Research Progress on Probiotics in Alleviating Cow’s Milk Allergy: A Review
Abstract
1. Introduction
2. Major Allergens in Cow’s Milk
2.1. Caseins
2.2. Whey Proteins
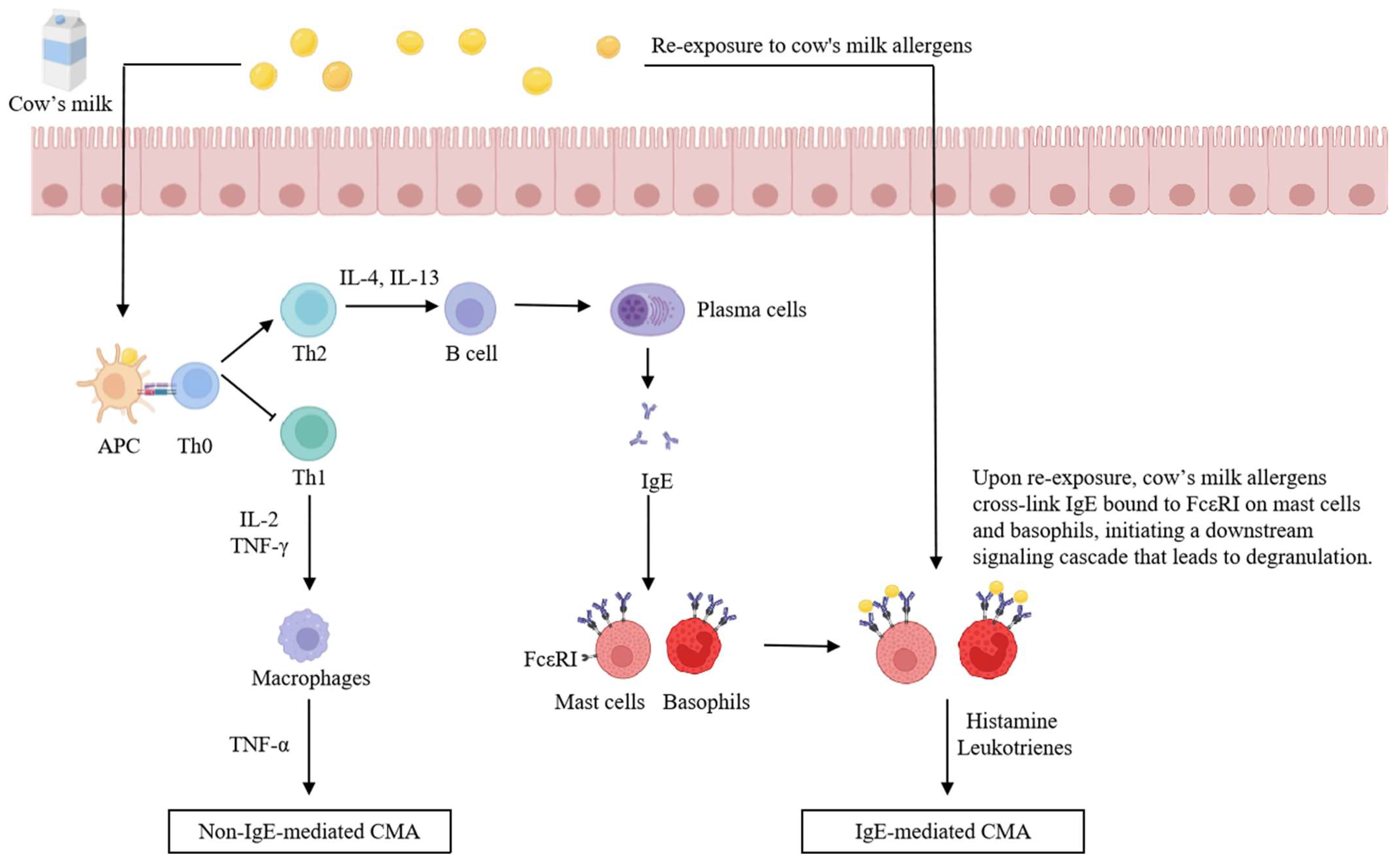
3. Mechanisms of Cow’s Milk Allergy
3.1. IgE-Mediated CMA
3.2. Non-IgE-Mediated CMA
4. Gut Microbiota Alterations in CMA Patients
5. Mechanisms of Probiotic Alleviation of CMA
5.1. Application of Probiotics in CMA
5.2. Mechanism of Action of Probiotics in Modulating Cow’s Milk Allergy
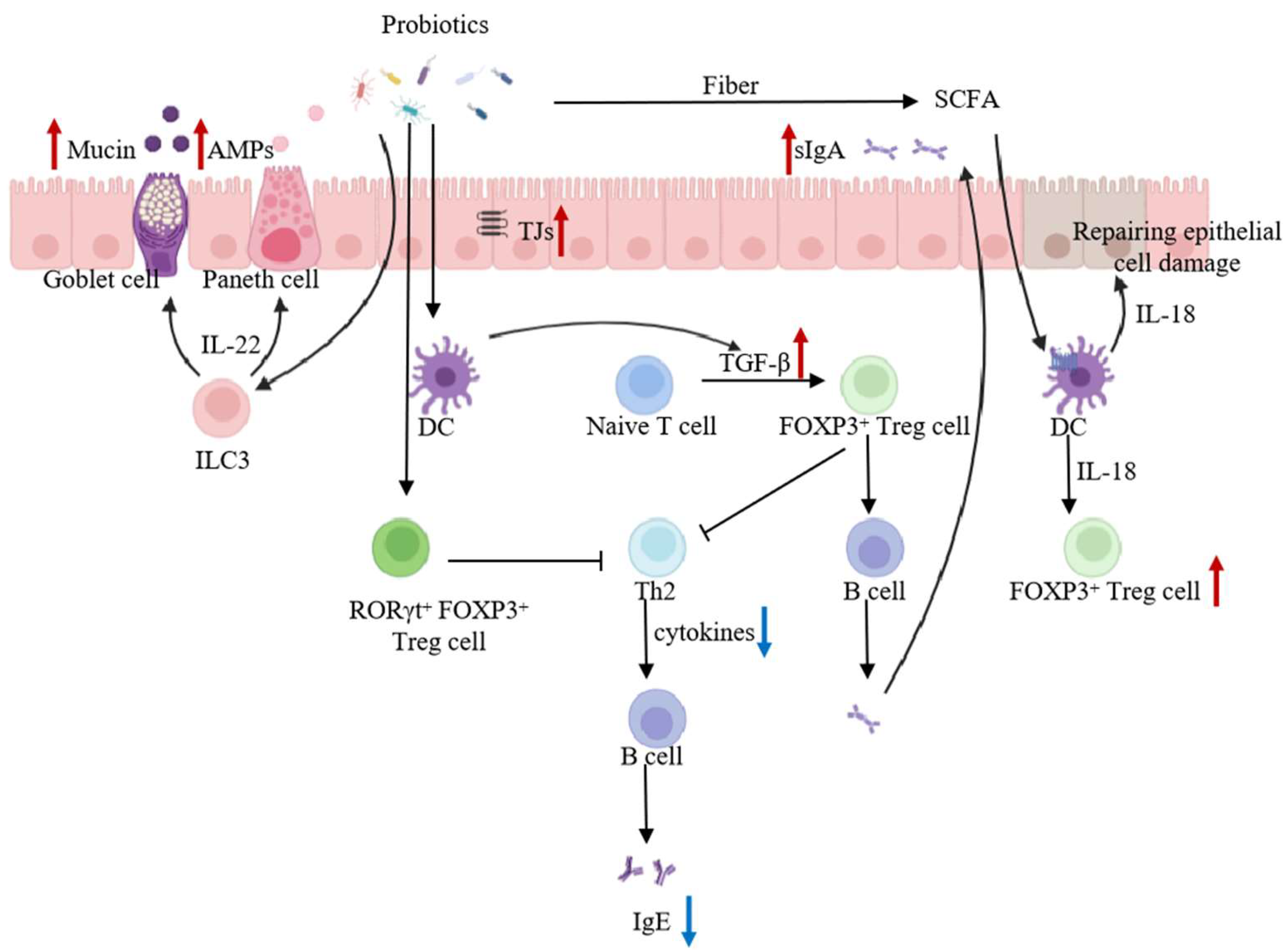
5.2.1. Regulation of Intestinal Microbiota
5.2.2. Enhancement of the Intestinal Barrier
5.2.3. Promotion of Intestinal Mucosal Immunity
5.2.4. Decomposition of Cow’s Milk Allergenic Proteins
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Poowutikul, P.; Pansare, M.; Kamat, D. Food allergy: A review. Pediatr. Ann. 2020, 49, e50–e58. [Google Scholar] [CrossRef]
- Bartha, I.; Almulhem, N.; Santos, A.F. Feast for thought: A comprehensive review of food allergy 2021–2023. J. Allergy Clin. Immunol. 2024, 153, 576–594. [Google Scholar] [CrossRef]
- Verhasselt, V. A newborn’s perspective on immune responses to food. Immunol. Rev. 2024, 326, 117–129. [Google Scholar] [CrossRef]
- Bognanni, A.; Chu, D.K.; Firmino, R.T.; Arasi, S.; Waffenschmidt, S.; Agarwal, A.; Dziechciarz, P.; Horvath, A.; Jebai, R.; Mihara, H.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s MilkAllergy (DRACMA) guideline update—XIII—Oral immunotherapy for CMA—Systematic review. World Allergy Organ. J. 2022, 15, 100682. [Google Scholar] [CrossRef]
- Cianferoni, A.; Muraro, A. Food-induced anaphylaxis. Immunol. Allergy Clin. N. Am. 2012, 32, 165–195. [Google Scholar] [CrossRef]
- Jaiswal, L.; Worku, M. Recent perspective on cow’s milk allergy and dairy nutrition. Crit. Rev. Food Sci. Nutr. 2022, 62, 7503–7517. [Google Scholar] [CrossRef]
- Xie, A.; Shen, X.; Hong, R.; Xie, Y.; Zhang, Y.; Chen, J.; Li, Z.; Li, M.; Yue, X.; Quek, S.Y. Unlocking the potential of donkey Milk: Nutritional composition, bioactive properties and future prospects. Food Res. Int. 2025, 209, 116307. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Brough, H.A.; Fiocchi, A.; Miqdady, M.; Munasir, Z.; Salvatore, S.; Thapar, N.; Venter, C.; Vieira, M.C.; Meyer, R. Current guidelines and future strategies for the management of cow’s milk allergy. J. Asthma Allergy. 2021, 14, 1243–1256. [Google Scholar] [CrossRef]
- Shen, X.; Xie, A.; Li, Z.; Jiang, C.; Wu, J.; Li, M.; Yue, X. Research Progress for probiotics regulating intestinal Flora to improve functional dyspepsia: A review. Foods 2024, 13, 151. [Google Scholar] [CrossRef]
- Xie, A.; Zhao, S.; Liu, Z.; Yue, X.; Shao, J.; Li, M.; Li, Z. Polysaccharides, proteins, and their complex as microencapsulation carriers for delivery of probiotics: A review on carrier types and encapsulation techniques. Int. J. Biol. Macromol. 2023, 242, 124784. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Naumovski, N.; Ajlouni, S.; Ayyash, M.; Silva, R.; Balthazar, C.F.; Esmerino, E.A.; Freitas, M.Q.; da Silva, M.C.; Sant’Ana, A.S. Nonbovine milk and its products as sources of probiotics delivery: An overview of its viability, functionality and product quality characteristics. Int. J. Dairy Technol. 2023, 76, 482–511. [Google Scholar] [CrossRef]
- Li, Z.; Kanwal, R.; Yue, X.; Li, M.; Xie, A. Polyphenols and intestinal microorganisms: A review of their interactions and effects on human health. Food Biosci. 2024, 62, 105220. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Shao, J.; Yue, X.; Li, M. Maillard reaction-based conjugates as carrier strategies for delivery of bioactive compounds: A review. Curr. Opin. Food Sci. 2025, 61, 101260. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, S.; Yao, K.; Liu, D.; Peng, X.; Huang, J.; Huang, Y.; Li, L. Physicochemical, microbiological, rheological, and sensory properties of yoghurts with new polysaccharide extracts from Lactarius volemus Fr. using three probiotics. Int. J. Dairy Technol. 2020, 73, 168–181. [Google Scholar] [CrossRef]
- Xie, A.; Dong, Y.; Liu, Z.; Li, Z.; Shao, J.; Li, M.; Yue, X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. [Google Scholar] [CrossRef]
- Hong, R.; Xie, A.; Jiang, C.; Guo, Y.; Zhang, Y.; Chen, J.; Shen, X.; Li, M.; Yue, X. A review of the biological activities of lactoferrin: Mechanisms and potential applications. Food Funct. 2024, 15, 8182–8199. [Google Scholar] [CrossRef]
- Hong, R.; Yang, H.; Guo, Y.; Liu, Q.; Xu, N.; Xie, Y.; Li, M.; Yue, X. The application and mechanism of polysaccharides, proteins and their complexes on enhancing yogurt gel stability: A review. Food Sci. Anim. Prod. 2024, 2, 9240066. [Google Scholar] [CrossRef]
- Ritchie, M.L.; Romanuk, T.N. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE 2012, 7, e34938. [Google Scholar] [CrossRef]
- Sharma, H.; Ramanathan, R. GC–MS-based metabolomics approach reveals metabolic variations between probiotics incorporated cow and goat milk yoghurt. Int. J. Dairy Technol. 2023, 76, 521–532. [Google Scholar] [CrossRef]
- Ajlouni, S.; Ranadheera, C.S.; Chua, E.L. Encapsulation increases the in vitro bioaccessibility of probiotics in yoghurt. Int. J. Dairy Technol. 2021, 74, 118–127. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Liu, H.; Guo, W.; Jiang, Z.; Han, Q.; Liu, Y. Proliferation of probiotics and antioxidant effects of functional oligosaccharides added in fermented dairy product. Int. J. Dairy Technol. 2024, 77, 893–904. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, C.; Wang, X.; Wang, Y.; Huang, D.; Shi, S.; Shen, Z.; Li, S.; He, Y.; Wang, Z. Probiotics ameliorates pulmonary inflammation via modulating gut microbiota and rectifying Th17/Treg imbalance in a rat model of PM2. 5 induced lung injury. Ecotoxicol. Environ. Saf. 2022, 244, 114060. [Google Scholar] [CrossRef]
- Wang, X.; Sun, B.; Wang, Y.; Gao, P.; Song, J.; Chang, W.; Xiao, Z.; Xi, Y.; Li, Z.; An, F. Research progress of targeted therapy regulating Th17/Treg balance in bone immune diseases. Front. Immunol. 2024, 15, 1333993. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wu, R.; Han, N.; Fu, J.; Luo, Z.; Guo, L.; Su, Y.; Du, J.; Liu, Y. Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin. Transl. Immunol. 2020, 9, e1213. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, Y.; Choi, J.-W.; Park, S.-H.; Cho, M.-L.; Kwok, S.-K. Lactobacillus acidophilus supplementation exerts a synergistic effect on tacrolimus efficacy by modulating Th17/Treg balance in lupus-prone mice via the SIGNR3 pathway. Front. Immunol. 2021, 12, 696074. [Google Scholar] [CrossRef]
- Xia, X.; Tobin, J.T.; Fenelon, M.A.; Mcsweeney, P.L.; Sheehan, J.J. Production, composition and preservation of micellar casein concentrate and its application in cheesemaking: A review. Int. J. Dairy Technol. 2022, 75, 46–58. [Google Scholar] [CrossRef]
- Starkl, P.; Watzenboeck, M.L.; Popov, L.M.; Zahalka, S.; Hladik, A.; Lakovits, K.; Radhouani, M.; Haschemi, A.; Marichal, T.; Reber, L.L.; et al. IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcusaureus. Immunity 2020, 53, 793–804.e799. [Google Scholar] [CrossRef]
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Jiang, C.; Xie, A.; Yue, X.; Li, M. A review of casein phosphopeptides: From enrichment identification to biological properties. Food Biosci. 2024, 59, 104217. [Google Scholar] [CrossRef]
- Wal, J.M. Cow’s milk proteins/allergens. Ann. Allergy Asthma Immunol. 2002, 89, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.; Créminon, C.; Yvon, M.; Wal, J.M. Specificity of the human IgE response to the different purified caseins in allergy to cow’s milk proteins. Int. Arch. Allergy. Immunol. 1998, 115, 235–244. [Google Scholar] [CrossRef]
- Spuergin, P.; Mueller, H.; Walter, M.; Schiltz, E.; Forster, J. Allergenic epitopes of bovine alpha S1-casein recognized by human IgE and IgG. Allergy 1996, 51, 306–312. [Google Scholar]
- Chatchatee, P.; Järvinen, K.M.; Bardina, L.; Vila, L.; Beyer, K.; Sampson, H.A. Identification of IgE and IgG binding epitopes on beta- and kappa-casein in cow’s milk allergic patients. Clin. Exp. Allergy 2001, 31, 1256–1262. [Google Scholar] [CrossRef]
- Järvinen, K.M.; Chatchatee, P.; Bardina, L.; Beyer, K.; Sampson, H.A. IgE and IgG binding epitopes on alpha-lactalbumin and beta-lactoglobulin in cow’s milk allergy. Int. Arch. Allergy. Immunol. 2001, 126, 111–118. [Google Scholar] [CrossRef]
- Cerecedo, I.; Zamora, J.; Shreffler, W.G.; Lin, J.; Bardina, L.; Dieguez, M.C.; Wang, J.; Muriel, A.; de la Hoz, B.; Sampson, H.A. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J. Allergy Clin. Immunol. 2008, 122, 589–594. [Google Scholar] [CrossRef]
- Cong, Y.; Yi, H.; Qing, Y.; Li, L. Identification of the critical amino acid residues of immunoglobulin E and immunoglobulin G epitopes on αs1-casein by alanine scanning analysis. J. Dairy Sci. 2013, 96, 6870–6876. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, R.; Zhang, J.; Zhou, P. Heat-induced denaturation and bioactivity changes of whey proteins. Int. Dairy J. 2021, 123, 105175. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Abdlla, R.; Chen, J.; Yue, X.; Quek, S.Y. Donkey whey proteins ameliorate dextran sulfate sodium-induced ulcerative colitis in mice by downregulating the S100A8-TRAF6-NF-κB axis-mediated inflammatory response. Food Sci. Hum. Wellness 2023, 12, 1809–1819. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Yu, H.; Zhang, X.; Li, D.; Song, W.; Zheng, Y.; Yue, X. Differentially expressed whey proteins of donkey and bovine colostrum revealed with a label-free proteomics approach. Food Sci. Hum. Wellness 2023, 12, 1224–1231. [Google Scholar] [CrossRef]
- Lam, H.Y.; Van Hoffen, E.; Michelsen, A.; Guikers, K.; Van Der Tas, C.; Bruijnzeel-Koomen, C.; Knulst, A. Cow’s milk allergy in adults is rare but severe: Both casein and whey proteins are involved. Clin. Exp. Allergy 2008, 38, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Costa, J.; Oliveira, M.; Mafra, I. Bovine Milk Allergens: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, Y.; Luo, Y.; Jiang, M. Detection and analysis of binding activities of IgE antibodies from child patients allergic to cow’s milk protein. J. China Agric. Univ. 2017, 22, 40–44. [Google Scholar]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Focke-Tejkl, M.; Civaj, V.; Balic, N.; Nystrand, M.; Härlin, A.; Thalhamer, J.; Scheiblhofer, S.; et al. Visualization of clustered IgE epitopes on alpha-lactalbumin. J. Allergy Clin. Immunol. 2010, 125, 1279–1285. [Google Scholar] [CrossRef]
- Li, X.; Yuan, S.; Huang, M.; Gao, J.; Wu, Z.; Tong, P.; Yang, A.; Chen, H. Identification of IgE and IgG epitopes on native Bos d 4 allergen specific to allergic children. Food Funct. 2016, 7, 2996–3005. [Google Scholar] [CrossRef]
- Cong, Y.; Li, L. Identification of the critical amino acid residues of immunoglobulin E and immunoglobulin G epitopes in β-lactoglobulin by alanine scanning analysis. J. Dairy Sci. 2012, 95, 6307–6312. [Google Scholar] [CrossRef]
- Schoemaker, A.; Sprikkelman, A.; Grimshaw, K.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M. Incidence and natural history of challenge-proven cow’s milk allergy in European children–EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, L.; Zhu, M.; Chen, Y. Clinical significance of determination of serum IgE in infants with milk allergy. Chin. J. Contemp. Pediatr. 2015, 17, 618–622. [Google Scholar]
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473. [Google Scholar] [CrossRef]
- Trudeau, J.; Hu, H.; Chibana, K.; Chu, H.W.; Westcott, J.Y.; Wenzel, S.E. Selective downregulation of prostaglandin E2–related pathways by the Th2 cytokine IL-13. J. Allergy Clin. Immunol. 2006, 117, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Anvari, S.; Miller, J.; Yeh, C.-Y.; Davis, C.M. IgE-mediated food allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef]
- Shek, L.; Bardina, L.; Castro, R.; Sampson, H.; Beyer, K. Humoral and cellular responses to cow milk proteins in patients with milk-induced IgE-mediated and non-IgE-mediated disorders. Allergy 2005, 60, 912–919. [Google Scholar] [CrossRef]
- Venter, C.; Brown, T.; Shah, N.; Walsh, J.; Fox, A.T. Diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy-a UK primary care practical guide. Clin. Transl. Allergy 2013, 3, 23. [Google Scholar] [CrossRef]
- Walsh, J.; Meyer, R.; Shah, N.; Quekett, J.; Fox, A.T. Differentiating milk allergy (IgE and non-IgE mediated) from lactose intolerance: Understanding the underlying mechanisms and presentations. Br. J. Gen. Pract. 2016, 66, e609–e611. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Winter, S.E.; Bäumler, A.J. Gut dysbiosis: Ecological causes and causative effects on human disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2316579120. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.; Sampson, H.; Sicherer, S. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Paparo, L.; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow’s milk allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar] [CrossRef]
- Dong, P.; Feng, J.-j.; Yan, D.-y.; Lyu, Y.-j.; Xu, X. Early-life gut microbiome and cow’s milk allergy-a prospective case-control 6-month follow-up study. Saudi J. Biol. Sci. 2018, 25, 875–880. [Google Scholar] [CrossRef]
- Mauras, A.; Wopereis, H.; Yeop, I.; Esber, N.; Delannoy, J.; Labellie, C.; Reygner, J.; Kapel, N.; Slump, R.; van Eijndthoven, T. Gut microbiota from infant with cow’s milk allergy promotes clinical and immune features of atopy in a murine model. Allergy 2019, 74, 1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, S.; Yang, X.; Huazeng, B.; Cheng, Q. Influences of non-IgE-mediated cow’s milk protein allergy-associated gut microbial dysbiosis on regulatory T cell-mediated intestinal immune tolerance and homeostasis. Microb. Pathog. 2021, 158, 105020. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Basturk, A.; Isik, İ.; Atalay, A.; Yılmaz, A. Investigation of the Efficacy of Lactobacillus rhamnosus GG in Infants With Cow’s Milk Protein Allergy: A Randomised Double-Blind Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 12, 138–143. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Vitale, A.; Perna, F.; Garziano, F.; Dolce, P.; Vitale, S.; Micillo, T.; Oglio, F.; del Giudice, M.M.; Matarese, G.; et al. Bifidobacteria modulate immune response in pediatric patients with cow’s milk protein allergy. Pediatr. Res. 2023, 94, 1111–1118. [Google Scholar] [CrossRef]
- Jing, W.; Liu, Q.; Wang, W. Bifidobacterium bifidumTMC3115 ameliorates milk protein allergy in by affecting gut microbiota: A randomized double-blind control trial. J. Food Biochem. 2020, 44, e13489. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Font, G.; Saavedra, L.; Mozzi, F. Hydrolysate of β-lactoglobulin by Lactobacillus delbrueckii subsp bulgaricus CRL 656 suppresses the immunoreactivity of β-lactoglobulin as revealed by in vivo assays. Int. Dairy J. 2019, 88, 71–78. [Google Scholar] [CrossRef]
- Wróblewska, B.; Kaliszewska-Suchodola, A.; Fuc, E.; Markiewicz, L.H.; Ogrodowczyk, A.M.; Zlotkowska, D.; Wasilewska, E. Effect of Low-Immunogenic Yogurt Drinks and Probiotic Bacteria on Immunoreactivity of Cow’s Milk Proteins and Tolerance Induction-In Vitro and In Vivo Studies. Nutrients 2020, 12, 3390. [Google Scholar] [CrossRef]
- Neau, E.; Delannoy, J.; Marion, C.; Cottart, C.-H.; Labellie, C.; Holowacz, S.; Butel, M.-J.; Kapel, N.; Waligora-Dupriet, A.-J. Three Novel Candidate Probiotic Strains with Prophylactic Properties in a Murine Model of Cow’s Milk Allergy. Appl. Environ. Microbiol. 2016, 82, 1722–1733. [Google Scholar] [CrossRef]
- Cortes-Perez, N.G.; Lozano-Ojalvo, D.; Maiga, M.A.; Hazebrouck, S.; Adel-Patient, K. Intragastric administration of Lactobacillus casei BL23 induces regulatory FoxP3+RORγt+ T cells subset in mice. Benefic. Microbes 2017, 8, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, B.; Kaliszewska-Suchodoła, A.; Markiewicz, L.H.; Szyc, A.; Wasilewska, E. Whey prefermented with beneficial microbes modulates immune response and lowers responsiveness to milk allergens in mouse model. J. Funct. Foods 2019, 54, 41–52. [Google Scholar] [CrossRef]
- Guo, Z.H.; Wang, Q.; Zhao, J.H.; Xu, Y.P.; Mu, G.Q.; Zhu, X.M. Lactic acid bacteria with probiotic characteristics in fermented dairy products reduce cow milk allergy. Food Biosci. 2023, 55, 103055. [Google Scholar] [CrossRef]
- Zhang, J.; Su, H.; Li, Q.; Wu, H.; Liu, M.; Huang, J.; Zeng, M.; Zheng, Y.; Sun, X. Oral administration of Clostridium butyricum CGMCC0313-1 inhibits β-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut Pathog. 2017, 9, 11. [Google Scholar] [CrossRef]
- Feng, L.; Chen, G.L.; Guo, Z.H.; Yao, W.P.; Li, X.L.; Mu, G.Q.; Zhu, X.M. Both live and heat killed Lactiplantibacillus plantarum DPUL-F232 alleviate whey protein-induced food allergy by regulating cellular immunity and repairing the intestinal barrier. Food Funct. 2024, 15, 5496–5509. [Google Scholar] [CrossRef]
- Feng, L.; Guo, Z.H.; Yao, W.P.; Mu, G.Q.; Zhu, X.M. Metagenomics and Untargeted Metabolomics Analysis Revealed the Probiotic and Postbiotic Derived from Lactiplantibacillus plantarum DPUL F232 Alleviate Whey Protein-Induced Food Allergy by Reshaping Gut Microbiota and Regulating Key Metabolites. J. Agric. Food Chem. 2024, 72, 25436–25448. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, F.; Ma, R.; Zhang, Z.; Chi, L.; Li, Y.; Xu, C.; Mu, G.; Zhu, X. Effects of Lacticaseibacillus paracasei XJ-003 on the allergenicity and antigenicity of milk proteins during fermentation. Food Biosci. 2024, 59, 103967. [Google Scholar] [CrossRef]
- Ogrodowczyk, A.M.; Kalicki, B.; Wróblewska, B. The effect of lactic acid fermentation with different bacterial strains on the chemical composition, immunoreactive properties, and sensory quality of sweet buttermilk. Food Chem. 2021, 353, 129512. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.P.; Li, X.L.; Chi, L.; Li, Y.; Xu, C.; Mu, G.Q.; Zhu, X.M. Modulating Whey Proteins Antigenicity with Lactobacillus delbrueckii subsp. bulgaricus DLPU F-36 Metabolites: Insights from Spectroscopic and Molecular Docking Studies. J. Agric. Food Chem. 2024, 72, 15198–15212. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zhang, Y.; Chen, F. Effects of fermentation by lactic acid bacteria on the antigenicity of bovine whey proteins. J. Sci. Food Agric. 2010, 90, 2015–2020. [Google Scholar] [CrossRef]
- Bertrandharb, C.; Ivanova, I.V.; Dalgalarrondo, M.; Haertllé, T. Evolution of β-lactoglobulin and α-lactalbumin content during yoghurt fermentation. Int. Dairy J. 2003, 13, 39–45. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, U.K.; Sharma, A.; Kapila, R.; Kansal, V.K. Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum modulates immunoglobulin levels and cytokines expression in whey proteins sensitised mice. J. Sci. Food Agric. 2016, 96, 3180–3187. [Google Scholar] [CrossRef]
- Walter, J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical Research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef]
- Faghfoori, Z.; Pourghassem Gargari, B.; Saber Gharamaleki, A.; Bagherpour, H.; Yari Khosroushahi, A. Cellular and molecular mechanisms of probiotics effects on colorectal cancer. J. Funct. Foods 2015, 18, 463–472. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Wen, R.; Zhu, X.; Liu, L.; Li, C. 2′-Fucosyllactose promotes Bifidobacterium bifidum DNG6 adhesion to Caco-2 cells. J. Dairy Sci. 2020, 103, 9825–9834. [Google Scholar] [CrossRef]
- Abdulqadir, R.; Engers, J.; Al-Sadi, R. Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives. Curr. Dev. Nutr. 2023, 7, 102026. [Google Scholar] [CrossRef]
- Mennini, M.; Reddel, S.; Del Chierico, F.; Gardini, S.; Quagliariello, A.; Vernocchi, P.; Valluzzi, R.L.; Fierro, V.; Riccardi, C.; Napolitano, T.; et al. Gut Microbiota Profile in Children with IgE-Mediated Cow’s Milk Allergy and Cow’s Milk Sensitization and Probiotic Intestinal Persistence Evaluation. Int. J. Mol. Sci. 2021, 22, 1649. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Storey, K.B.; Lushchak, V.I. Intestinal barrier permeability: The influence of gut microbiota, nutrition, and exercise. Front. Physiol. 2024, 15, 1380713. [Google Scholar] [CrossRef]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Gustafsson, J.K.; Knoop, K.A.; McDonald, K.G.; Bidani, S.S.; Davis, J.E.; Floyd, A.N.; Hogan, S.P.; Hsieh, C.-S.; Newberry, R.D. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol. 2020, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.F.; Yu, W.R.; Sun, S.Y.; Lei, Y.M.; Li, Y.X.; Lu, C.X.; Zhai, J.N.; Bai, F.R.; Ren, F.; et al. Roles of Probiotics, Prebiotics, and Postbiotics in B-Cell-Mediated Immune Regulation. J. Nutr. 2025, 155, 37–51. [Google Scholar] [CrossRef]
- Giovanna, V.; Carla, C.; Alfina, C.; Domenico, P.; Elena, L. The immunopathogenesis of cow’s milk protein allergy (CMPA). Ital. J. Pediatr. 2012, 38, 35. [Google Scholar] [CrossRef]
- Jensen, S.A.; Fiocchi, A.; Baars, T.; Jordakieva, G.; Nowak-Wegrzyn, A.; Pali-Schöll, I.; Passanisi, S.; Pranger, C.L.; Roth-Walter, F.; Takkinen, K.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update—III—Cow’s milk allergens and mechanisms triggering immune activation. World Allergy Organ. J. 2022, 15, 100668. [Google Scholar] [CrossRef]
- Gavrovic-Jankulovic, M.; Willemsen, L.E.M. Epithelial models to study food allergen-induced barrier disruption and immune activation. Drug Discov. Today Dis. Models 2015, 17–18, 29–36. [Google Scholar] [CrossRef]
- Donald, K.; Petersen, C.; Turvey, S.E.; Finlay, B.B.; Azad, M.B. Secretory IgA: Linking microbes, maternal health, and infant health through human milk. Cell Host Microbe 2022, 30, 650–659. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The Immune System Bridges the Gut Microbiota with Systemic Energy Homeostasis: Focus on TLRs, Mucosal Barrier, and SCFAs. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Kluger, M.A.; Nosko, A.; Ramcke, T.; Goerke, B.; Meyer, M.C.; Wegscheid, C.; Luig, M.; Tiegs, G.; Stahl, R.A.K.; Steinmetz, O.M. RORγt expression in Tregs promotes systemic lupus erythematosus via IL-17 secretion, alteration of Treg phenotype and suppression of Th2 responses. Clin. Exp. Immunol. 2017, 188, 63–78. [Google Scholar] [CrossRef][Green Version]
- Sardecka-Milewska, I.; Łoś-Rycharska, E.; Gawryjołek, J.; Toporowska-Kowalska, E.; Krogulska, A. Role of FOXP3 Expression and Serum Vitamin D and C Concentrations When Predicting Acquisition of Tolerance in Infants With Cow’s Milk Allergy. J. Investig. Allergol. Clin. Immunol. 2020, 30, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qu, Y.; Gao, Y.; Sun, S.; Wu, R.; Wu, J. Research Progress on the Correlation between the Intestinal Microbiota and Food Allergy. Foods 2022, 11, 2913. [Google Scholar] [CrossRef]
- Noh, J.; Noh, G.; Kim, H.S.; Kim, A.R.; Choi, W.S. Allergen-specific responses of CD19(+)CD5(+)Foxp3(+) regulatory B cells (Bregs) and CD4(+)Foxp3(+) regulatory T cell (Tregs) in immune tolerance of cow milk allergy of late eczematous reactions. Cell. Immunol. 2012, 274, 109–114. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Zhu, Z.; Zhao, Q.; Zhu, L.; Jiang, L. Efficient reduction of β-lactoglobulin allergenicity in milk using Clostridium tyrobutyricum Z816. Food Sci. Hum. Wellness 2023, 12, 809–816. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Haertlé, T.; Chobert, J.-M.; Mozzi, F.; Font de Valdez, G. Lactobacillus delbrueckii subsp. bulgaricus CRL 454 cleaves allergenic peptides of β-lactoglobulin. Food Chem. 2015, 170, 407–414. [Google Scholar] [CrossRef]
- El Mecherfi, K.-E.; Todorov, S.D.; Cavalcanti de Albuquerque, M.A.; Denery-Papini, S.; Lupi, R.; Haertlé, T.; Dora Gombossy de Melo Franco, B.; Larré, C. Allergenicity of Fermented Foods: Emphasis on Seeds Protein-Based Products. Foods 2020, 9, 792. [Google Scholar] [CrossRef]
- Biscola, V.; Tulini, F.L.; Choiset, Y.; Rabesona, H.; Ivanova, I.; Chobert, J.M.; Todorov, S.D.; Haertlé, T.; Franco, B.D.G.M. Proteolytic activity of Enterococcus faecalis VB63F for reduction of allergenicity of bovine milk proteins. J. Dairy Sci. 2016, 99, 5144–5154. [Google Scholar] [CrossRef]
- Mu, G.; Zhang, Z.; Wang, J.; Jiang, S.; Wang, H.; Xu, Y.; Li, X.; Chi, L.; Li, Y.; Tuo, Y.; et al. Antigenicity and Safety Evaluation of Lactiplantibacillus plantarum 7-2 Screened to Reduce α-Casein Antigen. Foods 2021, 11, 88. [Google Scholar] [CrossRef]
| Species | Allergen | Probiotics | Treatment | Results | Reference |
|---|---|---|---|---|---|
| Infant | Cow’s milk | Lacticaseibacillus rhamnosus GG (LGG) | Infants aged 0–12 months with CMA were administered an extensively hydrolyzed casein formula (EHCF) supplemented with LGG, and were followed up for a period of 36 months. | Reduce the incidence of other allergic manifestations and accelerate the development of oral tolerance. | [63] |
| Infant | Cow’s milk | LGG | Infants aged 0–12 months with CMA were administered an EHCF supplemented with LGG for a period of 36 months. | Improving infant gut microbial composition, diversity, and metabolites to promote tolerance | [64] |
| Infant | Cow’s milk protein | LGG | Infants aged 0–12 months with cow’s milk protein allergy were administered LGG alongside a milk-free diet for a period of 4 weeks. | Improving bloody stools, diarrhoea, restlessness, and bloating. | [65] |
| Infant | Cow’s milk protein | Bifidobacteria | Infants aged 6–12 months with cow’s milk protein allergy were administered Bifidobacteria alongside a milk-free diet for a period of 45 days. | Reduce naive and activated CD4+ T cells as well as degranulated basophilic granulocytes. | [66] |
| Infant | Cow’s milk protein | Bifidobacterium bifidum TMC3115 | Infants aged 0–12 months with cow’s milk protein allergy were administered Bifidobacterium bifidum TMC3115 for a period of 6 months. | Reduce allergy scores, enhance anti-inflammatory responses, decrease serum IgE levels, increase IgG2 levels, and regulate the gut microbiota. | [67] |
| Mice | β-LG | Lactobacillus delbrueckii subsp bulgaricus CRL 656 (H656) | After hydrolysis of β-LG with H656, BALB/c mice were gavaged. | After hydrolyzing β-LG with H656, the allergic response induced by β-LG was suppressed by increasing the secretion of IL-6, IL-10, and IFN-γ, reducing IL-4 levels, improving intestinal mucosal damage, and decreasing leukocyte infiltration. | [68] |
| Mice | α-Caseins and β-LG | yogurt beverage (Lactiplantibacillus plantarum and Bifidobacterium animalis subsp. lactis) | Administer the yogurt beverage to BALB/c mice via gavage for a period of 4 weeks | Enhanced the secretion of IL-10, TGF-β, and IgA, while reducing the levels of IL-4, IgE, and IgG1. | [69] |
| Mice | β-LG | Lactobacillus salivarius LA307, Bifidobacterium longum subsp. infantis LA308, Lacticaseibacillus rhamnosus LA305 | Administer probiotics to BALB/c mice via gavage for a period of 6 weeks. | Lactobacillus salivarius LA307 blocked Th1 and Th2 responses; Bifidobacterium longum subsp. infantis LA308 induced a pro-Th1 response; Lacticaseibacillus rhamnosus LA305 induced both a pro-Th1 and an immunoregulatory response. | [70] |
| Mice | Cow’s milk | Lacticaseibacillus casei BL23 | Administer Lacticaseibacillus casei BL23 to BALB/cJ mice via gavage continuously for 5 days. | Induces both local and systemic Foxp3+ RORγt+ type 3 regulatory T cells (Tr3). | [71] |
| Mice | α-Caseins and β-LG | Fermented whey (Streptococcus salivarius subsp. thermophilus 2 K, Lactobacillus delbrueckii subsp. bulgaricus BK—FW whey, S. thermophilus 2 K, L. bulgaricus BK, Lactiplantibacillus plantarum W42, and Bifidobacterium animalis ssp. lactis Bi30—FW-LB) | Administer the fermented whey to BALB/c mice via gavage for a period of 4 weeks | Altered the Th1/Th2 balance towards a Th1 response, enhanced the secretion of IL-10 and TGF-β, and reduced the levels of allergy markers. | [72] |
| Mice | skimmed milk | Fermented milk beverage (L. plantarum DPUL-F20, L. paracasei DPUL-F29, L. bulgaricus DPUL-F36 and S. thermophilus BD0453) | Administer the Fermented milk beverage to BALB/c mice via gavage for a period of 7 weeks | Regulated the Th1/Th2 and Th17/Treg immune balance, reduced the levels of total IgG, total IgG1, and total IgE antibodies, serum mast cell protease, and plasma histamine levels, and modulated the composition of the gut microbiota. | [73] |
| Mice | β-LG | Clostridium butyricum CGMCC0313-1 | Administer Clostridium butyricum CGMCC0313-1 to BALB/cJ mice via gavage for a period of 3 weeks. | Improved intestinal allergic reaction symptoms. Increased levels of sIgA and CD4+ CD25+ Foxp3+ Treg cells. Reversed the imbalance between Th1/Th2 and Th17/Treg. | [74] |
| Rat | Whey protein | Lactiplantibacillus plantarum DPUL-F232 | Administer Lactiplantibacillus plantarum DPUL-F232 to SD rat via gavage. | Alleviated allergic symptoms, reduced intestinal inflammation, and lowered serum antibody and histamine levels in rats; regulated the Th1/Th2 balance, promoted the secretion of IL-10, and inhibited mast cell degranulation. Upregulated the expression of tight junction proteins to restore the integrity of the intestinal barrier; modulated the gut microbiota and its metabolic products to alleviate allergies. | [75,76] |
| In Vitro | Cow’s milk protein | Lacticaseibacillus paracasei XJ-003 | Fermented | Significantly reduced the antigenicity of milk proteins. | [77] |
| In Vitro | Cow’s milk and butter milk | Lactic acid bacteria and Bifidobacterium | Fermented | Fermentation with L. casei LcY bacteria significantly reduced the immunoreactivity of BLG, α-CN, β-CN, κ-CN, and raw milk by 98%, 96%, 89%, 75%, and 93%, respectively. Similarly, fermentation with L. delbrueckii ssp. bulgaricus 151 resulted in reductions in the immunoreactivity of the same proteins by 98%, 95%, 90%, 71%, and 89%. A significant reduction in IgE reactivity was only observed in the products fermented by both bacterial strains together. | [78] |
| In Vitro | α-LA and β-LG | Lactic acid bacteria | Fermented | Reduced the allergenic potential of α-LA and β-LG | [79] |
| In Vitro | α-LA and β-LG | Lactobacillus helveticus and Streptococcus thermophilus | Fermented | Reduced the allergenic potential of α-LA and β-LG | [80] |
| In Vitro | α-LA and β-LG | Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. | Fermented | Block allergenic epitopes, produce bioactive peptides; modulate the immune system. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Chen, J.; Qu, Y.; Wu, X.; Zhang, S.; Wang, J.; Yue, X.; Liu, Z.; Xie, A.; Li, M. Research Progress on Probiotics in Alleviating Cow’s Milk Allergy: A Review. Foods 2025, 14, 1879. https://doi.org/10.3390/foods14111879
Guo Y, Chen J, Qu Y, Wu X, Zhang S, Wang J, Yue X, Liu Z, Xie A, Li M. Research Progress on Probiotics in Alleviating Cow’s Milk Allergy: A Review. Foods. 2025; 14(11):1879. https://doi.org/10.3390/foods14111879
Chicago/Turabian StyleGuo, Yangze, Jiali Chen, Yezhi Qu, Xilin Wu, Shengyi Zhang, Jiale Wang, Xiqing Yue, Zhenmin Liu, Aijun Xie, and Mohan Li. 2025. "Research Progress on Probiotics in Alleviating Cow’s Milk Allergy: A Review" Foods 14, no. 11: 1879. https://doi.org/10.3390/foods14111879
APA StyleGuo, Y., Chen, J., Qu, Y., Wu, X., Zhang, S., Wang, J., Yue, X., Liu, Z., Xie, A., & Li, M. (2025). Research Progress on Probiotics in Alleviating Cow’s Milk Allergy: A Review. Foods, 14(11), 1879. https://doi.org/10.3390/foods14111879










