Ultrasound-Assisted Extraction Optimization and Cultivar Screening for Polyphenol Recovery from Thinned Peach Fruit: A Comprehensive Evaluation of 179 Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Optimization of Conditions for Ultrasonic Assisted Extraction of Thinned Peach Polyphenolics (TPPs)
2.2.1. Ultrasound-Assisted Extraction of Phenolic Compounds
2.2.2. Single Factor Experiments
2.2.3. Response Surface Design Experiments
2.3. Quantitative Polyphenolic Analysis
2.3.1. Total Phenolic Content (TPC)
2.3.2. Total Flavonoid Content (TFC)
2.3.3. Total Anthocyanin Content (TAC)
2.4. Antioxidant Activity
2.4.1. Ferric Reducing Antioxidant Power (FRAP)
2.4.2. 2,2′-Azino-di-3-ethylbenzthiazoline Sulfonic Acid (ABTS) Radical Scavenging Assay
2.4.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Capacity
2.5. Statistical Analysis
3. Results
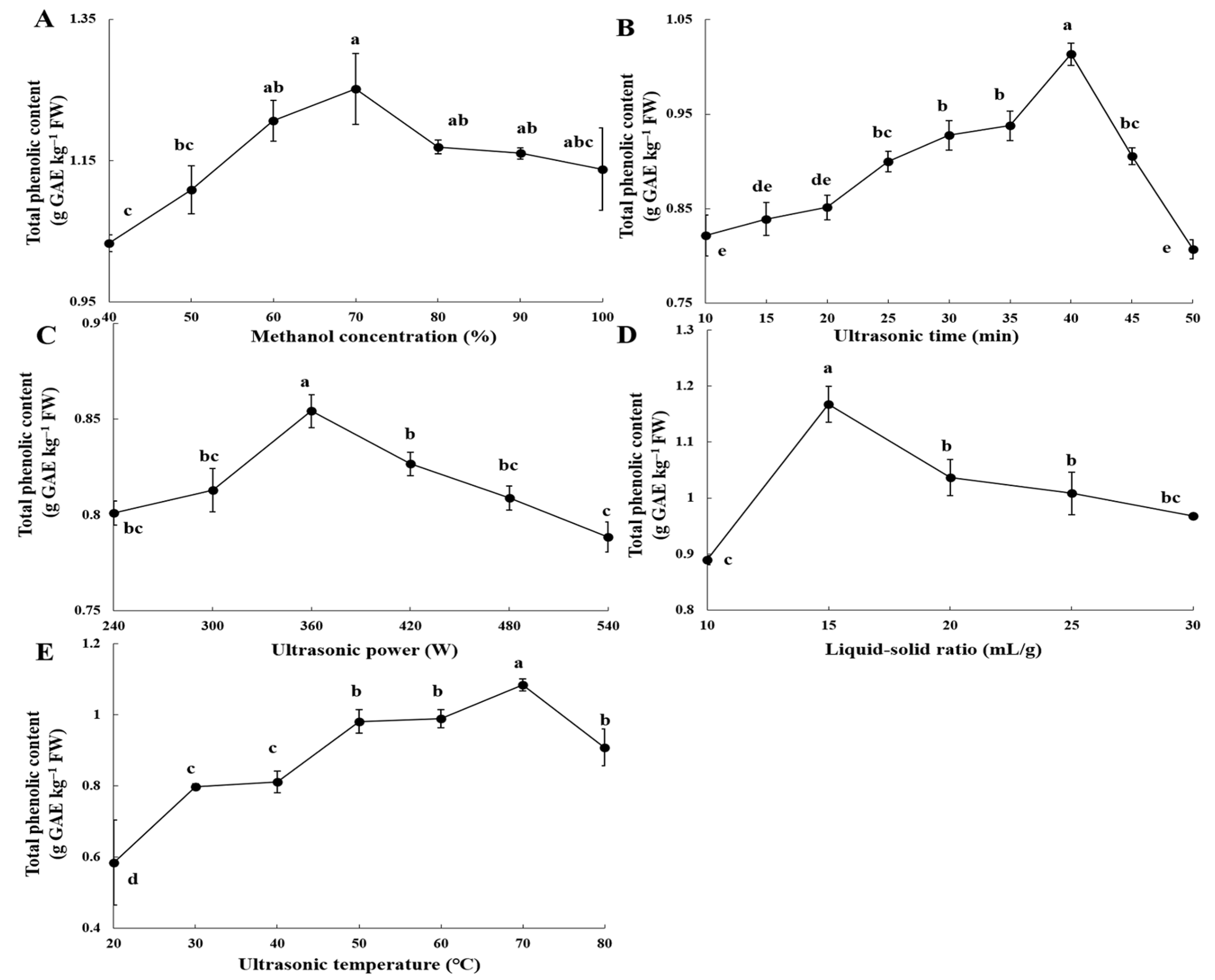
3.1. Impact of Key Ultrasound Extraction Parameters on the Recovery Efficiency of Thinned Peach Polyphenols (TPPs)
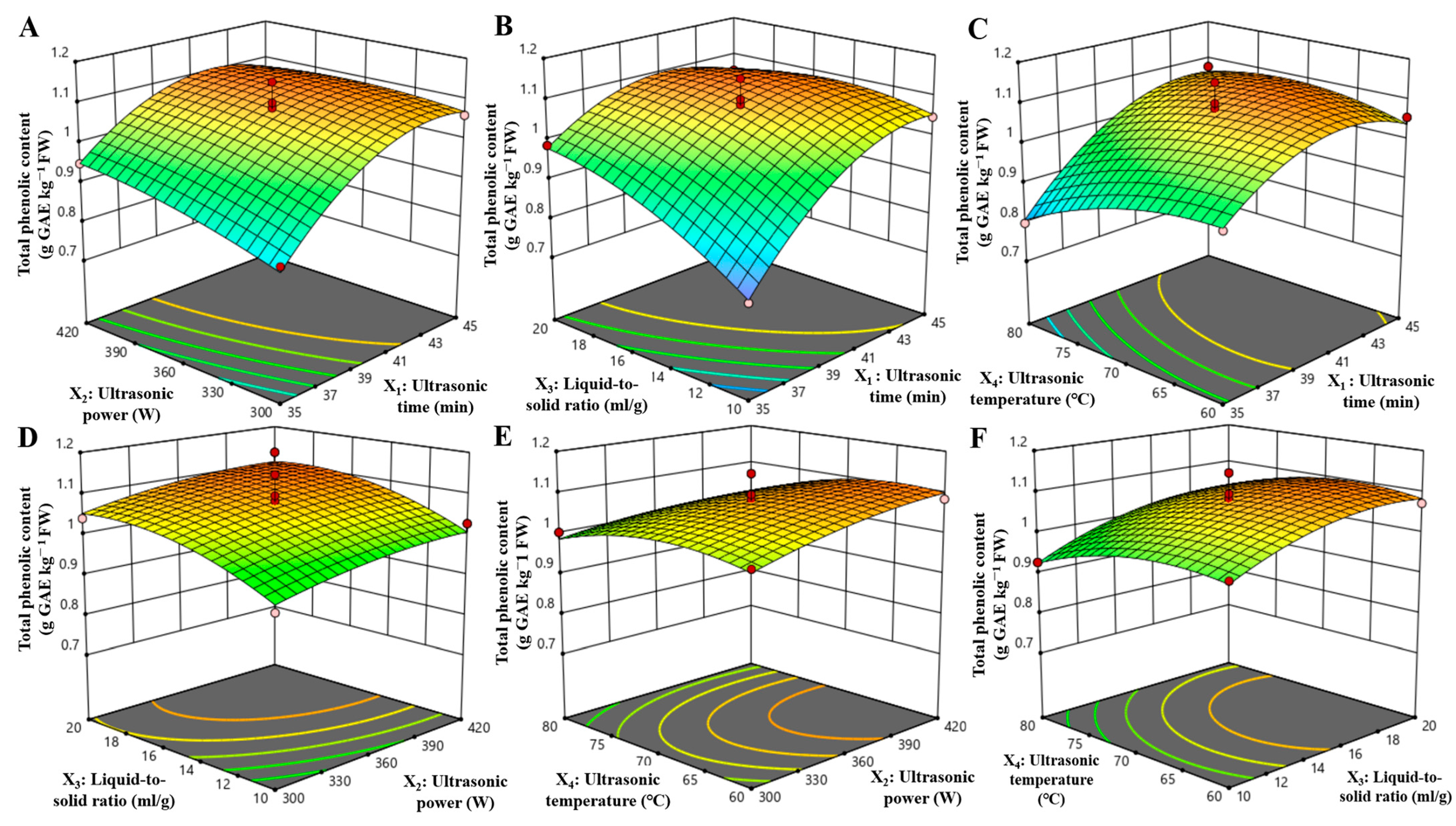
3.2. Optimization of Extraction Conditions Using a Response Surface Model
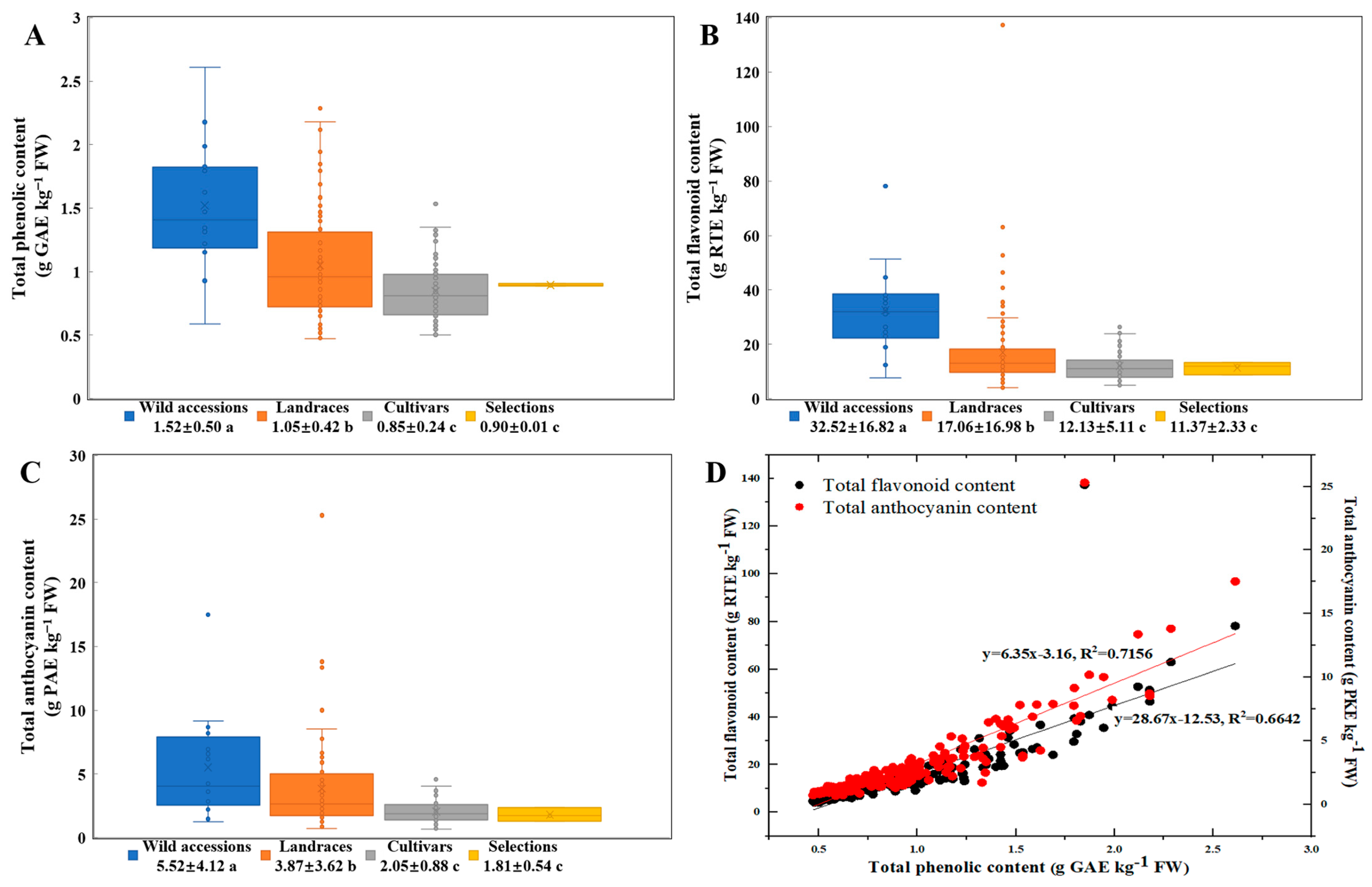
3.3. The Analysis of Polyphenolic Content in Thinned Peaches Across Various Germplasm Resources
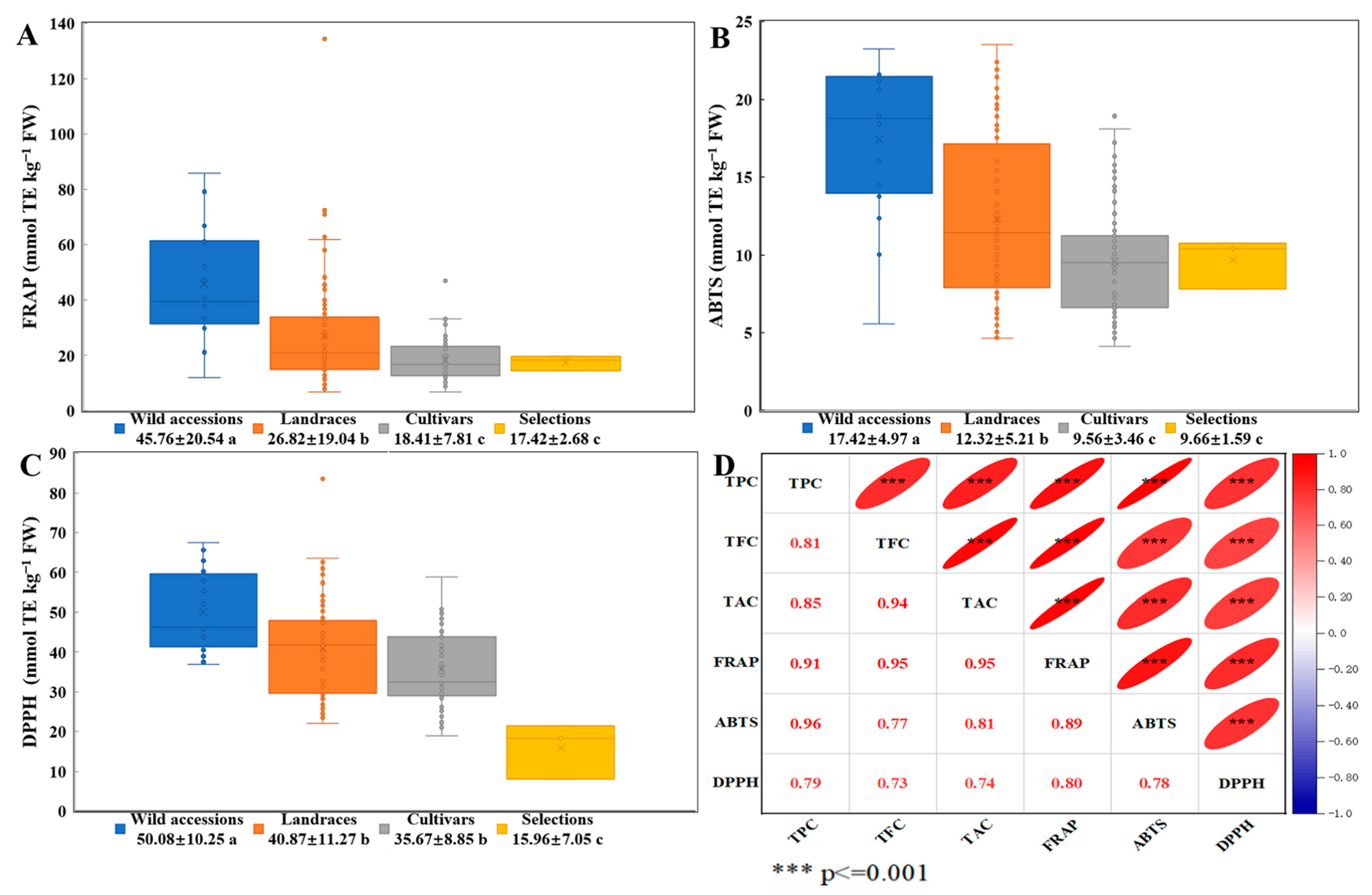
3.4. Comparative Analysis of Antioxidant Activity and Polyphenol-Antioxidant Correlations in TPPs Across Germplasm Types
3.5. High-Quality Germplasm Resources of Peach Thinned Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, M.Y.; Wang, H.L.; Ma, T.T.; Ge, Q.; Fang, Y.L.; Sun, X.Y. Comprehensive utilization of thinned unripe fruits from horticultural crops. Foods 2021, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Chen, H.; Zhang, Y.Y.; Guo, S.L.; Wang, X.J.; Sun, M.; Yu, M.L.; Ma, R.J. Effects of blooming and fruit thinning on the yield, fruit quality, and leaf photosynthesis of peach cultivar ‘Xiahui 5’ in China. Food Qual. Saf. 2024, 8, fyae019. [Google Scholar] [CrossRef]
- Guo, C.T.; Li, X.; Bi, J.F.; Lü, J.; Wu, X.Y.; Lü, Y.; Xu, Y. Recent achievements in bioactive components, functional properties and applications of thinned fruits. Food Sci. 2020, 41, 303–309. [Google Scholar] [CrossRef]
- Hitaj, C. Peaches. In The Economics of Food Loss in the Produce Industry; Routledge: London, UK, 2019; pp. 197–204. [Google Scholar]
- Hong, C.X.; Holtz, B.A.; Morgan, D.P.; Michailides, T.J. Significance of thinned fruit as a source of the secondary inoculum of Monilinia fructicola in California nectarine orchards. Plant Dis. 1997, 81, 519–524. [Google Scholar] [CrossRef]
- Dai, K.; Song, Y.; Zhang, D.C.; Wei, Y.Y.; Jiang, S.; Xu, F.; Wang, H.F.; Zhang, X.; Shao, X.F. Thinned peach polyphenols alleviate obesity in high fat mice by affecting gut microbiota. Food Res. Int. 2022, 157, 111255. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Song, Y.; Jiang, Y.; Wei, Y.Y.; Jiang, S.; Chen, Y.; Ye, J.F.; Shao, X.F. Physicochemical properties and anti-diabetic ability of polysaccharides from a thinned peach fruit. Food Funct. 2023, 19, 8876–8892. [Google Scholar] [CrossRef]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Costa, G.D.; Temsamani, H.; Renouf, E.; Mérillon, J.M.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic contents and bioactive potential of peach fruit extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef]
- Ding, T.Y.; Cao, K.; Fang, W.C.; Zhu, G.R.; Chen, C.W.; Wang, X.W.; Wang, L.R. Evaluation of phenolic components (anthocyanins, flavanols, phenolic acids, and flavonols) and their antioxidant properties of peach fruits. Sci. Hortic. 2020, 268, 109365. [Google Scholar] [CrossRef]
- Guo, C.T.; Bi, J.F.; Li, X.; Lyu, J.; Zhou, M.; Wu, X.Y. Antioxidant profile of thinned young and ripe fruits of Chinese peach and nectarine varieties. Int. J. Food Prop. 2020, 23, 1272–1286. [Google Scholar] [CrossRef]
- Dai, K.; Wei, Y.Y.; Jiang, S.; Xu, F.; Wang, H.F.; Zhang, X.; Shao, X.F. Lab scale extracted conditions of polyphenols from thinned peach fruit have antioxidant, hypoglycemic, and hypolipidemic properties. Foods 2021, 11, 99. [Google Scholar] [CrossRef]
- Gong, W.F.; Li, J.; Zhu, G.Y.; Wang, Y.C.; Zheng, G.Y.; Kan, Q.C. Chlorogenic acid relieved oxidative stress injury in retinal ganglion cells through IncRNA-TUG1/Nrf2. Cell Cycle 2019, 18, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, S.Y.; Lee, P.; Hur, J.Y. Neochlorogenic acid inhibits lipopolysaccharide-induced activation and pro-inflammatory responses in BV2 microglial cells. Neurochem. Res. 2015, 40, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Inhibitory potential against digestive enzymes linked to obesity and type 2 diabetes and content of bioactive compounds in 20 cultivars of the peach fruit grown in Poland. Plant Foods Hum. Nutr. 2018, 73, 314–320. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Abderrahmane, M.; Khodir, M. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Mihaylova, D.; Terziyska, M.; Desseva, I.; Popova, A.; Lante, A. Optimization of ultrasound-assisted extraction of free phenolic compounds and in vitro biological activity from peach fruit using response surface methodology. Appl. Sci. 2024, 14, 4286. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Xiao, Z.L.; Gu, Q.Y.; Dou, L.P.; Bo, H. Comprehensive evaluation of the fruit quality of yellow-flesh peaches in Ningxian. J. Fruit. Sci. 2024, 41, 65–75. [Google Scholar] [CrossRef]
- Redondo, D.; Arias, E.; Oria, R.; Venturini, M.E. Thinned stone fruits are a source of polyphenols and antioxidant compounds. J. Sci. Food Agric. 2017, 97, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 16, 1231–1237. [Google Scholar] [CrossRef]
- Guo, C.T.; Bi, J.F.; Li, X.; Lyu, J.; Wu, X.Y.; Xu, Y. Polyphenol metabolic diversity of Chinese peach and nectarine at thinned and ripe stages by UPLC-ESI-Q-TOF-MS combined with multivariate statistical analysis. J. Food Compos. Anal. 2020, 90, 103502. [Google Scholar] [CrossRef]
- Yonezawa, T.; Momota, R.; Iwano, H.; Zhao, S.; Hakozaki, T.; Soh, C.; Sawaki, S.; Toyama, K.; Oohashi, T. Unripe peach (Prunus Persica) extract ameliorates damage from UV irradiation and improved collagen XVIII expression in 3D skin model. J. Cosmet. Dermatol. 2019, 18, 1507–1515. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- Eva, D.; Gloria, L.M.; Monica, G. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef]
- Patricia, G.S.; Aranzazu, M.S.; Antonio, S.C.; Alberto, F.G. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Altemimi, A.; Watson, D.G.; Choudhary, R.; Dasari, M.R.; Lightfoot, D.A. Ultrasound assisted extraction of phenolic compounds from peaches and pumpkins. PLoS ONE 2016, 11, e0148758. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.M.; Kim, S.Y.; Lee, G.W.; Kim, I.D.; Park, Y.S.; Dhungana, S.K.; Kim, J.H.; Shin, D.H. Quality characteristics and antioxidant activity of unripe peach (Prunus Persica L. Batsch) extracts with distilled water coupled with ultrasonication and prethanol-A. Int. J. Fruit Sci. 2020, 20, S111–S122. [Google Scholar] [CrossRef]
- Tsiaka, T.; Lantzouraki, D.Z.; Polychronaki, G.; Sotiroudis, G.; Kritsi, E.; Sinanoglou, V.J.; Kalogianni, D.P.; Zoumpoulakis, P. Optimization of ultrasound- and microwave-assisted extraction for the determination of phenolic compounds in peach byproducts using experimental design and liquid chromatography-tandem mass spectrometry. Molecules 2023, 28, 518. [Google Scholar] [CrossRef]
- Baltacıoğlu, C.; Baltacıoğlu, H.; Okur, İ.; Yetişen, M.; Alpas, H. Recovery of phenolic compounds from peach pomace using conventional solvent extraction and different emerging techniques. J. Food Sci. 2024, 89, 1672–1683. [Google Scholar] [CrossRef]
- Redondo, D.; Gimeno, D.; Calvo, H.; Venturini, M.E.; Oria, R.; Arias, E. Antioxidant activity and phenol content in different tissues of stone fruits at thinning and at commercial maturity stages. Waste Biomass Valorization 2021, 12, 1861–1875. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Desseva, I.; Petkova, N.; Stoyanova, M.; Vrancheva, R.; Slavov, A.; Slavchev, A.; Lante, A. Comparative study of early- and mid-ripening peach (Prunus persica L.) varieties: Biological activity, macro-, and micro-nutrient profile. Foods 2021, 10, 164. [Google Scholar] [CrossRef]
- Cai, Z.X.; Yan, J.; Su, Z.W.; Xu, Z.Y.; Zhang, M.H.; Shen, Z.J.; Yang, J.; Ma, R.J.; Yu, M.L. Evaluation of main phenolic compounds in different types of peach germplasm resources. Acta Hortic. Sin. 2022, 49, 1008–1022. [Google Scholar] [CrossRef]
- Shen, Z.J.; Tian, Y.; Cai, Z.X.; Xu, Z.Y.; Yan, J.; Sun, M.; Ma, R.J.; Yu, M.L. Evaluation of brown rot resistance in peach based on genetic resources conserved in national germplasm repository of peach in Nanjing. Sci. Agric. Sin. 2022, 55, 3018–3028. [Google Scholar] [CrossRef]
- Villarino, M.; Sandín-España, P.; Melgarejo, P.; De Cal, A. High chlorogenic and neochlorogenic acid levels in immature peaches reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. J. Agric. Food Chem. 2011, 59, 3205–3213. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, X.P.; Mu, Q.E.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.X.; Yu, X.Y. Differences in total phenolics, antioxidant activity and metabolic characteristics in peach fruits at different stages of ripening. LWT 2023, 178, 114586. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Shen, Z.J.; Ma, R.J.; Jiang, H.; Yan, J.; Yu, M.L. Antioxidant capacity evaluation of peach fruit based on flesh individual phenol and total phenol content. Acta Hortic. Sin. 2018, 45, 931–942. [Google Scholar] [CrossRef]
- Shen, Z.J.; Ma, R.J.; Yu, M.L.; Xu, J.L.; Cai, Z.X.; Ni, L.J.; Yan, S.B. Evaluation of antioxidant factors in peach with three types of flesh color. Sci. Agric. Sin. 2012, 45, 2232–2241. [Google Scholar] [CrossRef]
- Liao, X.; Greenspan, P.; Pegg, R.B. Characterizing the phenolic constituents and antioxidant capacity of Georgia peaches. Food Chem. 2019, 271, 345–353. [Google Scholar] [CrossRef]
- Giuliana, N.; Weston, P.; David, B.; Luis, C.Z. Polyphenolics from peach (Prunus persica var. Rich Lady) inhibit tumor growth and metastasis of MDA-MB-435 breast cancer cells in vivo. J. Nutr. Biochem. 2014, 25, 796–800. [Google Scholar] [CrossRef]
- Kan, J.; Chen, C.C.; Huo, T.B.; Xie, W.J.; Hui, Y.Y.; Liu, J.; Jin, C.H. Polyphenolic-enriched peach peels extract regulates lipid metabolism and improves the gut microbiota composition in high fat diet-fed mice. J. Funct. Foods 2020, 72, 104082. [Google Scholar] [CrossRef]
| Number | X1/Ultrasonic Time (min) | X2/Ultrasonic Power (W) | X3/Liquid-to-Solid (mL/g) | X4/Ultrasonic Temperature (°C) | Total Phenolic Content (g GAE kg−1 FW) |
|---|---|---|---|---|---|
| 1 | 40 (0) | 360 (0) | 10:1 (−1) | 60 (−1) | 0.994 ± 0.003 hij |
| 2 | 40 (0) | 420 (1) | 15:1 (0) | 80 (1) | 1.006 ± 0.001 ghi |
| 3 | 35 (−1) | 360 (0) | 15:1 (0) | 60 (−1) | 0.938 ± 0.001 kl |
| 4 | 35 (−1) | 360 (0) | 10:1 (−1) | 70 (0) | 0.764 ± 0.000 n |
| 5 | 40 (0) | 360 (0) | 20:1 (1) | 80 (1) | 1.015 ± 0.002 fghi |
| 6 | 40 (0) | 420 (1) | 10:1 (−1) | 70 (0) | 1.027 ± 0.001 defghi |
| 7 | 45 (1) | 420 (1) | 15:1 (0) | 70 (0) | 1.052 ± 0.001 cdefgh |
| 8 | 35 (−1) | 360 (0) | 20:1 (1) | 70 (0) | 0.986 ± 0.003 ijk |
| 9 | 35 (−1) | 420 (1) | 15:1 (0) | 70 (0) | 0.949 ± 0.001 jkl |
| 10 | 40 (0) | 420 (1) | 20:1 (1) | 70 (0) | 1.131 ± 0.000 ab |
| 11 | 45 (1) | 360 (0) | 10:1 (−1) | 70 (0) | 1.047 ± 0.000 cdefgh |
| 12 | 40 (0) | 300 (−1) | 15:1 (0) | 80 (1) | 1.003 ± 0.003 ghij |
| 13 | 45 (1) | 300 (−1) | 15:1 (0) | 70 (0) | 1.060 ± 0.002 cdefg |
| 14 | 35 (−1) | 300 (−1) | 15:1 (0) | 70 (0) | 0.856 ± 0.000 m |
| 15 | 45 (1) | 360 (0) | 15:1 (0) | 60 (−1) | 1.056 ± 0.002 cdefg |
| 16 | 40 (0) | 360 (0) | 15:1 (0) | 70 (0) | 1.082 ± 0.001 bcd |
| 17 | 45 (1) | 360 (0) | 15:1 (0) | 80 (1) | 1.077 ± 0.000 bcde |
| 18 | 40 (0) | 360 (0) | 15:1 (0) | 70 (0) | 1.045 ± 0.002 cdefghi |
| 19 | 40 (0) | 360 (0) | 10:1 (−1) | 80 (1) | 0.927 ± 0.001 l |
| 20 | 40 (0) | 300 (−1) | 10:1 (−1) | 70 (0) | 0.929 ± 0.001 l |
| 21 | 40 (0) | 360 (0) | 15:1 (0) | 70 (0) | 1.034 ± 0.002 defghi |
| 22 | 40 (0) | 300 (−1) | 20:1 (1) | 70 (0) | 1.040 ± 0.000 cdefghi |
| 23 | 40 (0) | 360 (0) | 15:1 (0) | 70 (0) | 1.196 ± 0.002 a |
| 24 | 40 (0) | 300 (−1) | 15:1 (0) | 60 (−1) | 1.021 ± 0.002 efghi |
| 25 | 40 (0) | 420 (1) | 15:1 (0) | 60 (−1) | 1.084 ± 0.001 bcd |
| 26 | 40 (0) | 360 (0) | 20:1 (1) | 60 (−1) | 1.072 ± 0.003 cdef |
| 27 | 40 (0) | 360 (0) | 15:1 (0) | 70 (0) | 1.095 ± 0.037 abc |
| 28 | 45 (1) | 360 (0) | 20:1 (1) | 70 (0) | 1.056 ± 0.001 cdefg |
| 29 | 35 (−1) | 360 (0) | 15:1 (0) | 80 (1) | 0.798 ± 0.001 n |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 0.2126 | 14 | 0.0152 | 17.49 | <0.0001 | ** |
| X1 (Ultrasonic time) | 0.0932 | 1 | 0.0932 | 107.36 | 0.005 | ** |
| X2 (Ultrasonic power) | 0.0097 | 1 | 0.0097 | 11.12 | 0.0049 | ** |
| X3 (Liquid-to-solid ratio) | 0.0311 | 1 | 0.0311 | 35.83 | <0.0001 | ** |
| X4 (Ultrasonic temperature) | 0.0096 | 1 | 0.0096 | 11.04 | <0.0001 | ** |
| X1X2 | 0.0025 | 1 | 0.0025 | 2.92 | 0.1098 | |
| X1X3 | 0.0113 | 1 | 0.0113 | 13.00 | 0.0029 | ** |
| X1X4 | 0.0065 | 1 | 0.0065 | 7.45 | 0.0163 | * |
| X2X3 | 0.0000 | 1 | 0.0000 | 0.0147 | 0.9052 | |
| X2X4 | 0.0009 | 1 | 0.0009 | 1.03 | 0.3263 | |
| X3X4 | 0.0000 | 1 | 0.0000 | 0.0320 | 0.8606 | |
| X12 | 0.0422 | 1 | 0.0422 | 48.62 | <0.0001 | ** |
| X22 | 0.0017 | 1 | 0.0017 | 1.92 | 0.1873 | |
| X32 | 0.0089 | 1 | 0.0089 | 10.31 | 0.0063 | ** |
| X42 | 0.0087 | 1 | 0.0087 | 10.04 | 0.0068 | ** |
| Residual | 0.0122 | 14 | 0.0009 | |||
| Lack of Fit | 0.0042 | 10 | 0.0004 | 0.2088 | 0.9797 | |
| Cor Total | 0.2248 | 28 | ||||
| R2 = 0.9459, R2adj = 0.8918, R2pre = 0.8376 | ||||||
| Germplasm Type | Name | TPC | TFC | TAC | FRAP | ABTS | DPPH |
|---|---|---|---|---|---|---|---|
| g GAE kg−1 FW | g RTE kg−1 FW | g PAE kg−1 FW | mmol TE kg−1 FW | mmol TE kg−1 FW | mmol TE kg−1 FW | ||
| WA | Gansu Peach 2 | 2.6 ± 0.1 a | 78.2 ± 10.4 b | 17.5 ± 1.6 b | 85.8 ± 11.0 b | 23.2 ± 0.1 a | 67.5 ± 0.1 b |
| LD | Nanshan Sweet Peach 1 | 2.3 ± 0.1 b | 63.0 ± 6.7 c | 13.8 ± 0.8 c | 72.4 ± 2.2 cd | 22.6 ± 0.2 b | 63.4 ± 1.1 c |
| LD | Yanwohong | 2.2 ± 0.1 bc | 46.4 ± 2.2 de | 8.5 ± 0.5 ef | 58.1 ± 3.9 fgh | 22.1 ± 0.2 bc | 61.0 ± 0.9 de |
| WA | Fujian Hairy Peach 1 | 2.2 ± 0.1 bc | 51.3 ± 7.5 d | 8.7 ± 0.7 ef | 79.2 ± 8.4 bc | 21.6 ± 0.2 cd | 62.9 ± 1.3 cd |
| LD | Nanshan Sweet Peach | 2.1 ± 0.1 cd | 52.7 ± 2.2 d | 13.4 ± 0.3 c | 70.9 ± 10.2 cde | 22.4 ± 0.1 b | 62.6 ± 0.6 cd |
| WA | Guanghetao | 2.0 ± 0.1 de | 44.5 ± 2.5 def | 8.2 ± 0.2 ef | 66.8 ± 3.7 def | 21.7 ± 0.3 cd | 65.6 ± 0.8 b |
| LD | Purple Nectarine 9 | 2.0 ± 0.1 ef | 35.5 ± 3.6 fghi | 10.0 ± 0.1 d | 62.0 ± 3.0 ef | 21.4 ± 0.3 cd | 59.5 ± 0.2 e |
| LD | Lianyungang Winter Peach | 1.9 ± 0.2 ef | 40.8 ± 10.5 efg | 10.2 ± 1.7 d | 62.8 ± 4.8 def | 21.9 ± 0.4 bc | 57.3 ± 0.8 f |
| LD | Early White Blossom Peach | 1.9 ± 0.1 efg | 137.3 ± 12.5 a | 25.3 ± 0.0 a | 134.5 ± 2.5 a | 23.5 ± 0.1 a | 83.6 ± 0.6 a |
| WA | Red Flower Mountain Peach | 1.8 ± 0.1 efg | 38.2 ± 3.9 efgh | 6.9 ± 0.5 ghi | 61.1 ± 7.0 efg | 21.1 ± 0.9 de | 52.0 ± 2.7 h |
| WA | Thai Hairy Peach | 1.8 ± 0.1 fg | 32.9 ± 0.7 ghij | 6.6 ± 0.2 hi | 47.1 ± 2.7 ij | 20.65 ± 0.39 ef | 46.1 ± 1.4 j |
| WA | Guanghetao 24-1 | 1.8 ± 0.1 fg | 39.5 ± 1.6 efgh | 9.2 ± 0.6 de | 61.5 ± 2.6 efg | 21.60 ± 0.18 cd | 60.3 ± 1.1 e |
| LD | Zhanghuang 3 | 1.8 ± 0.2 fg | 29.6 ± 4.0 hij | 7.8 ± 0.8 fgh | 45.5 ± 6.9 ij | 20.70 ± 0.80 ef | 54.9 ± 0.5 g |
| LD | Yexian Yellow Peach 8 | 1.7 ± 0.1 gh | 24.1 ± 2.1 j | 7.9 ± 0.3 fg | 49.2 ± 6.7 hi | 20.13 ± 0.28 fg | 54.1 ± 0.4 g |
| WA | Hairy Peach | 1.6 ± 0.1 hi | 36.7 ± 2.8 efgh | 4.2 ± 0.5 j | 37.9 ± 2.5 j | 18.92 ± 0.76 hi | 40.5 ± 0.4 k |
| LD | Purple Nectarine 5 | 1.6 ± 0.1 hi | 27.2 ± 2.4 ij | 7.8 ± 0.4 fg | 48.7 ± 2.9 hi | 19.91 ± 0.27 g | 50.6 ± 1.9 hi |
| LD | Red Leaf Winter Peach | 1.6 ± 0.1 hi | 26.6 ± 1.6 ij | 6.9 ± 0.3 ghi | 48.5 ± 4.2 hi | 19.67 ± 0.17 g | 48.6 ± 2.5 i |
| CV | Shuangbai | 1.5 ± 0.1 hi | 24.1 ± 1.6 j | 3.7 ± 0.3 j | 46.9 ± 2.0 ij | 17.21 ± 0.26 j | 58.8 ± 1.9 ef |
| LD | Rugao Purple Nectarine 3 | 1.5 ± 0.1 i | 24.9 ± 0.4 j | 7.8 ± 0.1 fgh | 44.3 ± 3.8 ij | 18.03 ± 0.13 i | 50.7 ± 0.1 hi |
| WA | Red Leaf Flat Peach peach | 1.5 ± 0.1 i | 35.2 ± 4.5 fghi | 6.2 ± 0.5 i | 52.1 ± 0.6 ghi | 20.62 ± 0.02 ef | 45.8 ± 0.8 j |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xu, J.; Cai, Z.; Guo, S.; Yu, M.; Shen, Z. Ultrasound-Assisted Extraction Optimization and Cultivar Screening for Polyphenol Recovery from Thinned Peach Fruit: A Comprehensive Evaluation of 179 Cultivars. Foods 2025, 14, 1897. https://doi.org/10.3390/foods14111897
Li S, Xu J, Cai Z, Guo S, Yu M, Shen Z. Ultrasound-Assisted Extraction Optimization and Cultivar Screening for Polyphenol Recovery from Thinned Peach Fruit: A Comprehensive Evaluation of 179 Cultivars. Foods. 2025; 14(11):1897. https://doi.org/10.3390/foods14111897
Chicago/Turabian StyleLi, Shenge, Jianlan Xu, Zhixiang Cai, Shaolei Guo, Mingliang Yu, and Zhijun Shen. 2025. "Ultrasound-Assisted Extraction Optimization and Cultivar Screening for Polyphenol Recovery from Thinned Peach Fruit: A Comprehensive Evaluation of 179 Cultivars" Foods 14, no. 11: 1897. https://doi.org/10.3390/foods14111897
APA StyleLi, S., Xu, J., Cai, Z., Guo, S., Yu, M., & Shen, Z. (2025). Ultrasound-Assisted Extraction Optimization and Cultivar Screening for Polyphenol Recovery from Thinned Peach Fruit: A Comprehensive Evaluation of 179 Cultivars. Foods, 14(11), 1897. https://doi.org/10.3390/foods14111897





