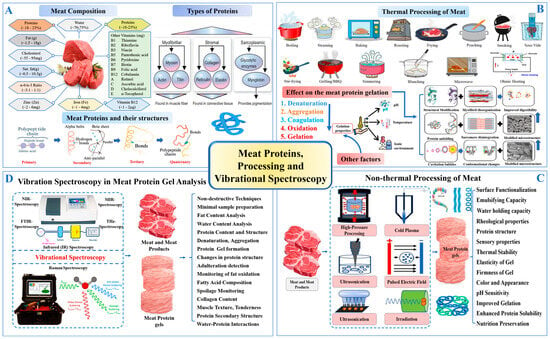
Figure 1.
(A) A representation of meat composition, types of meat proteins, and their structure. (B) The thermal processing of meat and its effect on meat protein gel formation. (C) The non-thermal meat processing and its effect on meat protein gel. (D) The application of vibrational spectroscopy in meat protein and meat protein gel analysis.
Lately, various non-thermal techniques, including high-pressure processing (HPP), pulsed electric fields (PEFs), ultrasound, and cold atmospheric plasma (CAP), have been investigated for their ability to enhance the functionality of meat proteins while preserving nutritional and sensory qualities. Among these, HPP has been shown to enhance gel strength and WHC in myofibrillar proteins by promoting protein–protein interactions without causing extensive denaturation. Baldi et al. [7] and Li et al. [8] have demonstrated that PEF and ultrasound treatments can improve the texture and WHC of meat products by altering protein structures and increasing solubility. Meanwhile, CAP has emerged as a promising technique for enhancing protein gelation through oxidative modifications that increase cross-linking and protein aggregation [9]. As shown in Figure 1C, these innovative non-thermal methods help mitigate the limitations of traditional thermal treatments, offering improved texture, flavor retention, and extended shelf life while maintaining nutritional integrity. Thus, to optimize meat product quality, it is crucial to track the structural changes in proteins throughout the processing stages. Integrating advanced analytical methods with conventional processing is vital for understanding the impact on texture, WHC, and overall meat quality. Despite extensive research into individual non-thermal techniques and analytical methods, there remains a lack of comprehensive reviews addressing their combined effects on the structural and functional attributes of MPGs. In addition to vibrational spectroscopy, other viable tools for assessing protein structure include fluorescence spectroscopy, nuclear magnetic resonance (NMR), differential scanning calorimetry (DSC), and electron microscopy. Nonetheless, the synergistic use of these methods, particularly vibrational spectroscopy, for real-time protein structure monitoring has not been fully explored.
Vibrational spectroscopy techniques, including Near-Infrared (NIR) [10], Fourier Transform Infrared (FTIR) [11], and Raman spectroscopy [12], are widely used to develop non-destructive monitoring strategies, offering a rapid alternative to conventional destructive methods such as chemical extraction or histological analysis. Significant advancements in these techniques have broadened the scope of meat protein analysis. As illustrated in Figure 2A, the timeline of advancements from 2017 to 2024 presents technological milestones, including innovations like Raman Chemical Imaging (RCI) and Surface-Enhanced Raman Spectroscopy (SERS), as well as advancements in Mid-infrared (MIR) and Attenuated Total Reflectance (ATR) methodologies. Collectively, these innovations indicate a growing trend toward applying vibrational spectroscopy in the analysis of meat proteins. Figure 2B reflects the increasing research focus for the past 2 decades, highlighting the rise in scientific publications utilizing these spectroscopic techniques, which accentuates their expanding role in enhancing meat quality assessment.
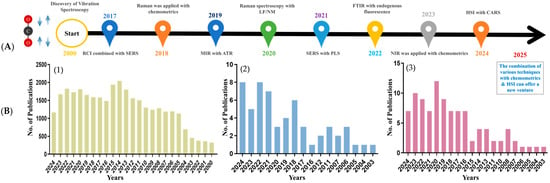
Figure 2.
(A) The time-based evolution of vibrational spectroscopic techniques (2017 to 2024) [13]. (B) (1) Number of publications for vibration spectroscopy for meat protein analysis. (2) IR spectroscopy for meat protein analysis. (3) Raman spectroscopy for meat protein analysis. Abbreviations: RCI = Raman Chemical Imaging, SERS = Surface-enhanced Raman Spectroscopy, MIR = Mid-infrared, ATR = Attenuated Total Reflectance, LF/NMR = Low-field Nuclear Magnetic Resonance, PLS = Partial Least Square, FTIR = Fourier Transform Infrared, NIR = Near Infrared, HIS = Hyperspectral Imaging, CARS = Competitive Adaptive Reweighted Sampling.
Nevertheless, the integration of vibrational spectroscopy with advanced chemometric analysis allows for precise monitoring of MPGs during processing. Advanced computational techniques, such as Partial Least Square (PLS) modeling, machine learning algorithms like Extreme Learning Machine (ELM) and Support Vector Machine (SVM), alongside deep learning models [14], are transforming the precision of predicting protein structural changes and assessing water content in meat products. These methods leverage data-driven analyses combined with spectroscopic data to significantly enhance meat quality evaluations. For instance, Nunekpeku et al. successfully employed a Long Short-Term Memory (LSTM) deep learning model integrated with fused Raman and NIR spectra to accurately predict the gel strength of minced chicken, showcasing the synergy of spectral data with advanced computational tools [12]. In a similar study, Li et al. utilized an SVM algorithm to optimize the gel quality of pork by incorporating various starches, effectively predicting gel strength and WHC, thereby demonstrating the potential of machine learning in improving the functional properties of meat gels [15].
Accordingly, this review explores recent advancements in non-thermal processing techniques, particularly focusing on methods such as HPP, ultrasound, CAP, and PEF and their impact on the structural and functional attributes of meat myofibrillar proteins. Furthermore, it highlights the use of vibrational spectroscopy techniques, such as FTIR and Raman spectroscopy, for real-time monitoring of protein gelation processes. By leveraging these non-invasive analytical methods, the industry can achieve consistent production of high-quality meat products, meeting consumer expectations for both safety and nutritional value while optimizing processing efficiency.
2. Role of Meat Proteins in Nutrition and Meat Processing
The functional attributes of meat proteins are key to the appeal and utility of meat and its derivatives, including their role in defining texture, flavor, and nutritional quality [16]. Meat proteins are typically categorized into three main types: myofibrillar, sarcoplasmic, and connective tissue proteins, each contributing uniquely to the quality and performance of meat products [17].
Myofibrillar proteins, which comprise about 55% to 60% of muscle proteins, are the most abundant and are critical for muscle contraction as well as MPG formation. These proteins include key structural components such as myosin, actin, tropomyosin, the troponin complex, nebulin, and titin Figure 1A. Myosin, being the most prevalent myofibrillar protein, is essential in meat gelation and WHC due to its ability to form strong, elastic gels upon thermal processing [18]. Actin collaborates with myosin to preserve structural integrity, while proteins such as tropomyosin and the troponin complex regulate muscle contraction. Additionally, nebulin and titin provide stability and elasticity to the sarcomere, forming the foundation of the muscle framework. During processing, these myofibrillar proteins undergo denaturation, exposing hydrophobic regions that promote protein aggregation. This aggregation leads to the development of a cohesive gel network, which is critical for achieving the desired texture and firmness in processed meat products.
Sarcoplasmic proteins, which are water-soluble and make up approximately 25% to 30% of muscle proteins, are critical for the biochemical and sensory attributes of meat [19]. These proteins include enzymes and pigments like myoglobin, which significantly influence meat color, a key factor in consumer acceptance. The oxidation state of myoglobin dictates the color of both fresh and processed meats. Additionally, sarcoplasmic proteins include enzymes such as proteases, which are vital in tenderization and flavor development during postmortem aging. While these proteins do not directly contribute to gel formation, they are crucial for preserving the appearance, flavor, and shelf life of meat products [20,21].
Connective tissue proteins, mainly collagen and elastin, provide structural support to muscle fibers and significantly affect meat texture and tenderness. The ratio of myofibrillar to connective tissue proteins determines gel elasticity, with higher collagen content requiring pretreatment to achieve optimal texture [22]. Collagen is abundant in connective tissues and can convert to gelatin upon heating, thereby enhancing meat tenderness and juiciness (Figure 1A). Conversely, elastin contributes to the elasticity and firmness of meat but is more resistant to thermal denaturation than collagen. The distribution and amount of collagen within meat influence its tenderness; higher collagen content is generally associated with tougher meat, especially in heavily exercised muscles. However, during cooking, the breakdown of collagen significantly contributes to the gelation process, improving the texture and WHC of certain processed meat products.
3. Thermal Processing of Meat and Its Impact on MP and MPG’s Structure
Thermal processing remains the cornerstone of traditional MPG formation, leveraging three key techniques: (1) heat-induced gelation, (2) chemical cross-linking, and (3) mechanical manipulation. Among these, heat-induced gelation is the most employed [23], particularly for myofibrillar proteins. The process typically involves solubilizing proteins using salts to enhance ionic strength, followed by controlled heating therefore causing the proteins to denature, exposing hydrophobic regions that promote aggregation and gel formation [24,25]. However, while effective, this method can result in over-denaturation if heating is excessive, leading to brittle gels with reduced WHC, undesirable textures, and diminished digestibility. As demonstrated in Figure 3, non-uniform heating may further compromise gel consistency, affecting the structural integrity and mouthfeel of the meat products [26,27].
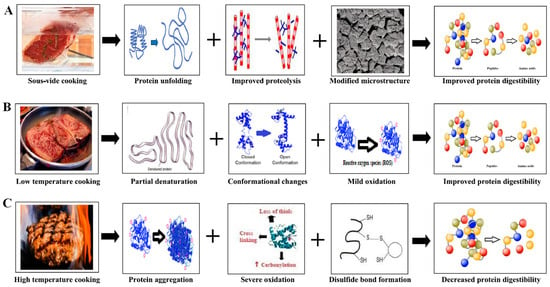
Figure 3.
(A) Effect of sous-vide cooking on meat protein’s unfolding, proteolysis, microstructure, and protein digestibility. (B) Effect of low-temperature cooking on denaturation, oxidation, conformational and protein digestibility. (C) Effect of high-temperature cooking induces protein aggregation, promotes oxidation, and facilitates disulfide bond formation [28].
Chemical cross-linking, using agents such as transglutaminase, enhances gel strength and cohesiveness by creating covalent bonds between protein molecules, thus stabilizing the gel matrix [29]. However, this approach is not without challenges. While effective in improving gel stability, the use of chemical additives raises concerns regarding food safety, potential allergenicity, and regulatory scrutiny. There are also implications for consumer perception, as these additives may alter the natural qualities of the meat, leading to hesitancy in adoption [30].
On the other hand, mechanical techniques such as chopping, mixing, and emulsifying are widely applied to improve the homogeneity of the meat matrix, enhance protein–water interactions, and promote the formation of stable gel networks during subsequent thermal processing [31,32]. These processes physically disrupt muscle fibers, exposing hydrophobic regions that enhance protein aggregation during subsequent heat treatment. Nonetheless, excessive mechanical action can degrade product quality, resulting in rubbery or overly dense gels, which negatively affect consumer satisfaction. Additionally, the energy-intensive nature of these methods can escalate production costs, especially in large-scale meat processing operations [33].
As the demand for high-quality, sustainable, and minimally processed meat products grows, the exploration of innovative approaches to MPG formation has become increasingly critical. This shift is driven not only by technological advancements but also by evolving consumer preferences for safer, more natural, and environmentally conscious food production. Addressing these demands requires the development of processing methods that are gentler, more precise, and capable of preserving the native structure of proteins while enhancing gel functionality. The next step in this exploration focuses on leveraging novel non-thermal approaches to overcome the constraints of thermal processing, offering a pathway to optimize MPG formation and improve the quality and appeal of meat products.
4. Non-Thermal Processing of Meat and Its Impact on MP and MPG’s Structure
Non-thermal processing methods, such as HPP, PEF, ultrasound, and CAP, offer significant advantages in enhancing MPGs without the drawbacks of heat-induced damage [34]. These techniques not only preserve the structural integrity of meat proteins but also enhance gel formation, WHC, and texture, thereby improving the overall quality of meat products. By preventing excessive protein denaturation, non-thermal methods help maintain the nutritional value of meat, offering a healthier alternative to traditional thermal treatments while ensuring superior sensory characteristics. Additionally, packaging technologies like modified atmospheric packaging (MAP), also referred to as protective gas packaging, are employed in meat processing to extend shelf life and preserve product quality by reducing oxidation and microbial growth [35]. The following sections discussed the effect of various non-thermal processing techniques on MP and MPG’s structure.
4.1. Effect of HPP on MP and MPG’s Structure
HPP is an innovative non-thermal technology that leverages pressures ranging from 100 to 600 MPa to enhance the quality and safety of meat products without the detrimental effects associated with traditional thermal treatments. Unlike heat-based methods, which can lead to excessive protein denaturation and nutrient loss, HPP preserves the nutritional and sensory properties of meat while effectively inactivating microbial contaminants. This technique offers a unique advantage by maintaining the fresh-like characteristics of meat products, thereby extending shelf life and improving overall product quality.
HPP works by exerting intense pressure on meat proteins, leading to structural modifications that influence the functionality of myofibrillar proteins, which are critical for gelation [36]. At pressures above 300 MPa, there are significant alterations in the protein network, resulting in the partial unfolding and denaturation of proteins. This controlled denaturation enhances protein coagulation, aggregation, and gelation, as depicted in Figure 4A. The application of high pressure can thereby optimize meat texture, improve WHC, and enhance gel strength, which is crucial for processed products like sausages and restructured meats [37]. These structural effects of HPP on myofibrillar proteins, particularly α-helix to β-sheet transitions and enhanced gelation, are summarized in Table 1.
Moreover, HPP contributes to a notable increase in product yield of approximately 15%, as it stabilizes the gel structure of proteins and preserves the meat’s natural moisture content. This increase in yield is in stark contrast to thermal treatments, which often result in moisture loss and reduced meat yield due to excessive heat exposure [3]. HPP achieves microbial inactivation by disrupting cell membranes, altering enzyme functionality, and impairing cellular metabolic pathways, which enhances food safety without compromising quality [38]. HPP is not only effective in meat processing but also shows the potential to improve the texture and shelf life of gel-based fish products [39]. Techniques like high-pressure shift freezing and thawing have been demonstrated to prolong the storage life of frozen muscle foods while minimizing quality deterioration typically associated with conventional freezing methods [40]. Interestingly, combining HPP with natural polysaccharides has been shown to enhance the textural attributes and water-retention properties of meat gels, providing a novel approach to improving product consistency and juiciness. However, the effectiveness of HPP depends on several critical factors, including pressure intensity, processing temperature, and duration. While HPP is highly efficient at inactivating vegetative microorganisms, it requires pressures above 600 MPa combined with temperatures exceeding 100 °C to inactivate highly resistant microbial spores. This dual requirement poses challenges, as elevated temperatures can degrade heat-sensitive compounds like vitamins and flavor components. Therefore, integrating HPP with complementary preservation methods, such as mild heating or natural antimicrobials, is essential to ensure effective spore control while preserving product quality [41]. One limitation of HPP is its reduced effectiveness in low-moisture environments, making it less suitable for dry meat products where insufficient water content limits the impact of high pressure. Thus, while HPP is a versatile technique, its application is best suited for products with moderate to high water content, where it can maximize both safety and quality without compromising the nutritional integrity of the meat [42].
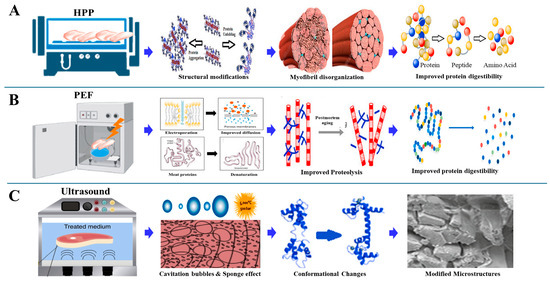
Figure 4.
(A) Effect of HPP on meat protein and digestibility. (B) PEF improves meat proteolysis and increases meat protein and digestibility. (C) Ultrasound generates cavitation, induces conformational changes, and modifies microstructures, meat protein and digestibility [43].
4.2. Effect of PEF on MP and MPG’s Structure
PEF technology has emerged as a non-thermal alternative that enhances the functional properties of meat proteins without the drawbacks associated with heat-based treatments [44,45]. Through the application of short, high-voltage electric fields, typically between 10 and 80 kV/cm, PEF triggers electroporation—a mechanism that disrupts cell membranes-leading to improvements in texture, tenderness, and WHC of meat products. This technique not only preserves the nutritional and sensory attributes of meat but also extends its shelf life by ensuring microbial safety while maintaining high product quality [46]. PEF operates through the generation of intense electric fields that permeabilize cell membranes, either reversibly or irreversibly. Reversible electroporation enables cells to regain functionality after exposure, while irreversible electroporation results in permanent membrane disruption, causing intracellular content leakage and microbial cell inactivation. At higher field strengths (10 to 50 kV/cm), PEF effectively inactivates microorganisms with minimal adverse effects on meat proteins compared with conventional thermal methods [47]. The ability to inactivate microbes while preserving protein structures makes PEF particularly appealing for enhancing gel formation in MPGs [48].
Notably, lower field strengths (0.5 to 5 kV/cm) are utilized to enhance mass transfer processes during freezing, drying, and osmotic dehydration, thereby improving the functional attributes of meat [7]. This enhancement is due to the microstructural changes induced by PEF, which ionizes carboxyl and amino groups in proteins, disrupting peptide interactions. This electroporation effect promotes protein unfolding, aggregation, and the reorganization of secondary and tertiary structures, thereby improving the gelation properties of myofibrillar proteins [49,50,51]. As depicted in Figure 4B, PEF causes significant modifications in protein structure, leading to changes in protein network formation and gel strength. The ionization and unfolding of protein molecules facilitate the exposure of hydrophobic regions, which enhances protein–protein interactions essential for gel formation. This not only improves the gel’s texture but also optimizes the water-binding capacity, contributing to superior product quality. Additionally, PEF has shown promise in applications beyond microbial inactivation. For instance, optimized PEF treatments have proven effective for tasks such as enzyme inactivation, dehydration, and bioactive compound extraction while maintaining the aroma, color, and nutritional value of meat products [52]. This versatile approach demonstrates that PEF can improve both the functional properties and sensory quality of meat, establishing its importance in contemporary meat processing. Table 1 further illustrates how PEF alters protein solubility and gel-forming ability through localized membrane disruption and moderate structural unfolding.
Recent developments have investigated the combined application of PEF and low-frequency magnetic fields (LF-MF) as a strategy to improve the quality of myofibrillar protein gels. LF-MF treatment, combined with pH adjustments, has been found to modify protein structures by increasing α-helix and β-sheet formations, which enhances the WHC and gel strength of pork proteins. This approach could reduce reliance on chemical additives like phosphates, which are traditionally used to improve meat quality [53].
4.3. Effect of Ultrasound on MP and MPG’s Structure
Ultrasound treatment has emerged as a powerful non-thermal processing technique to enhance the structural and functional properties of MPs and MPGs [54]. It operates through high-frequency sound waves that induce mechanical and biochemical changes, significantly improving protein unfolding, gel strength, WHC, and texture [55]. This non-invasive technique optimizes meat quality while preserving the nutritional and sensory characteristics, making it a promising alternative to conventional thermal treatments. Studies have demonstrated that ultrasound can enhance protein solubility, denaturation, and aggregation [56], which are critical for effective gel formation. For instance, Saleem and Ahmad investigated the impact of ultrasonication on the secondary structure and heat-induced gelation of chicken myofibrils, noting enhancements in gel strength and texture [57]. Similarly, it was found that ultrasound treatment improved the functional properties of reduced-salt chicken breast meat batter, leading to better textural outcomes and enhanced WHC [57,58,59]. These enhancements are especially advantageous for creating healthier meat products with lower sodium levels. Low-intensity, high-frequency ultrasound (<1 W/cm2; 100 KHz–10 MHz) has been widely adopted for its ability to maintain food composition and structure without causing thermal damage. The cavitation effect generated by ultrasound (Figure 4C) promotes nucleation and the formation of fine ice crystals during freezing, thereby reducing freezing time and minimizing damage to muscle tissues. This aligns with findings by Alarcon-Rojo et al., where ultrasound was used to reduce freezing-induced protein denaturation, preserving meat integrity [60].
This causes the nucleation of crystals and encourages the development of fine ice crystals, ultimately enhancing the freezing rate. Furthermore, the collapse of cavitating bubbles produces forces strong enough to break the existing ice crystals into small pieces, which act as new nuclei and foster secondary ice nucleation. This cavitation also enhances the heat transfer rate. Consequently, ultrasound can facilitate the formation of small, uniform ice crystals, reduce freezing-induced damage to muscle tissue, and decrease protein denaturation [60]. Furthermore, ultrasound treatment has been shown to accelerate mass transfer processes, such as brining and marination. For example, Ojha et al. investigated the use of ultrasound-assisted diffusion for sodium salt replacers, significantly enhancing the physicochemical properties of pork meat [61]. Additionally, ultrasound can improve emulsification and gelling properties, thereby reducing the need for chemical additives like phosphates. Zhao et al. demonstrated that ultrasound-assisted cooking of pork meatballs improved their texture and microstructure, enhancing product quality while reducing processing times [62]. As demonstrated in Figure 5, ultrasound treatment significantly enhances MPGs by improving WHC, optimizing protein structure, and promoting robust gel network formation.
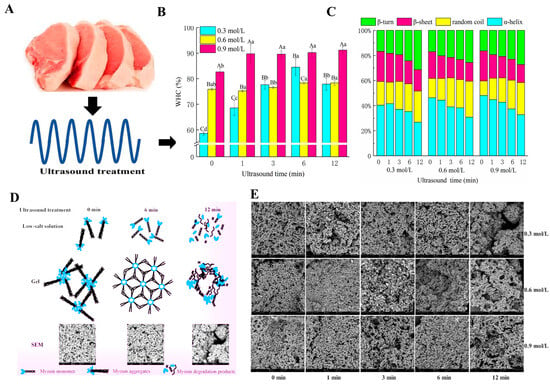
Figure 5.
(A) Ultrasound treatment on pork meat. (B) Effects at different times on the WHC of myosin gel. Different letters (a–d) within the same NaCl concentration indicates significant difference (p < 0.05). Different capital letters (A–C) within the same treatment time of ultrasound indicates significant difference (p < 0.05). (C) Effects of 0.3–0.9 mol/L NaCl concentration and illustration of how ultrasound treatment on myosin gel (D) and potential effects on the secondary structure of myosin gel. (E) SEM analysis of myosin gel [63].
The ultrasound-induced cavitation process not only improves water diffusion rates but also facilitates the formation of a denser gel network [62]. Ultrasound treatment promotes protein unfolding, exposing hydrophilic groups that improve water retention. This effect is particularly pronounced when higher salt concentrations (e.g., 0.9 mol/L) are used, combined with treatment times between 6–12 min, resulting in structural changes from α-helices to β-sheets, which enhance the gel’s stability. However, prolonged ultrasound treatment beyond the optimal range (e.g., exceeding 12 min) can lead to protein degradation, which weakens the gel structure and diminishes its functional properties. Sun et al.’s Scanning Electron Microscopy (SEM) analyses reveal that precise ultrasound parameters result in denser and more cohesive gel networks, confirming its efficacy in enhancing MPG quality, while overexposure causes the gel network to deteriorate [63]. Additionally, recent studies have shown that these ultrasound-assisted modifications can be effectively applied across various meat products, as summarized in Table 1, further supporting its versatility in meat processing.

Table 1.
Effect of non-thermal treatment on meat protein of gel quality.
Table 1.
Effect of non-thermal treatment on meat protein of gel quality.
| Method | Protein Source | Parameters | Effects | References |
|---|---|---|---|---|
| HPP | Whitebait | 100, 200, 300, 400 MPa, 10 min, 25 °C | Improved emulsification with molecular stretching increased the gel strength (4.8 fold) and myofibrillar protein gel’s digestibility (1.8 fold). α-helix changed into β-sheet with increased elastic and viscous modulus and a decrease in the sulfhydryl groups with increased hydrophobicity of protein and dense microstructure. | [64] |
| HPP | Pork | 150 MPa | Uniform, homogenous, low viscosity, small-sized stable protein particles with emulsifying properties, increased hydrophobicity due to protein unfolding, and high dispersion stability. Fragmentation of high molecular weight proteins (myosin) occurs. | [65] |
| HPP | Pork | 150 MPa | High-pressure homogenization-modified soy 11S globulin enhanced cooking yield, whiteness, hardness, texture, cohesiveness, shear stress, viscosity, storage, and loss modulus of myofibrillar protein. Improved water holding capacity, gel, and rheological properties of protein with the addition of soy 11S globulin. | [66] |
| HPP | Pork | 200, 300, 400 MPa, 4 °C, 5 min | 300, 400 MPa: improved drip loss with no free water with an increased fraction trapped in the myofibrillar network with no effect on water mobility and water holding capacity. The highly dynamic protein–water system with high competition between protein and water molecules, while myosin and actin are highly affected during storage. | [67] |
| HPP | Pork | 200 MPa, 2 °C, 10 min | Soy protein (2%) increases cooking yield, gel hardness, storage modulus, sulfhydryl groups, hydrophobicity, thermal stability, water holding capacity, and immobilized water of 1% NaCl pork meat myofibrillar protein. | [37] |
| HPP | Beef | 100, 200, 400, 600 MPa, 4 °C, 10 min | 200 MPa improved cooking loss, and 400–600 MPa improved protein structure as heavy chain dissociates, reduces the density of low molecular weight bands and increases insoluble proteins due to protein aggregation. Enhanced water holding capacity, increased hardness and breaking stress of meatballs, increased elastic modulus with decreased aerobic bacteria count. No color change after cooking, improved water content. | [68] |
| HPP | Beef | 0.1, 100, 200, 300 MPa, 21 °C 3 min | Conformational changes at 300 MPa in myosin and actin. 200, 300 MPa: reduced actomyosin content and enhance protein digestibility and degradation through the activity of cathepsin B. | [69] |
| HPP | Chicken | 100, 150, 200, 250, 300 MPa, 10 min | 200 MPa gives maximum water holding capacity, gel strength, solubility, and decreased aggregation potential of protein, increases display of Tyr and Trp residues and solubility of actin and myosin due to unfolding of the tertiary structure of the protein. 300 MPa induces disulfide cross-linking of myosin, hydrophobicity, and improved gel properties of reduced sodium myofibrillar protein. | [70] |
| HPP | Crab | 100, 300, 500 MPa, 20 min | 100 MPa partially denatures protein and forms flexible networks and stable, flexible protein structures. 500 MPa reduces myosin denaturation, suggesting protein unfolding, reduces α-helix structure, develops β-sheet portion and increases ionic, hydrogen, and hydrophobic bonds. | [3] |
| PEF | Chicken | 0–28 kV/cm, 80–1000 Hz | Improved the solubility of myofibrillar proteins, increased rheological properties, protein aggregation, surface hydrophobicity, and sulfhydryl groups, stable tertiary structure, and increased α-helix content. | [71] |
| PEF | Pork | 3.8 mT, 50 Hz, 3 h, 4 °C | High pH increases the WHC and electrostatic repulsion of myofibrillar proteins and decreases free water content. α-helix unfolding led to stability, tryptophan and tyrosine residues exposed from inside and contributed to hydrogen bonding, thus increasing hydrophobic interactions, uniform network, and reduced phosphate levels, which improves tenderness and texture of meat. | [53] |
| PEF | Beef | 99 kJ/kg, 24 h, 60 °C | Increased release of ninhydrin amino nitrogen and proteolysis led to improved protein digestion due to muscle disruption. Increased penetration of digestive juices due to pores in the membrane, thus facilitating the digestion and improved protein digestibility of the meat. | [72] |
| PEF | Beef | 0.63–0.78 kV/cm, 88.73–112 kJ/kg, 50 Hz, 60 °C, 24 h | No effect on cooking loss increases tenderness and water holding capacity, releases the rigid myofibril structure, and aggregation of sarcoplasmic proteins that rupture fat globules, ultimately resulting in juicier ribs. | [73] |
| PEF | Beef | 0.85 kV/cm, 110.96 kJ/kg, 50 Hz, 60 °C, 24 h | Less cooking loss and hardness, with increased soluble collagen and tenderness. | [74] |
| PEF | Duck | 2 kV/cm, 50 Hz, 12 °C | It reduces the loss of myofibrillar protein gel by 40%, with increased WHC and surface hydrophobicity, like fresh samples. Myosin actin activities were also like those of fresh duck meat, with reduced protein denaturation during thawing, maintaining gelation and emulsification. | [75] |
| Ultrasound | Chicken | 450 W, 20 kHz | Enhanced immobilized water and β-fold content of gels, more dense, porous, and uniform gels, enhanced texture, preheating increases myosin assembly and exposes hydrophobic groups. | [76] |
| Ultrasound | Chicken | 750 W, 20 kHz, 2 to 4 s | 1% NaCl decreases particle size, suggesting enhanced protein interaction; HIU increased water holding capacity and altered the α-helical structure to the random coil and β-sheet at 1 and 2% NaCl, respectively. β-sheet increases hydrogen bond facilitated protein–protein interactions. | [77] |
| Ultrasound | Chicken | 165 W, 30 kHz, 30 s | UF165 reduces the loss of the elastic modulus, improves gel strength and WHC due to reduced mobility, forms an intact and homogenous myofibrillar protein gel network, and maintains primary, tertiary, and secondary protein structures and gel whiteness. Fast-freezing. | [78] |
| Ultrasound | Chicken | 200 W, 40 kHz | Ultrasound-based slightly acidic electrolyzed water (EUT) can improve the rheological properties, gel quality, and water holding capacity and effectively maintain the gel whiteness. | [79] |
| Ultrasound | Pork | 240 W, 20 kHz, 6 min | Enhanced fat myofibrillar protein gel properties, decreased porous gel networks, and particle size of fats. The changes in hardness, springiness, and WHC of mixed gels under different MP and fat ratios as affected by HIU are shown in Figure 1. HIU decreases the hardness of the gels at a protein/fat ratio higher than 1:5, while springiness and water holding capacity increase at a ratio < 1:10, improving texture. | [80] |
| Ultrasound | Pork | 250 W, 20 kHz, 0 to 12 min | While improving the quality of the low-salt gel while damaging the medium and high-salt gels, prolonged ultrasound treatment causes myosin degradation by increasing its solubility. Improved texture and WHC of gel. | [63] |
| Ultrasound | Silver carp (Hypophthalmichthys molitrix) | 400 W, 25 kHz, 5, 10, and 15 min | Myofibrillar protein expands via the cavitation effect, which facilitates interaction between protein and water, ultimately decreases thiol, α-helix and the random coil content and increases β-sheet and solubility. Decreased gel pores with improved gel strength and WHC. | [81] |
| Ultrasound | Litopenaeus vannamei | 25–29, 74–80, 123–130 W/cm2, 20 kHz, 60 min | Oxidation and aggregation of myofibrillar protein by increased carbonyl and free radical contents improved gelling emulsification, rheological properties, and viscoelasticity. Decreased sulfhydryl content, with increased surface hydrophobicity, fluorescence intensity, and secondary structure of protein. | [82] |
| Ultrasound | Litopenaeus vannamei | 400 W, 20 kHz, 15 min | It increased the surface hydrophobicity, gel strength, WHC, emulsifying capacity, and stability of frozen shrimp’s myofibrillar protein. The cavitation effect changes the α-helix protein structure to β-sheet and β-turn. Protein unfolding increased the sulfhydryl groups. | [8] |
4.4. Effect of CAP on MP and MPG’s Structure
Cold atmospheric plasma (CAP) has emerged as a promising non-thermal processing technique that enhances the quality and safety of meat products while preserving their structural integrity [83]. CAP operates at atmospheric pressure and low temperatures, generating reactive plasma species that effectively inactivate pathogens without significantly affecting meat proteins’ structure or functional properties. This method is particularly advantageous in meat processing, as it maintains the product’s texture, color, and nutritional value, unlike traditional thermal treatments that often lead to excessive protein denaturation and nutrient loss. By targeting cell membranes, CAP induces oxidative stress, which disrupts microbial cells and improves the shelf life of meat products, making it a viable alternative for microbial safety [84].
Recent studies have explored CAP’s application in enhancing MPGs, focusing on its ability to improve gelation properties without the drawbacks associated with heat. Qian and colleagues showed that plasma-activated water effectively enhances the gelation properties of chicken myofibrillar proteins, resulting in improved WHC and a longer shelf life. The reactive species generated by CAP, such as nitric oxide radicals, interact with meat proteins, altering their structure by increasing the formation of disulfide bonds. This results in the formation of a more compact and cohesive gel network, as shown in Figure 6, which is essential for preserving the texture and juiciness of processed meat products [85].
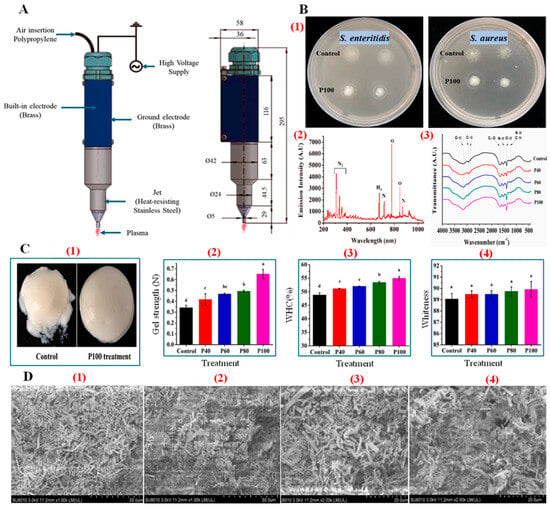
Figure 6.
(A) Schematic representation of the plasma jet device [86]. (B) (1) Antimicrobial effects of plasma treatment on S. Enteritidis and S. aureus, showing inhibition zones for control and P100 treatment. (2) Emission spectra of the plasma, highlighting reactive species. (3) FTIR spectra of samples under different plasma treatments, showing changes in functional groups and bond vibrations. (C) (1) Visual comparison of gel appearance for control and P100-treated samples. (2) Gel strength comparison across different plasma treatments (P0, P40, P60, P100). (3) WHC results for each treatment. (4) Whiteness values after different plasma treatments, (a–d): different letters indicate significant differences (p < 0.05). (D) SEM micrographs of gel structure showing microstructural differences in the gel network under different plasma treatments (1, 3): Control, (2, 4): P100 treatment [85].
CAP treatment has been shown to trigger conformational modifications in myofibrillar proteins, enhancing gel strength and texture. The decrease in pH induced by plasma ions (like H3O+ and HNO3) leads to protein aggregation, enhancing the gel structure by promoting intramolecular and intermolecular interactions. This effect is particularly beneficial for meat products, as it strengthens the protein matrix, leading to a firmer gel that retains moisture, thereby improving the product’s overall texture and quality.
However, optimizing CAP parameters is crucial to avoid over-oxidation, which can degrade meat proteins and negatively affect gel formation [9]. Excessive exposure to reactive plasma species can lead to protein fragmentation, resulting in weaker gels with reduced water retention. Studies by Jiang et al. indicate that prolonged CAP treatment may cause protein over-oxidation, reducing gel integrity and potentially impacting the sensory qualities of meat products [87].
CAP’s potential to reduce the activity of proteolytic enzymes, such as cathepsin and calpain, further supports its application in processed or aquatic meat products. For example, CAP treatment has been shown to suppress these enzymes (Figure 7), helping to stabilize protein structure and delay degradation, thereby improving texture retention and shelf life [88]. This differs from postmortem aging, where controlled protease activity is typically beneficial for tenderness development. Moreover, the use of CAP has demonstrated improvements in the gel strength of various meat products, such as sausages and restructured meats, by enhancing protein cross-linking and reducing sulfhydryl content, leading to better textural properties [89]. A comparison of CAP-induced conformational changes with other techniques is also presented in Table 1, highlighting its potential for improving gel strength while preserving nutritional quality.
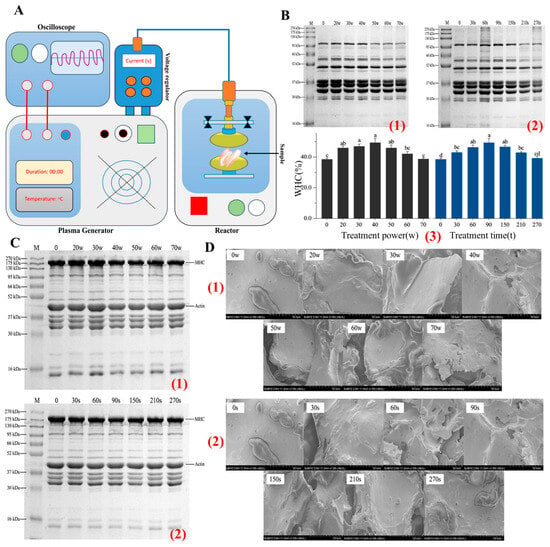
Figure 7.
(A) Schematic representation of dielectric barrier discharge setup. (B) SDS-PAGE analysis of crude proteases from golden pomfret after CAP treatment: (1) different power levels; (2) different treatment times. (3) Water holding capacity of meat after CAP treatment, a–d: Different letters indicate significant differences (p < 0.05). (C) (1) and (2) SDS-PAGE of muscle proteins after regular pressure CAP treatment: (1) different power levels; (2) different treatment times. (D) SEM micrographs of plasma-treated proteins: (1) different power levels; (2) different treatment times [87].
However, the industrial adoption of CAP still faces challenges, including high equipment costs and the need for precise process control. Regulatory standards for food applications remain inconsistent across countries, limiting widespread approval [90,91]. Additionally, extended or suboptimal CAP treatment may affect meat aroma profiles and lead to minor sensory changes during storage.
4.5. Effect of MAP on MP and MPG’s Structure
Modified atmospheric packaging (MAP) is widely employed in the meat industry to extend the shelf life and preserve the quality of meat products by controlling the gaseous environment within the packaging [92]. This technique typically uses a combination of gases, such as carbon dioxide, nitrogen, and oxygen, to reduce spoilage and improve the visual appeal of meat [93]. High oxygen levels, often around 80%, are particularly popular for meat packaging because they enhance the redness of meat by stabilizing oxymyoglobin, thus maintaining an attractive color for consumers. Nevertheless, the benefits of high oxygen levels come with potential drawbacks, such as increased lipid oxidation, which can negatively impact the nutritional and sensory qualities of meat [94]. Recent studies have explored the effects of varying oxygen concentrations in MAP on the structural properties of meat proteins. For example, Liu et al. investigated pork treated with different oxygen levels (20%, 40%, 60%, and 80%) and found that an oxygen concentration of 60% significantly improved gelation properties by increasing carbonyl content, disulfide bond formation, and gel strength [95], as shown in Figure 8. This enhancement is attributed to the oxidative modifications that strengthen protein–protein interactions, thus increasing particle size while decreasing sulfhydryl content and protein solubility. These structural changes also involved a reduction in α-helix content with a corresponding increase in β-sheet structures, suggesting stronger hydrophobic interactions that stabilize the gel network.
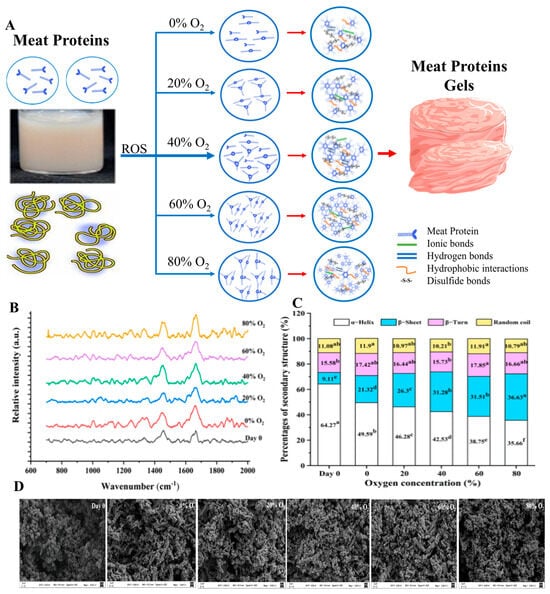
Figure 8.
(A) Illustration of pork gels stored under a modified atmosphere of varying O2 concentrations. (B,C) Raman spectra of secondary structures of proteins, a–f: Different letters indicate significant differences (p < 0.05). (D) 1 SEM images of the microstructure of pork paste gels modified and adapted from [95].
Moreover, the study by Liu et al. showed that while a 60% oxygen level optimizes gelation and textural properties, a lower concentration of 40% is more effective for maintaining the redness and tenderness of pork over a 14-day refrigerated period [95]. The study also highlighted that higher oxygen levels could lead to increased carbonylation and higher shear force in sarcoplasmic and myofibrillar proteins, indicating that myoglobin-rich sarcoplasmic proteins are more prone to oxidation than myofibrillar proteins. This increased susceptibility is likely due to the localization of myoglobin in the sarcoplasm, which makes lysine residues vulnerable to oxidative changes, such as the formation of α-aminoadipic semialdehyde. Interestingly, studies have also shown that MAP can be combined with other non-thermal techniques to enhance its effectiveness [96]. For instance, Jiang et al. found that combining MAP (70% nitrogen, 30% carbon dioxide) with ultrasound treatment led to a significant decrease in β-sheet and α-helix content while improving protein solubility and digestion efficiency. This combination maintained the nutritional quality of pork meat while reducing oxidative damage. However, these findings highlight the complexity of MAP’s effects on meat proteins, as different gas compositions and processing conditions can lead to varied impacts on protein structure and functionality [97]. Despite its advantages, MAP is not without its limitations. While it extends the shelf life and maintains meat quality, the high oxygen levels can accelerate protein oxidation, leading to the formation of oxidized derivatives that may affect meat texture and nutritional value. For example, Shen et al. reported that beef steaks stored under 80% oxygen MAP exhibited increased protein carbonylation, resulting in cross-linking of lysine residues into lysinonorleucine, which may affect the meat’s tenderness and flavor [98].
There is a growing need for more research to fully understand the implications of MAP on meat protein structures, especially regarding the health effects of consuming oxidized proteins. Future studies should focus on in vivo models to explore the potential impacts of these oxidation products on human health, thus providing a more comprehensive understanding of MAP’s benefits and drawbacks in meat preservation.
4.6. Effect of Irradiation on MP and MPG’s Structure
Irradiation, especially through electron-beam (E-beam) technology, serves as an efficient non-thermal method to improve meat product safety and quality by prolonging shelf life and eliminating pathogens [99,100]. However, it also induces structural changes in MPs and MPGs, depending on the irradiation dose, as illustrated in Figure 9. The impact of irradiation on meat proteins is dose-dependent, with varying effects on protein crosslinking, solubility, and gelation properties. At low doses (e.g., 0 kGy), proteins maintain their native structure with minimal crosslinking and weak gelation, as they remain largely intact [101].
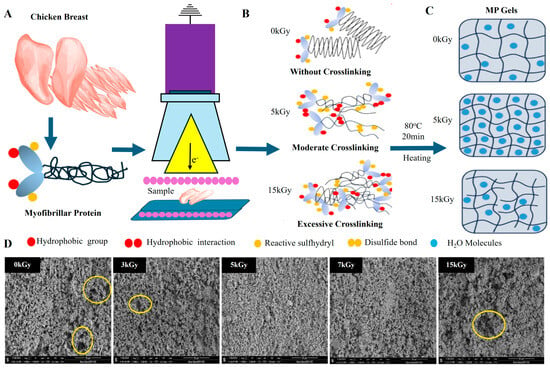
Figure 9.
(A) Schematic of E-beam irradiation on chicken myofibrillar protein structure. (B) Protein crosslinking stages at different irradiation doses, no crosslinking (0 kGy), moderate crosslinking (5 kGy), and excessive crosslinking (15 kGy) after heating at 80 °C for 20 min. (C) MP gel structures represent increased network density from 0 to 15 kGy. (D) SEM images displaying microstructural changes in MP gels across irradiation doses [101].
At moderate doses (around 5 kGy), significant improvements in protein functionality are observed. Irradiation at this level induces partial denaturation, which exposes hydrophobic and sulfhydryl groups on the protein surface. This exposure facilitates hydrophobic interactions and disulfide bond formation, enhancing protein–protein interactions and strengthening the gel network. SEM images at 5 kGy reveal a more compact and uniform gel structure, indicating better organization of the gel matrix. This enhanced network structure translates into improved WHC and gel texture as the proteins become more soluble and better dispersed in solution, which contributes to a more cohesive gel formation. Interestingly, moderate irradiation also boosts protein solubility, which is crucial for the development of strong, elastic gels with desirable textural attributes. The exposure at 5 kGy optimizes the balance between denaturation and crosslinking, resulting in improved gelation and structural integrity of MPGs. However, as the irradiation dose increases beyond optimal levels, adverse effects begin to surface. For instance, high doses (around 15 kGy) lead to excessive protein crosslinking, which results in over-aggregation and reduced solubility. SEM micrographs of meat proteins treated at 15 kGy show a more aggregated, dense, and irregular gel network with larger pores, which compromises the flexibility and WHC of the gel.
Further analysis using SDS-PAGE highlights the impact of high-dose irradiation on protein degradation [101]. At doses of 15 kGy, there is a noticeable reduction in the myosin heavy chain (MHC) band, accompanied by the formation of high-molecular-weight aggregates. These changes indicate that excessive irradiation leads to substantial protein degradation, affecting the functional properties of MPGs by reducing their ability to form cohesive gels. Therefore, optimizing irradiation doses is critical in meat processing to achieve the desired balance between microbial safety and product quality. Moderate doses (3–5 kGy) are found to enhance the structural and functional characteristics of MPGs by promoting controlled crosslinking and improving solubility, leading to better gel texture and WHC. However, excessive doses (e.g., 15 kGy) should be avoided, as they can compromise gel quality by causing undesirable aggregation and structural degradation [101]. Thus, careful optimization of irradiation parameters is essential to harness the benefits of this technology.
Despite its proven efficacy, irradiation is limited by high infrastructure costs and varying regulatory acceptance. For example, while approved in countries like the U.S. and China, it remains controversial in the EU due to labeling requirements and consumer concerns. High-dose treatments can also promote lipid oxidation, which may result in off-odors during storage.
5. Advances in Vibrational Spectroscopy
Non-thermal processing techniques, such as HPP, PEF, ultrasound, and cold plasma, provide a controlled approach to enhancing the structural and functional properties of MPGs while preserving the meat’s nutritional quality. However, real-time monitoring of these molecular-scale transitions conventional quality assessment methods face significant challenges [102,103].
Conventional techniques, including Texture Profile Analysis (TPA), WHC tests, and rheological measurements [104,105,106], provide macroscopic insights into the mechanical properties of MPGs, such as hardness and cohesiveness [107,108,109]. Despite their utility, these methods inherently struggle to resolve the molecular interactions that govern gelation dynamics [110,111]. This gap underscores the need for advanced, non-destructive tools capable of real-time molecular analysis, a role uniquely served by vibrational spectroscopy.
Vibrational spectroscopy has become a cornerstone in optimizing non-thermal food processing, primarily due to its capacity for real-time monitoring of protein dynamics—including denaturation, gelation, and aggregation—which enables data-driven process control for consistent product quality [112], as illustrated in Figure 10. As a non-destructive analytical platform, these techniques provide critical insights into molecular interactions and structural transformations, thereby driving innovation in food science to meet growing consumer demands for minimally processed, nutritionally enhanced, and safer meat products [113]. Within this technological framework, Raman spectroscopy, infrared spectroscopy, and hyperspectral imaging have emerged as particularly versatile modalities, each offering unique advantages in spatial resolution, chemical specificity, or multidimensional data acquisition during processing [114].
Figure 10.
The representation of vibrational spectroscopic techniques in assessing meat properties obtained from various sources.
5.1. Raman Spectroscopy
Raman spectroscopy is a vibrational technique based on inelastic light scattering, where incident photons interact with molecular vibrations, resulting in frequency shifts known as Raman shifts [115] (Figure 11A,B). These shifts provide a unique molecular fingerprint for identifying structural and chemical changes. While the majority of scattered photons are elastically scattered (Rayleigh), the small fraction that undergoes Raman scattering carries valuable structural information [116].
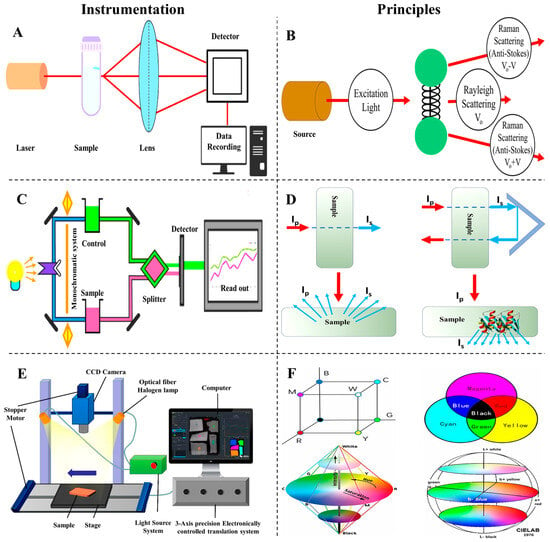
Figure 11.
(A) Instrumentation and components of Raman spectrometer. (B) The working principle of Raman spectrometer. (C) Instrumentation and components of NIR spectrometer. (D) The working principle and mechanism of NIR. (E) Instrumentation and components and elements of hyperspectral imaging. (F) The working mechanism elements of hyperspectral imaging.
In the context of meat proteins, Raman spectroscopy is particularly useful for non-destructive analysis of secondary structures such as α-helices, β-sheets, and random coils. Key spectral regions, including the amide I and III bands, allow the assessment of protein folding, unfolding, and aggregation [117,118,119]. This is crucial for evaluating processing effects like heating, pH adjustment, and additive incorporation on MPGs.
For example, Raman analysis has been used to monitor the structural transition of myofibrillar proteins during gelation, revealing how changes in tryptophan and tyrosine microenvironments reflect hydrophobic interactions and crosslinking behavior [115]. In one study, incorporating 2% camellia seed oil into MP–lipid composite gels increased α-helix content and disulfide bond formation, thereby enhancing gel strength [120]. Additionally, combined Raman and NMR studies have shown that pH-driven changes in hydrogen bonding can modulate protein network formation in pork myofibrillar gels [53]. These applications highlight Raman spectroscopy’s potential as a rapid, label-free tool for monitoring molecular-level changes in meat processing and ensuring product quality.
5.2. Infrared Spectroscopy
Infrared (IR) spectroscopy is a widely adopted and efficient technique for rapid, non-invasive analysis in fields such as agriculture, pharmaceuticals, and food products [121,122,123]. It measures molecular vibrations based on the absorption of IR radiation by chemical bonds, producing spectral patterns that reveal the presence and structure of key functional groups. Figure 11C,D illustrate the instrumentation setup and working principle of IR spectroscopy, providing visual context for its analytical framework.
In meat protein systems, the Mid-infrared (MIR) region (4000–400 cm−1) is particularly informative. Key absorption bands, especially amide I, II, and III, are directly related to protein secondary structures such as α-helices and β-sheets [124]. These bands are highly sensitive to conformational changes, protein unfolding, and aggregation, making IR spectroscopy a powerful tool for monitoring myofibrillar protein behavior during processing. The use of attenuated total reflectance (ATR) further enhances its utility by allowing direct analysis of hydrated or intact samples with minimal preparation [125].
Numerous studies have demonstrated the effectiveness of IR spectroscopy in tracking protein transitions under thermal and non-thermal treatments. For example, HPP and ultrasound have been shown to induce β-sheet enrichment and reduce α-helical content, correlating with improved gel strength and WHC in MPGs [126]. These structural insights are crucial for optimizing the texture and stability of meat products. Together, these capabilities establish IR spectroscopy as a valuable technique for evaluating structural integrity and processing effects in meat protein systems.
5.3. Hyperspectral Imaging
Hyperspectral imaging combines spectroscopic analysis with imaging techniques, offering enhanced spatial and spectral data for assessing the quality of agricultural, food, and pharmaceutical products [127,128,129,130]. This technique captures images across hundreds of narrow, contiguous spectral bands, typically spanning the visible, NIR, and short-wave IR regions [131]. The system comprises an illumination source, an imaging spectrograph, and a detector [132]. As shown in Figure 11E, the spectrograph collects light that interacts with the sample, disperses it into its spectral components, and directs it to a detector like a CCD camera. This results in a 3D hypercube containing 2D spatial information (x and y coordinates) and 1D spectral data (wavelengths) for each pixel. The generated hypercube allows the visualization of the spectral signatures of each pixel, enabling precise identification and quantification of chemical constituents and their spatial distribution within a sample. Unlike conventional imaging systems that only capture surface characteristics, hyperspectral imaging provides a detailed assessment of internal chemical compositions, such as moisture, fat, and protein content, along with their distribution [126]. This technique is particularly beneficial for heterogeneous samples, as it overcomes the limitations of traditional spectroscopy, which only offers averaged measurements that might not fully represent the sample’s variability.
Hyperspectral imaging has demonstrated its effectiveness in various applications, including detecting defects in meat and meat products, assessing quality parameters, classifying products, inspecting poultry carcasses, and evaluating freshness [133,134]. Its ability to simultaneously analyze spatial and spectral data enables comprehensive inspection of entire surfaces, making it a powerful tool for quality control. To further illustrate the application of vibrational spectroscopic techniques in MPG analysis, Table 2 presents a comparative overview of commonly used methods, such as Raman spectroscopy, IR spectroscopy, NIR, MIR, and FTIR. The table highlights key aspects like spectral range, detection mechanisms, chemical specificity, and spectral modes, along with the benefits and constraints associated with each technique. This comparison helps clarify their roles in analyzing MPGs, especially in evaluating the effects of non-thermal processing technologies.

Table 2.
Comparison of IR and Raman spectroscopy adapted from [126].
6. Application of Vibrational Spectroscopy in MPG Analysis
Vibrational spectroscopy techniques, such as Raman, IR spectroscopy, and hyperspectral imaging, provide critical advantages in characterizing MPGs by enabling non-destructive analysis of molecular interactions and structural transitions. These methods effectively decode key mechanisms, including protein denaturation kinetics, disulfide bond formation, and gel network assembly, thereby offering insights into MPG functionality such as water retention and texture modulation. By capturing real-time chemical changes during processing, vibrational spectroscopy supports the optimization of parameters for industrial applications while preserving meat’s native protein conformations. The subsequent sections first outline fundamental factors influencing MP/MPG quality, then detail how spectroscopic tools advance precision control in meat processing.
6.1. Characteristics and Factors Affecting MP and MPGs
The quality of meat products is heavily influenced by the structural and functional properties of MPs, and their gel-forming ability is regulated by various intrinsic and extrinsic factors [135].
One such critical factor is pH, which influences protein stability and gel-forming ability. A rapid decline in pH during post-mortem muscle conversion can disrupt the polypeptide chain network, reducing the WHC of the meat and impacting the functionality of myofibrillar and sarcoplasmic proteins. WHC influences several meat attributes, such as color, firmness, juiciness, texture, and tenderness. For instance, drip loss, which refers to the release of extracellular water as droplets on the cut surface, is a common issue linked to changes in the muscle structure post-rigor mortis, where the narrowing of filamentary space and alterations in cellular membranes [136]. Color is another vital indicator of meat quality, serving as the first visual cue for consumers. A bright red color is generally associated with freshness and consumer acceptance, particularly in red meats [137]. Factors like aging, shelf life, and moisture levels can significantly influence meat color and, consequently, its perceived quality. Meat tenderness, which is critical to consumer satisfaction, depends on the composition and structural integrity of skeletal muscle. The primary contributors to muscle strength are myofibrillar proteins and connective tissues. Collagen, the most abundant protein in connective tissue, varies in form and function across different muscle tissues. Its content and structure contribute to meat toughness. During postmortem aging, tenderness improvement is primarily due to the degradation of myofibrillar proteins, with minimal enzymatic degradation of collagen. However, interventions such as ultrasound can modify collagen structure, increasing its solubility and reducing thermal stability, thereby indirectly enhancing meat tenderness [138]. Vibrational spectroscopic techniques, such as NIR, FTIR, and Raman spectroscopy, are highly effective for assessing these properties, offering insights into meat’s chemical and physical attributes.
Overall, the gelation of myofibrillar proteins is influenced by factors such as pH, temperature, salt concentration, and mechanical processing, which regulate protein unfolding, solubility, and aggregation. These processes determine key quality attributes like water retention and texture. Advances in analytical techniques and the use of functional additives, along with clean-label and non-thermal innovations, continue to expand the functionality and applications of MP-based gels.
6.2. Raman Spectroscopy in MP and MPG Analysis
Raman spectroscopy is a robust, non-destructive technique widely applied in meat protein analysis. As previously discussed, Raman spectroscopy enables molecular-level analysis. In meat processing, it is particularly effective for detecting protein structural changes such as unfolding and aggregation [13]. Unlike other spectroscopic techniques, Raman spectroscopy is not hindered by water interference, making it ideal for analyzing the effects of thermal and non-thermal processing on MPGs. This capability allows for optimizing product texture, WHC, and overall quality. Additionally, Raman spectroscopy, when integrated with chemometric methods, offers rapid and high-throughput analysis, proving itself a versatile tool for assessing meat quality.
For instance, combining Raman spectroscopy with low-field nuclear magnetic resonance (LF-NMR) has proven effective in evaluating the quality of surimi gels in relation to calcium lactate addition [139]. This combination provided critical information on water-binding capabilities, with results indicating that calcium lactate improves gel strength, whiteness, and WHC by influencing protein secondary structures. Calcium ions facilitate protein cross-linking, leading to a more compact gel network that significantly enhances the gelation properties of fish and meat proteins [140]. Figure 12 illustrates these structural changes, demonstrating the role of calcium in enhancing gel quality. Moreover, Raman spectroscopy has been used to study protein–lipid interactions in meat products. In a study, adding lipids led to hydrophobic interactions that enhanced gel properties, resulting in improved whiteness, WHC, and sensory attributes [141]. Further analysis showed that lipid addition modifies protein secondary structures, increasing β-sheet content, which contributes to a more stable gel network. Similarly, Wang and colleagues demonstrated that incorporating lipids into meat emulsions stabilized by chickpea protein led to changes in myosin microenvironment, enhancing water retention and oxidative stability [118].
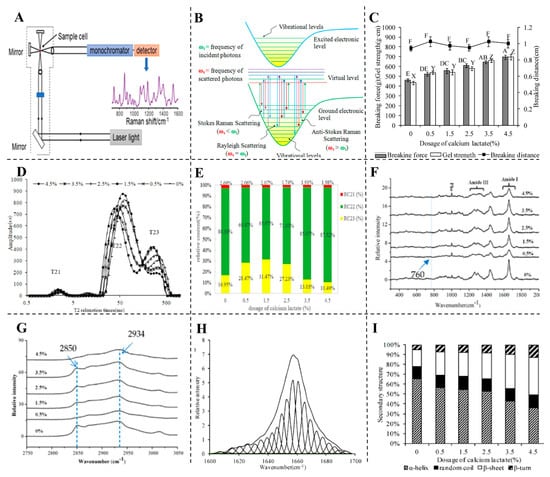
Figure 12.
(A) The components of the Raman spectrometer [13]. (B) Representation of the Raman scattering mechanism adapted from [142]. (C) The effect of calcium lactate on the breaking force, distance, and gel strength. A–E: different letters at breaking force differ significantly (p < 0.05). X–Z: different letters at gel strength differ significantly (p < 0.05). F: indicates no significant difference (p > 0.05) at breaking distance. (D) The relaxation time and bound water contents. (E) Immobilized and free water contents. (F) Raman spectrum 300–2000 cm−1, (G) 2750–3050 cm−1, (H) and amide I band. (I) The secondary structure of proteins in surimi gels with different doses of calcium lactate adapted from [140].
Raman spectroscopy has also been utilized in combination with machine learning algorithms for real-time quality assessment. For example, Li’s group applied Raman spectroscopy and a deep learning model to predict pork batter quality during heating. The CNN-LSTM hybrid model excelled in predicting gel strength and whiteness, achieving high correlation coefficients (Rp > 0.93) and RPD values above 3, indicating robust predictive capabilities [143]. This integration highlights the potential of combining Raman spectroscopy with advanced algorithms to optimize meat processing quality control. Additionally, Raman spectroscopy aids in understanding the impact of non-thermal treatments on MPGs. For example, incorporating citric acid into low-salt meat products has been shown to modify protein structures, enhancing gel stability and water retention [144].
Lastly, Raman spectroscopy’s ability to detect subtle changes in protein conformations is crucial for monitoring MPG formation. As shown in Figure 13, the gelation process involves interactions between meat proteins and additives like starch, leading to structural shifts in secondary protein elements, such as α-helices and β-sheets. Raman spectra effectively capture these alterations, highlighting the influence of processing parameters on the gel’s functional properties, such as gel strength and WHC [15].
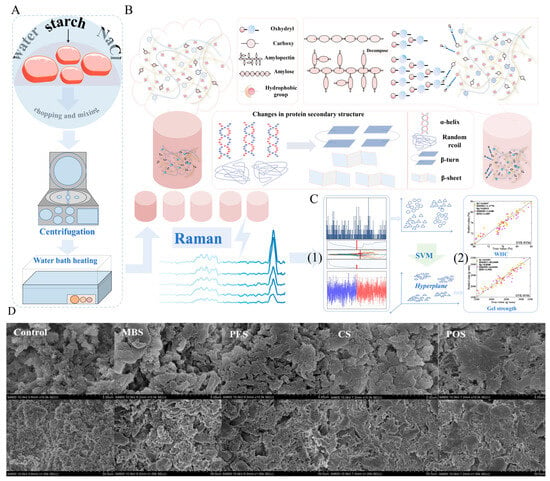
Figure 13.
(A) Preparation of pork meat protein gel through mixing of starch with meat proteins, followed by centrifugation and heating. (B) Structural changes in proteins and starch interactions during gelation, leading to transformations in secondary structures such as α-helix, β-sheet, and random coil formations. (C) (1) Raman spectroscopy used to analyze the gel structure, (2) Use SVM to predict key properties like WHC and gel strength. (D) SEM images showing microstructural differences in gels subjected to various starch treatments (Control, MBS, PES, CS, and POS), highlighting variations in the gel matrix morphology [15].
6.3. Infrared Spectroscopy in MP and MPG Analysis
IR spectroscopy, encompassing techniques such as FTIR and MIR, is extensively applied in meat science to study the structural and functional attributes of proteins. By measuring the absorption of infrared light by molecular bonds, IR spectroscopy provides detailed insights into protein structures, particularly those involving amide bonds, C=O, N-H, and O-H groups. This technique is highly effective in identifying changes in meat proteins during both thermal and non-thermal processing, making it an indispensable tool for optimizing key meat quality parameters like WHC, texture, and protein gel formation. Figure 12C,D illustrate the instrumentation setup and principles behind IR spectroscopy, respectively. In meat protein analysis, FTIR is particularly useful for tracking structural changes in myofibrillar proteins. For example, studies have shown that FTIR can detect alterations in the amide I band, which correlates with shifts in protein secondary structures [145], such as increases in β-sheets and β-turns and decreases in α-helix content [146]. Additionally, FTIR with fluorescence spectroscopy has shown that dietary pterostilbene prevents the unfolding of protein structures in chicken, thereby maintaining α-helix structures. These structural changes are crucial for understanding gel formation and stability, particularly under processing conditions like high temperature, pressure or cold storage [147,148,149].
Furthermore, IR spectroscopy, combined with chemometric techniques, offers a powerful approach to analyzing the textural and structural properties of meat products [150,151]. For instance, NIR spectroscopy has been utilized to evaluate attributes like WHC, chewiness, and hardness in meat gels. The incorporation of NIR with chemometric models such as Principal Component Analysis (PCA) enhances the differentiation and prediction of textural properties [129]. Additionally, recent advancements highlight the integration of spectroscopy with machine learning algorithms for real-time quality monitoring. In practical applications, machine learning models driven by chemometric data, including Support Vector Machine (SVM) and Extreme Learning Machine (ELM), have been successfully employed alongside spectroscopic methods to accurately predict meat gel strength and texture. For example, combining NIR spectroscopy with ultrasound treatment was shown to optimize meat gel properties by modifying protein structures. The Genetic Algorithm (GA)-ELM model achieved high prediction accuracy (Rp2 = 0.8772), demonstrating the effectiveness of this integrated approach for non-destructive quality control in meat processing [152]. Figure 14 illustrates how ultrasonic treatment (ranging from 0 to 50 min) enhances minced chicken gel properties, including texture (hardness, cohesiveness, and springiness) and gel strength, with optimal results around 30 min due to improved protein interactions.
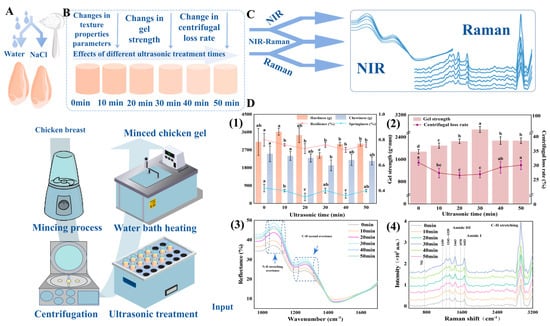
Figure 14.
(A) Preparation of minced chicken gel with water and NaCl, followed by heating and ultrasonic treatment. (B) Effects of varying ultrasonic treatment times (0–50 min) on texture properties, gel strength, and centrifugal loss rate of minced chicken gel. (C) Raman, NIR, and combined NIR-Raman spectroscopy are used to analyze structural changes in the gels. (D) (1) Changes in hardness, chewiness, springiness, and resilience with different ultrasonic times. (2) Gel strength and centrifugal loss rate as a function of ultrasonic treatment duration. (3) NIR spectra showing changes in reflectance at different ultrasonic times. (4) Raman spectra illustrating molecular changes in the gel structure across various ultrasonic durations. Different letters indicate significant differences (p < 0.05) among treatments [12].
Moreover, combining NIR and Raman spectroscopy has demonstrated significant success in improving predictive accuracy for evaluating gel quality, as illustrated in Figure 13. Utilizing data fusion techniques and advanced models like Convolutional Neural Networks (CNNs) and Long Short-Term Memory (LSTM), these predictive approaches achieved exceptional precision [12]. This integrated approach holds significant potential for advancing the meat processing industry by enabling rapid, reliable, and non-invasive quality checks, ensuring consistent product quality while reducing reliance on labor-intensive testing methods. Chemometric techniques have shown great potential in transforming raw spectral data into actionable insights. By filtering noise, selecting critical variables, and enhancing model accuracy, chemometric-driven machine learning models support the optimization of MPGs. Table 3 summarizes current applications of chemometric analysis combined with spectroscopic techniques for meat protein analysis, highlighting advances in non-invasive quality assessment.

Table 3.
Application of different spectroscopic techniques and chemometric algorithms on meat from multiple sources.
6.4. Hyperspectral Imaging in MP and MPG Analysis
Hyperspectral imaging (HSI) is an advanced, non-destructive analytical technique that integrates imaging with spectroscopy to assess meat protein properties [165,166]. By capturing detailed spectral data across a wide range of wavelengths, HSI allows for a comprehensive evaluation of multiple quality attributes, such as protein content, WHC, and structural changes, in a spatially resolved manner [167]. This capability is particularly useful for monitoring protein denaturation, texture changes, and gel formation during meat processing, providing rapid and high-throughput insights into the effects of both thermal and non-thermal treatments on meat quality [168,169]. HSI’s ability to combine spatial and spectral information makes it a powerful tool for real-time quality assessment, optimizing meat product characteristics efficiently [170].
Recently, HSI was employed to evaluate the freshness of frozen beef [171], combined with SVM models. The spectral images provided indices of meat deterioration by analyzing muscle tissue, offering a fast, cost-effective, and minimally invasive method compared to traditional techniques. As depicted in Figure 15, this approach efficiently assesses meat quality during storage, especially under freezing and thawing conditions. Key factors affecting quality, such as cell rupture, fat oxidation, and the denaturation of myofibrillar proteins, are closely monitored. Given that meat is primarily composed of water, proteins, fats, and carbohydrates, changes in the spectroscopic properties of these biomolecules are crucial for distinguishing between fresh, frozen-stored, and frozen-thawed samples [172,173]. In the context of meat protein analysis, HSI, alongside other spectroscopic methods like Raman and IR spectroscopy, plays a vital role. Raman spectroscopy provides detailed insights into molecular vibrations, while IR spectroscopy focuses on functional groups and protein denaturation. Together, these techniques offer a comprehensive analysis of protein structure, conformation, and interactions, which are essential for monitoring changes during meat processing. The integration of these methods can significantly enhance the ability to monitor the role of meat proteins in optimizing quality, texture, and safety across various thermal and non-thermal processing methods. This multi-modal approach ensures a deeper understanding of protein functionality, leading to improved meat processing strategies and product consistency.
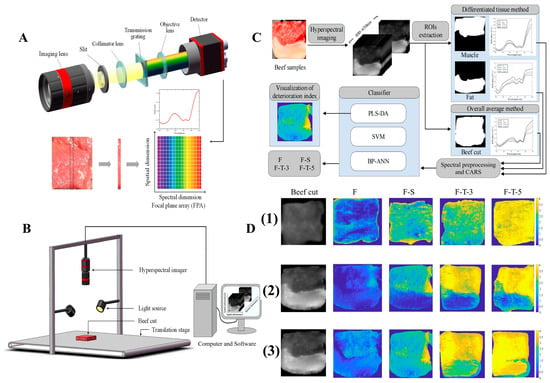
Figure 15.
(A,B) The representation of hyperspectral imaging. (C) Illustrative representation of steps involved in the classification model. (D) Visualization of beef cut classification maps under four treatments: frozen (F), frozen-stored (F-S), and frozen-thawed for 3 days (F-T-3) and 5 days (F-T-5), based on (1) original images, (2) the overall average method, and (3) the differentiated tissue method.
7. Limitations and Challenges
Despite the significant advantages and diverse applications of vibrational spectroscopy in assessing meat quality, its industrial adoption faces several limitations and challenges [174,175]. One major hurdle is the complex and heterogeneous chemical composition of meat, which can lead to interference in the resultant spectrum, producing inaccurate or non-selective results. This complexity complicates sample analysis, making pre-processing essential. Combining multiple techniques, such as hyperspectral imaging and multi-spectral imaging with Raman scattering, could enhance sample detection by providing more detailed insights [176,177,178]. However, even with advancements, real-time monitoring of meat quality remains challenging. Thus, there is an urgent need for automated, on-site detection systems using spectroscopic techniques to enable real-time quality control during meat processing.
Furthermore, while non-thermal processing techniques hold great promise for maintaining meat quality, they can inadvertently cause the oxidation of biomolecules, leading to discoloration. This issue could be mitigated by optimizing the combination of non-thermal treatments to achieve the desired effects at lower intensities [179,180,181]. Additionally, the high cost of Raman spectroscopy systems remains a significant barrier to widespread adoption in the industry. Although recent developments have led to portable and handheld Raman devices, these systems are still limited in performance, making it difficult to meet the demands of large-scale production. Introducing robust, industrial-grade Raman systems on production lines would be necessary to fully realize the potential of this technology in meat processing.
Moreover, the presence of other components in meat, such as lipids, water, and carbohydrates, can interfere with the detection of subtle structural changes in proteins. Overlapping spectra from these biomolecules can complicate the interpretation of results. While chemometric techniques have been applied to address this issue, further refinement and sophistication in these analytical methods are needed to enhance accuracy and reliability [182]. These challenges highlight the need for continued research and technological advancements to fully leverage vibrational spectroscopy for industrial applications in meat quality assessment [183].
8. Future Prospects and Instrumentation Directions
As non-thermal technologies continue to evolve, future research must address both equipment-level innovation and process-level optimization [184]. While substantial progress has been made in improving meat protein gelation and safety through methods such as HPP, PEF, and ultrasound, many challenges remain in ensuring precise control, real-time monitoring, and industrial scalability. Instrumentation advances will be essential to bridge this gap. The increasing global demand for meat products, combined with the absence of efficient methods for in situ, rapid, precise, and high-throughput analysis, underlines the necessity for advancements in spectroscopic techniques specifically designed for the meat industry. Despite the current utility of these techniques, their limitations necessitate innovation and refinement to fully realize their potential in ensuring meat quality and safety [185,186]. Future developments in applying spectroscopy to meat processing should focus on the following areas:
- Enhancing real-time and accurate detection: To achieve reliable real-time analysis, advancements in Raman spectroscopy are crucial. Incorporating chemometric methods can effectively address issues like signal overlapping and spectral misinterpretation. Employing advanced mathematical algorithms such as PCA, Partial Least Square (PLS), and SVM can optimize data extraction, leading to more precise quality assessments.
- Integration of visual techniques: Combining spectroscopic methods with visual analysis offers significant potential for advancing food quality and safety assessments. Techniques like SERS [187], RCI [188], and spectral imaging [189] could be integrated to enhance tissue and microstructural assessment. This approach would not only improve monitoring of meat quality but also enhance insights into factors like animal health and nutritional status, ensuring safer meat products.
- Development of cost-effective, high-performance systems: There is a pressing need to develop efficient, real-time, and in situ detection systems that are both cost-effective and high-performing. By leveraging advancements in modern optics, computer technologies, and chemometric algorithms, spectroscopy can soon provide comprehensive and accurate evaluations of meat quality, thereby elevating the standards of protein quality and safety in the meat industry.
Looking ahead, interdisciplinary collaboration across food science, analytical chemistry, and machine learning will be key to building intelligent, robust, and scalable quality monitoring platforms for the meat industry.
9. Conclusions
Meat proteins significantly influence the quality, texture, and nutritional value of meat products, particularly through their involvement in MPG formation. While traditional thermal processing ensures food safety, it often results in excessive protein denaturation and aggregation, adversely affecting WHC, texture, and nutritional quality. This has increased the need for alternative processing methods that preserve the functional and structural properties of meat proteins. Non-thermal techniques, including HPP, PEF, ultrasound, and CAP, have proven to be effective substitutes for conventional approaches. These technologies enhance MPG formation by maintaining the structural and functional properties of meat proteins, thereby improving texture and preserving nutritional value. To optimize MPGs further, future research should focus on fine-tuning non-thermal processing parameters, such as HPP, PEF, ultrasound, and CAP, to achieve stronger gels with enhanced WHC, cohesiveness, and stability. Crucially, such optimization requires monitoring structural changes in proteins during processing. Vibrational spectroscopy techniques, such as Raman and infrared spectroscopy (FTIR, MIR, and NIR), provide powerful tools for real-time, non-invasive monitoring of protein conformational changes, denaturation, and gelation processes. These spectroscopic methods offer critical insights that are essential for ensuring the production of high-quality meat products. Additionally, integrating vibrational spectroscopy with advanced analytical approaches such as chemometrics, hyperspectral imaging, and machine learning can significantly improve the accuracy and specificity of meat quality assessments. This integration allows for rapid, high-throughput evaluations of complex meat systems, facilitating better predictions of processing outcomes. The combined use of non-thermal processing techniques with advanced vibrational spectroscopy and complementary technologies presents a promising strategy for producing high-quality, functional meat products that meet consumer demand for healthier, minimally processed foods. Continued research and technological innovations in this area will be pivotal in enhancing the efficiency, accuracy, and sustainability of meat production and quality control systems, ultimately aligning with industry trends toward more sustainable and consumer-friendly food processing solutions.
Author Contributions
H.L.: conceptualization, investigation, resources, writing—original draft, and writing—review and editing. C.L.: supervision, writing—review and editing. M.S.: writing—review and editing. W.Z.: writing—review and editing. A.M.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Foreign Experts Program (H20240213) and Jiangsu Province “333 High-level Talents” Cultivation Support and Funding Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gastaldello, A.; Giampieri, F.; De Giuseppe, R.; Grosso, G.; Baroni, L.; Battino, M. The Rise of Processed Meat Alternatives: A Narrative Review of the Manufacturing, Composition, Nutritional Profile and Health Effects of Newer Sources of Protein, and Their Place in Healthier Diets. Trends Food Sci. Technol. 2022, 127, 263–271. [Google Scholar] [CrossRef]
- Kutsanedzie, F.Y.H.; Guo, Z.; Chen, Q. Advances in Nondestructive Methods for Meat Quality and Safety Monitoring. Food Rev. Int. 2019, 35, 536–562. [Google Scholar] [CrossRef]
- Velazquez, G.; Méndez-Montealvo, M.G.; Welti-Chanes, J.; Ramírez, J.A.; Martínez-Maldonado, M.A. Effect of High Pressure Processing and Heat Treatment on the Gelation Properties of Blue Crab Meat Proteins. LWT 2021, 146, 111389. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of High Pressure Modification on Conformation and Gelation Properties of Myofibrillar Protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef]
- Zhou, C.; Okonkwo, C.E.; Inyinbor, A.A.; Yagoub, A.E.A.; Olaniran, A.F. Ultrasound, Infrared and Its Assisted Technology, a Promising Tool in Physical Food Processing: A Review of Recent Developments. Crit. Rev. Food Sci. Nutr. 2023, 63, 1587–1611. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M. Thermal and Non-Thermal Processing Affect Maillard Reaction Products, Flavor, and Phytochemical Profiles of Ginkgo Biloba Seed. Food Biosci. 2021, 41, 101044. [Google Scholar] [CrossRef]
- Baldi, G.; D’Elia, F.; Soglia, F.; Tappi, S.; Petracci, M.; Rocculi, P. Exploring the Effect of Pulsed Electric Fields on the Technological Properties of Chicken Meat. Foods 2021, 10, 241. [Google Scholar] [CrossRef]
- Li, J.; Dai, Z.; Chen, Z.; Hao, Y.; Wang, S.; Mao, X. Improved Gelling and Emulsifying Properties of Myofibrillar Protein from Frozen Shrimp (Litopenaeus vannamei) by High-Intensity Ultrasound. Food Hydrocoll. 2023, 135, 108188. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Frimpong, E.B.; Muhammad, U.; Qian, J.; Mustapha, A.T.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric Barrier Discharge Cold Atmospheric Plasma: Influence of Processing Parameters on Microbial Inactivation in Meat and Meat Products. Comp. Rev. Food Sci. Food Safe 2021, 20, 2626–2659. [Google Scholar] [CrossRef]
- Zareef, M.; Chen, Q.; Hassan, M.M.; Arslan, M.; Hashim, M.M.; Ahmad, W.; Kutsanedzie, F.Y.H.; Agyekum, A.A. An Overview on the Applications of Typical Non-Linear Algorithms Coupled with NIR Spectroscopy in Food Analysis. Food Eng. Rev. 2020, 12, 173–190. [Google Scholar] [CrossRef]
- Berthomieu, C.; Hienerwadel, R. Fourier Transform Infrared (FTIR) Spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Nunekpeku, X.; Zhang, W.; Gao, J.; Adade, S.Y.-S.S.; Li, H.; Chen, Q. Gel Strength Prediction in Ultrasonicated Chicken Mince: Fusing near-Infrared and Raman Spectroscopy Coupled with Deep Learning LSTM Algorithm. Food Control 2025, 168, 110916. [Google Scholar] [CrossRef]
- Qu, C.; Li, Y.; Du, S.; Geng, Y.; Su, M.; Liu, H. Raman Spectroscopy for Rapid Fingerprint Analysis of Meat Quality and Security: Principles, Progress and Prospects. Food Res. Int. 2022, 161, 111805. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Jiewen, Z.; Povey, M.J.W.; Holmes, M.; Hanpin, M. Variables Selection Methods in Near-Infrared Spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Nunekpeku, X.; Sheng, W.; Chen, Q. Investigating the Change Mechanism and Quantitative Analysis of Minced Pork Gel Quality with Different Starches Using Raman Spectroscopy. Food Hydrocoll. 2025, 159, 110634. [Google Scholar] [CrossRef]
- Wu, X.; Liang, X.; Wang, Y.; Wu, B.; Sun, J. Non-Destructive Techniques for the Analysis and Evaluation of Meat Quality and Safety: A Review. Foods 2022, 11, 3713. [Google Scholar] [CrossRef]
- Lee, S.; Jo, K.; Choi, Y.-S.; Jung, S. Tracking Bioactive Peptides and Their Origin Proteins during the in Vitro Digestion of Meat and Meat Products. Food Chem. 2024, 454, 139845. [Google Scholar] [CrossRef]
- Fang, T.; Han, M.; Wang, Y.; Xiang, X.; Chen, L.; Yang, H.; Kang, Z.; Huang, F.; Fan, X.; Han, M.; et al. Effects of Heating Rates on the Self-assembly Behavior and Gelling Properties of Beef Myosin. J. Sci. Food Agric. 2023, 103, 2473–2482. [Google Scholar] [CrossRef]
- Warren, D.; Soule, L.; Taylor, K.; Skinner, R.C.; Ku, K.M.; Matak, K.; Benedito, V.A.; Tou, J.C. Protein Quality and Safety Evaluation of Sarcoplasmic Protein Derived from Silver Carp (Hypophthalmichthys molitrix) Using a Rat Model. J. Food Sci. 2020, 85, 2544–2553. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P.; Estévez, M.; Yuan, L.; Gao, R. Freezing of Meat and Aquatic Food: Underlying Mechanisms and Implications on Protein Oxidation. Comp. Rev. Food Sci. Food Safe 2021, 20, 5548–5569. [Google Scholar] [CrossRef]
- Rong, Y.; Zareef, M.; Liu, L.; Din, Z.U.; Chen, Q.; Ouyang, Q. Application of Portable Vis-NIR Spectroscopy for Rapid Detection of Myoglobin in Frozen Pork. Meat Sci. 2023, 201, 109170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, T.; Wang, Y.; Liu, J.; Shi, W.; Hu, H.; Meng, Y.; Meng, X.; He, R. Influence of Multi-Frequency Ultrasound Treatment on Conformational Characteristics of Beef Myofibrillar Proteins with Different Degrees of Doneness. Foods 2023, 12, 2926. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shi, T.; Sun, Q.; Li, X.; McClements, D.J.; Yuan, L. Effects of L-Arginine and l-Histidine on Heat-Induced Aggregation of Fish Myosin: Bighead Carp (Aristichthys nobilis). Food Chem. 2019, 295, 320–326. [Google Scholar] [CrossRef]
- Gao, R.; Feng, X.; Li, W.; Yuan, L.; Ge, J.; Lu, D.; Chen, B.; Yu, G. Changes in Properties of White Shrimp (Litopenaeus vannamei) Protein during Thermal Denaturation. Food Sci. Biotechnol. 2016, 25, 21–26. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Mu, J.; Shi, T.; Yuan, L. Effect of L-Histidine on the Heat-Induced Aggregation of Bighead Carp (Aristichthys nobilis) Myosin in Low/High Ionic Strength Solution. Food Hydrocoll. 2018, 75, 174–181. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, D.; Tu, J.; Zhong, Y.; Zhang, D.; Wang, Z.; Tao, X. Mechanisms of Change in Gel Water-Holding Capacity of Myofibrillar Proteins Affected by Lipid Oxidation: The Role of Protein Unfolding and Cross-Linking. Food Chem. 2021, 344, 128587. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Wang, J.; Wang, Y.; Jin, G.; Teng, W.; Geng, F.; Cao, J. Myofibrillar Protein Denaturation/Oxidation in Freezing-Thawing Impair the Heat-Induced Gelation: Mechanisms and Control Technologies. Trends Food Sci. Technol. 2023, 138, 655–670. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.A.; Kumar, S.; Bhat, H.F. Thermal Processing Implications on the Digestibility of Meat, Fish and Seafood Proteins. Comp. Rev. Food Sci. Food Safe 2021, 20, 4511–4548. [Google Scholar] [CrossRef]
- Wang, R.-X.; Li, Y.-Q.; Sun, G.-J.; Wang, C.-Y.; Liang, Y.; Hua, D.-L.; Chen, L.; Mo, H.-Z. Effect of Transglutaminase on Structure and Gelation Properties of Mung Bean Protein Gel. Food Biophys. 2023, 18, 421–432. [Google Scholar] [CrossRef]
- Monto, A.R.; Li, M.; Wang, X.; Wijaya, G.Y.A.; Shi, T.; Xiong, Z.; Yuan, L.; Jin, W.; Li, J.; Gao, R. Recent Developments in Maintaining Gel Properties of Surimi Products under Reduced Salt Conditions and Use of Additives. Crit. Rev. Food Sci. Nutr. 2022, 62, 8518–8533. [Google Scholar] [CrossRef]
- Chang, J.; Liu, R.; Zheng, B.; Gao, X.; Li, B.; Zhang, Y.; Wang, T.; Wang, H. Amelioration of Myofibrillar Protein Emulsion Gel Properties by Mildly Oxidized Sunflower Oil. Food Chem. 2025, 467, 142253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, L.; Gao, P.; Yu, P.; Yang, F.; Yu, D.; Chen, H.; Xia, W. Study on the Effect and Mechanism of Chicken Breast on the Gel Properties of Silver Carp (Hypophthalmichtys molitrix) Surimi. J. Sci. Food Agric. 2024, 104, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Q.; Guo, L.; Ho, H.; Wang, B.; Sun, J.; Xu, X.; Huang, M. Effects of Ultrafine Comminution Treatment on Gelling Properties of Myofibrillar Proteins from Chicken Breast. Food Hydrocoll. 2019, 97, 105199. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zeng, X.; Han, Z.; Brennan, C.S. Non-thermal Technologies and Its Current and Future Application in the Food Industry: A Review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, L.; Qin, Z.; Wang, T. Review of Recent Advances in Intelligent and Antibacterial Packaging for Meat Quality and Safety. Foods 2025, 14, 1157. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Z.; Wu, D.; Fei, X.; Ei-Seedi, H.R.; Wang, C. High-Pressure Homogenization Influences the Functional Properties of Protein from Oyster (Crassostrea gigas). LWT 2021, 151, 112107. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Sukmanov, V.; Ma, H. Effects of Soy Protein Isolate on Gel Properties and Water Holding Capacity of Low-Salt Pork Myofibrillar Protein under High Pressure Processing. Meat Sci. 2021, 176, 108471. [Google Scholar] [CrossRef]
- Sun, L.; Lv, J.; Liu, Y.; Zang, M.; Li, P.; Wang, D.; Zhu, Y.; Xu, W. Effects of Combined Carnosine and Ultra-High Pressure on the Inhibition of Fishy off-Odor of Snakehead Fillets and the Possible Mechanism. Food Chem. 2022, 395, 133615. [Google Scholar] [CrossRef]
- Xiong, Z.; Shi, T.; Jin, W.; Bao, Y.; Monto, A.R.; Yuan, L.; Gao, R. Gel Performance of Surimi Induced by Various Thermal Technologies: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 3075–3090. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Bhandari, B.; Sun, J.; Gao, Z. Infusion of CO2 in a Solid Food: A Novel Method to Enhance the Low-Frequency Ultrasound Effect on Immersion Freezing Process. Innov. Food Sci. Emerg. Technol. 2016, 35, 194–203. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, F.; Zhang, Y.; Zhou, C.; Zhang, W.; Shi, J.; Zou, X.; Xiao, J. Freezing Characteristics and Relative Permittivity of Rice Flour Gel in Pulsed Electric Field Assisted Freezing. Food Chem. 2022, 373, 131449. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Rao, L.; Zhao, L.; Wu, X.; Wang, Y.; Liao, X. High Pressure Processing Combined with Selected Hurdles: Enhancement in the Inactivation of Vegetative Microorganisms. Comp. Rev. Food Sci. Food Safe 2021, 20, 1800–1828. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.-D.A.; Kumar, S.; Bhat, H.F. Emerging Processing Technologies for Improved Digestibility of Muscle Proteins. Trends Food Sci. Technol. 2021, 110, 226–239. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Z.; Mintah, B.K.; Xu, H.; Dabbour, M.; Cheng, Y.; Dai, C.; He, R.; Ma, H. Effects of Nonthermal Physical Processing Technologies on Functional, Structural Properties and Digestibility of Food Protein: A Review. J. Food Process Eng. 2022, 45, e14010. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Huang, X.; Zou, X.; Li, Z.; Zhang, D.; Zhang, W.; Xu, Y. Effects of Pulsed Electric Field on Freeze-Thaw Quality of Atlantic Salmon. Innov. Food Sci. Emerg. Technol. 2020, 65, 102454. [Google Scholar] [CrossRef]
- Guo, L.; Azam, S.M.R.; Guo, Y.; Liu, D.; Ma, H. Germicidal Efficacy of the Pulsed Magnetic Field against Pathogens and Spoilage Microorganisms in Food Processing: An Overview. Food Control 2022, 136, 108496. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current Applications and New Opportunities for the Use of Pulsed Electric Fields in Food Science and Industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Al-Hilphy, A.R.; Al-Asadi, M.H.; Al-Hmedawy, N.K.; Khalil, A.A.; Roobab, U.; Ranjha, M.M.A.N.; Manzoor, M.F. Effects of Electrical Field Stimulation on the Physicochemical and Sensory Attributes of Aged Chicken Meat. J. Food Process Eng. 2022, 45, e14032. [Google Scholar] [CrossRef]
- Hong, J.; An, D.; Liu, C.; Li, L.; Han, Z.; Guan, E.; Xu, B.; Zheng, X.; Bian, K. Rheological, Textural, and Digestible Properties of Fresh Noodles: Influence of Starch Esterified by Conventional and Pulsed Electric Field-assisted Dual Technique with Full Range of Amylose Content. J. Food Process Preserv. 2020, 44, e14567. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Fang, W.; Huang, X.; Shi, J.; Zou, X. Effects of Variety and Pulsed Electric Field on the Quality of Fresh-Cut Apples. Agriculture 2023, 13, 929. [Google Scholar] [CrossRef]
- Wang, B.; Khir, R.; Pan, Z.; Wood, D.; Mahoney, N.E.; El-Mashad, H.; Wu, B.; Ma, H.; Liu, X. Simultaneous Decontamination and Drying of Rough Rice Using Combined Pulsed Light and Holding Treatment: Simultaneous Decontamination and Drying of Rough Rice. J. Sci. Food Agric. 2016, 96, 2874–2881. [Google Scholar] [CrossRef] [PubMed]
- Astráin-Redín, L.; Raso, J.; Cebrián, G.; Álvarez, I. Potential of Pulsed Electric Fields for the Preparation of Spanish Dry-Cured Sausages. Sci. Rep. 2019, 9, 16042. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhou, Y.; Guo, J.; Feng, X.; Wang, X.; Wang, L.; Ma, J.; Sun, W. Low Frequency Magnetic Field plus High pH Promote the Quality of Pork Myofibrillar Protein Gel: A Novel Study Combined with Low Field NMR and Raman Spectroscopy. Food Chem. 2020, 326, 126896. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, Y.; Wang, D.; Xiong, G.; Xu, W. Effects of Ultrasound Pretreatment on the Extent of Maillard Reaction and the Structure, Taste and Volatile Compounds of Chicken Liver Protein. Food Chem. 2020, 331, 127369. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Liu, X.; He, L.; Zhang, M.; Huang, S.; Huang, S.; Liu, Y.; Zhang, Y.; Jin, G. A Multivariate Insight into the Organoleptic Properties of Porcine Muscle by Ultrasound-Assisted Brining: Protein Oxidation, Water State and Microstructure. LWT 2022, 159, 113136. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Guo, X.; Gao, S.; Wu, D.; Liu, Z.; Wu, P.; Xu, Z.; Zou, X.; Meng, X. Changes in Textural Quality and Water Retention of Spiced Beef under Ultrasound-Assisted Sous-Vide Cooking and Its Possible Mechanisms. Foods 2022, 11, 2251. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, R. Effect of Ultrasonication on Secondary Structure and Heat Induced Gelation of Chicken Myofibrils. J. Food Sci. Technol. 2016, 53, 3340–3348. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Yan, J.-K.; Ding, Y.; Rashid, M.T.; Ma, H. Effect of Sweep Frequency Ultrasound and Fixed Frequency Ultrasound Thawing on Gelling Properties of Myofibrillar Protein from Quick-Frozen Small Yellow Croaker and Its Possible Mechanisms. LWT 2021, 150, 111922. [Google Scholar] [CrossRef]
- Cheng, Y.; Donkor, P.O.; Ren, X.; Wu, J.; Agyemang, K.; Ayim, I.; Ma, H. Effect of Ultrasound Pretreatment with Mono-Frequency and Simultaneous Dual Frequency on the Mechanical Properties and Microstructure of Whey Protein Emulsion Gels. Food Hydrocoll. 2019, 89, 434–442. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and Meat Quality: A Review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Ojha, K.S.; Keenan, D.F.; Bright, A.; Kerry, J.P.; Tiwari, B.K. Ultrasound Assisted Diffusion of Sodium Salt Replacer and Effect on Physicochemical Properties of Pork Meat. Int. J. Food Sci. Technol. 2016, 51, 37–45. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Lai, B.; Liu, R.; Wu, M.; Ge, Q.; Yu, H. Effects of Ultrasound-Assisted Cooking on the Physicochemical Properties and Microstructure of Pork Meatballs. Meat Sci. 2024, 208, 109382. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, L.; Fu, Y.; Dai, H.; Zhang, Y. The Improvement of Gel and Physicochemical Properties of Porcine Myosin under Low Salt Concentrations by Pulsed Ultrasound Treatment and Its Mechanism. Food Res. Int. 2021, 141, 110056. [Google Scholar] [CrossRef]
- Xu, M.; Ni, X.; Liu, Q.; Chen, C.; Deng, X.; Wang, X.; Yu, R. Ultra-High Pressure Improved Gelation and Digestive Properties of Tai Lake Whitebait Myofibrillar Protein. Food Chem. X 2024, 21, 101061. [Google Scholar] [CrossRef]
- Lee, M.H.; In Yong, H.; Kim, Y.J.; Choi, Y.-S. High-Pressure Induced Structural Modification of Porcine Myofibrillar Protein and Its Relation to Rheological and Emulsifying Properties. Meat Sci. 2023, 196, 109032. [Google Scholar] [CrossRef]
- Ge, Q.; Wu, Y.; Yuan, N.; Jia, Z.; Liu, R.; Lu, F.; Ma, H.; Kang, Z. Effect of High Pressure Homogenization-Modified Soy 11S Globulin on the Gel and Rheological Properties of Pork Myofibrillar Protein. Foods 2023, 12, 810. [Google Scholar] [CrossRef]
- Jia, G.; Orlien, V.; Liu, H.; Sun, A. Effect of High Pressure Processing of Pork (Longissimus dorsi) on Changes of Protein Structure and Water Loss during Frozen Storage. LWT 2021, 135, 110084. [Google Scholar] [CrossRef]
- Okur, G.; Tavman, S.; Tsutsuura, S.; Nishiumi, T. Effect of High Pressure Processing on Traditional Turkish Meatballs Properties and Microbiological Safety during Frozen Storage. LWT 2023, 185, 115110. [Google Scholar] [CrossRef]
- Lee, S.; Jo, K.; Jeong, S.-K.-C.; Choi, Y.-S.; Jung, S. High-Pressure Processing of Beef Increased the in Vitro Protein Digestibility in an Infant Digestion Model. Meat Sci. 2023, 205, 109318. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, X.; Ma, F.; Xu, B.; Li, P.; Chen, C. Origin of High-Pressure Induced Changes in the Properties of Reduced-Sodium Chicken Myofibrillar Protein Gels Containing CaCl2: Physicochemical and Molecular Modification Perspectives. Food Chem. 2020, 319, 126535. [Google Scholar] [CrossRef]
- Dong, M.; Xu, Y.; Zhang, Y.; Han, M.; Wang, P.; Xu, X.; Zhou, G. Physicochemical and Structural Properties of Myofibrillar Proteins Isolated from Pale, Soft, Exudative (PSE)-like Chicken Breast Meat: Effects of Pulsed Electric Field (PEF). Innov. Food Sci. Emerg. Technol. 2020, 59, 102277. [Google Scholar] [CrossRef]
- Chian, F.M.; Kaur, L.; Oey, I.; Astruc, T.; Hodgkinson, S.; Boland, M. Effects of Pulsed Electric Field Processing and Sous Vide Cooking on Muscle Structure and In Vitro Protein Digestibility of Beef Brisket. Foods 2021, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Oey, I.; Bremer, P.; Silcock, P. Understanding the Effect of Meat Electrical Conductivity on Pulsed Electric Field (PEF) Process Parameters and the Ability of PEF to Enhance the Quality and Shorten Sous Vide Processing for Beef Short Ribs. Food Res. Int. 2023, 163, 112251. [Google Scholar] [CrossRef]
- Karki, R.; Oey, I.; Bremer, P.; Leong, S.Y.; Silcock, P. Effect of Pulsed Electric Fields (PEF) Pre-Treatment on the Quality of Sous Vide (SV) Processed Beef Short Ribs and Optimisation of PEF and SV Process Parameters Using Multiple Polynomial Regression Model. Food Bioprocess Technol. 2023, 16, 216–231. [Google Scholar] [CrossRef]
- Chang, C.-K.; Lung, C.-T.; Gavahian, M.; Yudhistira, B.; Chen, M.-H.; Santoso, S.P.; Hsieh, C.-W. Effect of Pulsed Electric Field-Assisted Thawing on the Gelling Properties of Pekin Duck Meat Myofibrillar Protein. J. Food Eng. 2023, 350, 111482. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, Y.; Sun, J.; Qiao, C. Preheating and High-Intensity Ultrasound Synergistically Affect the Physicochemical, Structural, and Gelling Properties of Chicken Wooden Breast Myofibrillar Protein. Food Res. Int. 2022, 162, 111975. [Google Scholar] [CrossRef]
- Xing, T.; Xu, Y.; Qi, J.; Xu, X.; Zhao, X. Effect of High Intensity Ultrasound on the Gelation Properties of Wooden Breast Meat with Different NaCl Contents. Food Chem. 2021, 347, 129031. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Wang, H.; Xia, X.; Kong, B. Ultrasound-Assisted Immersion Freezing Reduces the Structure and Gel Property Deterioration of Myofibrillar Protein from Chicken Breast. Ultrason. Sonochem. 2020, 67, 105137. [Google Scholar] [CrossRef]
- Kong, D.; Quan, C.; Xi, Q.; Han, R.; Li, P.; Du, Q.; Yang, Y.; Sun, J.; Tamplin, M.; Wang, J. Effects of Ultrasound-Assisted Slightly Acidic Electrolyzed Water Thawing on Myofibrillar Protein Conformation and Gel Properties of Chicken Breasts. Food Chem. 2023, 404, 134738. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Lorenzo, J.M.; Zhang, W. Effects of Ultrasound Emulsification on the Properties of Pork Myofibrillar Protein-Fat Mixed Gel. Food Chem. 2021, 345, 128751. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Shi, W. Ultrasound-Assisted Processing: Changes in Gel Properties, Water-Holding Capacity, and Protein Aggregation of Low-Salt Hypophthalmichthys molitrix Surimi by Soy Protein Isolate. Ultrason. Sonochem. 2023, 92, 106258. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, K.-X.; Du, J.-Y.; Tan, Z.-F.; Xu, Y.-P.; Liu, X.-Y.; Zhou, D.-Y.; Li, D.-Y. High-Intensity Ultrasound Improved the Physicochemical and Gelling Properties of Litopenaeus vannamei Myofibrillar Protein. Ultrason. Sonochem. 2022, 90, 106217. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Sultana, T.; Xun, S.; Jabbar, S.; Riaz Rajoka, M.S.; Albahi, A.; Abid, M.; Ranjha, M.M.A.N.; El-Seedi, H.R.; Xie, F.; et al. Advances in the Application of Functional Nanomaterial and Cold Plasma for the Fresh-keeping Active Packaging of Meat. Food Sci. Nutr. 2023, 11, 5753–5772. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Shi, T.; Xiong, Z.; Jin, W.; Bao, Y.; Monto, A.R.; Yuan, L.; Gao, R. Mechanism of Plasma-Activated Water Promoting the Heat-Induced Aggregation of Myofibrillar Protein from Silver Carp (Aristichthys Nobilis). Innov. Food Sci. Emerg. Technol. 2024, 91, 103555. [Google Scholar] [CrossRef]
- Qian, J.; Wang, Y.; Zhuang, H.; Yan, W.; Zhang, J.; Luo, J. Plasma Activated Water-Induced Formation of Compact Chicken Myofibrillar Protein Gel Structures with Intrinsically Antibacterial Activity. Food Chem. 2021, 351, 129278. [Google Scholar] [CrossRef]
- Qian, J.; Zhuang, H.; Nasiru, M.M.; Muhammad, U.; Zhang, J.; Yan, W. Action of Plasma-Activated Lactic Acid on the Inactivation of Inoculated Salmonella Enteritidis and Quality of Beef. Innov. Food Sci. Emerg. Technol. 2019, 57, 102196. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, R.A.; Hu, X.; Zhang, R.; Ding, T.; Zhou, J. Changes in Physicochemical and Conformational Properties of Myofibrillar Proteins Isolated from Mandarin Fish (Siniperca chuatsi) Treated by Atmospheric Pressure Plasma Jet. CyTA—J. Food 2023, 21, 625–633. [Google Scholar] [CrossRef]
- Situ, H.; Li, Y.; Gao, J.; Zhang, C.; Qin, X.; Cao, W.; Chen, Z.; Lin, H. Effects of Cold Atmospheric Plasma on Endogenous Enzyme Activity and Muscle Protein Oxidation in Trachinotus Ovatus. Food Chem. 2023, 407, 135119. [Google Scholar] [CrossRef]
- Miao, W.; Nyaisaba, B.M.; Koddy, J.K.; Chen, M.; Hatab, S.; Deng, S. Effect of Cold Atmospheric Plasma on the Physicochemical and Functional Properties of Myofibrillar Protein from Alaska Pollock (Theragra chalcogramma). Int. J. Food Sci. Technol. 2020, 55, 517–525. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Feasibility of Cold Plasma for the Control of Biofilms in Food Industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Shao, S.; Ye, Z.; Sun, J.; Liu, C.; Yan, J.; Liu, T.; Li, X.; Zhang, H.; Xiao, R. A Review on the Application of Non-Thermal Plasma (NTP) in the Conversion of Biomass: Catalyst Preparation, Thermal Utilization and Catalyst Regeneration. Fuel 2022, 330, 125420. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Kamal, M.M.; Karim, N.; Saifullah, M.; Khan, S.; Zhang, K.; Marappan, G.; Aalim, H.; Hashim, S.B.H.; Zhai, X.; et al. Cold Plasma and Integrated Approaches for Fresh Produce Preservation: Mechanisms, Quality Attributes, Challenges, and Future Directions. Trends Food Sci. Technol. 2025, 159, 104988. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased Materials for Active Food Packaging: A Review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Gurunathan, K.; Tahseen, A.; Manyam, S. Effect of Aerobic and Modified Atmosphere Packaging on Quality Characteristics of Chicken Leg Meat at Refrigerated Storage. Poult. Sci. 2022, 101, 102170. [Google Scholar] [CrossRef]
- Liu, R.; Guan, W.; Lv, W.; Kang, Z.; Wang, Q.; Jin, D.; Zhao, X.; Ge, Q.; Wu, M.; Yu, H. Oxidative Modification, Structural Conformation, and Gel Properties of Pork Paste Protein Mediated by Oxygen Concentration in Modified Atmosphere Packaging. Foods 2024, 13, 391. [Google Scholar] [CrossRef]
- Li, S.; Guo, X.; Shen, Y.; Pan, J.; Dong, X. Effects of Oxygen Concentrations in Modified Atmosphere Packaging on Pork Quality and Protein Oxidation. Meat Sci. 2022, 189, 108826. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, M.; Liu, H.; Li, Q.; Xue, D.; Nian, Y.; Zhao, D.; Shan, K.; Dai, C.; Li, C. Ultrasound Treatment Can Increase Digestibility of Myofibrillar Protein of Pork with Modified Atmosphere Packaging. Food Chem. 2022, 377, 131811. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, X.; Li, X.; Wang, W.; Wang, S.; Pan, J.; Dong, X.; Li, S. Effect of Cooking Temperatures on Meat Quality, Protein Carbonylation and Protein Cross-Linking of Beef Packed in High Oxygen Atmosphere. LWT 2022, 154, 112633. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Liu, J.; Sun, Z.; Yu, Z.; Battino, M.; El-Seedi, H.; Guan, X. Mechanistic Insights into the Changes of Enzyme Activity in Food Processing under Microwave Irradiation. Comp. Rev. Food Sci. Food Safe 2023, 22, 2465–2487. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, W.; Ding, X.; Wu, Z.; Li, Y. Effects of Dual-Frequency Ultrasound with Different Energy Irradiation Modes on the Structural and Emulsifying Properties of Soy Protein Isolate. Food Bioprod. Process. 2020, 123, 419–426. [Google Scholar] [CrossRef]
- Bai, R.; Han, J.; Ye, X.; Yu, J.; Jiang, S.; Li, Z.; Zhang, L.; Yang, C.; Chen, Y.; Wang, S.; et al. Improvement on Gel Properties of Chicken Myofibrillar Protein with Electron Beam Irradiation: Based on Protein Structure, Gel Quality, Water State. Int. J. Biol. Macromol. 2024, 280, 135806. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Kairam, N.; Ahmad, T.; Yadav, D.N. Physico Chemical, Microstructural and Sensory Characteristics of Low-fat Meat Emulsion Containing Aloe Gel as Potential Fat Replacer. Int. J. Food Sci. Technol. 2016, 51, 309–316. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Gao, S.; Bao, Y.; Tan, Y.; Luo, Y.; Li, X.; Hong, H. Effect of Protein Oxidation in Meat and Exudates on the Water Holding Capacity in Bighead Carp (Hypophthalmichthys nobilis) Subjected to Frozen Storage. Food Chem. 2022, 370, 131079. [Google Scholar] [CrossRef]
- Yan, J.-K.; Cai, W.-D.; Wang, C.; Yu, Y.-B.; Zhang, H.-N.; Yang, Y.; Wang, W.-H. Macromolecular Behavior, Structural Characteristics and Rheological Properties of Alkali-Neutralization Curdlan at Different Concentrations. Food Hydrocoll. 2020, 105, 105785. [Google Scholar] [CrossRef]
- Shi, T.; Xiong, Z.; Jin, W.; Yuan, L.; Sun, Q.; Zhang, Y.; Li, X.; Gao, R. Suppression Mechanism of L-Arginine in the Heat-Induced Aggregation of Bighead Carp (Aristichthys nobilis) Myosin: The Significance of Ionic Linkage Effects and Hydrogen Bond Effects. Food Hydrocoll. 2020, 102, 105596. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, C.; Qiu, W.-Y.; Chen, T.-T.; Yang, Y.; Wang, W.-H.; Zhang, H.-N. Ultrasonic Treatment at Different pH Values Affects the Macromolecular, Structural, and Rheological Characteristics of Citrus Pectin. Food Chem. 2021, 341, 128216. [Google Scholar] [CrossRef]
- Liu, Y.; Mubango, E.; Dou, P.; Bao, Y.; Tan, Y.; Luo, Y.; Li, X.; Hong, H. Insight into the Protein Oxidation Impact on the Surface Properties of Myofibrillar Proteins from Bighead Carp. Food Chem. 2023, 411, 135515. [Google Scholar] [CrossRef]
- Wu, D.; Wang, H.; Guo, X.; Zhang, Z.; Gao, Z.; Gao, S.; Liu, Z.; Rao, S.; Meng, X. Insight into the Mechanism of Enhancing Myofibrillar Protein Gel Hardness by Ultrasonic Treatment Combined with Insoluble Dietary Fiber from Oat. LWT 2023, 178, 114539. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Yang, H.; Xue, J.; Li, Y.; Wang, S.; Ge, L.; Shen, Q.; Zhang, M. Comparative Study on the Rheological Properties of Myofibrillar Proteins from Different Kinds of Meat. LWT 2022, 153, 112458. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Bai, F.; Wang, J.; Zhou, X.; Gao, R.; Xu, X.; Zhao, Y. Influence of Thermal Processing on Flavor and Sensory Profile of Sturgeon Meat. Food Chem. 2022, 374, 131689. [Google Scholar] [CrossRef]
- Ye, T.; Chen, X.; Lu, Y.; Zhao, H.; Chen, Z.; Lin, L.; Zheng, Z.; Lu, J. Masterly Substitution of Plant Proteins Can Achieve Acceptable Sensory and Nutritional Attributes in Meat-Reduced Surimi Gel. LWT 2024, 208, 116668. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman Spectroscopy for Food Quality Assurance and Safety Monitoring: A Review. Curr. Opin. Food Sci. 2022, 47, 100910. [Google Scholar] [CrossRef]
- Yan, H.; Neves, M.D.G.; Wise, B.M.; Moraes, I.A.; Barbin, D.F.; Siesler, H.W. The Application of Handheld Near-Infrared Spectroscopy and Raman Spectroscopic Imaging for the Identification and Quality Control of Food Products. Molecules 2023, 28, 7891. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Yang, Z.; Wang, X.; Wang, W.; Hui, T. Rapid Non-Destructive Detection Technology in the Field of Meat Tenderness: A Review. Foods 2024, 13, 1512. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Sun, D.-W. Raman Spectroscopic Techniques for Detecting Structure and Quality of Frozen Foods: Principles and Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 2623–2639. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman Spectroscopy to Characterize Biological Materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, T.; Wang, X.; Zou, Y.; Wang, D.; Xu, W. Effects of the Structure and Gel Properties of Myofibrillar Protein on Chicken Breast Quality Treated with Ultrasound-Assisted Potassium Alginate. Food Chem. 2021, 358, 129873. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Xu, Y.; Mi, H.; Yi, S.; Gao, R.; Li, X.; Li, J. Effects of Chickpea Protein-Stabilized Pickering Emulsion on the Structure and Gelling Properties of Hairtail Fish Myosin Gel. Food Chem. 2023, 417, 135821. [Google Scholar] [CrossRef]
- Shi, T.; Xiong, Z.; Liu, H.; Jin, W.; Mu, J.; Yuan, L.; Sun, Q.; McClements, D.J.; Gao, R. Ameliorative Effects of L-Arginine? On Heat-Induced Phase Separation of Aristichthys nobilis Myosin Are Associated with the Absence of Ordered Secondary Structures of Myosin. Food Res. Int. 2021, 141, 110154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, H.; Lyu, F.; Lin, H.; Zhang, Q.; Ding, Y. Physicochemical Properties and Microstructure of Fish Myofibrillar Protein-Lipid Composite Gels: Effects of Fat Type and Concentration. Food Hydrocoll. 2019, 90, 433–442. [Google Scholar] [CrossRef]
- Pasquini, C. Near Infrared Spectroscopy: A Mature Analytical Technique with New Perspectives—A Review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, X.; Liang, Z.; Qi, W.; Zheng, X.; Lu, D.; Chen, B. Application of NIR Spectral Standardization Based on Principal Component Score Evaluation in Wheat Flour Crude Protein Model Sharing. J. Food Qual. 2022, 2022, 9009756. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ali, S.; Ouyang, Q.; Li, H.; Wu, X.; Hashim, M.M.; Javaria, S.; Chen, Q. Application of Benchtop NIR Spectroscopy Coupled with Multivariate Analysis for Rapid Prediction of Antioxidant Properties of Walnut (Juglans regia). Food Chem. 2021, 359, 129928. [Google Scholar] [CrossRef]
- Karoui, R.; Downey, G.; Blecker, C. Mid-Infrared Spectroscopy Coupled with Chemometrics: A Tool for the Analysis of Intact Food Systems and the Exploration of Their Molecular Structure−quality Relationships—A Review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiao, L.; Wu, J.; Zhang, Y.; Zhu, Q.; Dong, D. Non-Destructive and in-Situ Detection of Shrimp Freshness Using Mid-Infrared Fiber-Optic Evanescent Wave Spectroscopy. Food Chem. 2023, 422, 136189. [Google Scholar] [CrossRef]
- Hassoun, A.; Ojha, S.; Tiwari, B.; Rustad, T.; Nilsen, H.; Heia, K.; Cozzolino, D.; Bekhit, A.E.-D.; Biancolillo, A.; Wold, J.P. Monitoring Thermal and Non-Thermal Treatments during Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances. Appl. Sci. 2020, 10, 6802. [Google Scholar] [CrossRef]
- Xu, M.; Sun, J.; Zhou, X.; Tang, N.; Shen, J.; Wu, X. Research on Nondestructive Identification of Grape Varieties Based on EEMD-DWT and Hyperspectral Image. J. Food Sci. 2021, 86, 2011–2023. [Google Scholar] [CrossRef]
- Yao, K.; Sun, J.; Chen, C.; Xu, M.; Zhou, X.; Cao, Y.; Tian, Y. Non-Destructive Detection of Egg Qualities Based on Hyperspectral Imaging. J. Food Eng. 2022, 325, 111024. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; You, J.; Xiong, S. Rapid Determination of the Textural Properties of Silver Carp (Hypophthalmichthys molitrix) Using near-Infrared Reflectance Spectroscopy and Chemometrics. LWT 2020, 129, 109545. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, C.; Sun, J.; Cao, Y.; Yao, K.; Xu, M. A Deep Learning Method for Predicting Lead Content in Oilseed Rape Leaves Using Fluorescence Hyperspectral Imaging. Food Chem. 2023, 409, 135251. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Pu, Y.; Wang, J.; Tang, B.; Dai, L.; Yu, S.; Chen, R. Identification of Transgenic Agricultural Products and Foods Using NIR Spectroscopy and Hyperspectral Imaging: A Review. Processes 2023, 11, 651. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Tian, Y.; Lu, B.; Hang, Y.; Chen, Q. Hyperspectral Technique Combined with Deep Learning Algorithm for Detection of Compound Heavy Metals in Lettuce. Food Chem. 2020, 321, 126503. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sun, J.; Yao, K.; Xu, M.; Zhou, X. Nondestructive Detection and Visualization of Protein Oxidation Degree of Frozen-Thawed Pork Using Fluorescence Hyperspectral Imaging. Meat Sci. 2022, 194, 108975. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, J.; Yao, K.; Xu, M.; Dai, C. Multi-Task Convolutional Neural Network for Simultaneous Monitoring of Lipid and Protein Oxidative Damage in Frozen-Thawed Pork Using Hyperspectral Imaging. Meat Sci. 2023, 201, 109196. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Zhou, G. Covalent Chemical Modification of Myofibrillar Proteins to Improve Their Gelation Properties: A Systematic Review. Comp. Rev. Food Sci. Food Safe 2021, 20, 924–959. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Alvarenga, T.I.R.C.; Hopkins, D.L. The Effect of Fibre Orientation, Measurement Interval and Muscle on Lamb Meat Drip Loss Values. Meat Sci. 2020, 161, 107959. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wu, D.; Gao, S.; Jiang, S.; Tang, H.; Lv, G.; Zou, X.; Meng, X. Changes in Physicochemical Quality and Protein Properties of Porcine Longissimus lumborum during Dry Ageing. Int. J. Food Sci. Technol. 2022, 57, 5954–5965. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Teng, S.; Zhang, W.; Purslow, P.P.; Zhang, R. Changes in Collagen Properties and Cathepsin Activity of Beef M. Semitendinosus by the Application of Ultrasound during Post-Mortem Aging. Meat Sci. 2022, 185, 108718. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. Factors Influencing Gel Formation by Myofibrillar Proteins in Muscle Foods. Comp. Rev. Food Sci. Food Safe 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Sang, S.; Chen, X.; Qin, Y.; Tong, L.; Ou, C. A Study on the Effects of Calcium Lactate on the Gelling Properties of Large Yellow Croaker (Pseudosciaena crocea) Surimi by Low-Field Nuclear Magnetic Resonance and Raman Spectroscopy. Foods 2022, 11, 3197. [Google Scholar] [CrossRef]
- Lan, M.; Li, L.; Peng, X.; Chen, J.; Cao, Q.; He, N.; Cai, J.; Li, B.; Zhang, X. Effects of Different Lipids on the Physicochemical Properties and Microstructure of Pale, Soft and Exudative (PSE)-like Chicken Meat Gel. LWT 2021, 145, 111284. [Google Scholar] [CrossRef]
- Mandal, P.; Tewari, B.S. Progress in Surface Enhanced Raman Scattering Molecular Sensing: A Review. Surf. Interfaces 2022, 28, 101655. [Google Scholar] [CrossRef]
- Li, H.; Sheng, W.; Adade, S.Y.-S.S.; Nunekpeku, X.; Chen, Q. Investigation of Heat-Induced Pork Batter Quality Detection and Change Mechanisms Using Raman Spectroscopy Coupled with Deep Learning Algorithms. Food Chem. 2024, 461, 140798. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, S.; Duan, Z.; Kang, R.; Zhao, M.; Xia, G.; Shen, X. Effect of Citric Acid on Physicochemical Properties and Protein Structure of Low-Salt Restructured Tilapia (Oreochromis mossambicus) Meat Products. J. Sci. Food Agric. 2021, 101, 1636–1645. [Google Scholar] [CrossRef]
- Shi, T.; Yuan, L.; Mu, J.; Gao, R. The Effect of Arginine, Lysine and Histidine in the Myosin Secondary Structure by Circular Dichroism and Raman Spectroscopy. CyTA—J. Food 2019, 17, 656–660. [Google Scholar] [CrossRef]
- Martínez-Maldonado, M.A.; Velazquez, G.; Ramírez De León, J.A.; Borderías, A.J.; Moreno, H.M. Effect of High Pressure Processing on Heat-Induced Gelling Capacity of Blue Crab (Callinectes sapidus) Meat. Innov. Food Sci. Emerg. Technol. 2020, 59, 102253. [Google Scholar] [CrossRef]
- Hameed, A.; Maan, A.A.; Khan, M.K.I.; Mahmood Khan, I.; Niazi, S.; Waheed Iqbal, M.; Riaz, T.; Manzoor, M.F.; Abdalla, M. Evaporation Kinetics and Quality Attributes of Grape Juice Concentrate as Affected by Microwave and Vacuum Processing. Int. J. Food Prop. 2023, 26, 1596–1611. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Shi, G.; Xiong, G.; Shi, L.; Kang, J.; Su, J.; Ding, A.; Li, X.; Qiao, Y.; et al. Quantitative Proteomics Insights into Gel Properties Changes of Myofibrillar Protein from Procambarus clarkii under Cold Stress. Food Chem. 2022, 372, 130935. [Google Scholar] [CrossRef]
- Tang, S.; Feng, G.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Zhao, Y.; Zeng, M. Thermal Gel Degradation (Modori) in Sturgeon (Acipenseridae) Surimi Gels. J. Food Sci. 2019, 84, 3601–3607. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Chen, Q.; Zhang, Y. Nondestructive Measurement of Total Volatile Basic Nitrogen (TVB-N) in Pork Meat by Integrating near Infrared Spectroscopy, Computer Vision and Electronic Nose Techniques. Food Chem. 2014, 145, 228–236. [Google Scholar] [CrossRef]
- Li, Q.; Wu, X.; Zheng, J.; Wu, B.; Jian, H.; Sun, C.; Tang, Y. Determination of Pork Meat Storage Time Using Near-Infrared Spectroscopy Combined with Fuzzy Clustering Algorithms. Foods 2022, 11, 2101. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Nunekpeku, X.; Zhang, W.; Adade, S.Y.-S.S.; Ahmad, W.; Sheng, W.; Chen, Q. Quantitative Prediction of Minced Chicken Gel Strength under Ultrasonic Treatment by NIR Spectroscopy Coupled with Nonlinear Chemometric Tools Evaluated Using APaRPs. Food Chem. 2025, 463, 141373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xie, Y.; Yu, H.; Guo, Y.; Yao, W. Non-Destructive Prediction of Colour and Water-Related Properties of Frozen/Thawed Beef Meat by Raman Spectroscopy Coupled Multivariate Calibration. Food Chem. 2023, 413, 135513. [Google Scholar] [CrossRef]
- Vilkova, D.; Sangaré, M.; Egorov, M.; Karoui, R. Mid-Infrared Spectroscopy Enabled Rapid Differentiation between Fresh and Frozen–Thawed Sevruga (Acipenser stellatus) Samples Presenting Different Raw Quality. Eur. Food Res. Technol. 2023, 249, 2299–2310. [Google Scholar] [CrossRef]
- Yang, H.; Hopkins, D.L.; Zhang, Y.; Zhu, L.; Dong, P.; Wang, X.; Mao, Y.; Luo, X.; Fowler, S.M. Preliminary Investigation of the Use of Raman Spectroscopy to Predict Beef Spoilage in Different Types of Packaging. Meat Sci. 2020, 165, 108136. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, P.; Fengou, L.-C.; Nychas, G.-J.; Fowler, S.M.; Mao, Y.; Luo, X.; Zhang, Y. Preliminary Investigation into the Prediction of Indicators of Beef Spoilage Using Raman and Fourier Transform Infrared Spectroscopy. Meat Sci. 2023, 200, 109168. [Google Scholar] [CrossRef]
- Leng, T.; Li, F.; Chen, Y.; Tang, L.; Xie, J.; Yu, Q. Fast Quantification of Total Volatile Basic Nitrogen (TVB-N) Content in Beef and Pork by near-Infrared Spectroscopy: Comparison of SVR and PLS Model. Meat Sci. 2021, 180, 108559. [Google Scholar] [CrossRef]
- León-Ecay, S.; López-Maestresalas, A.; Murillo-Arbizu, M.T.; Beriain, M.J.; Mendizabal, J.A.; Arazuri, S.; Jarén, C.; Bass, P.D.; Colle, M.J.; García, D.; et al. Classification of Beef Longissimus thoracis Muscle Tenderness Using Hyperspectral Imaging and Chemometrics. Foods 2022, 11, 3105. [Google Scholar] [CrossRef]
- Ruiz, M.; Beriain, M.J.; Beruete, M.; Insausti, K.; Lorenzo, J.M.; Sarriés, M.V. Application of MIR Spectroscopy to the Evaluation of Chemical Composition and Quality Parameters of Foal Meat: A Preliminary Study. Foods 2020, 9, 583. [Google Scholar] [CrossRef]
- Coria, M.S.; Castaño Ledesma, M.S.; Gómez Rojas, J.R.; Grigioni, G.; Palma, G.A.; Borsarelli, C.D. Prediction of Tenderness in Bovine Longissimus thoracis et Lumborum Muscles Using Raman Spectroscopy. Anim. Biosci. 2023, 36, 1435–1444. [Google Scholar] [CrossRef]
- Kucha, C.T.; Liu, L.; Ngadi, M.; Gariépy, C. Prediction and Visualization of Fat Content in Polythene-Packed Meat Using near-Infrared Hyperspectral Imaging and Chemometrics. J. Food Compos. Anal. 2022, 111, 104633. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W.; Xie, Y.; Ozaki, Y. Non-Destructive Prediction of Texture of Frozen/Thaw Raw Beef by Raman Spectroscopy. J. Food Eng. 2020, 266, 109693. [Google Scholar] [CrossRef]
- Hoffman, L.; Ingle, P.; Hemant Khole, A.; Zhang, S.; Yang, Z.; Beya, M.; Bureš, D.; Cozzolino, D. Discrimination of Lamb (Ovis aries), Emu (Dromaius novaehollandiae), Camel (Camelus dromedarius) and Beef (Bos taurus) Binary Mixtures Using a Portable near Infrared Instrument Combined with Chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 294, 122506. [Google Scholar] [CrossRef]
- Wachirattanapongmetee, K.; Katekaew, S.; Weerapreeyakul, N.; Thawornchinsombut, S. Differentiation of Protein Types Extracted from Tilapia Byproducts by FTIR Spectroscopy Combined with Chemometric Analysis and Their Antioxidant Protein Hydrolysates. Food Chem. 2024, 437, 137862. [Google Scholar] [CrossRef]
- Aheto, J.H.; Huang, X.; Tian, X.; Lv, R.; Dai, C.; Bonah, E.; Chang, X. Evaluation of Lipid Oxidation and Volatile Compounds of Traditional Dry-cured Pork Belly: The Hyperspectral Imaging and Multi-gas-sensory Approaches. J. Food Process Eng. 2020, 43, e13092. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, J.; Yao, K.; Dai, C. Generalized and Hetero Two-Dimensional Correlation Analysis of Hyperspectral Imaging Combined with Three-Dimensional Convolutional Neural Network for Evaluating Lipid Oxidation in Pork. Food Control 2023, 153, 109940. [Google Scholar] [CrossRef]
- Aheto, J.H.; Huang, X.; Tian, X.; Ren, Y.; Bonah, E.; Alenyorege, E.A.; Lv, R.; Dai, C. Combination of Spectra and Image Information of Hyperspectral Imaging Data for Fast Prediction of Lipid Oxidation Attributes in Pork Meat. J. Food Process Eng. 2019, 42, e13225. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, J.; Shi, L.; Dai, C. An Effective Method Fusing Electronic Nose and Fluorescence Hyperspectral Imaging for the Detection of Pork Freshness. Food Biosci. 2024, 59, 103880. [Google Scholar] [CrossRef]
- Tian, X.; Aheto, J.H.; Huang, X.; Zheng, K.; Dai, C.; Wang, C.; Bai, J. An Evaluation of Biochemical, Structural and Volatile Changes of Dry-cured Pork Using a Combined Ion Mobility Spectrometry, Hyperspectral and Confocal Imaging Approach. J. Sci. Food Agric. 2021, 101, 5972–5983. [Google Scholar] [CrossRef]
- Li, H.; Kutsanedzie, F.; Zhao, J.; Chen, Q. Quantifying Total Viable Count in Pork Meat Using Combined Hyperspectral Imaging and Artificial Olfaction Techniques. Food Anal. Methods 2016, 9, 3015–3024. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Hu, X.; Li, Z.; Huang, X.; Liang, J.; Zhang, X.; Zhang, D.; Zou, X.; Shi, J. Quantitative Characterization of the Diffusion Behavior of Sucrose in Marinated Beef by HSI and FEA. Meat Sci. 2023, 195, 109002. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, W.; Zhang, H.; Liu, R.; Li, C. Discrimination among Fresh, Frozen–Stored and Frozen–Thawed Beef Cuts by Hyperspectral Imaging. Foods 2024, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Qiu, C.; Guo, Y.; Zhang, C.; Guo, X.; Bouhile, Y.; Ma, H. Ultrasonic-Assisted Flowing Water Thawing of Frozen Beef with Different Frequency Modes: Effects on Thawing Efficiency, Quality Characteristics and Microstructure. Food Res. Int. 2022, 157, 111484. [Google Scholar] [CrossRef]
- Li, Y.; Pan, T.; Li, H.; Chen, S. Non-Invasive Quality Analysis of Thawed Tuna Using near Infrared Spectroscopy with Baseline Correction. J. Food Process Eng. 2020, 43, e13445. [Google Scholar] [CrossRef]
- Khan, I.A.; Khan, A.; Zou, Y.; Zongshuai, Z.; Xu, W.; Wang, D.; Huang, M. Heterocyclic Amines in Cooked Meat Products, Shortcomings during Evaluation, Factors Influencing Formation, Risk Assessment and Mitigation Strategies. Meat Sci. 2022, 184, 108693. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of Hyperspectral Imaging as a Nondestructive Technique for Foodborne Pathogen Detection and Characterization. Foodborne Pathog. Dis. 2019, 16, 712–722. [Google Scholar] [CrossRef]
- Aheto, J.H.; Huang, X.; Xiaoyu, T.; Bonah, E.; Ren, Y.; Alenyorege, E.A.; Chunxia, D. Investigation into Crystal Size Effect on Sodium Chloride Uptake and Water Activity of Pork Meat Using Hyperspectral Imaging. J. Food Process Preserv. 2019, 43, e14197. [Google Scholar] [CrossRef]
- Tian, X.; Aheto, J.H.; Dai, C.; Ren, Y.; Bai, J. Monitoring Microstructural Changes and Moisture Distribution of Dry-cured Pork: A Combined Confocal Laser Scanning Microscopy and Hyperspectral Imaging Study. J. Sci. Food Agric. 2021, 101, 2727–2735. [Google Scholar] [CrossRef]
- Wu, X.; Fu, H.; Tian, X.; Wu, B.; Sun, J. Prediction of Pork Storage Time Using Fourier Transform near Infrared Spectroscopy and adaboost-ULDA. J. Food Process Eng. 2017, 40, e12566. [Google Scholar] [CrossRef]
- Owusu-Ansah, P.; Yu, X.; Osae, R.; Zhou, C.; Zhang, R.; Mustapha, A.T.; Li, M.; Ma, H. Optimization of Thermosonication on Bacillus cereus from Pork: Effects on Inactivation and Physicochemical Properties. J. Food Process Eng. 2020, 43, e13401. [Google Scholar] [CrossRef]
- Owusu-Ansah, P.; Yu, X.; Osae, R.; Mustapha, A.T.; Zhang, R.; Zhou, C. Inactivation of Bacillus cereus from Pork by Thermal, Non-Thermal and Single-Frequency/Multi-Frequency Thermosonication: Modelling and Effects on Physicochemical Properties. LWT 2020, 133, 109939. [Google Scholar] [CrossRef]
- Yang, S.; Sen, C.; Thompson, R.; Zhou, J.-G.; Akkus, O. An in Vitro Raman Study on Compositional Correlations of Lipids and Protein with Animal Tissue Hydration. Vib. Spectrosc. 2020, 107, 103022. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Xiaobo, Z.; Jianbo, X.; Mahunu, G.K.; Jiyong, S.; Xu, J.-L.; Sun, D.-W. Recent Progress in Rapid Analyses of Vitamins, Phenolic, and Volatile Compounds in Foods Using Vibrational Spectroscopy Combined with Chemometrics: A Review. Food Anal. Methods 2019, 12, 2361–2382. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Yan, J.; Aadil, R.M. Editorial: Innovative Non-Thermal Technologies for the Extraction and Modification of Bioactive Compounds from Food Processing By-Products. Front. Nutr. 2023, 10, 1161957. [Google Scholar] [CrossRef]
- Ye, H.; Yang, J.; Xiao, G.; Zhao, Y.; Li, Z.; Bai, W.; Zeng, X.; Dong, H. A Comprehensive Overview of Emerging Techniques and Chemometrics for Authenticity and Traceability of Animal-Derived Food. Food Chem. 2023, 402, 134216. [Google Scholar] [CrossRef]
- Li, B.; Zhong, M.; Sun, Y.; Liang, Q.; Shen, L.; Qayum, A.; Rashid, A.; Rehman, A.; Ma, H.; Ren, X. Recent Advancements in the Utilization of Ultrasonic Technology for the Curing of Processed Meat Products: A Comprehensive Review. Ultrason. Sonochem. 2024, 103, 106796. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Zheng, Z.; Haruna, S.A.; Chen, Q. Flexible SERS Sensor Using AuNTs-Assembled PDMS Film Coupled Chemometric Algorithms for Rapid Detection of Chloramphenicol in Food. Food Chem. 2023, 418, 135998. [Google Scholar] [CrossRef]
- Holroyd, S.E.; Nickless, E. Understanding Mozzarella Cheese Microstructure with Raman Confocal Imaging. Int. Dairy. J. 2024, 152, 105893. [Google Scholar] [CrossRef]
- Dong, F.; Xu, Y.; Shi, Y.; Feng, Y.; Ma, Z.; Li, H.; Zhang, Z.; Wang, G.; Chen, Y.; Xian, J.; et al. Spectral Reconstruction from RGB Image to Hyperspectral Image: Take the Detection of Glutamic Acid Index in Beef as an Example. Food Chem. 2025, 463, 141543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).