Unlocking the Functional and Nutritional Potential of Microalgae Proteins in Food Systems: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Integrated Perspectives on Microalgae Proteins
3.1. Diversity and Biochemical Composition
3.2. Nutritional Quality and Health Benefits
3.3. Functional Attributes in Food Systems
4. Integrated Processing Strategies: Extraction, Purification, and Protein Modification
4.1. Extraction Techniques and Cell Disruption
4.2. Purification Strategies
4.3. Protein Modification Methods
4.4. Linking Processing to Functionality
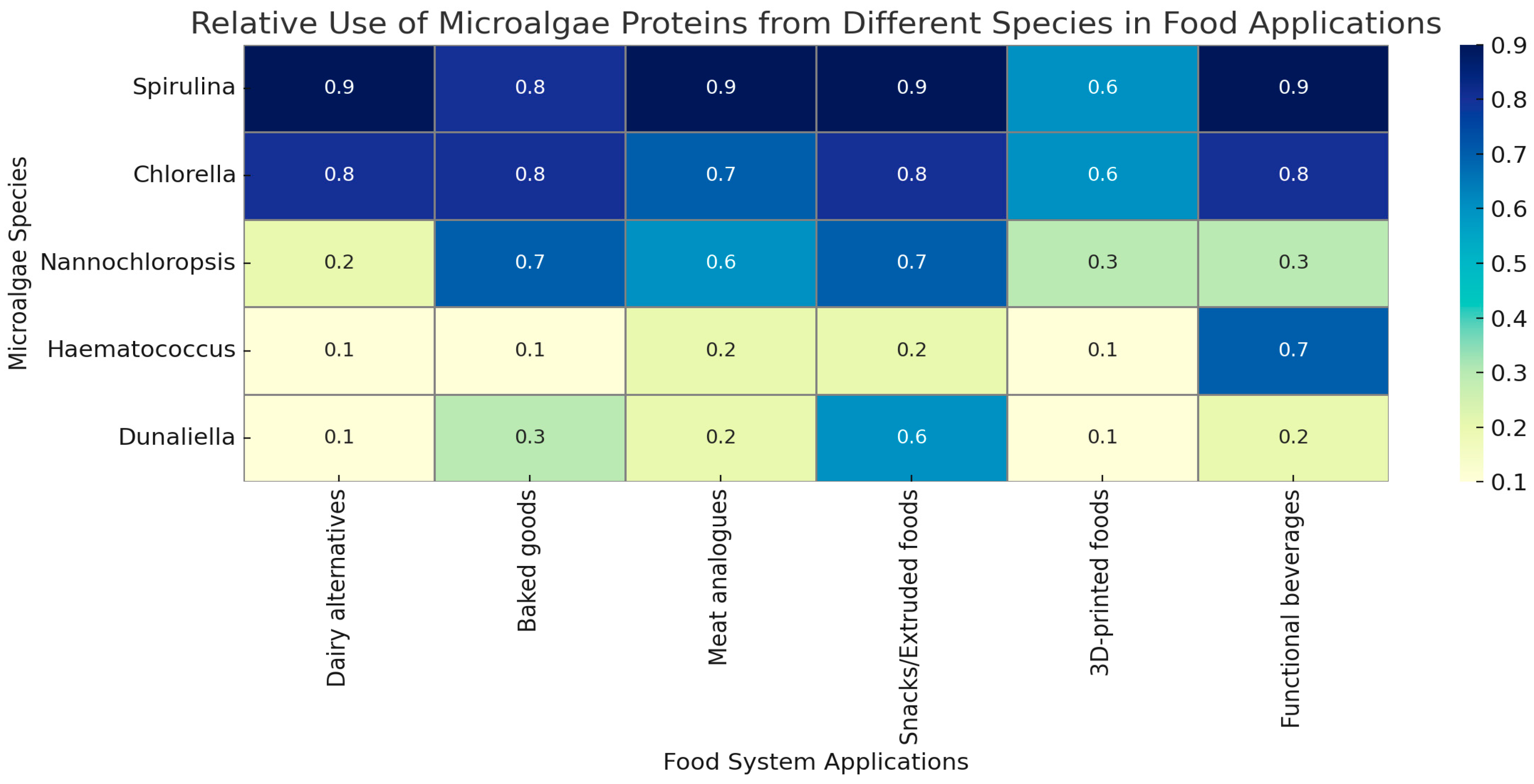
5. Applications of Microalgae Proteins in Food Systems
5.1. Dairy Alternatives and Fermented Products
5.2. Bakery, Pasta, and Extruded Snacks
5.3. Emerging Food Formats and Innovative Applications
5.4. Functional Foods and Nutraceuticals
5.5. Clean-Label Colourants and Aesthetic Enhancements
6. Sustainability, Scalability, and Economic Viability
6.1. Environmental Sustainability and Resource Efficiency
6.2. Technical and Operational Challenges in Scaling
6.3. Economic Considerations and Integrated Biorefinery Approaches
7. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williamson, E.; Ross, I.; Wall, B.; Hankamer, B. Microalgae: Potential novel protein for sustainable human nutrition. Trends Plant Sci. 2023, 29, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Avni, D. Microalgae as a sustainable source of protein and food ingredients. Open Access Gov. 2023, 39, 466–467. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Wang, J.; Cheng, K.; Liu, J.; He, Y.; Zhang, Y.; Mou, H.; Sun, H. Microalgal protein for sustainable and nutritious foods: A joint analysis of environmental impacts, health benefits and consumer’s acceptance. Trends Food Sci. Technol. 2023, 143, 104278. [Google Scholar] [CrossRef]
- Costa, M.M.; Prates, J.A.M. Sustainable livestock production and poverty alleviation. In Smart Technologies for Sustainable Development Goals: No Poverty; CRC Press: Boca Raton, FL, USA, 2024; pp. 109–124. [Google Scholar]
- Costa, M.; Coelho, D.; Alfaia, C.; Pestana, J.; Lopes, P.A.; Prates, J.A.M. Microalgae application in feeds for monogastrics. In Handbook of Food and Feed from Microalgae: Production, Application, Regulation, and Sustainability; Academic Press: New York, NY, USA, 2023; pp. 411–420. [Google Scholar]
- Guo, X.; Wang, Q.; Wu, Y.; Liu, X.; Gong, Z. Comprehensive Insights into Microalgae Proteins: Nutritional Profiles and Innovative Applications as Sustainable Alternative Proteins in Health and Food Sciences. Food Hydrocoll. 2024, 154, 110112. [Google Scholar] [CrossRef]
- Prates, J.A.M. Enhancing Meat Quality and Nutritional Value in Monogastric Livestock Using Sustainable Novel Feed Ingredients. Foods 2025, 14, 146. [Google Scholar] [CrossRef]
- Xu, Y.; Tong, X.; Lu, Y.; Lu, Y.; Wang, X.; Han, J.; Liu, Z.; Ding, J.; Diao, C.; Mumby, W.; et al. Microalgal proteins: Unveiling sustainable alternatives to address the protein challenge. Int. J. Biol. Macromol. 2024, 276, 133747. [Google Scholar] [CrossRef]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Chemical Compounds, Bioactivities, and Applications of Chlorella vulgaris in Food, Feed and Medicine. Appl. Sci. 2024, 14, 10810. [Google Scholar] [CrossRef]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a sustainable source of edible proteins and bioactive peptides—Current trends and future prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef]
- Spínola, M.P.; Mendes, A.R.; Prates, J.A.M. Chemical Composition, Bioactivities, and Applications of Spirulina (Limnospira platensis) in Food, Feed, and Medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, X.; Du, W.; Cai, Y.; Yang, Z.; Yin, Y.; Wakisaka, M.; Wang, J.; Zhou, Z.; Liu, D.; et al. Leveraging microalgae as a sustainable ingredient for meat analogues. Food Chem. 2024, 450, 139360. [Google Scholar] [CrossRef]
- Mohammadi, A.; Shahidi, S.-A.; Rafe, A.; Raeisi, S.N.; Ghorbani-HasanSaraei, A. Rheological properties of dairy desserts: Effect of rice bran protein and fat content. J. Food Sci. 2022, 87, 4977–4990. [Google Scholar] [CrossRef]
- Mosibo, O.K.; Ferrentino, G.; Udenigwe, C.C. Microalgae Proteins as Sustainable Ingredients in Novel Foods: Recent Developments and Challenges. Foods 2024, 13, 733. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Z.; Liu, S.; Luo, S.; He, R.; Yang, X.; Li, S.; Yang, X.; An, Y.; Lu, Y. Utilization of Microalgae and Duckweed as Sustainable Protein Sources for Food and Feed: Nutritional Potential and Functional Applications. J. Agric. Food Chem. 2025, 73, 4466–4482. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Siahbalaei, R.; Kavoosi, G.; Noroozi, M. Protein nutritional quality, amino acid profile, anti-amylase and anti-glucosidase properties of microalgae: Inhibition and mechanisms of action through in vitro and in silico studies. LWT 2021, 150, 112023. [Google Scholar] [CrossRef]
- Kaur, M.; Bhatia, S.; Bagchi, D.; Tak, Y.; Kaur, G.; Kaur, C.; Kaur, A.; Sharma, N. Enhancing Microalgal Proteins for Nutraceutical and Functional Food Applications. Future Foods 2025, 11, 100564. [Google Scholar] [CrossRef]
- Prates, J.A.M. The role of microalgae in providing essential minerals for sustainable swine nutrition. Front. Anim. Sci. 2025, 6, 1526433. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Acquah, C.; Tibbetts, S.M.; Pan, S.; Udenigwe, C. Nutritional quality and bioactive properties of proteins and peptides from microalgae. In Handbook of Microalgae-Based Processes and Products Handbook of Microalgae-Based Processes and Products; Academic Press: Cambridge, MA, USA, 2020; pp. 493–531. [Google Scholar] [CrossRef]
- Bernaerts, T.M.; Van Loey, A.M. Microalgae as structuring ingredients in food. In Cultured Microalgae for the Food Industry; Academic Press: New York, NY, USA, 2021; pp. 265–286. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Esquivel, P.; Meléndez-Martínez, A.J. Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry. Foods 2023, 12, 4080. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae biomass as an additional ingredient of gluten-free bread: Dough rheology, texture quality and nutritional properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- Vieira, R.; Medeiros, J.; Nascimento, M.D.; Abud, A.; Raymundo, A.; De Farias Silva, C.E. Microalgae as sustainable food: Incorporation as strategy in the formulation of functional food. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–30. [Google Scholar] [CrossRef]
- De Caro, V.; Murgia, D.; Seidita, F.; Bologna, E.; Alotta, G.; Zingales, M.; Campisi, G. Enhanced In Situ Availability of Aphanizomenon Flos-Aquae Constituents Entrapped in Buccal Films for the Treatment of Oxidative Stress-Related Oral Diseases. Pharmaceutics 2019, 11, 35. [Google Scholar] [CrossRef]
- Tomassi, E.; Arouna, N.; Caruso, M.G.; Girgenti, A.; Picone, P.; Nuzzo, D.; Pucci, L. Fermentation of Chlorella vulgaris and Aphanizomenon flos-aquae biomass improves the antioxidant profile. LWT 2024, 215, 117183. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Scoglio, S.; Serio, R. AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats. Nutrients 2020, 12, 3635. [Google Scholar] [CrossRef]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and Nutritional Evaluation of Chlorella and Auxenochlorella Biomasses Relevant for Food Application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef]
- Perez, B.; Zermatten, C.; Haberkorn, I.; Mathys, A. Enhancing protein extraction from heterotrophic Auxenochlorella protothecoides microalgae through emerging cell disruption technologies combined with incubation. Bioresour. Technol. 2024, 407, 131099. [Google Scholar] [CrossRef]
- Sägesser, C.; Kallfelz, J.M.; Boulos, S.; Dumpler, J.; Böcker, L.; Mair, T.; Nyström, L.; Mathys, A. Structurability of microalgae, soy and pea protein for extruded high-moisture meat analogues. Food Hydrocoll. 2024, 156, 110290. [Google Scholar] [CrossRef]
- Darwish, R.; Gedi, M.; Akepach, P.; Assaye, H.; Zaky, A.; Gray, D. Chlamydomonas reinhardtii Is a Potential Food Supplement with the Capacity to Outperform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Hammel, A.J.; Zimmer, D.; Sommer, F.; Mühlhaus, T.; Schroda, M. Absolute Quantification of Major Photosynthetic Protein Complexes in Chlamydomonas reinhardtii Using Quantification Concatamers (QconCATs). Front. Plant Sci. 2018, 9, 1265. [Google Scholar] [CrossRef]
- Wietrzynski, W. Rubisco Biogenesis and Assembly in Chlamydomonas Reinhardtii. Doctoral Dissertation, Université Pierre et Marie Curie, Paris, France, 2017. [Google Scholar]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef]
- Ng, J.W.; Teh, T.M.; Tan, C.F.; Bi, X.; Low, Z.E.; Talukder, M.M.R. Alkaline solubilization of microalgal protein and its impact on the functional properties of protein extract. Future Foods 2024, 9, 100368. [Google Scholar] [CrossRef]
- Truong, V.; Truong, T.V.; Ho, T.C.T.; Nguyen, Q.D.; Nguyen, T.T.T. Harvesting of Chlorella vulgaris grown in closed-photobioreactor with chitosan for use in food. J. Agric. Dev. 2018, 17, 102–111. [Google Scholar] [CrossRef]
- Davidi, L.; Levin, Y.; Ben-Dor, S.; Pick, U. Proteome Analysis of Cytoplasmatic and Plastidic β-Carotene Lipid Droplets in Dunaliella bardawil. Plant Physiol. 2014, 167, 60–79. [Google Scholar] [CrossRef]
- Sui, Y.; Vlaeminck, S.E. Dunaliella microalgae for nutritional protein: An undervalued asset. Trends Biotechnol. 2020, 38, 10–12. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y.; Liang, M.-H.; Yan, B.; Jiang, J.-G. Creatinine combined with light increases the contents of lutein and β-carotene, the main carotenoids of Dunaliella bardawil. Enzym. Microb. Technol. 2021, 151, 109913. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Sui, Y.; Mazzucchi, L.; Acharya, P.; Xu, Y.; Morgan, G.; Harvey, P. A Comparison of β-Carotene, Phytoene and Amino Acids Production in Dunaliella salina DF 15 (CCAP 19/41) and Dunaliella salina CCAP 19/30 Using Different Light Wavelengths. Foods 2021, 10, 2824. [Google Scholar] [CrossRef]
- Tomita, Y.; Takeya, M.; Suzuki, K.; Nitta, N.; Higuchi, C.; Marukawa-Hashimoto, Y.; Osanai, T. Amino acid excretion from Euglena gracilis cells in dark and anaerobic conditions. Algal Res. 2019, 37, 169–177. [Google Scholar] [CrossRef]
- Xie, W.; Li, X.; Xu, H.; Chen, F.; Cheng, K.-W.; Liu, H.; Liu, B. Optimization of Heterotrophic Culture Conditions for the Microalgae Euglena gracilis to Produce Proteins. Mar. Drugs 2023, 21, 519. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, M.; Wang, Y.; Zhu, Z.; Zhao, X. Euglena gracilis Protein: Effects of Different Acidic and Alkaline Environments on Structural Characteristics and Functional Properties. Foods 2024, 13, 2050. [Google Scholar] [CrossRef]
- Castillo, A.; Finimundy, T.C.; Heleno, S.A.; Rodrigues, P.C.A.; Fernandes, F.A.; Pereira, S.; Lores, M.; Barros, L.; García-Jares, C. The generally recognized as safe (GRAS) microalgae Haematococcus pluvialis (wet) as a multifunctional additive for coloring and improving the organoleptic and functional properties of foods. Food Funct. 2023, 14, 6023–6035. [Google Scholar]
- Yap, S.M.; Lan, J.C.-W.; Kee, P.E.; Ng, H.S.; Yim, H.S. Enhancement of protein production using synthetic brewery wastewater by Haematococcus pluvialis. J. Biotechnol. 2022, 350, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Gao, J. Selectively extraction of astaxanthin from Haematococcus pluvialis by aqueous biphasic systems composed of ionic liquids and deep eutectic solutions. Food Chem. 2023, 434, 137399. [Google Scholar] [CrossRef]
- Martínez, R.; García-Beltrán, A.; Kapravelou, G.; Mesas, C.; Cabeza, L.; Perazzoli, G.; Guarnizo, P.; Rodríguez-López, A.; Vallejo, R.A.; Galisteo, M.; et al. In Vivo Nutritional Assessment of the Microalga Nannochloropsis gaditana and Evaluation of the Antioxidant and Antiproliferative Capacity of Its Functional Extracts. Mar. Drugs 2022, 20, 318. [Google Scholar] [CrossRef]
- Paterson, S.; Alonso-Pintre, L.; Morato-López, E.; González de la Fuente, S.; Gómez-Cortés, P.; Hernández-Ledesma, B. Microalga Nannochloropsis gaditana as a Sustainable Source of Bioactive Peptides: A Proteomic and In Silico Approach. Foods 2025, 14, 252. [Google Scholar] [CrossRef]
- Verspreet, J.; Soetemans, L.; Gargan, C.; Hayes, M.; Bastiaens, L. Nutritional Profiling and Preliminary Bioactivity Screening of Five Micro-Algae Strains Cultivated in Northwest Europe. Foods 2021, 10, 1516. [Google Scholar] [CrossRef]
- Li, J.; Shen, S.-g.; Han, C.-F.; Liu, S.-t.; Zhang, L.-l.; Chen, N.; Jia, S.-R.; Han, P.-P. Nostoc flagelliforme capsular polysaccharides from different culture conditions improve hyperlipidemia and regulate intestinal flora in C57BL/6J mice to varying degrees. Int. J. Biol. Macromol. 2022, 202, 224–233. [Google Scholar] [CrossRef]
- Xu, L.; Yong, H.; Tu, X.; Wang, Q.; Fan, J. Physiological and proteomic analysis of Nostoc flagelliforme in response to alkaline pH shift for polysaccharide accumulation. Algal Res. 2019, 39, 101444. [Google Scholar] [CrossRef]
- Yue, S.J.; Jia, S.R.; Yao, J.; Dai, Y.-j. Nutritional Analysis of the Wild and Liquid Suspension Cultured Nostoc Flagelliforme and Antitumor Effects of the Extracellular Polysaccharides. Adv. Mater. Res. 2011, 345, 177–182. [Google Scholar] [CrossRef]
- Sørensen, M.; Kousoulaki, K.; Hammerø, R.; Kokkali, M.; Kleinegris, D.; Martí-Quijal, F.J.; Barba, F.J.; Palihawadana, A.M.; Egeland, E.S.; Johnsen, C.A.; et al. Mechanical processing of Phaeodactylum tricornutum and Tetraselmis chui biomass affects phenolic and antioxidant compound availability, nutrient digestibility and deposition of carotenoids in Atlantic salmon. Aquaculture 2023, 569, 739395. [Google Scholar] [CrossRef]
- Stack, J.; Le Gouic, A.V.; Tobin, P.R.; Guihéneuf, F.; Stengel, D.B.; Fitzgerald, R.J. Protein extraction and bioactive hydrolysate generation from two microalgae, Porphyridium purpureum and Phaeodactylum tricornutum. J. Food Bioact. 2018, 1, 153–165. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Zhu, Z.; Liu, M.; Zhao, X. Effects of Different pH Levels on the Structural and Functional Properties of Proteins of Phaeodactylum tricornutum. Molecules 2024, 29, 3139. [Google Scholar] [CrossRef]
- Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Ordenes, D.; Renato, G.; Ruiz-Domínguez, M.C. Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes 2020, 8, 1628. [Google Scholar] [CrossRef]
- González-Ramírez, E.J.; Andújar-Sánchez, M.; Ortiz-Salmerón, E.; Bacarizo, J.; Cuadri, C.; Mazzuca-Sobczuk, T.; Ibáñez, M.J.; Cámara-Artigas, A.; Martínez-Rodríguez, S. Thermal and pH Stability of the B-Phycoerythrin from the Red Algae Porphyridium cruentum. Food Biophys. 2014, 9, 184–192. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Elhadef, K.; Hentati, O.; Blavignac, C.; Fendri, I.; Smaoui, S.; Michaud, P.; Abdelkafi, S. B-phycoerythrin of Porphyridium cruentum UTEX 161: A multifunctional active molecule for the development of biodegradable films. Eur. Polym. J. 2024, 208, 112851. [Google Scholar] [CrossRef]
- Amiri, M.; Hosseini, S.E.; Asadi, G.; Khayambashi, B. Optimization of the alcalase and trypsin hydrolysis conditions of an isolated protein from Scenedesmus obliquus microalgae and characterization of its functional properties. LWT 2024, 210, 116819. [Google Scholar] [CrossRef]
- Da Silva, M.I.; Leal, M.A.; Resende, M.d.O.; Martins, M.; Coimbra, J. Scenedesmus obliquus protein concentrate: A sustainable alternative emulsifier for the food industry. Algal Res. 2021, 59, 102468. [Google Scholar] [CrossRef]
- Vendruscolo, R.G.; Deprá, M.C.; Pinheiro, P.N.; Furlan, V.J.M.; Barin, J.S.; Cichoski, A.J.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E.; Wagner, R. Food potential of Scenedesmus obliquus biomasses obtained from photosynthetic cultivations associated with carbon dioxide mitigation. Food Res. Int. 2022, 160, 111590. [Google Scholar] [CrossRef]
- Böcker, L.; Hostettler, T.; Diener, M.; Eder, S.; Demuth, T.; Adamcik, J.; Reineke, K.; Leeb, E.; Nyström, L.; Mathys, A. Time-temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira platensis/Spirulina. Food Chem. 2020, 316, 126374. [Google Scholar] [CrossRef]
- Vasudevan, S.; Seetharam, S.; Dohnalek, M.; Cartwright, E. Spirulina: A daily support to our immune system. Int. J. Noncommun. Dis. 2021, 6, S47–S54. [Google Scholar] [CrossRef]
- Moon, S.-H.; Cho, S.-J. Evaluation of the antioxidant activity of Tetraselmis chuii after in vitro gastrointestinal digestion and investigation of its antioxidant peptides. Algal Res. 2023, 76, 103328. [Google Scholar] [CrossRef]
- Prandi, B.; Boukid, F.; Van De Walle, S.; Cutroneo, S.; Comaposada, J.; Van Royen, G.; Sforza, S.; Tedeschi, T.; Castellari, M. Protein Quality and Protein Digestibility of Vegetable Creams Reformulated with Microalgae Inclusion. Foods 2023, 12, 2395. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Kousoulaki, K.; Uhlen, A.K.; Kleinegris, D.M.M.; Skjånes, K.; Rieder, A. Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris. Foods 2021, 10, 3078. [Google Scholar] [CrossRef]
- De Souza, A.T.; Souza, K.; De Amorim, A.; Bezerra, R.; Porto, A. Methods to protein and peptide extraction from microalgae: A systematic review. An. Acad. Bras. Cienc. 2024, 96, e20240113. [Google Scholar] [CrossRef]
- Zwander, S.; Chaturvedi, P.; Ghatak, A.; Weckwerth, W.; Marko, D.; Castejón, N. Integrating eco-friendly approaches to produce protein extracts and hydrolysates with antioxidant properties from Microchloropsis gaditana. Algal Res. 2023, 77, 103368. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Wilken, L.R.; Mallawarachchi, S.; Nikolov, Z.L. Process development of enzymatically-generated algal protein hydrolysates for specialty food applications. Algal Res. 2021, 55, 102248. [Google Scholar] [CrossRef]
- Spínola, M.P.; Costa, M.M.; Simões, R.S.; Fernandes, V.O.; Cardoso, V.; Pires, V.M.R.; Afonso, C.; Cardoso, C.; Bandarra, N.M.; Fontes, C.M.G.A.; et al. Improving protein hydrolysis and digestibility in Arthrospira platensis biomass through recombinant peptidases (EC 3.4): Opportunities for monogastric animal diets. Heliyon 2025, 11, e41460. [Google Scholar] [CrossRef]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Enhancing Digestibility of Chlorella vulgaris Biomass in Monogastric Diets: Strategies and Insights. Animals 2023, 13, 1017. [Google Scholar] [CrossRef]
- Nunes, E.; Odenthal, K.; Nunes, N.; Fernandes, T.; Fernandes, I.A.; De Carvalho, M.A.A.P. Protein extracts from microalgae and cyanobacteria biomass. Techno-functional properties and bioactivity: A review. Algal Res. 2024, 82, 103638. [Google Scholar] [CrossRef]
- Costa, M.M.; Spínola, M.P.; Alves, V.D.; Mestre Prates, J.A. Improving protein extraction and peptide production from Chlorella vulgaris using combined mechanical/physical and enzymatic pre-treatments. Heliyon 2024, 10, e32704. [Google Scholar] [CrossRef]
- Mohammad, S.S.; Da Silva Ferreira, M.; Barbosa, M.; Júnior, J. Characteristics of enzymatic hydrolysis of protein from different food sources and potential separation techniques. Curr. Nutr. Food Sci. 2022, 19, 590–601. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Pekkoh, J.; Kamngoen, A.; Wichaphian, A.; Zin, M.T.; Chaipoot, S.; Yakul, K.; Pathom-Aree, W.; Maneechote, W.; Cheirsilp, B.; Khoo, K.S.; et al. Production of ACE Inhibitory Peptides via Ultrasonic-Assisted Enzymatic Hydrolysis of Microalgal Chlorella Protein: Process Improvement, Fractionation, Identification, and In Silico Structure-Activity Relationship. Future Foods 2025, 11, 100548. [Google Scholar] [CrossRef]
- Samarathunga, J.; Le, T.P.L.; Gabard, M.; Strazdins, K.; Rens, J.; Adhikari, B. Microalgal proteins as ingredients for crating dairy mimetic products: Prospects for substituting bovine milk proteins. Future Foods 2025, 11, 100556. [Google Scholar] [CrossRef]
- Hernández, N.; Nunes, M.C.; Prista, C.; Raymundo, A. Innovative and Healthier Dairy Products Through the Incorporation of Microalgae: A Review. Foods 2022, 11, 755. [Google Scholar] [CrossRef]
- Kafyra, M.-S.-G.; Papadaki, S.; Chronis, M.; Krokida, M. Microalgae based innovative animal fat and proteins replacers for application in functional baked products. Open Agric. 2018, 3, 427–436. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Punoo, H.A.; Haddad, M.; Ghnamat, S.A.; Manzoor, N.; Goksen, G.; Dar, B.N. Sustainable algal proteins, novel extraction techniques and applications in the bakery, dairy and pharmaceutical industries: A comprehensive review. Food Chem. 2024, 465 Pt 2, 141828. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Zhang, L.; Noort, M.W.J.; Schutyser, M.A.I.; García-Segovia, P.; Martínez-Monzó, J. Printability and Physicochemical Properties of Microalgae-Enriched 3D-Printed Snacks. Food Bioprocess Technol. 2020, 13, 2029–2042. [Google Scholar] [CrossRef]
- Ejike, C.E.C.C.; Ezeorba, T.P.C.; Ajah, O.; Udenigwe, C.C. Big Things, Small Packages: An Update on Microalgae as Sustainable Sources of Nutraceutical Peptides for Promoting Cardiovascular Health. Glob. Chall. 2023, 7, 2200162. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Maneechote, W.; Srinuanpan, S.; Angelidaki, I. Microalgae as Tools for Bio-Circular-Green Economy: Zero-waste Approaches for Sustainable Production and Biorefineries of Microalgal Biomass. Bioresour. Technol. 2023, 387, 129620. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Yunus Khan, T.M.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Fal, S.; Smouni, A.; El Arroussi, H. Integrated microalgae-based biorefinery for wastewater treatment, industrial CO2 sequestration and microalgal biomass valorization: A circular bioeconomy approach. Environ. Adv. 2023, 12, 100365. [Google Scholar] [CrossRef]
- Sarker, N.K.; Kaparaju, P. Microalgal Bioeconomy: A Green Economy Approach Towards Achieving Sustainable Development Goals. Sustainability 2024, 16, 11218. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Upadhyaya, H.M.; Gupta, V.K.; Verma, P. Microalgae conversion to alternative energy, operating environment and economic footprint: An influential approach towards energy conversion, and management. Energy Convers. Manag. 2022, 269, 116118. [Google Scholar] [CrossRef]
- Gorry, P.-L.; Sánchez, L.; Morales, M. Microalgae Biorefineries for Energy and Coproduct: Can Microalgae Be Part of the Bioeconomy? In Energy from Microalgae; Green Energy and Technology; Jacob-Lopes, E., Zepka, L.Q., Queiroz, M.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
| Microalgae Species | Protein/Extract Name | Physical Properties | Chemical Properties | Nutritional Properties | Food Applications | References |
|---|---|---|---|---|---|---|
| Aphanizomenon flos-aquae | Whole microalga protein (contains C-phycocyanin pigment) | Water-dispersible cyanobacterial biomass; similar functional traits to Spirulina (good water solubility, contributes blue colour); moderate stability (must monitor microcystin toxin contamination) | Rich in phycobiliproteins (e.g., C-phycocyanin ~α: 17 kDa, β: 18 kDa subunits); ~60–70% protein content (dry weight) | Complete AA profile (all essential AAs present); high protein (60–70% dw) with all 20 standard AAs; essential AAs-rich (score ~1.0) and contains bioactive compounds (e.g., phenylethylamine) | Dietary supplements (dried algae powders, tablets); added to smoothies and health foods for protein and micronutrients; historical use as food (e.g., harvested from Klamath Lake) | [27,28,29] |
| Auxenochlorella protothecoides (syn. Chlorella protothecoides) | Whole Algal Protein, heterotrophic Chlorella protein isolate | Extremely high solubility across a broad pH 2–12 (84–100% protein solubility); forms stable emulsions (oil-in-water) up to 7–14 days, outperforming whey protein; emulsions remain stable in high salt (0.5 M) and pH 2–9 | Predominantly polar, hydrophilic AAs in its proteins; many proteins are glycosylated, contributing a negative charge and water-binding; isoelectric precipitation yields pI ~4–5 for major fractions | Protein >60% dw; provides all essential AAs (high lysine, leucine); essential AA index >1.0 (exceeds FAO requirements); digestibility improved by cell disruption | Approved as novel protein ingredient (Canada, EU); used in protein supplements and blended into baked goods, beverages, and sauces for its neutral colour and high protein; studied in vegan mayonnaise and dressings | [30,31,32] |
| Chlamydomonas reinhardtii | Chlamydomonas biomass protein (rich in RuBisCO enzyme) | Green algal cells (motile) with protein largely in soluble form; moderate water solubility when cell is lysed; no known specialised extract; functional properties presumed similar to other chlorophytes | RuBisCO (ribulose-1,5-bisphosphate carboxylase) is ~560 kDa complex; protein ~48% dw in C. reinhardtii; balanced AA profile | Protein ~48% dw; complete AA profile (meets FAO requirements); high nutritive value and considered GRAS for use in foods | Emerging as a novel food ingredient; potential for use in nutrient-rich shakes, fermented foods, and as a host to produce recombinant proteins in edible form | [33,34,35] |
| Chlorella vulgaris | Chlorella protein (algal protein isolate or concentrate) | Fine green powder (cell-wall ruptured) disperses in water; solubility is lowest around pH ~4–5, highest at pH 7–8; good emulsifying capacity | Protein ~50–60% dw; contains RuBisCO (large subunit ~55 kDa) and light-harvesting chlorophyll-binding proteins (~20–30 kDa); all essential AAs present | High-quality protein with balanced profile (all 9 essential AAs); digestibility ~70–80% (cell wall hinders full digestibility) | Commercially popular in supplements (tablets, powders); incorporated into pastas, crackers, breads, and beverages for protein fortification | [36,37,38] |
| Dunaliella bardawil | Dunaliella protein (from β-carotene-rich algae) | Lacks a rigid cell wall; moderate water solubility; stability influenced by the high salt environment of growth | Protein ~10–35% dw; complete essential AA profile (AA score ~1.06) | Protein ~10–35% dw; all essential AAs present in balanced proportions | Used mainly as a natural β-carotene source in supplements and food colourant; whole biomass can enrich foods with protein | [39,40,41] |
| Dunaliella salina | Dunaliella protein (algal biomass protein) | No cellulosic cell wall, so cells are easily ruptured: proteins are water-soluble and accessible | Protein content ranges from ~20% up to 57% dw depending on growth conditions | Protein ~20–57% dw; rich in essential AAs; good digestibility due to lack of a hard wall | Primarily used for β-carotene (as a natural colourant and supplement); dried biomass has been added to specialty foods | [40,42,43] |
| Euglena gracilis | Euglena protein (from Euglena biomass) | Grown heterotrophically or photoautotrophically; fairly soluble proteins; mild extraction yields light beige powder | Notable for high content of sulphur AAs (cysteine, methionine); contains all 20 AAs | Protein ~30–39% dw; well-balanced essential AA profile; highly digestible | Incorporated into health foods and supplements; used in protein shakes and bars | [44,45,46] |
| Haematococcus pluvialis | Haematococcus protein (from astaxanthin-rich alga) | High water-holding capacity; good emulsifier; exhibits excellent foaming | Proteins include stress-related enzymes and RuBisCO; protein ~30–45% dw | Protein ~40% dw; high-quality AA profile; digestibility ~80% after cell breakage | Used commercially for astaxanthin; the remaining protein-rich meal is studied for use in foods | [47,48,49] |
| Nannochloropsis gaditana | Nannochloropsis protein (algal protein concentrate or hydrolysate) | Good emulsifying capacity; stable foams; moderate solubility | Protein ~19% to 45% dw depending on strain and growth; balanced AA profile | Protein ~20–45% dw; rich in glutamate and aspartate; good digestibility | Gaining approval as a novel food ingredient; used in breads and crackers | [50,51,52] |
| Nostoc flagelliforme | Nostoc protein (edible cyanobacterial biomass) | Forms filamentous colonies; physical functionality as isolated protein not well-studied | Moderate protein content (est. 20–30% dw); complete AA profile | Protein ~20–30% dw; all essential AAs present; digestibility presumed good when cooked | Eaten as a culinary ingredient in East Asia for centuries; modern use is limited due to overharvesting | [53,54,55] |
| Phaeodactylum tricornutum | Phaeodactylum protein (diatom biomass protein) | Silica frustule encases cells, needs disruption; moderate solubility | Lower protein content (~15–25% dw); complete but slightly limited in lysine and tryptophan | Protein ~15–20% dw; decent essential AA profile; digestibility ~70% | Incorporated into experimental foods for omega-3 and protein content | [56,57,58] |
| Porphyridium cruentum | R-Phycoerythrin (red protein pigment from Porphyridium) | Highly water-soluble; exhibits fluorescence; soluble across pH ~5–8 | Multimeric protein (~240 kDa as (αβ)_6 complex); moderate protein content (~25–30% dw) | Protein ~25% dw; complete AA profile; low digestibility due to polysaccharide matrix | Used as a natural red food colourant and antioxidant protein | [59,60,61] |
| Scenedesmus obliquus | Scenedesmus protein (algal protein from Scenedesmus) | Good water-holding and gelation ability; excellent emulsifying properties | Protein ~50–60% dw; balanced AA profile | Protein ~50–56% dw; complete essential AA profile; digestibility improves with cell wall breakage | Used in bread, pasta, and snack crackers; promising for meat analogues | [62,63,64] |
| Spirulina (Limnospira platensis, formerly Arthrospira platensis) | Spirulina protein (e.g., C-phycocyanin; Spirulina protein isolate) | Highly soluble in water except near pI (~pH 3.5); thermal stability up to ~70 °C for short times; excellent emulsifier and foaming agent at neutral–alkaline pH | Isoelectric point ~3.5; major proteins are phycobiliproteins and RuBisCO; phycocyanin pigment (blue) is ~30–40 kDa per subunit, forming ~210 kDa complexes; low in sulphur AAs (methionine, cysteine) | Protein ~50–70% dw; complete protein with ~51–71% of AAs as essential; very digestible (80–90% digestibility); PDCAAS ~0.81 | Widely used in foods and beverages: smoothies, nutritional bars, pasta, crackers; meat analogues and dairy-free cheese for protein fortification; C-phycocyanin from Spirulina is a natural blue colourant used in confections and drinks | [11,65,66] |
| Tetraselmis chui | Tetraselmis protein (algal protein from Tetraselmis) | Moderate solubility; good foaming and emulsifying capacity | Protein ~30–40% dw; high lysine and threonine content | Protein ~35% dw; complete essential AA profile; good digestibility | Approved as a novel food ingredient; used in sauces, seasonings, and protein shakes | [67,68,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prates, J.A.M. Unlocking the Functional and Nutritional Potential of Microalgae Proteins in Food Systems: A Narrative Review. Foods 2025, 14, 1524. https://doi.org/10.3390/foods14091524
Prates JAM. Unlocking the Functional and Nutritional Potential of Microalgae Proteins in Food Systems: A Narrative Review. Foods. 2025; 14(9):1524. https://doi.org/10.3390/foods14091524
Chicago/Turabian StylePrates, José A. M. 2025. "Unlocking the Functional and Nutritional Potential of Microalgae Proteins in Food Systems: A Narrative Review" Foods 14, no. 9: 1524. https://doi.org/10.3390/foods14091524
APA StylePrates, J. A. M. (2025). Unlocking the Functional and Nutritional Potential of Microalgae Proteins in Food Systems: A Narrative Review. Foods, 14(9), 1524. https://doi.org/10.3390/foods14091524






