Flavor Quality and Lipid-Lowering Function of Mixed Fermented Pu-erh Tea with Various Monascus Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
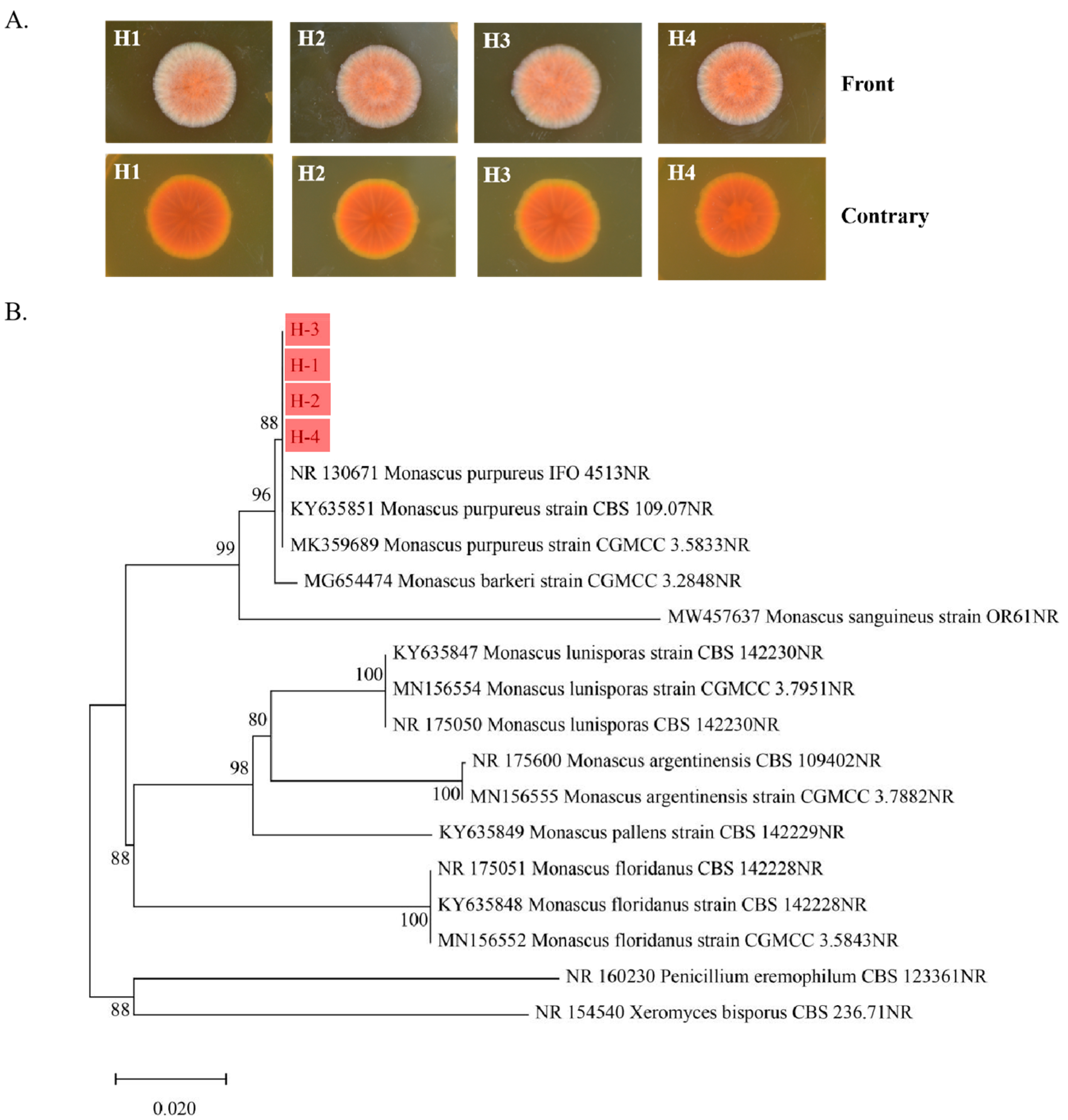
2.2. Methods of Isolation and Identification of Monascus
2.3. Solid Fermentation
2.4. Determination of Main Biochemical Components
2.5. Determination of Volatile Substances
2.6. Determination of Non-Volatile Substances
2.7. Sensory Review Method
2.8. Cell Experiment
2.9. Data Analysis
3. Results and Discussion
3.1. Results of Isolation and Identification of Monascus
3.2. Sensory Evaluation of UT and FT
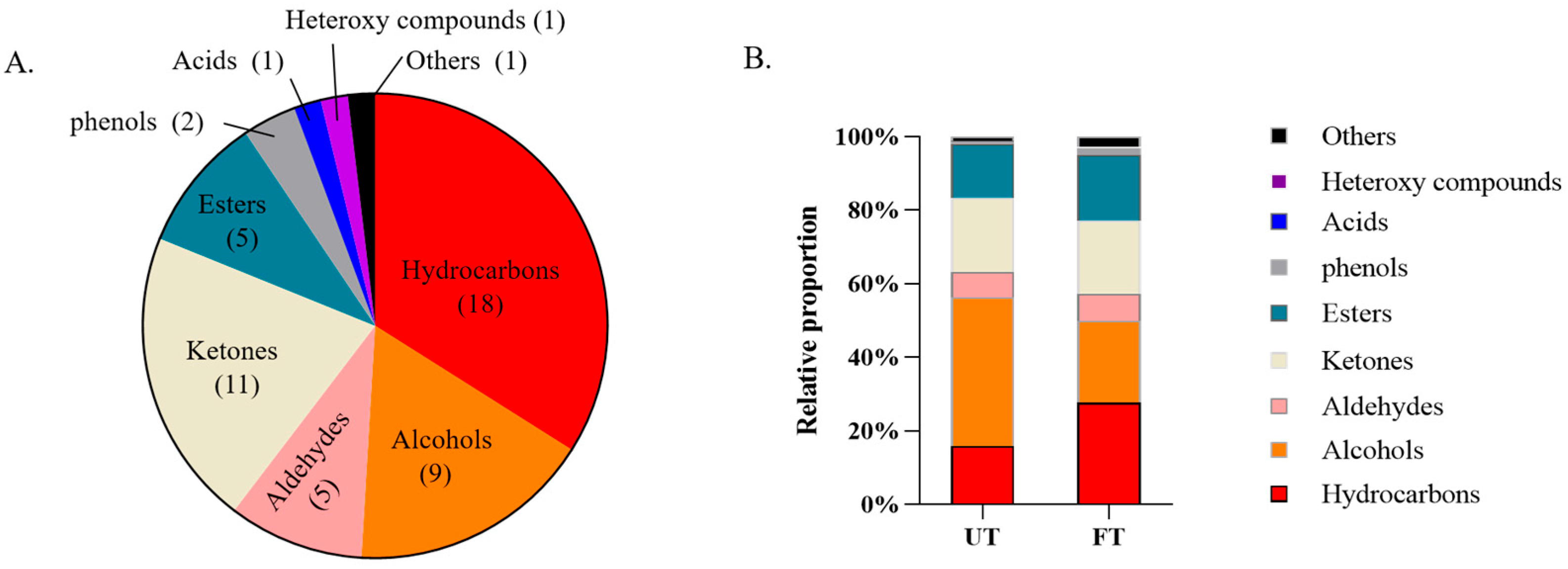
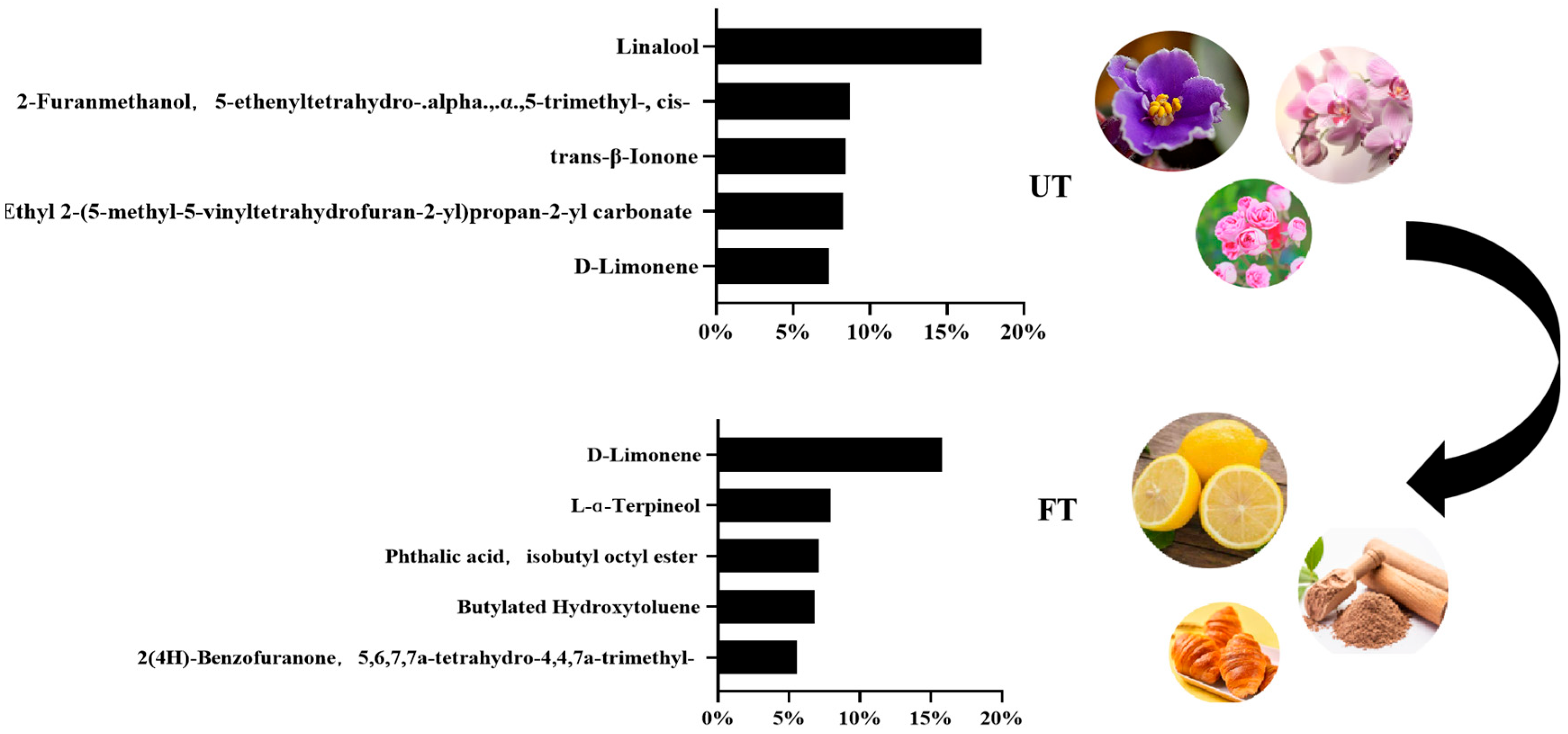
3.3. Comparative Analysis of Volatile Substances in UT and FT
3.4. Comparative Analysis of Non-Volatile Substances in UT and FT
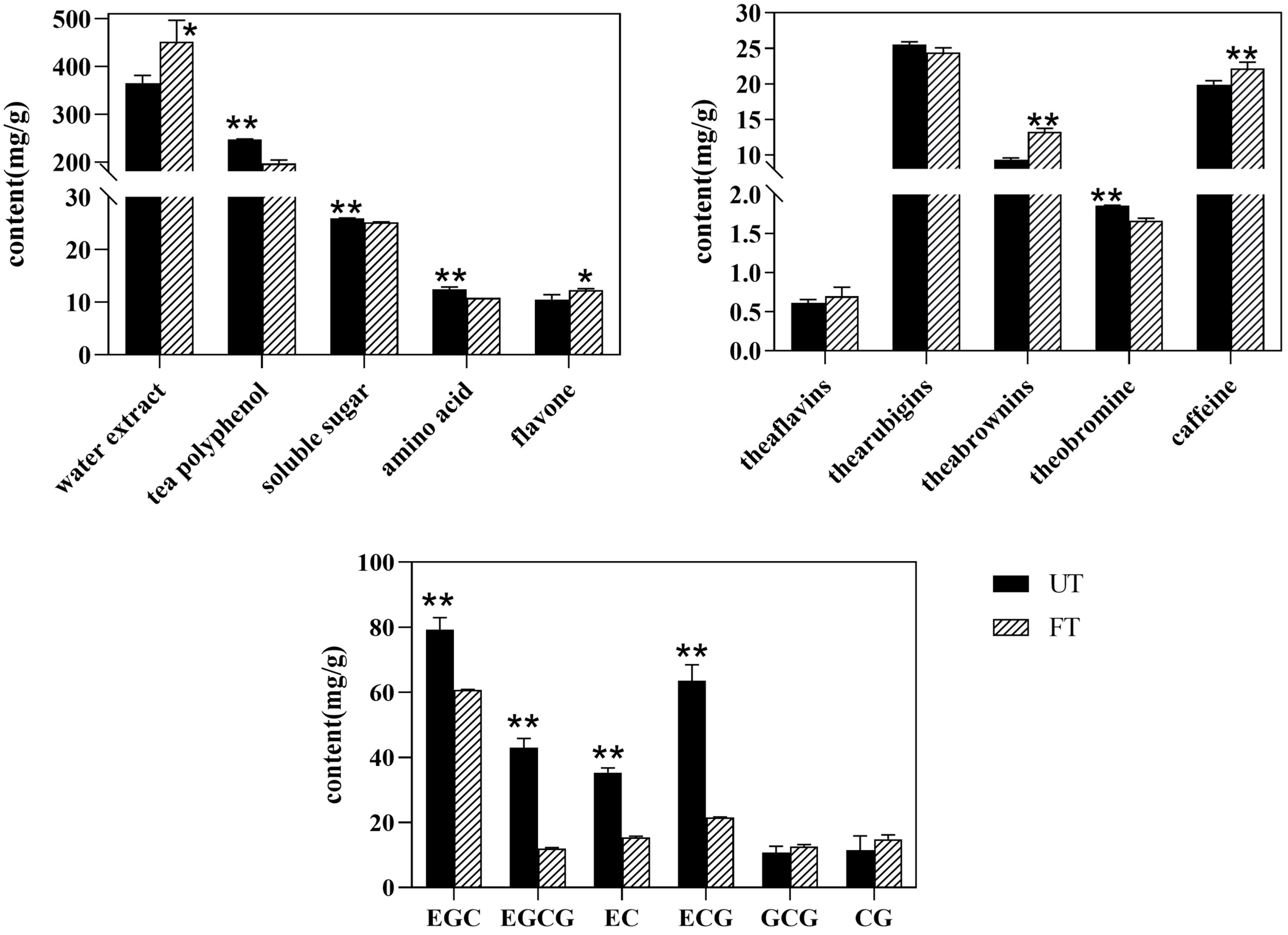
3.4.1. Main Biochemical Components of UT and FT
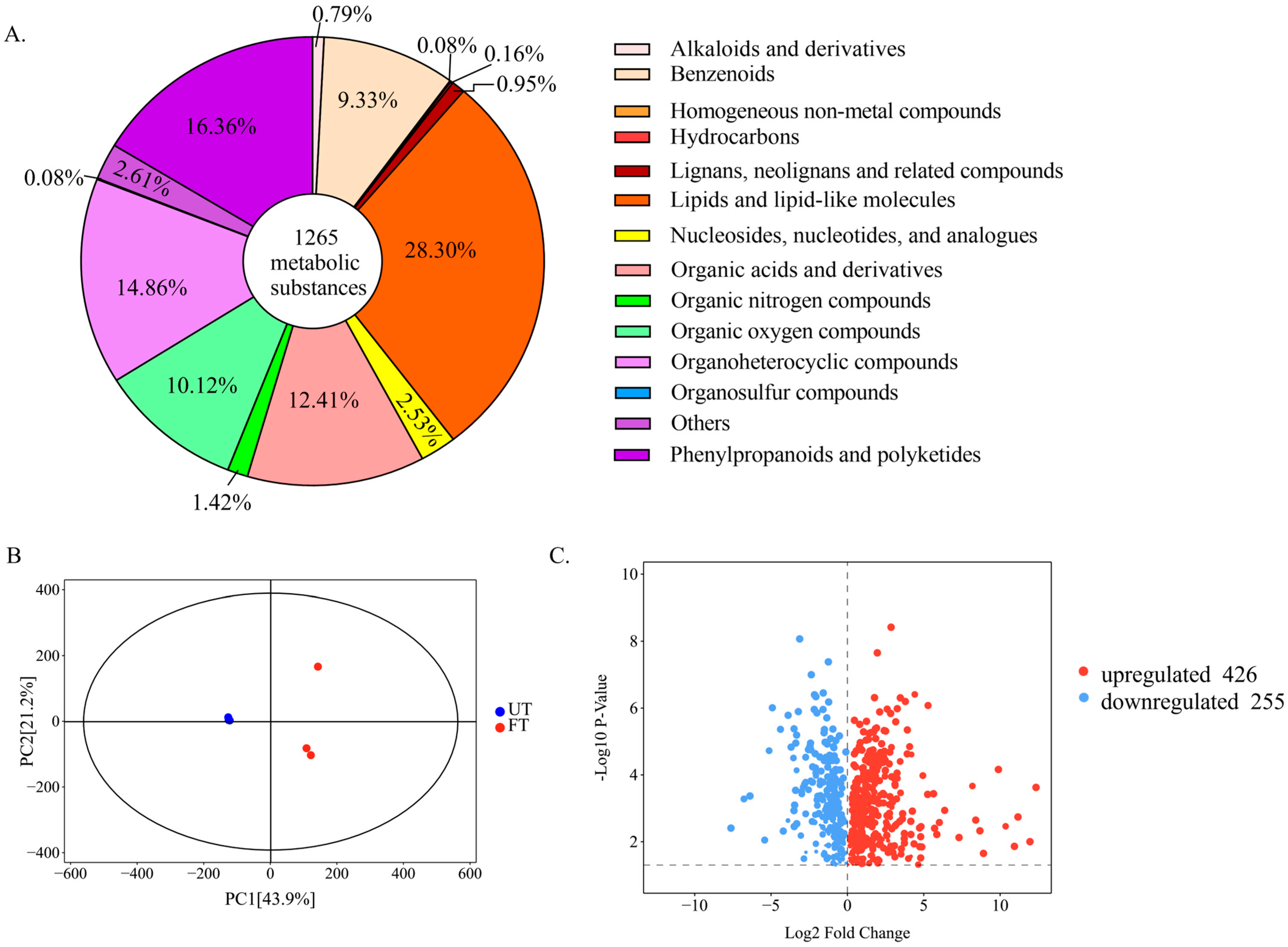
3.4.2. Overall Changes and Screening of Characteristic Substances in UT and FT
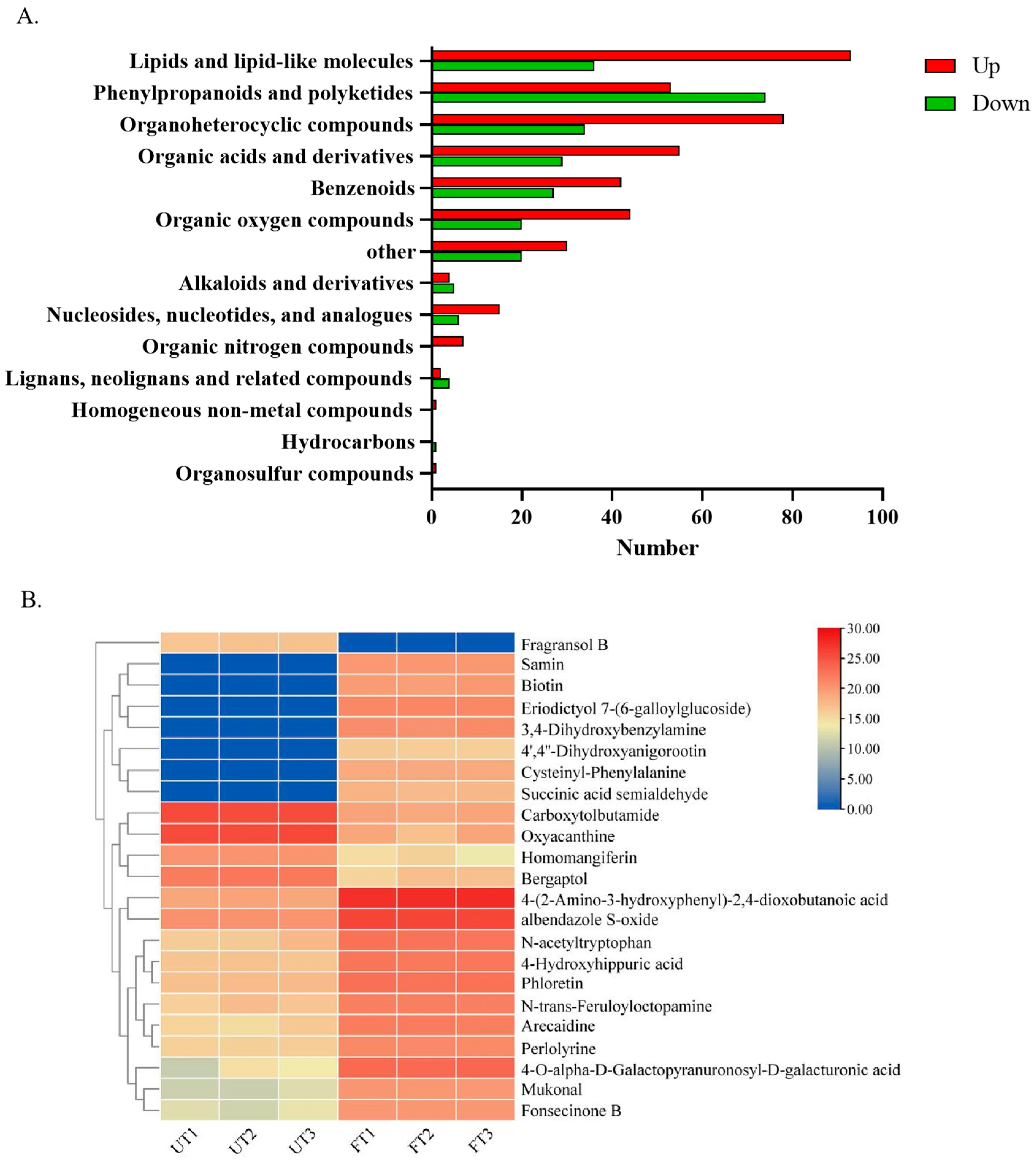
3.4.3. Changes in Flavor-Related Substances of UT and FT
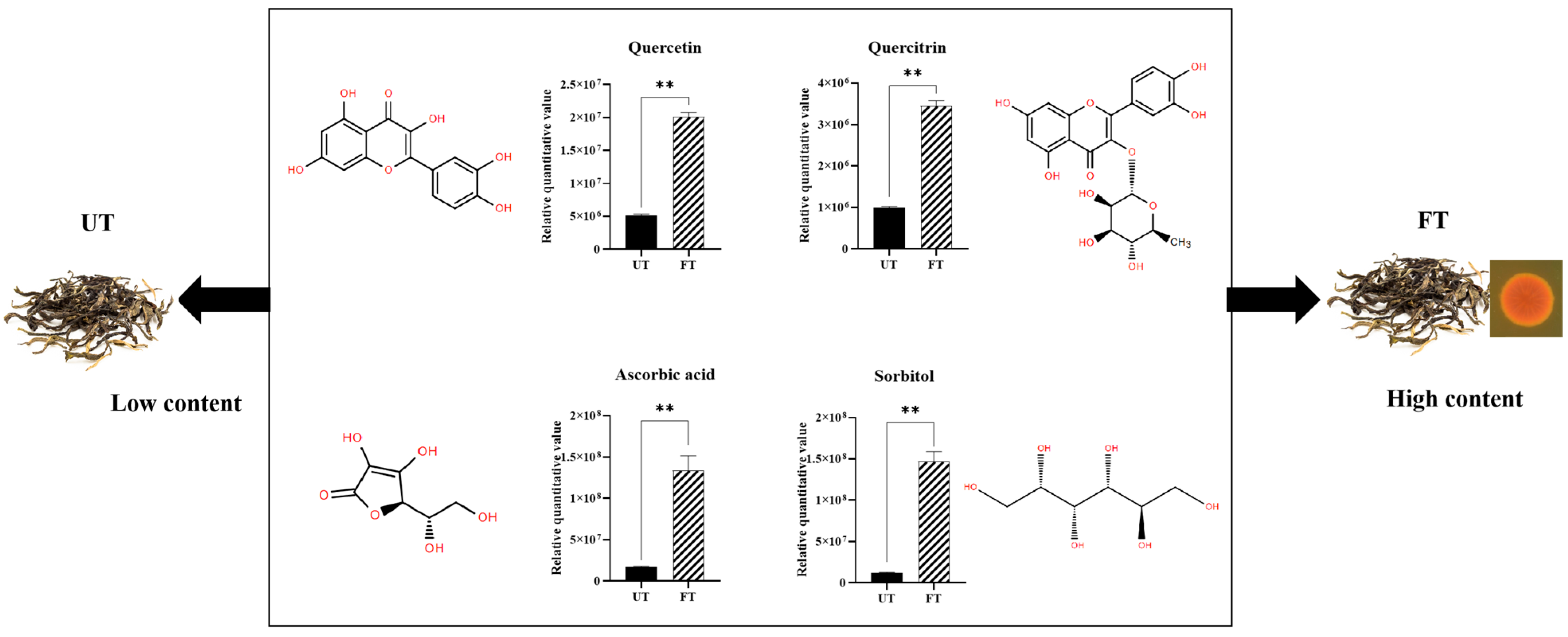
3.4.4. Changes in Potential Lipid-Lowering Substances of UT and FT
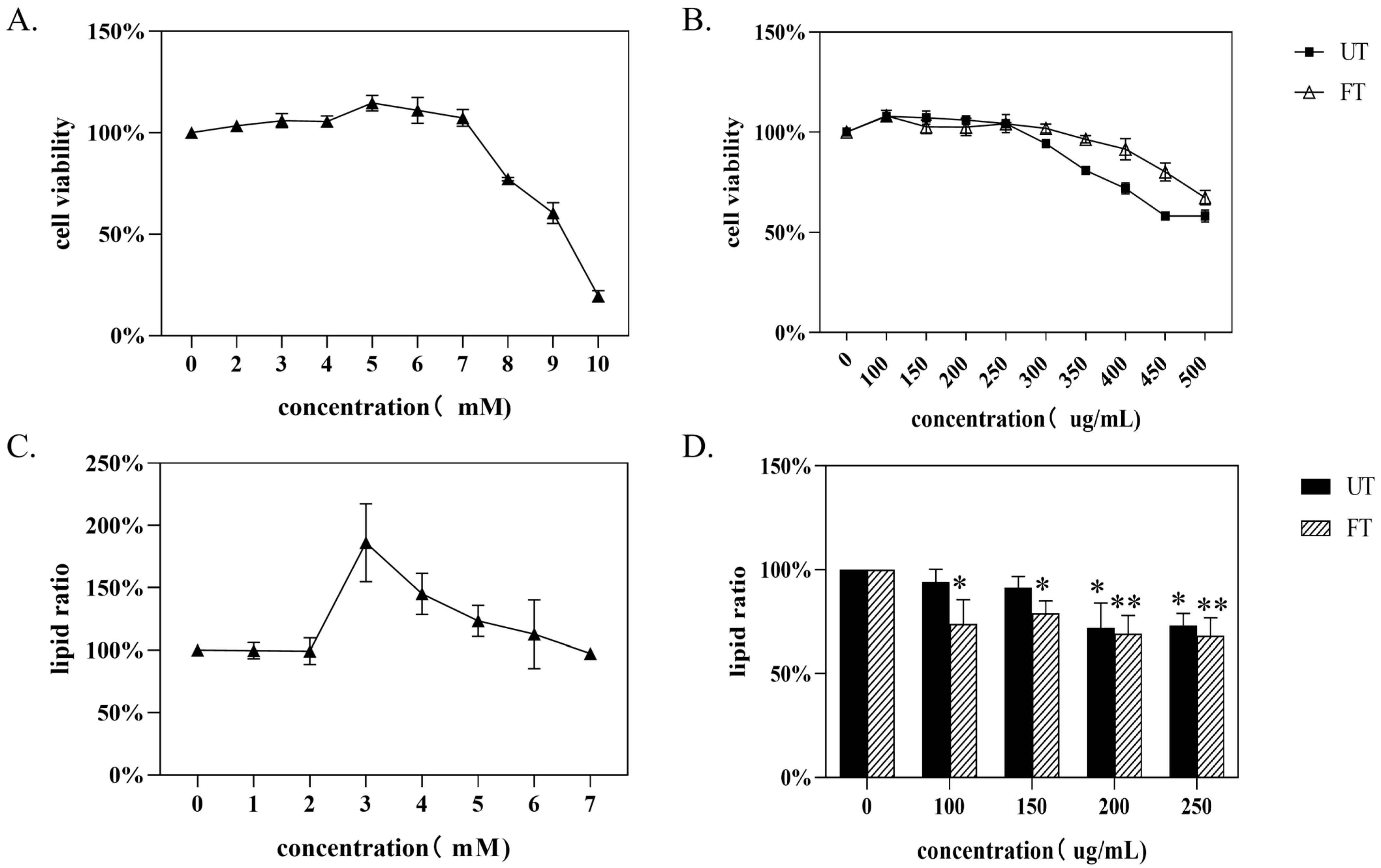
3.5. Study on Lipid-Lowering Function of UT and FT In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaczynski, P.; Iwaniuk, P.; Jankowska, M.; Orywal, K.; Socha, K.; Perkowski, M.; Farhan, J.A.; Lozowicka, B. Pesticide residues in common and herbal teas combined with risk assessment and transfer to the infusion. Chemosphere 2024, 367, 143550. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Niu, M.; Yang, H.; Zhou, X.; Ding, J.; Xu, Y.; Lv, C.; Li, J. Analysis of Metabolite Differences in Different Tea Liquors Based on Broadly Targeted Metabolomics. Foods 2024, 13, 2800. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bo, N.; Sha, G.; Guan, Y.; Yang, D.; Shan, X.; Lv, Z.; Chen, Q.; Yang, G.; Gong, S.; et al. Identification and molecular mechanism of novel hypoglycemic peptide in ripened pu-erh tea: Molecular docking, dynamic simulation, and cell experiments. Food Res. Int. 2024, 194, 114930. [Google Scholar] [CrossRef]
- Weng, Y.; Chen, L.; Kun, J.; He, S.; Tong, H.; Chen, Y. The unique aroma of ripened Pu-erh tea, Liupao tea and Tietban tea: Associated post-fermentation condition and dominant microorganism with key aroma-active compound. Food Chem. 2025, 464, 141788. [Google Scholar] [CrossRef]
- Yan, X.; Tian, Y.; Zhao, F.; Wang, R.; Zhou, H.; Zhang, N.; Wang, Y.; Shan, Z.; Zhang, C. Analysis of the key aroma components of Pu’er tea by synergistic fermentation with three beneficial microorganisms. Food Chem. X 2024, 21, 101048. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, Y.; Teng, J.; Xia, N.; Huang, L.; Wei, B.; Chen, Q. Evaluation of Mycoflora and Citrinin Occurrence in Chinese Liupao Tea. J. Agric. Food Chem. 2020, 68, 12116–12123. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, Z.; Wu, B.; Wang, L.; Liu, X. Bacterial and fungal communities in Pu’er tea samples of different ages. J. Food Sci. 2013, 78, M1249–M1256. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, L.; Aaqil, M.; Wang, Q.; Peng, W.; Zhuang, L.; Gong, W.; Zheng, T.; Zhao, M.; Wang, C.; et al. Enhanced Fermentation of Pu-Erh Tea with Aspergillus niger: Quality and Microbial Community Analysis. Molecules 2024, 29, 5647. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, C.; Zhang, X.; Wang, Y.; Li, Z.; Chen, Y.; Liu, Z.; Zhu, M.; Xiao, Y. Effects of solid-state fermentation with Bacillus subtilis LK-1 on the volatile profile, catechins composition and antioxidant activity of dark teas. Food Chem. X 2023, 19, 100811. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Jia, H.; Zhang, J.; Wei, Y.; Deng, W.W.; Ning, J. Effects of Microbial Action and Moist-Heat Action on the Nonvolatile Components of Pu-Erh Tea, as Revealed by Metabolomics. J. Agric. Food Chem. 2022, 70, 15602–15613. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Adin, S.N.; Gupta, I.; Panda, B.P.; Mujeeb, M. Monascin and ankaflavin-Biosynthesis from Monascus purpureus, production methods, pharmacological properties: A review. Biotechnol. Appl. Biochem. 2023, 70, 137–147. [Google Scholar] [CrossRef]
- Chen, D.; Li, H. Mannitol improves Monascus pigment biosynthesis with rice bran as a substrate in Monascus purpureus. Front. Microbiol. 2023, 14, 1300461. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; He, Z.; Lin, X.; Lin, X.; Liang, Z.; Liu, D.; Huang, Y.; Fang, Z. Screening and evaluation of Monascus purpureus FJMR24 for enhancing the raw material utilization rate in rice wine brewing. J. Sci. Food Agric. 2021, 101, 185–193. [Google Scholar] [CrossRef]
- Farawahida, A.H.; Palmer, J.; Flint, S. Monascus spp. and citrinin: Identification, selection of Monascus spp. isolates, occurrence, detection and reduction of citrinin during the fermentation of red fermented rice. Int. J. Food Microbiol. 2022, 379, 109829. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Huang, G.; Ren, L.; Li, Y.; Yu, J.; Lu, Q.; Yang, Y.; Deng, X.; Li, Y.; Zhou, H. Effects of Monascus purpureus on ripe Pu-erh tea in different fermentation methods and identification of characteristic volatile compounds. Food Chem. 2024, 440, 138249. [Google Scholar] [CrossRef]
- Deng, X.; Hou, Y.; Zhou, H.; Li, Y.; Xue, Z.; Xue, X.; Huang, G.; Huang, K.; He, X.; Xu, W. Hypolipidemic, anti-inflammatory, and anti-atherosclerotic effects of tea before and after microbial fermentation. Food Sci. Nutr. 2021, 9, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- National Health and Family Planning Commission of the People’s Republic of China. National Food Safety Standard—Determination of Moisture in Foods: GB 5009.3-2016; Standards Press of China: Beijing, China, 2016.
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China. Determination of Water Extracts in Tea: GB/T 8305-2013; Standards Press of China: Beijing, China, 2013. [Google Scholar]
- State Administration for Market Regulation, Standardization Administration of China. Determination of Tea Polyphenols and Catechins Content in Tea: GB/T 8313-2018; Standards Press of China: Beijing, China, 2018.
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Determination of Total Free Amino Acids in Tea: GB/T 8314-2013; Standards Press of China: Beijing, China, 2013.
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China. Methodology for Sensory Evaluation of Tea: GB/T 23776-2018; Standards Press of China: Beijing, China, 2018.
- Shen, S.; Fu, J.; Fan, R.; Zhang, J.; Sun, H.; Wang, Y.; Ning, J.; Yue, P.; Zhang, L.; Gao, X. Changes in the key odorants of loose-leaf dark tea fermented by Eurotium cristatum during aging for one year: Focus on the stale aroma. Food Res. Int. 2024, 197, 115244. [Google Scholar] [CrossRef]
- Wu, M.D.; Chen, J.J.; Cheng, M.J. Secondary Metabolites with Antifungal Activities from Mangrove Derived Fungus Monascus purpureus WMD2424. Mar. Drugs 2023, 21, 200. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.S.; Lee, S.I. Quality Characteristics and Content of Polysaccharides in Green Tea Fermented by Monascus pilosus. Prev. Nutr. Food Sci. 2012, 17, 293–298. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, T.H.; Pan, T.M. Alleviation of metabolic syndrome by monascin and ankaflavin: The perspective of Monascus functional foods. Food Funct. 2017, 8, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qiu, Y.; Gan, R.Y.; Zhu, F. Chemical constituents and biological properties of Pu-erh tea. Food Res. Int. 2022, 154, 110899. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, A.; Du, H.; Liu, Y.; Qi, B.; Yang, X. Theabrownin from Fu Brick Tea Exhibits the Thermogenic Function of Adipocytes in High-Fat-Diet-Induced Obesity. J. Agric. Food Chem. 2021, 69, 11900–11911. [Google Scholar] [CrossRef]
- Gahr, M. Caffeine, the most frequently consumed psychostimulant: A narrative review article. Fortschr. Neurol. Psychiatr. 2020, 88, 318–330. [Google Scholar]
- He, S.; Wang, Y.; Xie, J.; Gao, H.; Li, X.; Huang, Z. 1H NMR-based metabolomic study of the effects of flavonoids on citrinin production by Monascus. Food Res. Int. 2020, 137, 109532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, N.; Chen, M.; Wang, H.; Shi, J.; Wang, B.; Sun, B.; Wang, C. Metabolomics Analysis of the Effect of Glutamic Acid on Monacolin K Synthesis in Monascus purpureus. Front. Microbiol. 2020, 11, 610471. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Zhang, J.; Xu, H.; Bai, J.; Zhang, H.; Jiang, X.; Yuan, J.; Lu, G.; Jiang, L.; et al. Integrative Metabolomic and Transcriptomic Analyses Uncover Metabolic Alterations and Pigment Diversity in Monascus in Response to Different Nitrogen Sources. Msystems 2021, 6, e80721. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, C.; Flon, V.; Mann, S.; Leleu, S.; Prado, S.; Franck, X. Biosynthesis of azaphilones: A review. Nat. Prod. Rep. 2021, 38, 1058–1071. [Google Scholar] [CrossRef]
- Huang, Z.R.; Zhou, W.B.; Yang, X.L.; Tong, A.J.; Hong, J.L.; Guo, W.L.; Li, T.T.; Jia, R.B.; Pan, Y.Y.; Lin, J.; et al. The regulation mechanisms of soluble starch and glycerol for production of azaphilone pigments in Monascus purpureus FAFU618 as revealed by comparative proteomic and transcriptional analyses. Food Res. Int. 2018, 106, 626–635. [Google Scholar] [CrossRef]
- Qiao, J.; He, X.; Wang, C.; Yang, H.; Xin, Z.; Xin, B.; Wang, J.; Dong, R.; Zeng, H.; Li, F. Transcriptome analysis revealing molecular mechanisms of enhanced pigment yield by succinic acid and fluconazole. Prep. Biochem. Biotechnol. 2022, 52, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Everett, J.M.; D’Cunha, N.M.; Sergi, D.; Georgousopoulou, E.N.; Keegan, R.J.; McKune, A.J.; Mellor, D.D.; Anstice, N.; Naumovski, N. The Effects of Green Tea Amino Acid L-Theanine Consumption on the Ability to Manage Stress and Anxiety Levels: A Systematic Review. Plant Foods Hum. Nutr. 2020, 75, 12–23. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Han, Z.X.; Rana, M.M.; Liu, G.F.; Gao, M.J.; Li, D.X.; Wu, F.G.; Li, X.B.; Wan, X.C.; Wei, S. Green tea flavour determinants and their changes over manufacturing processes. Food Chem. 2016, 212, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.; Fogacci, F.; Stoian, A.P.; Toth, P.P. Red Yeast Rice for the Improvement of Lipid Profiles in Mild-to-Moderate Hypercholesterolemia: A Narrative Review. Nutrients 2023, 15, 2288. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Zhu, C.; Sun, Y.; Yan, Q.; Yi, G. Monascus purpureus M-32 fermented soybean meal improves the growth, immunity parameters, intestinal morphology, disease resistance, intestinal microbiota and metabolome in Pacific white shrimp (Litopenaeus vannamei). Anim. Nutr. 2024, 17, 283–296. [Google Scholar] [CrossRef]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Y.; Zhang, C.; Sullivan, M.A.; Chen, W.; Jing, X.; Yu, H.; Li, F.; Wang, Q.; Zhou, Z.; et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J. Nutr. Biochem. 2023, 120, 109414. [Google Scholar] [CrossRef]
- Pohanka, M.; Pejchal, J.; Snopkova, S.; Havlickova, K.; Karasova, J.Z.; Bostik, P.; Pikula, J. Ascorbic acid: An old player with a broad impact on body physiology including oxidative stress suppression and immunomodulation: A review. Mini Rev. Med. Chem. 2012, 12, 35–43. [Google Scholar] [CrossRef]
- Qiong, S.; Xishuang, Y. History of Pu’er Tea and comparative study for the effect of its various extracts on lipid-lowering diet. Pak. J. Pharm. Sci. 2014, 27, 1015–1022. [Google Scholar] [PubMed]


| Appearance (20%) | Soup Color (15%) | Aroma (25%) | Taste (30%) | Tea Residue (10%) | Gross Score | |
|---|---|---|---|---|---|---|
| UT | Loose, many stems | Bright orange | Fragrance of a flower | Pure and refreshing | Bluish yellow, diastolic | 88.97 |
| Score | 75 | 93 | 96 | 89 | 93 | |
| FT | Bluish brown with white mildew spots | Orange with a little brightness | Toasty fragrance, floral with bacteria | Peaceful and slightly bitter | Black with yellow green, soft | 87.79 |
| Score | 80 | 91 | 86 | 91 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hu, Y.; Zeng, Z.; Zhang, X.; Huang, Y. Flavor Quality and Lipid-Lowering Function of Mixed Fermented Pu-erh Tea with Various Monascus Species. Foods 2025, 14, 1894. https://doi.org/10.3390/foods14111894
Chen X, Hu Y, Zeng Z, Zhang X, Huang Y. Flavor Quality and Lipid-Lowering Function of Mixed Fermented Pu-erh Tea with Various Monascus Species. Foods. 2025; 14(11):1894. https://doi.org/10.3390/foods14111894
Chicago/Turabian StyleChen, Xiaomin, Yao Hu, Zhen Zeng, Xu Zhang, and Yahui Huang. 2025. "Flavor Quality and Lipid-Lowering Function of Mixed Fermented Pu-erh Tea with Various Monascus Species" Foods 14, no. 11: 1894. https://doi.org/10.3390/foods14111894
APA StyleChen, X., Hu, Y., Zeng, Z., Zhang, X., & Huang, Y. (2025). Flavor Quality and Lipid-Lowering Function of Mixed Fermented Pu-erh Tea with Various Monascus Species. Foods, 14(11), 1894. https://doi.org/10.3390/foods14111894







