Sustainable Exploitation of Apple By-Products: A Retrospective Analysis of Pilot-Scale Extraction Tests Using Hydrodynamic Cavitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Production of Apple Extracts
2.3. Analysis of Raw Material and Extracts
- Sugars: Glucose, fructose, sucrose, xylose and sorbitol were quantified according to the method developed by Di Lella et al. [51] using commercial standards provided by Merck (Darmstadt, Germany) 1 g of the raw apple material and 1 g of the extracted sample were dissolved in 40 mL of water, centrifuged, and the supernatant was subsequently diluted 25-fold for the raw material and 5-fold for the extracted samples. Quantification was performed using an ICS 5000 ion chromatograph (Dionex, Thermo Fisher Scientific, Waltham, MA, USA) equipped with a pulsed amperometric detector (PAD) consisting of a gold working electrode and a palladium reference electrode. The sugar content was calculated by summing the individual sugars. Linearity of the sugars was confirmed between 0.02 and 20 mg/L, and R2 were always >0.99. Repeatability (calculated as RSD% on three replicates) of 5% and uncertainty (σ/√2) of 4%.
- ORAC: Oxygen Radical Absorbance capacity was evaluated in according to Ou et al. [52], by dissolving 1 g of samples in 50 mL of an acetone:water mixture (50:50, v/v) for raw materials and 5 mL for extracted samples and appropriately diluting them with 10 mM potassium phosphate buffer (pH 7.4) for analysis. Subsequently, 150 µL of fluorescein working solution (1.2 µM) was added to microplate wells along with 50 µL of diluted buffer, standard (Trolox, 100 µM, Merck), control, and samples. The kinetic reaction with AAPH (2,2′-Azobis(2-methylpropionamidine) dihydrochloride) solution (41 g/L) took place in a fluorescence microplate reader (Varioskan Lux, Thermo Fisher Scientific, Waltham, MA, USA) and was measured every minute for 35 min (excitation at 485 nm and emission at 530 nm). Repeatability (calculated as RSD% on three replicates) of 11% and uncertainty (σ/√2) of 7%.
- TPC: Total polyphenol content was quantified adapting the protocol elaborated by Ceci et al. [53]. 10 g of the raw material were extracted with 40 mL of a water:methanol mixture (80:20, v/v) acidified with 0.85% H3PO4. The mixture was shaken for 15 min and centrifuged at 4 °C and 4000 rpm for 5 min (Rotina 380, Hettich, Germany). The supernatant was collected and stored at −20 °C until analysis. Extracts were diluted 25 times with the same solvent mixture. TPC was determined using the Folin–Ciocalteu method. 2 mL of the extract was added to 1 mL of Folin–Ciocalteu reagent, and the mixture was incubated for 5 min. Then, 5 mL of sodium carbonate solution (20% w/v) was added. After 90 min, the absorbance was recorded at 740 nm using a Cary 60 UV–Vis spectrophotometer (Agilent Technologies, Palo Alto, CA, USA) and compared to a standard curve of catechin [54]. Repeatability (calculated as RSD% on three replicates) of 16% and uncertainty (σ/√2) of 11%. TPC was expressed in (+)-catechin equivalent as recently used for the antioxidant capacity of flesh and peel of several apple cultivars [55].
- Phenolic profile: Individual phenolic compounds were quantified with a liquid chromatograph coupled to a heated electrospray ionization source (HESI-II) and a high-resolution Q-Exactive™ hybrid mass spectrometer (HPLC-HQOMS/Orbitrap; Thermo Fisher Scientific, Waltham, MA, USA) adapted from the method of Barnaba et al. [56]. Chromatographic separation was performed using an ACCLAIM Vanquish PA 2 column (150 × 3 mm, 2.7 µm particle size). The mobile phase consisted of water/formic acid 100 mM/NH4HCO2 20 mM 10% (A), acetonitrile 5% (B), and water 85% (C). Eluent A remains constant throughout the entire analytical run, while eluent B reaches 85% after 17 min, then returns to 5% to recondition the column. Total run time 21 min, with a flow rate of 0.4 mL/min. Mass spectrometric analysis was conducted with a Full MS scan—data dependent (MS/MS) experiment setting a resolution of 70,000 FWHM (m/z 200, 1.5 Hz) over a scan range of 200–2000 m/z. Raw material samples were prepared as for TPC analysis while extracted samples were appropriately diluted and filtered with a PTFE membrane. Phenolic compounds, shown in Table 2, were identified by comparison with authentic standards based on retention time, accurate mass (mass error < 5 ppm), and whenever possible, MS/MS fragmentation patterns. Linearity was confirmed in the range between 0.01 and 7 mg/L. Repeatability (calculated as RSD% on three replicates) of 10% and uncertainty (σ/√2) of 7%. Figure S1 in Supplementary Materials shows the LC-HRMS chromatograms of the apple pomace sample used in test REP4. Targeted analysis was performed using analytical standards (Merck Life Science, Milan, Italy) and solvent calibration possibly corrected with matrix addition, whereas compounds identified through suspect screening approach were quantified using the calibration curve of structurally similar compounds.
- TDS: thermobalance, model MA 110.R (Radwag, Radom, Poland).
2.4. Statistical Analysis
3. Results
3.1. AP Biochemical Characterization
3.2. APE Biochemical Characterization and Extraction Yield
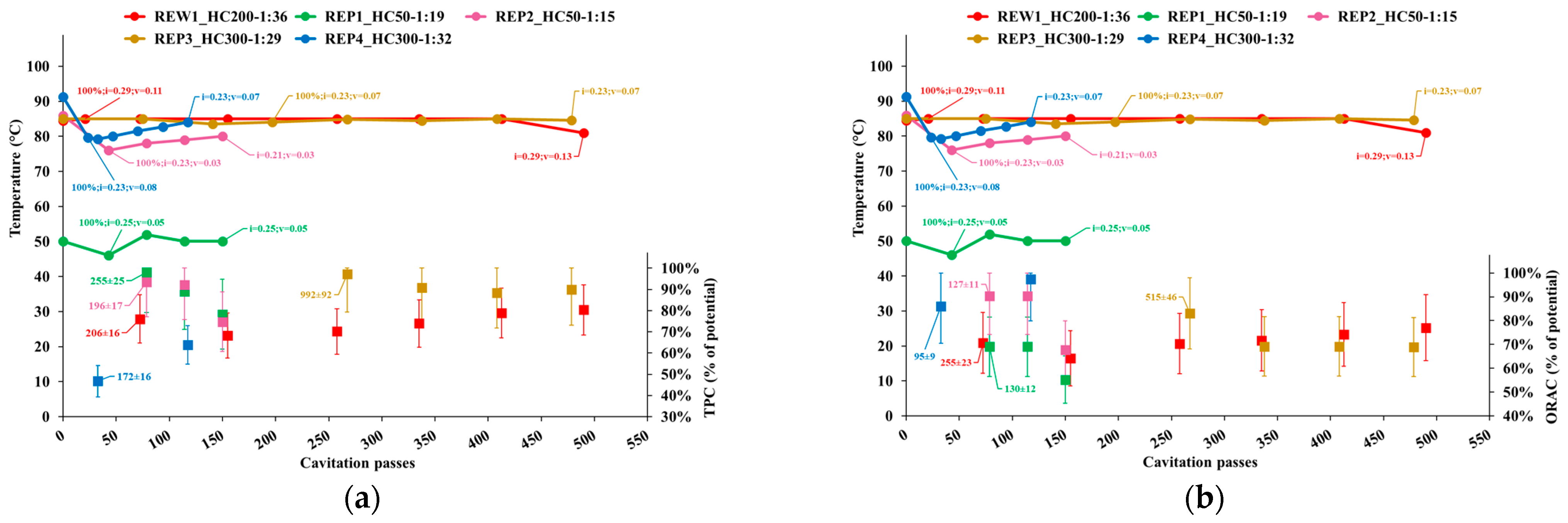
3.2.1. TPC and ORAC
- Temperature;
- Cavitation number in the impeller (i) and Venturi-shaped reactor (v) zones, depicted as tags to the temperature curve;
- Extraction yield, computed as the ratio of TPC content or ORAC level in APEs to the corresponding levels in AP, normalized to the dry biomass;
- Peak process yield, depicted as tags to extraction yield data points and computed as the consumed energy (Wh) needed to obtain 1 mgCAT of TPC, or 1 mgTE of ORAC, from 1 g of dry AP. Hence, the process yield increases with the decrease of the computed quantity.
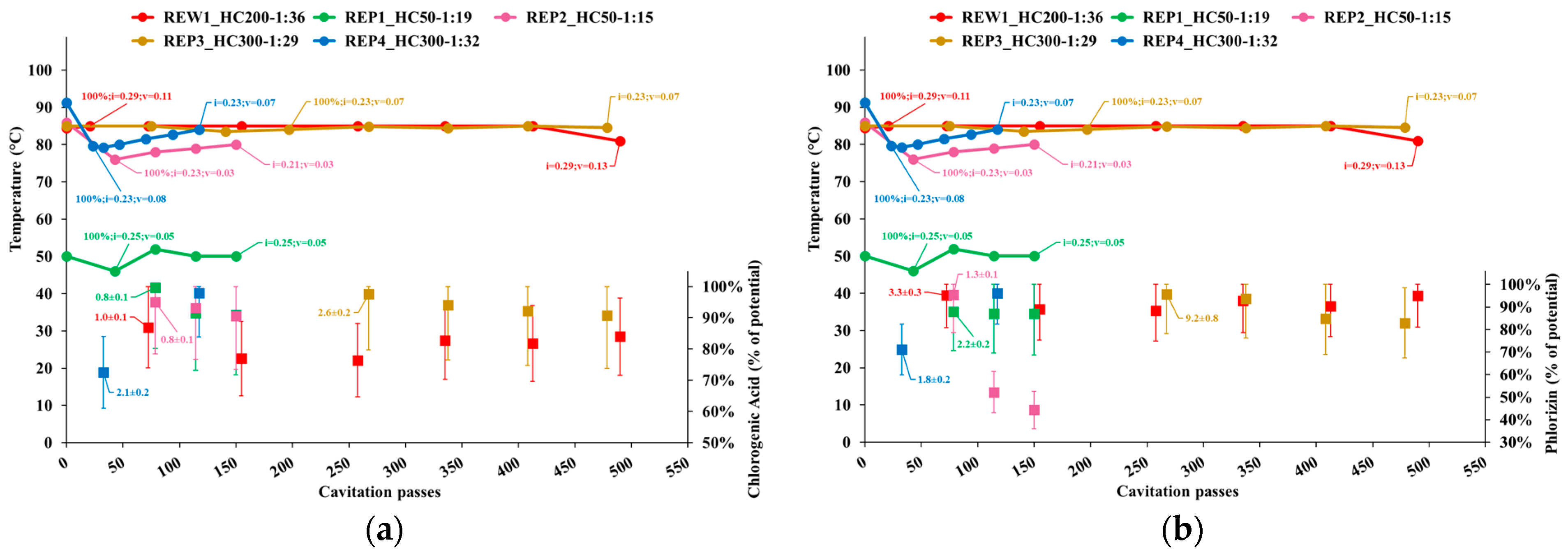
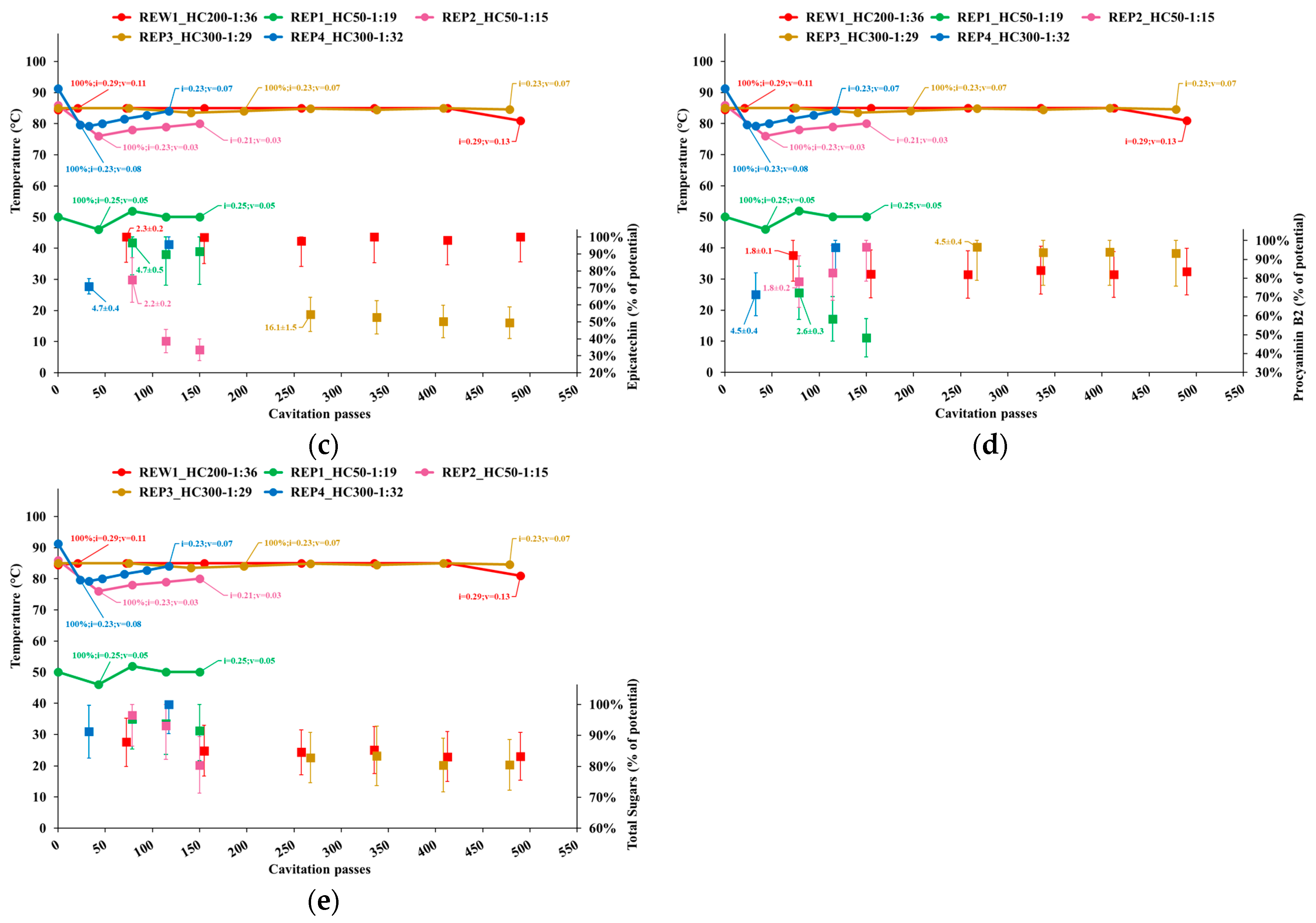
3.2.2. Individual Phenolics and Total Sugars
- Temperature;
- Cavitation number in the impeller (i) and Venturi-shaped reactor (v) zones, depicted as tags to the temperature curve;
- Extraction yield, computed as the ratio of the content of individual phenolic compounds or total sugars in APEs to the corresponding levels in AP, normalized to the dry biomass;
- Peak process yield, depicted as tags to extraction yield data points and computed as the consumed energy (Wh) needed to obtain 1 mg of individual phenols from 1 kg of dry AP. Hence, the process yield increases with the decrease of the computed quantity.
3.3. Mass Extraction Yield and Estimated Composition of Dry Extracts
4. Discussion
- This was a retrospective study of experiments originally aimed at investigating the feasibility and general performance of the HC-based processing of apple by-products, thus the extraction tests, were carried out without a proper design of experiments and with different lots of apples, leading to a remarkable variability of AP composition, as shown in Table 3, and to levels and content of the different properties and compounds, either relative to the dry raw biomass or the TDS (Table 4 and Table 5, respectively), generally unrelated to the actual extraction yield. This was the reason why Figure 2 and Figure 3, showing the extraction yield, are more representative of the actual performance and sensitivity of the HC-based processes.
- In tests REP1, REP2, REP3 and REP4, AP was produced from the whole fruit using a pilot-scale hydropress. The use of industrial by-products could improve the standardization of AP, reduce the total sugars content and enhance the reproducibility of the results, which is planned as a subject of further research.
- The structure of the extracted phytocomplexes was not investigated. For example, HC-based extracts of red orange by-products were found to consist of stable phytocomplexes with flavonoids adsorbed onto the surface of pectin [45,62]. The mechanisms underlying the generation of pectin-polyphenols conjugates, using both citrus and apple commercial pectin, were identified, for example in the case of hydroxytyrosol, as the adsorption onto the surface of pectin, resulting in relatively weak non-covalent bonds, and the free radical method that produces stronger covalent bonds [63]. It can be hypothesized that HC processes intensify both conjugation mechanisms: adsorption, due to the greatly enhanced mass transfer rate produced by the HC-induced turbulence; likely more important, the formation of strong covalent bonds, which can be boosted due to the HC-based effective generation of hydroxyl radicals (•OH) [39,61]. However, the extracted red orange pectin showed a very low degree of esterification of 17.05% [62], while pectin extracted from Renetta variety apples showed a substantially higher degree of esterification of 74.2% [27], associated with a higher degree of hydrophobicity [64]. While a stable conjugation of apple polyphenols and pectin could improve metabolic and cardiovascular outcomes [17,18,28,65], whether our HC-based process could lead to such conjugation remains to be investigated and will be the subject of further research, including in vivo experiments.
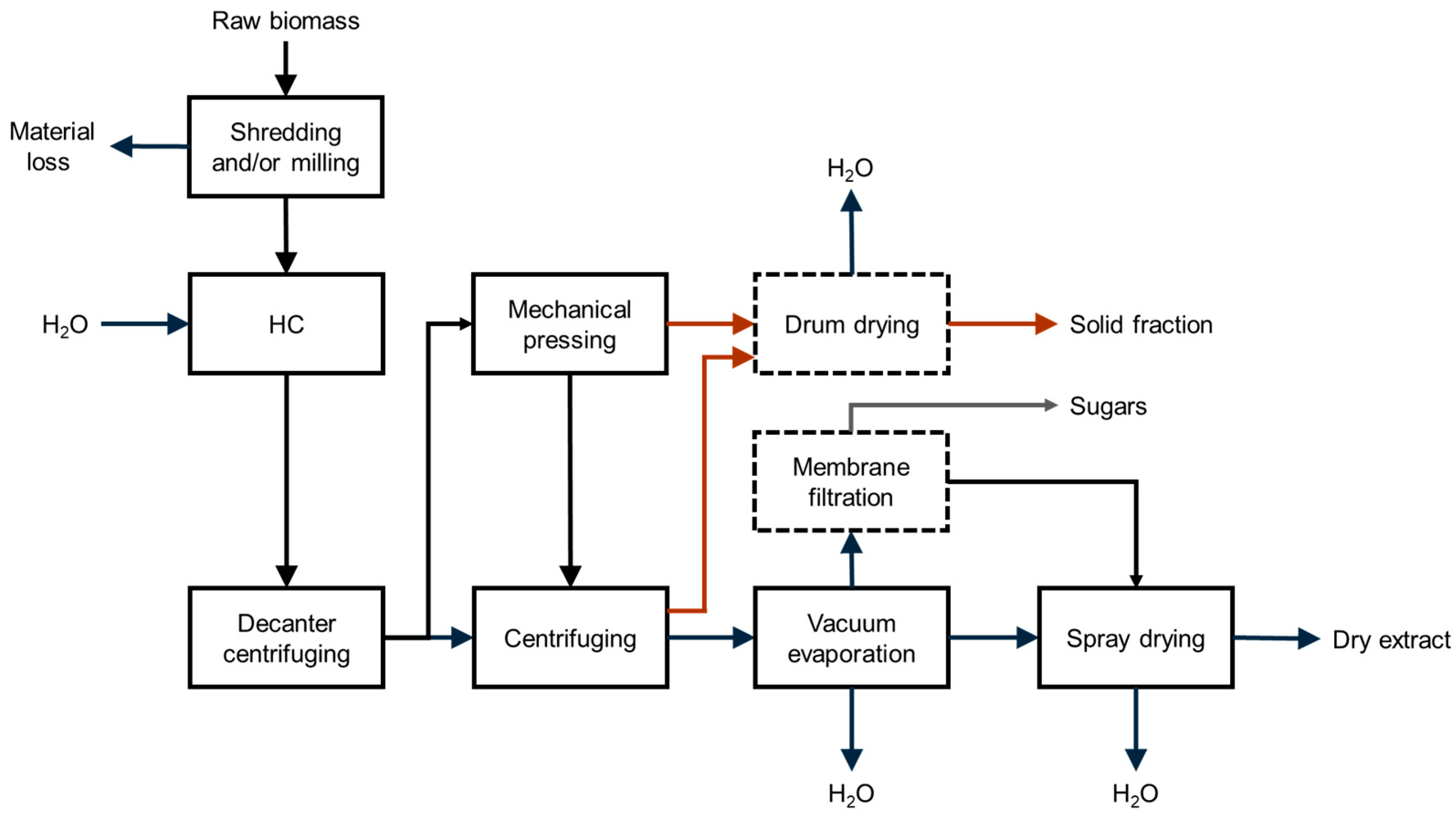
Scaled-Up Production of Dry Extracts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus Domestica)—A Review. Food Bioprocess Technol. 2023, 17, 2519–2560. [Google Scholar] [CrossRef]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An Apple a Day Keeps the Doctor Away”: The Potentials of Apple Bioactive Constituents for Chronic Disease Prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Liu, R.H. Apple Phytochemicals and Their Health Benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Koutsos, A.; Tuohy, K.M.; Lovegrove, J.A. Apples and Cardiovascular Health—Is the Gut Microbiota a Core Consideration? Nutrients 2015, 7, 3959–3998. [Google Scholar] [CrossRef]
- Caliceti, C.; Malaguti, M.; Marracino, L.; Barbalace, M.C.; Rizzo, P.; Hrelia, S. Agri-Food Waste from Apple, Pear, and Sugar Beet as a Source of Protective Bioactive Molecules for Endothelial Dysfunction and Its Major Complications. Antioxidants 2022, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, M.; Ghimire, S.; Karki, G.; Deokar, G.S.; Al-Asmari, F.; Nirmal, N.P. Fruit Waste: Potential Bio-Resource for Extraction of Nutraceuticals and Bioactive Compounds. In Bioactive Extraction and Application in Food and Nutraceutical Industries. Methods and Protocols in Food Science; Sarkar, T., Pati, S., Eds.; Springer: New York, NY, USA, 2024; pp. 299–332. [Google Scholar]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of Apple By-Products as Source of New Ingredients: Current Situation and Perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Szabo, K.; Mitrea, L.; Călinoiu, L.F.; Teleky, B.E.; Martău, G.A.; Plamada, D.; Pascuta, M.S.; Nemeş, S.A.; Varvara, R.A.; Vodnar, D.C. Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato-Processing By-Products and Related Health-Promoting Properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of Phenolic Compounds from Fresh Apple Pomace by Different Non-Conventional Techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and Functional Compounds in Apple Pomace from Juice and Cider Manufacturing: Potential Use in Dermal Formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liao, X.; Zhu, Z.; Huang, R.; Chen, M.; Huang, A.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y. Antioxidant and Anti-Inflammation Effects of Dietary Phytochemicals: The Nrf2/NF-ΚB Signalling Pathway and Upstream Factors of Nrf2. Phytochemistry 2022, 204, 113429. [Google Scholar] [CrossRef]
- Gado, F.; Ferrario, G.; Della Vedova, L.; Zoanni, B.; Altomare, A.; Carini, M.; Aldini, G.; D’Amato, A.; Baron, G. Targeting Nrf2 and NF-ΚB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals. Molecules 2023, 28, 1356. [Google Scholar] [CrossRef]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.R.; et al. Intake of Whole Apples or Clear Apple Juice Has Contrasting Effects on Plasma Lipids in Healthy Volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef]
- Kim, S.J.; Anh, N.H.; Jung, C.W.; Long, N.P.; Park, S.; Cho, Y.H.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Son, E.Y.; et al. Metabolic and Cardiovascular Benefits of Apple and Apple-Derived Products: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 766155. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Phenolic Compounds from Apples: From Natural Fruits to the Beneficial Effects in the Digestive System. Molecules 2024, 29, 568. [Google Scholar] [CrossRef]
- Jakobek, L.; Pöc, K.; Valenteković, M.; Matić, P. The Behavior of Phenolic Compounds from Apples during Simulated Gastrointestinal Digestion with Focus on Chlorogenic Acid. Foods 2024, 13, 693. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for Antioxidant and Antibacterial Activities of Phenolics from Golden Delicious Apple Pomace. Chem. Cent. J. 2016, 10, 47. [Google Scholar] [CrossRef]
- Birru, R.L.; Bein, K.; Bondarchuk, N.; Wells, H.; Lin, Q.; Di, Y.P.; Leikauf, G.D. Antimicrobial and Anti-Inflammatory Activity of Apple Polyphenol Phloretin on Respiratory Pathogens Associated with Chronic Obstructive Pulmonary Disease. Front. Cell. Infect. Microbiol. 2021, 11, 652944. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Molecular Pharmacology of Phloretin: Anti-Inflammatory Mechanisms of Action. Biomedicines 2023, 11, 143. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, S.-J.; Jung, U.; Ryu, R.; Choi, M.-S. Phlorizin Supplementation Attenuates Obesity, Inflammation, and Hyperglycemia in Diet-Induced Obese Mice Fed a High-Fat Diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Bangert, A.; Kottra, G.; Geillinger, K.E.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Inhibition of the Intestinal Sodium-coupled Glucose Transporter 1 (SGLT1) by Extracts and Polyphenols from Apple Reduces Postprandial Blood Glucose Levels in Mice and Humans. Mol. Nutr. Food Res. 2014, 58, 1795–1808. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, M.; Radha; Rais, N.; Puri, S.; Sharma, K.; Natta, S.; Dhumal, S.; Damale, R.D.; Kumar, S.; et al. Exploring Apple Pectic Polysaccharides: Extraction, Characterization, and Biological Activities—A Comprehensive Review. Int. J. Biol. Macromol. 2024, 255, 128011. [Google Scholar] [CrossRef]
- D’Agostino, S.; Cecchi, L.; Khatib, M.; Domizio, P.; Zoccatelli, G.; Civa, V.; Mainente, F.; Breschi, C.; Ugolini, T.; Meneguzzo, F.; et al. 1H-NMR, HPSEC-RID, and HPAEC-PAD Characterization of Polysaccharides Extracted by Hydrodynamic Cavitation from Apple and Pomegranate By-Products for Their Valorization: A Focus on Pectin. Processes 2024, 12, 2113. [Google Scholar] [CrossRef]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Rémésy, C.; Demigné, C. Apple Pectin and a Polyphenol-Rich Apple Concentrate Are More Effective Together than Separately on Cecal Fermentations and Plasma Lipids in Rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of Antioxidants from Apple Pomace by Supercritical Fluid Extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Zaky, A.A.; Witrowa-Rajchert, D.; Nowacka, M. Turning Apple Pomace into Value: Sustainable Recycling in Food Production—A Narrative Review. Sustainability 2024, 16, 7001. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K.; Meletharayil, G.H. Application of Hydrodynamic Cavitation to Improve Antioxidant Activity in Sorghum Flour and Apple Pomace. Food Bioprod. Process. 2016, 100, 335–343. [Google Scholar] [CrossRef]
- Manoharan, D.; Radhakrishnan, M.; Tiwari, B.K. Cavitation Technologies for Extraction of High Value Ingredients from Renewable Biomass. TrAC—Trends Anal. Chem. 2024, 174, 117682. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, G.; Petkovšek, M.; Long, K.; Coutier-Delgosha, O. Intensity and Regimes Changing of Hydrodynamic Cavitation Considering Temperature Effects. J. Clean. Prod. 2022, 338, 130470. [Google Scholar] [CrossRef]
- Acciardo, E.; Tabasso, S.; Cravotto, G.; Bensaid, S. Process Intensification Strategies for Lignin Valorization. Chem. Eng. Process.—Process Intensif. 2022, 171, 108732. [Google Scholar] [CrossRef]
- Arya, S.S.; More, P.R.; Ladole, M.R.; Pegu, K.; Pandit, A.B. Non-Thermal, Energy Efficient Hydrodynamic Cavitation for Food Processing, Process Intensification and Extraction of Natural Bioactives: A Review. Ultrason. Sonochem. 2023, 98, 106504. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Pagliaro, M. Natural Product Extraction via Hydrodynamic Cavitation. Sustain. Chem. Pharm. 2023, 33, 101083. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, J.; Rahimi, M.; Halim, R.; Shen, H.; Tiwari, B.K.; Zhao, L. Chemistry of Hydrodynamic Cavitation Technology. In Chemistry of Thermal and Non-Thermal Food Processing Technologies; Brijesh, K.T., Mysore, B.L., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 259–287. ISBN 9780443221828. [Google Scholar]
- Capocelli, M.; Musmarra, D.; Prisciandaro, M.; Lancia, A. Chemical Effect of Hydrodynamic Cavitation: Simulation and Experimental Comparison. AIChE J. 2014, 60, 2566–2572. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater Treatment Using Hybrid Treatment Schemes Based on Cavitation and Fenton Chemistry: A Review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.; Xiang, C.; Mou, J.; Qian, H.; Duan, Z.; Zhang, S.; Zhou, P. Numerical Study of Rotating Cavitation and Pressure Pulsations in a Centrifugal Pump Impeller. AIP Adv. 2024, 14, 105024. [Google Scholar] [CrossRef]
- Shen, X.; Wu, H.; Yang, G.; Tang, R.; Chang, C.; Xu, B.; Lin, S.; Zhang, D. Experimental Study on the Classification and Evolution of the Tip Cavitation Morphology in Axial Waterjet Pumps with Two Different Blade Numbers. J. Mar. Sci. Eng. 2024, 12, 1898. [Google Scholar] [CrossRef]
- Sun, X.; Xu, H.; Xuan, X.; Manickam, S.; Boczkaj, G.; Wang, B. Assessing the Industrialization Progress of Hydrodynamic Cavitation Process Intensification Technology: A Review. Curr. Opin. Chem. Eng. 2024, 45, 101037. [Google Scholar] [CrossRef]
- Mathijssen, A.J.T.M.; Lisicki, M.; Prakash, V.N.; Mossige, E.J.L. Culinary Fluid Mechanics and Other Currents in Food Science. Rev. Mod. Phys. 2023, 95, 25004. [Google Scholar] [CrossRef]
- Vesci, L.; Martinelli, G.; Liu, Y.; Tagliavento, L.; Dell’Agli, M.; Wu, Y.; Soldi, S.; Sagheddu, V.; Piazza, S.; Sangiovanni, E.; et al. The New Phytocomplex AL0042 Extracted from Red Orange By-Products Inhibits the Minimal Hepatic Encephalopathy in Mice Induced by Thioacetamide. Biomedicines 2025, 13, 686. [Google Scholar] [CrossRef]
- Benedetti, G.; Flori, L.; Spezzini, J.; Miragliotta, V.; Lazzarini, G.; Pirone, A.; Meneguzzo, C.; Tagliavento, L.; Martelli, A.; Antonelli, M.; et al. Improved Cardiovascular Effects of a Novel Pomegranate Byproduct Extract Obtained through Hydrodynamic Cavitation. Nutrients 2024, 16, 506. [Google Scholar] [CrossRef]
- Breschi, C.; D’Agostino, S.; Meneguzzo, F.; Zabini, F.; Chini, J.; Lovatti, L.; Tagliavento, L.; Guerrini, L.; Bellumori, M.; Cecchi, L.; et al. Can a Fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in a Gluten-Free and Vegan Cookie Recipe? Molecules 2024, 29, 1102. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of Polyphenols in Different Apple Varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.M.; Franceschi, P.; Trošt, K.; Galvin, A.; Braune, T.; Fava, F.; Perenzoni, D.; Mattivi, F.; et al. Two Apples a Day Lower Serum Cholesterol and Improve Cardiometabolic Biomarkers in Mildly Hypercholesterolemic Adults: A Randomized, Controlled, Crossover Trial. Am. J. Clin. Nutr. 2020, 111, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Albanese, L.; Bonetti, A.; D’Acqui, L.P.; Meneguzzo, F.; Zabini, F. Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies alba Mill.) Needles. Foods 2019, 8, 65. [Google Scholar] [CrossRef]
- Di Lella, S.; Tognetti, R.; La Porta, N.; Lombardi, F.; Nardin, T.; Larcher, R. Characterization of Silver Fir Wood Decay Classes Using Sugar Metabolites Detected with Ion Chromatography. J. Wood Chem. Technol. 2019, 39, 90–110. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of Total Antioxidant Capacity by Oxygen Radical Absorbance Capacity (ORAC) Using Fluorescein as the Fluorescence Probe: First Action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Ceci, A.T.; Bassi, M.; Guerra, W.; Oberhuber, M.; Robatscher, P.; Mattivi, F.; Franceschi, P. Metabolomic Characterization of Commercial, Old, and Red-Fleshed Apple Varieties. Metabolites 2021, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, R.; Cravero, M.C. Metodi per Lo Studio Dei Polifenoli Dell’ Uva. Riv. Vitic. Enol. 1991, 44, 37–45. [Google Scholar]
- Zielińska, D.; Turemko, M. Electroactive Phenolic Contributors and Antioxidant Capacity of Flesh and Peel of 11 Apple Cultivars Measured by Cyclic Voltammetry and HPLC–DAD–MS/MS. Antioxidants 2020, 9, 1054. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Malacarne, M.; Larcher, R. Identification and Quantification of 56 Targeted Phenols in Wines, Spirits, and Vinegars by Online Solid-Phase Extraction—Ultrahigh-Performance Liquid Chromatography—Quadrupole-Orbitrap Mass Spectrometry. J. Chromatogr. A 2015, 1423, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the In Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.M.; Amiot, M.; Aubert, S.Y. Enzymatic Browning Reactions in Apple and Apple Products. Crit. Rev. Food Sci. Nutr. 1994, 34, 109–157. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Fu, G.; Xu, W.; Zhai, Y.; Bai, L.; Li, J.; Qu, T. Experimental Study on Attenuation Effect of Liquid Viscosity on Shockwaves of Cavitation Bubbles Collapse. Ultrason. Sonochem. 2024, 111, 107063. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, W.G.; Han, X.D. Effects of Solid Particles at Varying Concentrations on Hydrodynamic Cavitation Evolution in a Nozzle. J. Appl. Fluid Mech. 2025, 18, 485–503. [Google Scholar] [CrossRef]
- Ding, W.; Hong, F.; Ying, D.; Huang, Y.; Nawaz Khan, S.; Jia, J. A Comprehensive Study on the Effects of Annular Protrusion for Cavitation Intensification in Venturi Tubes. Chem. Eng. J. 2024, 498, 155306. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Garcia, A.R.; Pagliaro, M.; Avellone, G.; Fidalgo, A.; Albanese, L.; Meneguzzo, F.; Ciriminna, R.; Ilharco, L.M. Red Orange and Bitter Orange IntegroPectin: Structure and Main Functional Compounds. Molecules 2022, 27, 3243. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Structural and Antioxidant Properties of Hydroxytyrosol-Pectin Conjugates: Comparative Analysis of Adsorption and Free Radical Methods and Their Impact on in Vitro Gastrointestinal Process. Food Hydrocoll. 2025, 162, 110954. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Dar, B.N.; Manzoor, N. Effect of Degree of Esterification on the Encapsulation Properties and Gut Health Potential of Apple Pectin. J. Polym. Environ. 2023, 31, 2424–2433. [Google Scholar] [CrossRef]
- Zaitseva, O.; Khudyakov, A.; Sergushkina, M.; Solomina, O.; Polezhaeva, T. Pectins as a Universal Medicine. Fitoterapia 2020, 146, 104676. [Google Scholar] [CrossRef]
- Psakis, G.; Lia, F.; Valdramidis, V.P.; Gatt, R. Exploring Hydrodynamic Cavitation for Citrus Waste Valorisation in Malta: From Beverage Enhancement to Potato Sprouting Suppression and Water Remediation. Front. Chem. 2024, 12, 1411727. [Google Scholar] [CrossRef]
- Sebastian Raj, A. Advancing Phytonutrient Extraction via Cavitation-Based Methodology: Exploring Catechin Recovery from Camellia Sinensis Leaves. Biocatal. Agric. Biotechnol. 2023, 54, 102895. [Google Scholar] [CrossRef]
- Tienaho, J.; Liimatainen, J.; Myllymäki, L.; Kaipanen, K.; Tagliavento, L.; Ruuttunen, K.; Rudolfsson, M.; Karonen, M.; Marjomäki, V.; Hagerman, A.E.; et al. Pilot Scale Hydrodynamic Cavitation and Hot-Water Extraction of Norway Spruce Bark Yield Antimicrobial and Polyphenol-Rich Fractions. Sep. Purif. Technol. 2025, 360, 130925. [Google Scholar] [CrossRef]
- Nopens, M.; Sazama, U.; Krause, A.; Fröba, M. Specific Heat Capacity of Wood between −140 and 50 °C in Dry and Wet State. Holzforschung 2021, 75, 779–785. [Google Scholar] [CrossRef]
- Leone, A.; Perone, C.; Berardi, A.; Tamborrino, A. Energy Analysis and Numerical Evaluation of the Decanter Centrifuge for Wastewater Management to Allow a Sustainable Energy Planning of the Process. Energy Convers. Manag. X 2024, 22, 100596. [Google Scholar] [CrossRef]
- Szepessy, S.; Thorwid, P. Low Energy Consumption of High-Speed Centrifuges. Chem. Eng. Technol. 2018, 41, 2375–2384. [Google Scholar] [CrossRef]
- Allegrosta, V.; Santoso, B.; Siswantoro, D.H. Power Modeling in Rotary Vacuum Evaporator for Meniran Extraction Process. AIP Conf. Proc. 2024, 3124, 070018. [Google Scholar]
- Baker, C.G.J.; McKenzie, K.A. Energy Consumption of Industrial Spray Dryers. Dry. Technol. 2005, 23, 365–386. [Google Scholar] [CrossRef]
- Liu, D.; Ji, Y.; Guo, Y.; Wang, H.; Wu, Z.; Li, H.; Wang, H. Dietary Supplementation of Apple Phlorizin Attenuates the Redox State Related to Gut Microbiota Homeostasis in C57BL/6J Mice Fed with a High-Fat Diet. J. Agric. Food Chem. 2021, 69, 198–211. [Google Scholar] [CrossRef] [PubMed]
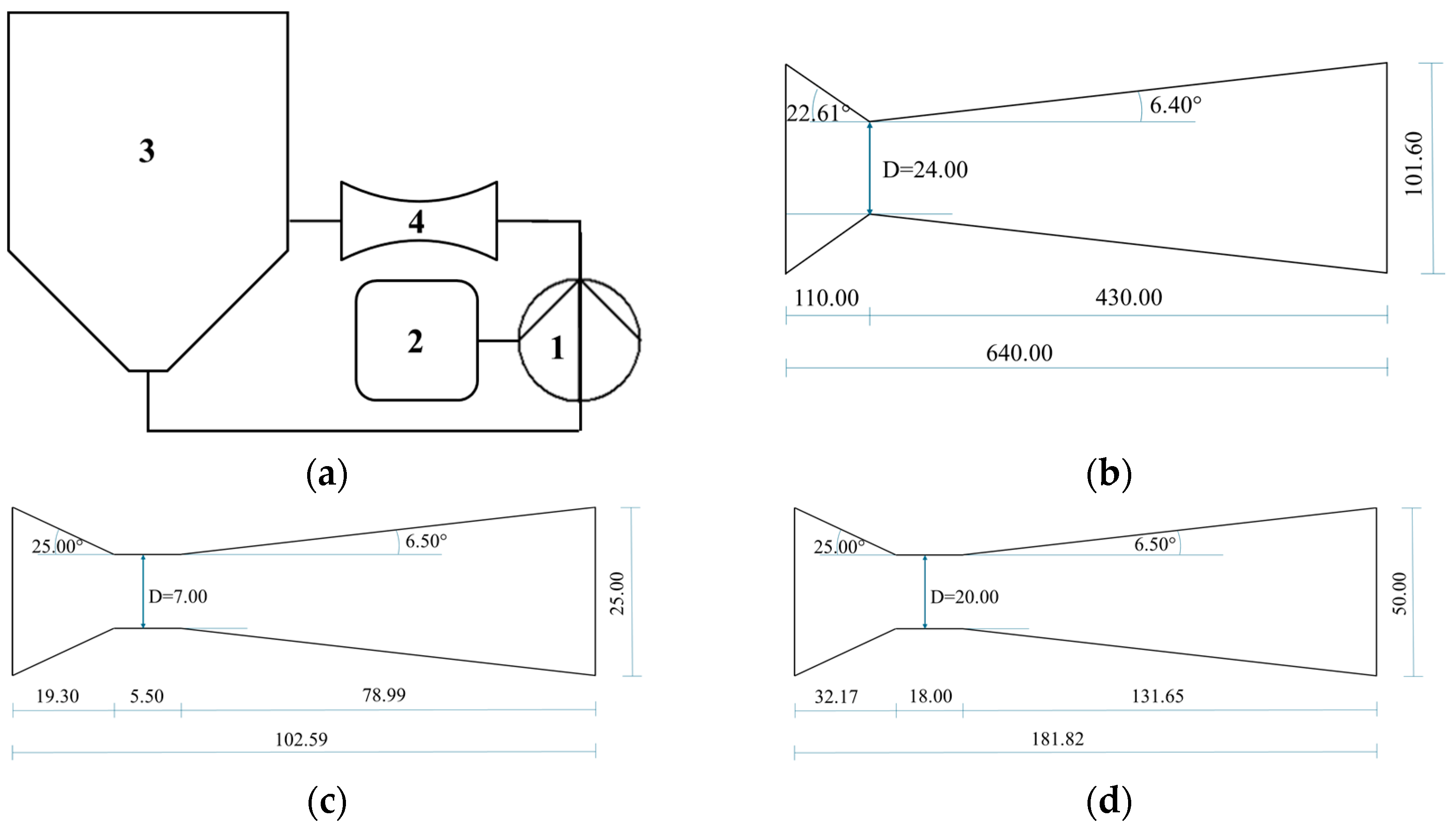
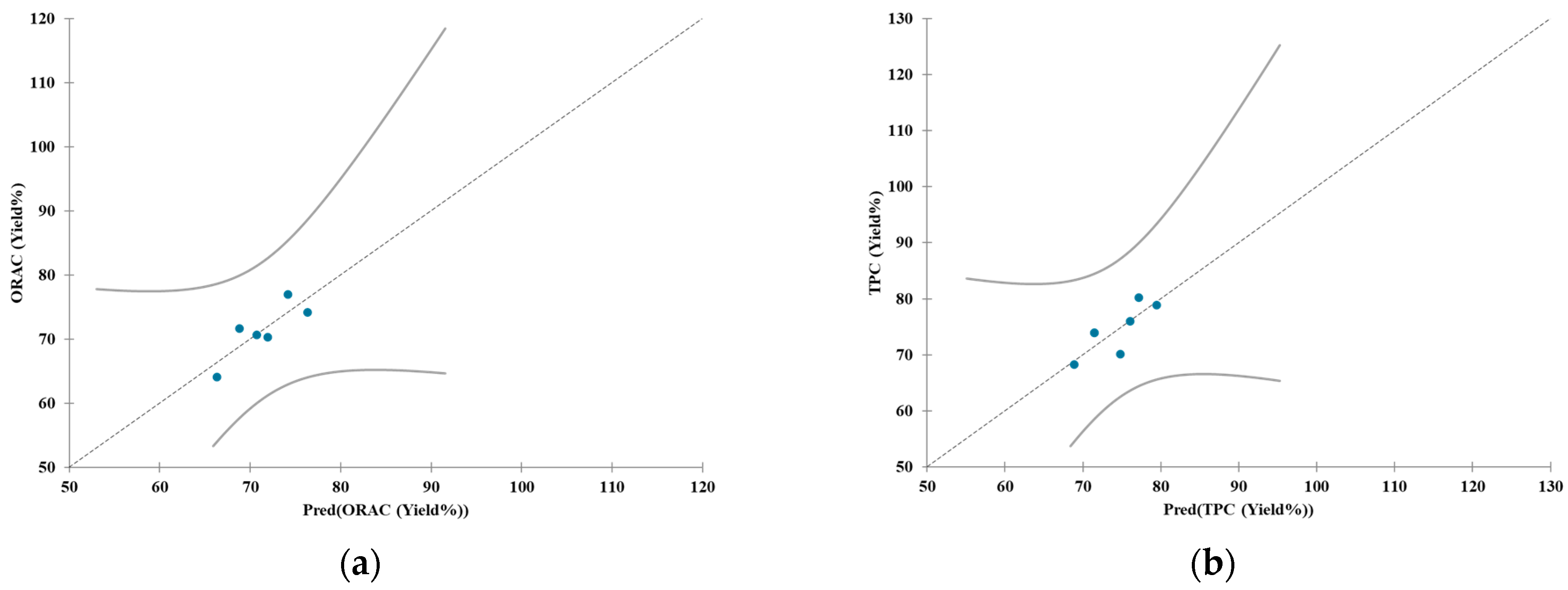
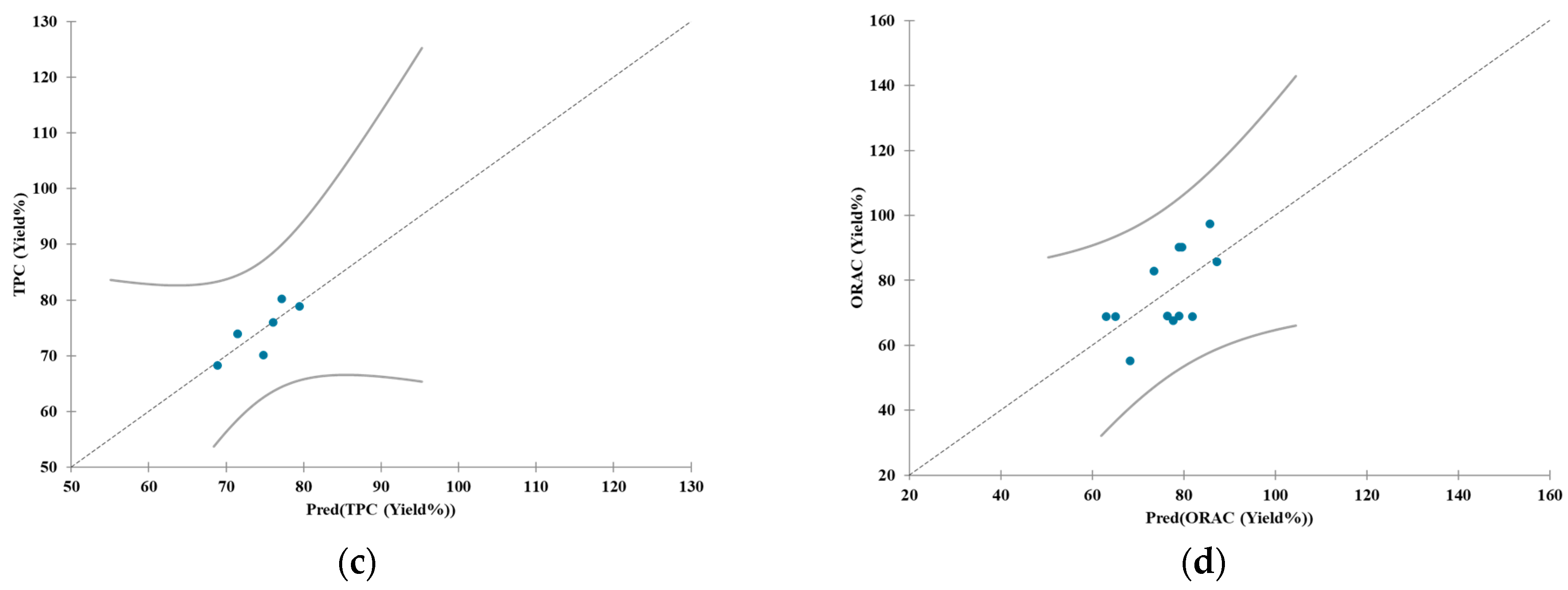

| Test ID | Date | Biomass a | Device | Fresh Biomass (kg) | Dry Biomass (kg) | Concentration (Dry Biomass to Water) b | Time (min) | Temp. (°C) |
|---|---|---|---|---|---|---|---|---|
| REW1 | October 2023 | Whole | HC200 | 28.7 | 4.8 | 1:36 | 95 | 84.4 ± 1.4 |
| REP1 | December 2023 | Pomace | HC50 | 11.4 | 2.1 | 1:19 | 42 | 49.6 ± 1.2 |
| REP2 | December 2023 | Pomace | HC50 | 17.4 | 2.9 | 1:15 | 42 | 79.8 ± 3.8 |
| REP3 | January 2024 | Pomace | HC300 | 48.9 | 8.4 | 1:29 | 102 | 84.5 ± 0.6 |
| REP4 | May 2024 | Pomace | HC300 | 37.3 | 6.0 | 1:32 | 25 | 82.6 ± 4.2 |
| Compound | RT (min) | [M-H]−1 (m/z) | Fragments (m/z) | R2 | C0 (Offset) | C1 (Slope) |
|---|---|---|---|---|---|---|
| Targeted analysis | ||||||
| Caffeic acid | 7.8 | 179.0350 | 135.044 | 0.999 | −490,716 | 16,664,740 |
| Catechin | 7.0 | 289.0718 | 245.083; 109.029 | 0.995 | 58,088 | 324,723 |
| Chlorogenic acid | 7.1 | 353.0878 | 191.056 | 0.997 | 24,340 | 6,207,023 |
| Cinnamic acid | 11.9 | 147.0452 | 87.924 | 0.981 | 32,156 | 38,267 |
| Epicatechin | 7.4 | 289.0723 | 245.082; 109.029 | 0.997 | 85,095 | 3,277,577 |
| Phloretin | 14.9 | 273.0769 | 167.034; 119.049 | 0.997 | 2,664,383 | 49,980,513 |
| Phlorizin | 10.2 | 435.1297 | 273.077; 167.034 | 0.998 | 177,148 | 4,406,812 |
| Procyanidin A1 | 9.4 | 575.1182 | 285.042; 125.025 | 0.997 | −152,227 | 1,590,501 |
| Procyanidin A2 | 9.9 | 575.1182 | 285.040; 125.025 | 0.997 | −81,492 | 1,706,784 |
| Procyanidin B1 | 6.7 | 577.1357 | 289.074; 125.023 | 1.000 | −4476 | 1,458,512 |
| Procyanidin B2 | 7.8 | 577.1357 | 289.070; 125.023 | 0.998 | 3857 | 1,549,692 |
| Procyanidin B3 | 7.1 | 577.1357 | 289.072; 125.023 | 0.999 | −76,337 | 1,835,583 |
| Quercetin | 14.2 | 301.0354 | 151.003; 178.999 | 0.999 | 109,125 | 11,965,356 |
| Quercetin-3-glucosyde/Hyperoside | 10.0 | 463.0882 | 300.029; 271.026 | 1.000 | −845,470 | 14,229,433 |
| Quercitrin | 10.7 | 447.0933 | 300.030; 271.026 | 0.995 | 59,526 | 5,185,775 |
| Quinic acid | 7.3 | 191.0561 | 85.028 | 0.999 | −18,145 | 137,859 |
| Rutin | 9.6 | 609.1461 | 300.030; 271.026 | 0.998 | 24,427 | 3,295,895 |
| Suspect screening analysis | ||||||
| Glucosyl-quinic acid | 5.9 | 515.1617 | - | Use Quinic acid | ||
| Dehydrodicaffeoylquinic acids | 9.0 | 513.1039 | - | Use Caffeic acid | ||
| Di-O-caffeoylquinic acid | 10.7 | 515.1195 | 191.046; 135.046 | Use Caffeic acid | ||
| Trimers C-C-C | 8.5 | 865.1985 | 125.025; 289.074 | Use Catechin | ||
| Dimers C-F | 8.5 | 561.1402 | 289.074; 125.026 | Use Catechin |
| Test ID | TPC (mgCAT/g_DW) a | ORAC (mgTE/g_DW) b | Phlorizin | Chlorogenic Acid | Epicatechin | Procyanidin B2 | Total Sugars (mg/g_DW) |
|---|---|---|---|---|---|---|---|
| (mg/kg_DW) | |||||||
| REW1 | 9.8 ± 0.7 ab | 8.5 ± 0.8 b | 492 ± 34 b | 1712 ± 119 ab | 672 ± 47 ab | 918 ± 64 ab | 695 ± 31 ab |
| REP1 | 4.9 ± 0.5 b | 13.7 ± 1.2 a | 628 ± 61 ab | 1628 ± 158 ab | 269 ± 26 b | 650 ± 63 ab | 697 ± 32 ab |
| REP2 | 6.5 ± 0.6 ab | 10.4 ± 0.9 ab | 959 ± 82 a | 1627 ± 140 ab | 728 ± 43 a | 858 ± 74 ab | 738 ± 32 a |
| REP3 | 5.8 ± 0.5 ab | 13.1 ± 1.2 ab | 640 ± 58 ab | 2209 ± 201 a | 640 ± 58 ab | 1298 ± 118 a | 646 ± 34 ab |
| REP4 | 12.4 ± 0.8 a | 12.2 ± 1.1 ab | 798 ± 51 ab | 658 ± 42 b | 325 ± 21 ab | 314 ± 20 b | 466 ± 25 b |
| Test ID | TPC (mgCAT/g_DW) a | ORAC (mgTE/g_DW) b | Phlorizin | Chlorogenic Acid | Epicatechin | Procyanidin B2 | Total Sugars (mg/g_DW) |
|---|---|---|---|---|---|---|---|
| (mg/kg_DW) | |||||||
| REW1 | 7.4 ± 0.6 ab | 6.0 ± 0.5 b | 468 ± 38 b | 1488 ± 119 a | 677 ± 54 a | 846 ± 68 ab | 611 ± 35 ab |
| REP1 | 4.8 ± 0.5 b | 9.4 ± 0.9 ab | 552 ± 54 ab | 1622 ± 159 a | 260 ± 25 b | 470 ± 46 ab | 665 ± 27 ab |
| REP2 | 6.1 ± 0.5 ab | 9.4 ± 0.8 ab | 914 ± 82 a | 1547 ± 138 a | 543 ± 49 ab | 829 ± 85 ab | 712 ± 36 a |
| REP3 | 5.6 ± 0.5 ab | 10.9 ± 1.0 ab | 612 ± 57 ab | 1474 ± 151 a | 348 ± 32 ab | 1254 ± 116 a | 535 ± 24 ab |
| REP4 | 7.9 ± 0.6 a | 11.9 ± 1.1 a | 767 ± 52 ab | 644 ± 51 b | 289 ± 23 ab | 303 ± 24 b | 395 ± 22 b |
| Test ID | Passes a | TDS (mg/g_DW) | Phlorizin | Chlorogenic Acid | Epicatechin | Procyanidin B2 | Total Sugars (g/kg) |
|---|---|---|---|---|---|---|---|
| (mg/kg) | |||||||
| REP2 | 107 | 685 ± 3 b | 620 ± 64 b | 2153 ± 221 ab | 356 ± 37 a | 1211 ± 124 ab | 867 ± 45 a |
| REP3 | 281 | 692 ± 3 ab | 766 ± 74 ab | 2896 ± 281 a | 456 ± 44 a | 1740 ± 170 a | 751 ± 33 ab |
| REP4 | 84 | 720 ± 5 a | 1068 ± 85 a | 897 ± 71 b | 403 ± 32 a | 422 ± 33 b | 656 ± 30 b |
| Step | Quantity | Level | Unit | Source/Notes |
|---|---|---|---|---|
| Biomass | Specific heat of the dry biomass | 1370 | J/kgK | [69] |
| Milling | Material loss | 0 | % | data |
| Specific energy consumption a | 50 | kWh/ton | Personal experience with commercial bio-shredder | |
| HC | Process time | 20 | minutes | Evidence from this study |
| Temperature ramp | Constant at 80 °C | Evidence from this study | ||
| Energy consumption per unit time | 0.8 | kWh/min | Based on test REP4 b | |
| Yield of dry extract relative to dry biomass | 500 | g/kg_DW | Based on test REP4 (Table 4) | |
| Decanter centrifuging | Separation efficiency | 95% | [70] | |
| Moisture in separated material | 75% | |||
| Specific energy consumption c | 3.38 | kWh/ton | ||
| Mechanical pressing | Specific energy consumption d | 30 | kWh/ton | Personal experience |
| Moisture in separated material | 40% | |||
| Centrifuge | Energy consumption per unit mass of water | 15 | kWh/ton | [71] |
| Separation rate | 100 | % | Negligible errors due to 95% separation by the decanter | |
| Vacuum dryer | Water evaporation rate | 80% | [72] | |
| Energy consumption per unit mass of extract | 150 | kWh/ton | ||
| Spray dryer | Energy consumption per unit mass of extract | 1600 | kWh/ton | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliavento, L.; Nardin, T.; Chini, J.; Vighi, N.; Lovatti, L.; Testai, L.; Meneguzzo, F.; Larcher, R.; Zabini, F. Sustainable Exploitation of Apple By-Products: A Retrospective Analysis of Pilot-Scale Extraction Tests Using Hydrodynamic Cavitation. Foods 2025, 14, 1915. https://doi.org/10.3390/foods14111915
Tagliavento L, Nardin T, Chini J, Vighi N, Lovatti L, Testai L, Meneguzzo F, Larcher R, Zabini F. Sustainable Exploitation of Apple By-Products: A Retrospective Analysis of Pilot-Scale Extraction Tests Using Hydrodynamic Cavitation. Foods. 2025; 14(11):1915. https://doi.org/10.3390/foods14111915
Chicago/Turabian StyleTagliavento, Luca, Tiziana Nardin, Jasmine Chini, Nicola Vighi, Luca Lovatti, Lara Testai, Francesco Meneguzzo, Roberto Larcher, and Federica Zabini. 2025. "Sustainable Exploitation of Apple By-Products: A Retrospective Analysis of Pilot-Scale Extraction Tests Using Hydrodynamic Cavitation" Foods 14, no. 11: 1915. https://doi.org/10.3390/foods14111915
APA StyleTagliavento, L., Nardin, T., Chini, J., Vighi, N., Lovatti, L., Testai, L., Meneguzzo, F., Larcher, R., & Zabini, F. (2025). Sustainable Exploitation of Apple By-Products: A Retrospective Analysis of Pilot-Scale Extraction Tests Using Hydrodynamic Cavitation. Foods, 14(11), 1915. https://doi.org/10.3390/foods14111915










