Application of Smart Packaging on the Preservation of Different Types of Perishable Fruits
Abstract
1. Introduction
- (i).
- They must be suitable for the intended purpose.
- (ii).
- They must comply with general safety requirements for all food-contact materials and with specific requirements for all active and intelligent materials and articles.
- (iii).
- They must comply with the composition, labeling, and declaration requirements of the regulation.
- (a)
- The migration of the active and/or intelligent substance(s).
- (b)
- The migration of their degradation and/or reaction products.
- (c)
- Their toxicological properties.
- (i).
- Harms to human health.
- (ii).
- Undesirable changes in chemical composition and sensory characteristics.
- (i).
- Active materials may cause changes in sensory quality or composition of foods, provided that the changes comply with the community or national provisions applicable to food.
- (ii).
- The released substances from active packaging must be authorized, and their use must comply with the relevant community provisions applicable to food.
- (iii).
- The organoleptic changes caused by active materials should not mask the spoilage of food and mislead consumers.
- (iv).
- The information about intelligent materials should not refer to the condition of the food, which could mislead consumers.
- (v).
- The labelling must enable the discrimination of non-edible parts.
- (vi).
- The labelling must indicate that the materials are active and/or intelligent.
2. Application of a Smart Packaging System to Fruits
2.1. Tree Fruits
Apple
2.2. Berries
2.2.1. Kiwifruit
2.2.2. Avocado
2.2.3. Banana
2.2.4. Tomato
2.3. Stone Fruits
2.3.1. Mango
2.3.2. Sweet Cherry
2.4. Aggregate Accessory Fruits
Strawberry
3. Principles of the Operation of Smart Packaging, Advantages, and Toxicity
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Erginkaya, Z.; Kalkan, S.; Ünal, E. Use of Antimicrobial Edible Films and Coatings as Packaging Materials for Food Safety. In Food Processing: Strategies for Quality Assessment; Malik, A., Erginkaya, Z., Ahmad, S., Erten, H., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional Bio-Nanocomposite Coatings for Perishable Fruits. Adv. Mater. 2020, 32, 1908291. [Google Scholar] [CrossRef]
- Kuswandi, B.; Murdyaningsih, E.A. Simple on Package Indicator Label for Monitoring of Grape Ripening Process Using Colorimetric pH Sensor. J. Food Meas. Charact. 2017, 11, 2180–2194. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Kerry, J.P.; Hopkins, D.L. A Review of Patents for the Smart Packaging of Meat and Muscle-Based Food Products. Recent Pat. Food Nutr. Agric. 2018, 9, 3–13. [Google Scholar] [CrossRef]
- Misra, S.K.; Pathak, K. Legislation on Active and Intelligent Packaging. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 97–113. [Google Scholar]
- Code of Federal Regulations (C.F.R.). General Provisions Applicable to Indirect Food Additives, 2024. Part 174.5, eCFR: 21 CFR 174.5-General Provisions Applicable to Indirect Food Additives. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-174/section-174.5 (accessed on 14 April 2025).
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Official Journal of the European Union, L 12/1-89. Available online: https://eur-lex.europa.eu/eli/reg/2011/10/oj/eng (accessed on 18 May 2025).
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current Topics in Active and Intelligent Food Packaging for Preservation of Fresh Foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef]
- Pant, A.F.; Thielmann, J. Active Packaging of Fresh and Fresh-Cut Fruit and Vegetables. In Innovative Packaging of Fruits and Vegetables-Strategies for Safety and Quality Maintenance, 1st ed.; Mohammed, W.S., Mohamma, S.H., Ali, A.W., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2018; p. 3. [Google Scholar]
- Yahia, E.M.; Carrillo-Lopez, A. Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Ghoshal, G. Recent Trends in Active, Smart, and Intelligent Packaging for Food Products. In Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 3, pp. 343–374. [Google Scholar] [CrossRef]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef]
- Bian, X.; Sun, X.; Min, T.; Zhou, L.; Du, H.; Zhu, Z.; Bian, Y.; Jiao, X.; Wen, Y. Functionalized Polyvinyl Alcohol Nanofibers with Visible Light-Triggered Antibacterial and Ethylene Scavenging Capabilities for Food Packaging. Food Packag. Shelf Life 2023, 36, 101056. [Google Scholar] [CrossRef]
- Agriopoulou, S. Active Packaging for Food Applications. EC Nutr. 2016, 6, 86–87. [Google Scholar]
- Vermeiren, L.; Devlieghere, F.; van Beest, M.; de Kruijf, N.; Debevere, J. Developments in the Active Packaging of Foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Cichello, S.A. Oxygen Absorbers in Food Preservation: A Review. J. Food Sci. Technol. 2015, 52, 1889–1895. [Google Scholar] [CrossRef]
- Sängerlaub, S.; Witzgall, S.; Müller, K.; Wiegert, T.; Pecyna, M.J. Palladium-Based Oxygen Scavenger for Food Packaging: Choosing Optimal Hydrogen Partial Pressure. Food Packag. Shelf Life 2021, 28, 100666. [Google Scholar] [CrossRef]
- Cruz, R.S.; Camilloto, G.P.; dos Santos Pires, A.C. Oxygen scavengers: An approach on food preservation. Struct. Funct. Food Eng. 2012, 2, 21–42. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2017, 17, 165–199. [Google Scholar] [CrossRef]
- Kuswandi, B.; Kinanti, D.P.; Jayus, J.; Abdullah, A.; Heng, L.Y. Simple and low-cost freshness indicator for strawberries packaging. Acta Manil. 2018, 61, 147–159. [Google Scholar]
- Flath, R.A.; Black, D.R.; Guadagni, D.G.; McFadden, W.H.; Schultz, T. Identification and Organoleptic Evaluation of Compounds in Delicious Apple Essence. J. Agric. Food Chem. 1967, 15, 29–35. [Google Scholar] [CrossRef]
- Flath, R.A.; Black, D.R.; Forrey, R.R.; McDonald, G.M.; Mon, T.R.; Teranishi, R. Volatiles in Gravenstein Apple Essence Identified by GC-Mass Spectrometry. J. Chromatogr. Sci. 1969, 7, 508–512. [Google Scholar] [CrossRef]
- Komthong, P.; Hayakawa, S.; Katoh, T.; Igura, N.; Shimoda, M. Determination of Potent Odorants in Apple by Headspace Gas Dilution Analysis. LWT—Food Sci. Technol. 2006, 39, 472–478. [Google Scholar] [CrossRef]
- Song, J.; Gardner, B.D.; Holland, J.L.; Beaudry, R.M. Rapid Analysis of Volatile Flavor Compounds in Apple Fruit Using SPME and GC/Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 1997, 45, 1801–1807. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, J. Comparative Analyses of Apple Aroma by a Tin-Oxide Gas Sensor Array Device and GC/MS. Food Chem. 2008, 107, 120–128. [Google Scholar] [CrossRef]
- Muste, S. Materii Prime Vegetale în Industria Alimentară (Reeditare Ediția 2008); Editura Academicpres: Cluj-Napoca, Romania, 2018. [Google Scholar]
- Betemps, D.L.; Fachinello, J.C.; Galarca, S.P.; Portela, N.M.; Remorini, D.; Massai, R.; Agati, G. Non-destructive evaluation of ripening and quality traits in apples using a multiparametric fluorescence sensor. J. Sci. Food Agric. 2012, 92, 1855–1864. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; Proietti, E.; D’Amico, A. The Evaluation of Quality of Post-Harvest Oranges and Apples by Means of an Electronic Nose. Sens. Actuators B Chem. 2001, 78, 26–31. [Google Scholar] [CrossRef]
- Saevels, S.; Lammertyn, J.; Berna, A.Z.; Veraverbeke, E.A.; Di Natale, C.; Nicolaï, B.M. An Electronic Nose and a Mass Spectrometry-Based Electronic Nose for Assessing Apple Quality during Shelf Life. Postharvest Biol. Technol. 2004, 31, 9–19. [Google Scholar] [CrossRef]
- Young, H.; Rossiter, K.; Wang, M.; Miller, M. Characterization of Royal Gala Apple Aroma Using Electronic Nose Technology-Potential Maturity Indicator. J. Agric. Food Chem. 1999, 47, 5173–5177. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Hübert, T. A Colour Ripeness Indicator for Apples. Food Bioprocess. Technol. 2011, 5, 3244–3249. [Google Scholar] [CrossRef]
- Cabanillas-Galán, P.; Farmer, L.; Hagan, T.; Nieuwenhuyzen, M.; James, S.L.; Lagunas, M.C. A New Approach for the Detection of Ethylene Using Silica-Supported Palladium Complexes. Inorg. Chem. 2008, 47, 9035–9041. [Google Scholar] [CrossRef]
- Hu, X.G.; Li, X.; Park, S.H.; Kim, Y.; Yang, S.I. Nondestructive Monitoring of Kiwi Ripening Process Using Colorimetric Ethylene Sensor. Bull. Korean Chem. Soc. 2016, 37, 759–762. [Google Scholar] [CrossRef]
- Vo, E.; Murray, D.K.; Scott, T.L.; Attar, A.J. Development of a Novel Colorimetric Indicator Pad for Detecting Aldehydes. Talanta 2007, 73, 87–94. [Google Scholar] [CrossRef]
- Feng, L.; Musto, C.J.; Suslick, K.S. A Simple and Highly Sensitive Colorimetric Detection Method for Gaseous Formaldehyde. J. Am. Chem. Soc. 2010, 132, 4046–4047. [Google Scholar] [CrossRef]
- Ponce, P.; Carbonari, G.L.; Lugão, A.B. Active Packaging Using Ethylene Absorber to Extend Shelf-Life. In Proceedings of the International Nuclear Atlantic Conference—INAC 2009, Rio de Janeiro, Brazil, 27 September–2 October 2009; ISBN 978-85-99141-03-8. [Google Scholar]
- Kim, Y.H.; Yang, Y.J.; Kim, J.S.; Choi, D.S.; Park, S.H.; Jin, S.Y.; Park, J.S. Non-Destructive Monitoring of Apple Ripeness Using an Aldehyde Sensitive Colorimetric Sensor. Food Chem. 2018, 267, 149–156. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Steffens, C.A. Ethylene absorption sachets in postharvest of ‘Royal Gala’ apples. Rev. Bras. Frutic. 2009, 31, 71–77. [Google Scholar] [CrossRef]
- Awalgaonkar, G.; Beaudry, R.; Almenar, E. Ethylene-Removing Packaging: Basis for Development and Latest Advances. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3980–4007. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, J.; Zarrinbal, M. Comparison of the storage effect of straw and some ethylene absorbents in apricot fruit packaging. J. Food Res. 2022, 32, 93–108. [Google Scholar] [CrossRef]
- Foralosso, F.B.; Fronza, N.; dos Santos, J.H.Z.; Capeletti, L.B.; Quadri, M.G.N. The Use of Duo-Functional PVC Film for Conservation of Minimally Processed Apples. Food Bioprocess Technol. 2013, 7, 1483–1495. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Beaudry, R.; Maraschin, M.; Di Piero, R.M.; Almenar, E. Double-Bottom Antimicrobial Packaging for Apple Shelf-Life Extension. Food Chem. 2019, 279, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Morton, J. Kiwifruit: Actinidia deliciosa In Fruits of Warm Climates; Center for New Crops & Plant Products at Purdue University: West Lafayette, IN, USA, 2011. [Google Scholar]
- Stirk, B. Growing Kiwifruit; Pacific Northwest Extension Publishing: Nampa, ID, USA, 2005. [Google Scholar]
- Beutel, J.A. Kiwifruit. In Advances in New Crops; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 2007; pp. 309–316. [Google Scholar]
- Wang, Y.; Wang, D.; Lv, Z.; Zeng, Q.; Fu, X.; Chen, Q.; Luo, Z.; Luo, C.; Wang, D.; Zhang, W. Analysis of the Volatile Profiles of Kiwifruits Experiencing Soft Rot Using E-Nose and HS-SPME/GC–MS. LWT 2023, 173, 114405. [Google Scholar] [CrossRef]
- Fang, D.; Yu, K.; Deng, Z.; Hu, Q.; Zhao, L. Storage quality and flavor evaluation of Volvariella volvacea packaged with nanocomposite-based packaging material during commercial storage condition. Food Packag. Shelf Life 2019, 22, e100412. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent Packaging: Trends and Applications in Food Systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Shao, P.; Liu, L.; Yu, J.; Zheng, L.; Sun, P. Novel Aldehyde Sensitive Bio-Based Colorimetric Film for Kiwi Fruit Freshness Monitoring. LWT 2022, 159, 113177. [Google Scholar] [CrossRef]
- Abe, K.; Watada, A.E. Ethylene Absorbent to Maintain Quality of Lightly Processed Fruits and Vegetables. J. Food Sci. 1991, 56, 1589–1592. [Google Scholar] [CrossRef]
- Shin, D.U.; Park, B.J.; Cho, H.W.; Kim, S.W.; Kim, E.S.; Jung, Y.W.; Kim, D.H.; Lee, S.J. Potassium Permanganate-Based Ethylene Gas Indicator of Kiwifruit Ripeness. Postharvest Biol. Technol. 2023, 200, 112330. [Google Scholar] [CrossRef]
- Oh, T.G.; Jo, J.A.; Lee, S.J. Evaluation of Time–Temperature Integrator for Indicating the Ripeness of Kiwifruit in Plastic Container at Home. J. Food Sci. 2021, 86, 2872–2885. [Google Scholar] [CrossRef]
- Esser, B.; Swager, T.M. Detection of Ethylene Gas by Fluorescence Turn-on of a Conjugated Polymer. Angew. Chem. Int. Ed. 2010, 49, 8872–8875. [Google Scholar] [CrossRef]
- Morton, J. Avocado. In Fruits of Warm Climates; Creative Resource Systems, Inc.: Winterville, NC, USA; Center for New Crops & Plant Products, Department of Horticulture and Landscape Architecture, Purdue University: West Lafayette, IN, USA, 1987; pp. 91–102. [Google Scholar]
- Storey, W.B. What Kind of Fruit is the Avocado? In California Avocado Society Yearbook; Nabu Press: Charleston, SC, USA, 1973; pp. 70–71. [Google Scholar]
- Widayanti, S.M.; Syamsu, M.; Warsiki, E.; Yuliani, S. Effect of Natural Bayah Zeolite Particle Size Reduction to Physico-Chemical Properties and Absortion against Potassium Permanganate (KMnO4). AIP Conf. Proc. 2016, 1710, 030029. [Google Scholar] [CrossRef]
- Herianus, J.D.L.; Singh, Z.; Tan, S.C. Aroma volatiles production during fruit ripening of “Kesington Pride” mango. Postharvest Biol. Technol. 2003, 27, 323–336. [Google Scholar] [CrossRef]
- Putri, V.J.; Warsiki, E.; Syamsu, K.; Iskandar, A. Application Nano Zeolite-Molybdate For Avocado Ripeness Indicator. IOP Conf. Ser. Earth Environ. Sci. 2019, 347, 012063. [Google Scholar] [CrossRef]
- Poças, M.F.F.; Delgado, T.F.; Oliveira, F.A.R. Smart Packaging Technologies for Fruits and Vegetables. In Smart Packaging Technologies for Fast Moving Consumer Goods; Kerry, J., Butler, P., Eds.; John Wiley & Sons. Ltd.: London, UK, 2008; pp. 151–166. [Google Scholar] [CrossRef]
- Alam, A.U.; Rathi, P.; Beshai, H.; Sarabha, G.K.; Deen, M.J. Fruit Quality Monitoring with Smart Packaging. Sensors 2021, 21, 1509. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.; Yuliasih, I.; Warsiki, E. Performance Improvement of Fruit Ripeness Smart Label Based On Ammonium Molibdat Color Indicators. Sci. Technol. Indones. 2020, 3, 48–57. [Google Scholar] [CrossRef]
- Wei, H.; Rui, J.; You, M.; Wang, X.; Wang, Y.; Zhu, C.; Ma, M.; Xiao, H. Construction of Efficient Ethylene Removal and Antibacterial Cellulose Paper-Based Packaging Materials for Avocado Preservation. Int. J. Biol. Macromol. 2025, 299, 139763. [Google Scholar] [CrossRef]
- Morton, J.F. Banana. In Fruits of Warm Climates; Echo Point Books & Media: Brattleboro, VT, USA, 2013; pp. 29–46. ISBN 978-1-62654-976-0. [Google Scholar]
- Borkar, P.A.; Jadhao, S.D.; Bakane, P.H.; Borkar, S.L.; Murumkar, R.P. Effect of ethylene absorbent and different packaging materials on storage life of banana. Asian J. Biol. Sci. 2008, 3, 233–236. [Google Scholar]
- Chamara, D.; Illeperuma, K.; Galappatty, P.T. Effect of modified atmosphere and ethylene absorbers on extension of storage life of `Kolikuttu’ banana at ambient temperature. Fruits 2000, 55, 381–388. [Google Scholar]
- Bains, B.K.; Sharma, M.; Singh, S.K. Quality regulation in banana through post-harvest treatment with ethylene and ethylene inhibitors. Res. Crop. 2017, 18, 656–661. [Google Scholar] [CrossRef]
- Tzeng, J.; Weng, C.; Huang, J.; Shiesh, C.; Lin, Y.; Lin, Y. Application of Palladium-Modified Zeolite for Prolonging Post-Harvest Shelf Life of Banana. J. Sci. Food Agric. 2019, 99, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.L.; Szabó, G.; Hitka, G.; Zsom, T.; Tóth, A.; Németh, C.; Kókai, Z. Effect of ethylene absorber on banana during storage. Int. Soc. Hortic. Sci. 2018, 1216, 55–58. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Shang, Y.; Wen, Y. Electrospun Nanofibers Containing TiO2 for the Photocatalytic Degradation of Ethylene and Delaying Postharvest Ripening of Bananas. Food Bioprocess Technol. 2019, 12, 281–287. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Inhibitors of Ethylene Responses in Plants at the Receptor Level: Recent Developments. Physiol. Plant. 1997, 100, 577–582. [Google Scholar] [CrossRef]
- In, B.-C.; Strable, J.; Binder, B.M.; Falbel, T.G.; Patterson, S.E. Morphological and Molecular Characterization of Ethylene Binding Inhibition in Carnations. Postharvest Biol. Technol. 2013, 86, 272–279. [Google Scholar] [CrossRef]
- Balaguera-López, H.E.; Espinal-Ruiz, M.; Rodríguez-Nieto, J.M.; Herrera-Arévalo, A.; Zacarías, L. 1-Methylcyclopropene Inhibits Ethylene Perception and Biosynthesis: A Theoretical and Experimental Study on Cape Gooseberry (Physalis peruviana L.) Fruits. Postharvest Biol. Technol. 2021, 174, 111467. [Google Scholar] [CrossRef]
- Serek, M.; Sisler, E.C.; Reid, M.S. Novel Gaseous Ethylene Binding Inhibitor Prevents Ethylene Effects in Potted Flowering Plants. J. Am. Soc. Hortic. Sci. 1994, 119, 1230–1233. [Google Scholar] [CrossRef]
- Hall, A.E.; Findell, J.L.; Schaller, G.E.; Sisler, E.C.; Bleecker, A.B. Ethylene Perception by the ERS1 Protein in Arabidopsis. Plant Physiol. 2000, 123, 1449–1458. [Google Scholar] [CrossRef]
- Binder, B.M.; Bleecker, A.B. A model for ethylene receptor function and 1- methylcyclopropene action. Acta Hortic. 2003, 628, 177–187. [Google Scholar] [CrossRef]
- Blankenship, J.T.; Backovic, S.T.; Sanny, J.S.; Weitz, O.; Zallen, J.A. Multicellular Rosette Formation Links Planar Cell Polarity to Tissue Morphogenesis. Dev. Cell 2006, 11, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Sisler, E.C. The Discovery and Development of Compounds Counteracting Ethylene at the Receptor Level. Biotechnol. Adv. 2006, 24, 357–367. [Google Scholar] [CrossRef]
- Tatsuki, M.; Endo, A.; Ohkawa, H. Influence of time from harvest to 1-MCP treatment on apple fruit quality and expression of genes for ethylene biosynthesis enzymes and ethylene receptors. Postharvest Biol. Technol. 2007, 43, 28–35. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Z.; Li, Q.; Li, J.; Chen, W.; Li, X. Physiological and Transcriptomic Analysis Reveals the Roles of 1-MCP in the Ripening and Fruit Aroma Quality of Banana Fruit (Fenjiao). Food Res. Int. 2020, 130, 108968. [Google Scholar] [CrossRef]
- Golding, J.B.; Shearer, D.; Wyllie, S.G.; McGlasson, W.B. Application of 1-MCP and Propylene to Identify Ethylene-Dependent Ripening Processes in Mature Banana Fruit. Postharvest Biol. Technol. 1998, 14, 87–98. [Google Scholar] [CrossRef]
- Chang, L.-Y.; Brecht, J.K. Responses of 1-Methylcyclopropene (1-MCP)−Treated Banana Fruit to Pre− and Post−Treatment Ethylene Exposure. Sci. Hortic. 2023, 309, 111636. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D.C.; Macnish, A.J. Extension of the Shelf Life of Banana Fruit by 1-Methylcyclopropene in Combination with Polyethylene Bags. Postharvest Biol. Technol. 1999, 16, 187–193. [Google Scholar] [CrossRef]
- Harris, D.R.; Seberry, J.A.; Wills, R.B.H.; Spohr, L.J. Effect of Fruit Maturity on Efficiency of 1-Methylcyclopropene to Delay the Ripening of Bananas. Postharvest Biol. Technol. 2000, 20, 303–308. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Venkatachalam, T. Shelf Life Extension of Ethylene Treated Bananas at Different Storage Temperature with the Ethylene Action Inhibitor, 1-Methylcyclopropene. Trends Biosci. 2014, 7, 3673–3679. [Google Scholar]
- Zhu, X.; Shen, L.; Fu, D.; Si, Z.; Wu, B.; Chen, W.; Li, X. Effects of the Combination Treatment of 1-MCP and Ethylene on the Ripening of Harvested Banana Fruit. Postharvest Biol. Technol. 2015, 107, 23–32. [Google Scholar] [CrossRef]
- Cameron, A.C.; Reid, M.B. 1-MCP Blocks Ethylene-Induced Petal Abscission of Pelargonium Peltatum but the Effect Is Transient. Postharvest Biol. Technol. 2001, 22, 169–177. [Google Scholar] [CrossRef]
- Serek, M.; Woltering, E.J.; Sisler, E.C.; Frello, S.; Srikandarajah, S. Controlling ethylene responses in flower at the receptor level. Biotechnol. Adv. 2006, 24, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Elfving, D.C.; Drake, S.A.; Reed, A.N.; Visser, D.B. Preharvest Applications of Sprayable 1-Methylcyclopropene in the Orchard for Management of Apple Harvest and Postharvest Condition. Hortscience 2007, 42, 1192–1199. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Bailén, G.; Serrano, M.; Guillén, F.; Valverde, J.M.; Zapata, P.; Castillo, S.; Valero, D. Tools to Maintain Postharvest Fruit and Vegetable Quality through the Inhibition of Ethylene Action: A Review. Crit. Rev. Food Sci. Nutr. 2007, 47, 543–560. [Google Scholar] [CrossRef]
- Cools, K.; Chope, G.A.; Hammond, J.P.; Thompson, A.J.; Terry, L.A. Ethylene and 1-Methylcyclopropene Differentially Regulate Gene Expression during Onion Sprout Suppression. Plant Physiol. 2011, 156, 1639–1652. [Google Scholar] [CrossRef]
- Botondi, R.; De Sanctis, F.; Bartoloni, S.; Mencarelli, F. Simultaneous application of ethylene and 1-MCP affects banana ripening features during storage. J. Sci. Food Agric. 2014, 94, 2170–2178. [Google Scholar] [CrossRef]
- Mubarok, S.; Suwali, N.; Suminar, E.; Kamaluddin, N.N. 1-Methylcyclopropene as an Effective Ethylene Inhibitor to Extend Musa acuminata Colla ‘Muli’ Postharvest Quality. IOP Conf. Ser. Earth Environ. Sci. 2019, 334, 012051. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Li, W.; Shao, Y. Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage. Horticulturae 2023, 9, 126. [Google Scholar] [CrossRef]
- Valbuena-Tellez, E.Y.; Patiño-Guio, J.E.; Balaguera-López, H.E. Effect of applications of 1-MCP and ethylene on the ripening and degreening process of banana fruits cv. Barranquillo. Rev. UDCA Actual. Divulg. Científica 2023, 26, e1978. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Dole, J.M.; Wilkins, H.P. Floriculture: Principle and Species, 2nd ed.; Pearson/Prentice Hall: Saddle River, NJ, USA, 2005; pp. 726–739. ISBN 0-13-046250-0. [Google Scholar]
- Choi, S.T.; Huber, D.J. Influence of aqueous 1-methylcyclopropene concentration, immersion duration, and solution longevity on the postharvest ripening of breaker-turning tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2008, 49, 147–154. [Google Scholar] [CrossRef]
- Façanha, R.V.; Spricigo, P.C.; Purgatto, E.; Jacomino, A.P. Combined application of ethylene and 1-methylcyclopropene on ripening and volatile compound production of Golden papaya. Postharvest Biol. Technol. 2019, 151, 160–169. [Google Scholar] [CrossRef]
- Satuor, R.F.; Attia, M.M.; Kassem, H.A.; Mostafa, Y.S. Effect of postharvest aminoethoxyvinylglycine, 1-methylcyclopropene and jasmonic acid treatments on storability and quality maintenance of apricot fruit Cv. “Canino”. Alex. J. Agric. Sci. 2019, 64, 11–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Dai, W.; Jin, X.; Li, J. Calcium chloride and 1-methylcyclopropene treatments delay postharvest and reduce decay of New Queen melon. Sci. Rep. 2019, 9, 13563. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Wang, S.; Zhu, H.; Dun, W.; Zhang, L.; Wang, Y.; Fang, C. 1-Methylcyclopropene affects ethylene synthesis and chlorophyll degradation during cold storage of Comice pears. Sci. Hortic. 2020, 260, 108865. [Google Scholar] [CrossRef]
- Lwin, H.P.; Choi, J.-H.; Chun, J.-P.; Watkins, C.B.; Lee, J. 1-Methylcyclopropene treatment alters fruit quality attributes and targeted metabolites in Wonhwang pears during shelf life. Sci. Hortic. 2021, 284, 110125. [Google Scholar] [CrossRef]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Abdullahi, I.I.; Abdullahi, N.; Abdu, A.M.; Ibrahim, A.S. Proximate, Mineral and Vitamin Analysis of Fresh and Canned Tomato. Biosci. Biotechnol. Res. Asia 2016, 13, 1163–1169. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Romero-Gonzalez, R.; Gonzalez-Fernandes, M.J.; Guil-Guerrero, J.L. Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J. Sci. Food Agric. 2017, 97, 488–496. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Zekrehiwot, A.; Yetenayet, B.T.; Ali, M. Effects of edible coating materials and stages of maturity at harvest on storage life and quality of tomato (Lycopersicon esculentum Mill.) fruits. Afr. J. Agric. Res. 2017, 12, 550–565. [Google Scholar] [CrossRef]
- García-García, I.; Taboada-Rodríguez, A.; López-Gomez, A.; Marín-Iniesta, F. Active packaging of cardboard to extend the shelf life of tomatoes. Food Bioprocess Technol. 2013, 6, 754–761. [Google Scholar] [CrossRef]
- Charles, F.; Sanchez, J.; Gontard, N. Active modified atmosphere packaging of fresh fruits and vegetables: Modeling with tomatoes and oxygen absorber. J. Food Sci. 2003, 68, 1736–1742. [Google Scholar] [CrossRef]
- Azmai, W.N.S.M.; Latif, N.S.A.; Zain, N.M. Efficiency of edible coating chitosan and cinnamic acid to prolong the shelf life of tomatoes. J. Trop. Resour. Sustain. Sci. 2019, 7, 47–52. [Google Scholar] [CrossRef]
- Liu, H.; Meng, F.; Chen, S.; Yin, T.; Hu, S.; Shao, Z.; Liu, Y.; Zhu, C.; Ye, H.; Wang, Q. Ethanol treatment improves the sensory quality of cherry tomatoes stored at room temperature. Food Chem. 2019, 298, 125069. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Artés-Hernández, F. Potassium permanganate-based ethylene scavengers for fresh horticultural produce as an active packaging. Food Eng. Rev. 2019, 11, 159–183. [Google Scholar] [CrossRef]
- Mansourbahmani, S.; Ghareyazie, B.; Zarinnia, V.; Kalatejari, S.; Mohammadi, R.S. Study on the efficiency of ethylene scavengers on the maintenance of postharvest quality of tomato fruit. J. Food Meas. Charact. 2018, 12, 691–701. [Google Scholar] [CrossRef]
- Bailen, G.; Guillen, F.; Castillo, S. Use of activated carbon inside modified atmosphere packages to maintain tomato fruit quality during cold storage. J. Agric. Food Chem. 2006, 54, 2229–2235. [Google Scholar] [CrossRef]
- de Chiara, M.L.V.; Pal, S.; Licciulli, A.; Amodio, M.L.; Colelli, G. Photocatalytic degradation of ethylene on mesoporous TiO2/SiO2 nanocomposites: Effects on the ripening of mature green tomatoes. Biosyst. Eng. 2015, 132, 61–70. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.-E.; Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Simon, E.; Varvara, R.-A.; Vodnar, D.C. Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract. Coatings 2020, 10, 141. [Google Scholar] [CrossRef]
- Singh, S.; Maji, P.K.; Lee, Y.S.; Gaikwad, K.K. Applications of Gaseous Chlorine Dioxide for Antimicrobial Food Packaging: A Review. Environ. Chem. Lett. 2021, 19, 253–270. [Google Scholar] [CrossRef]
- Shirazi, A.; Cameron, A.C. Controlling relative humidity in modified atmosphere packages of tomato fruit. HortScience 1992, 27, 336–339. [Google Scholar] [CrossRef]
- Rux, G.; Mahajan, P.V.; Linke, M.; Pant, A.; Sängerlaub, S.; Caleb, O.J.; Geyer, M. Humidity-regulating trays: Moisture absorption kinetics and applications for fresh produce packaging. Food Bioprocess Technol. 2016, 9, 709–716. [Google Scholar] [CrossRef]
- Agudelo-Rodríguez, G.; Moncayo-Martínez, D.; Castellanos, D.A. Evaluation of a predictive model to configure an active packaging with moisture adsorption for fresh tomato. Food Packag. Shelf Life 2020, 23, 100458. [Google Scholar] [CrossRef]
- Isaka, T.; Ohta, S.U.S. Patent No. 4,876,146. Washington, DC, USA, 1989. Patent and Trademark Office. Available online: https://www.uspto.gov/web/offices/com/sol/og/2022/week31/TOC.htm?utm_source=chatgpt.com (accessed on 20 November 2024).
- Coles, R.; McDowell, D.; Kirwan, M.J. Food Packaging Technology; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Kuhn, D.N.; Bally, I.S.E.; Dillon, N.L.; Innes, D.; Groh, A.M.; Rahaman, J.; Ophir, R.; Cohen, Y.; Sherman, A. Genetic Map of Mango: A Tool for Mango Breeding. Front. Plant Sci. 2017, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Warschefsky, E.J.; Wettberg, E.J.B. Population Genomic Analysis of Mango (Mangifera indica) Suggests a Complex History of Domestication. New Phytol. 2019, 222, 2023–2037. [Google Scholar] [CrossRef]
- Sherman, A.; Rubinstein, M.; Eshed, R.; Benita, M.; Ish-Shalom, M.; Sharabi-Schwager, M.; Rozen, A.; Saada, D.; Cohen, Y.; Ophir, R. Mango (Mangifera indica L.) Germplasm Diversity Based on Single Nucleotide Polymorphisms Derived from the Transcriptome. BMC Plant Biol. 2015, 15, 277. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, A.J.; de Troconis, N.G. Volatile Flavour Components of Mango Fruit. Phytochemistry 1982, 21, 2523–2526. [Google Scholar] [CrossRef]
- Narain, N.; Bora, P.S.; Narain, R.; Shaw, P.E. Mango. In Tropical and Subtropical Fruits; Shaw, P.E., Chan, H.T., Nagy, S., Eds.; AgScience: Auburndale, FL, USA, 1997; pp. 1–77. [Google Scholar]
- Wilson, C.W.; Shaw, P.E.; Knight, R.J. Importance of selected volatile compounds to mango (Mangifera indica L.) flavor. Dev. Food Sci. 1988, 18, 283–294. [Google Scholar]
- Chaturvedi, P.K.; Bhui, K.; Shukla, Y. Lupeol: Connotations for Chemoprevention. Cancer Lett. 2008, 263, 1–13. [Google Scholar] [CrossRef]
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Screening of Mango (Mangifera indica L.) Cultivars for Their Contents of Flavonol O- and Xanthone C-Glycosides, Anthocyanins, and Pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. [Google Scholar] [CrossRef]
- Gouado, I.; Schweigert, F.J.; Ejeh, R.A.; Tchouanguep, M.F.; Camp, J.V. Systemic Levels of Carotenoids from Mangoes and Papaya Consumed in Three Forms (Juice, Fresh and Dry Slice). Eur. J. Clin. Nutr. 2007, 61, 1180–1188. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Manthey, J.A.; Luzio, G.A.; Talcott, S.T.; Goodner, K.L.; Baldwin, E.A. Total Antioxidant Activity and Fiber Content of Select Florida-Grown Tropical Fruits. J. Agric. Food Chem. 2006, 54, 7355–7363. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, D.P.; Singh, M.; Maurya, S.; Srivastava, J.S.; Singh, R.B.; Singh, S.P. Characterization of Phenolic Compounds in Some Indian Mango Cultivars. Int. J. Food Sci. Nutr. 2004, 55, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, Z. Packaging and the Shelf Life of Fruits and Vegetables. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, S.; Singh, D.B.; Singh, G. Effect of ethylene absorbent on quality and shelf-life of mango (Mangifera indica). Indian J. Agric. Sci. 2010, 80, 832–834. [Google Scholar]
- Warsiki, E.; Aprilliani, F.; Iskandar, A. The Effects of the Use of Corrugated Cardboards Covered with Ethylene Absorbers on Mango Fruit Quality after Short-Term Storage (Mangifera indica L.). J. Hortic. Res. 2019, 27, 65–70. [Google Scholar] [CrossRef]
- Syamsu, K.; Warsiki, E.; Yuliani, S.; Widayanti, S.M. Nano zeolite-KMnO4 as ethylene adsorber in active packaging of horticulture products (Musa paradisiaca). Int. J. Sci. Basic Appl. Res. 2016, 30, 93–103. [Google Scholar]
- Wills, R.B.H.; Warton, M.A. Efficacy of Potassium Permanganate Impregnated into Alumina Beads to Reduce Atmospheric Ethylene. J. Am. Soc. Hortic. Sci. 2004, 129, 433–438. [Google Scholar] [CrossRef]
- Blanke, M.M. Reducing Ethylene Levels along the Food Supply ChaIn A Key to Reducing Food Waste? J. Sci. Food Agric. 2014, 94, 2357–2361. [Google Scholar] [CrossRef]
- Araújo, F.F.; Silva, T.P.; Ramos, P.A.S.; Guimaraes, A.A.; Silva, F.C.; Finger, F.L. Longevity of Epidendrum ibaguense flowers affected by an ethylene absorber. Acta Hortic. 2015, 1071, 281–285. [Google Scholar] [CrossRef]
- Rahman, K.S.; Salehin, M.M.; Roy, R.; Swarna, J.B.; Rakib, M.R.I.; Saha, C.K.; Rahman, A. Prediction of Mango Quality during Ripening Stage Using MQ-Based Electronic Nose and Multiple Linear Regression. Smart Agric. Technol. 2024, 9, 100558. [Google Scholar] [CrossRef]
- Lebrun, M.; Ducamp, M.; Plotto, A.; Goodner, K.; Baldwin, E. Development of Electronic Nose Measurements for Mango (Mangifera indica) Homogenate and Whole Fruit. Proc. Fla. State Hort. Soc. 2004, 117, 421–425. [Google Scholar]
- Salim, S.N.M.; Shakaff, A.Y.M.; Ahmad, M.N.; Adom, A.H.; Husin, Z. Development of Electronic Nose for Fruits Ripeness Determination. In Proceedings of the International Conference on Sensing Technology, Palmerston North, New Zealand, 21–23 November 2005. [Google Scholar]
- Lebrun, M.; Plotto, A.; Goodner, K.; Ducamp, M.-N.; Baldwin, E. Discrimination of Mango Fruit Maturity by Volatiles Using the Electronic Nose and Gas Chromatography. Postharvest Biol. Technol. 2008, 48, 122–131. [Google Scholar] [CrossRef]
- Benedetti, S.; Buratti, S.; Spinardi, A.; Mannino, S.; Mignani, I. Electronic Nose as a Non-Destructive Tool to Characterise Peach Cultivars and to Monitor Their Ripening Stage during Shelf-Life. Postharvest Biol. Technol. 2008, 47, 181–188. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Juan, W.S.; Valdés, H.; Moya-León, M.A.; Infante, R.; Campos-Vargas, R. The Aroma Development during Storage of Castlebrite Apricots as Evaluated by Gas Chromatography, Electronic Nose, and Sensory Analysis. Postharvest Biol. Technol. 2009, 51, 212–219. [Google Scholar] [CrossRef]
- Zakaria, A.; Shakaff, A.Y.M.; Adom, A.H.; Ahmad, M.N.; Jaafar, M.N.; Abdullah, A.H.; Fikri, N.A.; Kamarudin, L.M. Magnifera Indica Cv. Harumanis Classification Using E-Nose. Sens. Lett. 2011, 9, 359–363. [Google Scholar] [CrossRef]
- Gonçalves, B.; Oliveira, I.; Bacelar, E.; Morais, M.C.; Aires, A.; Cosme, F.; Ventura-Cardoso, J.; Anjos, R.; Pinto, T. Aromas and Flavours of Fruits; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Pandit, S.S.; Chidley, H.G.; Kulkarni, R.S.; Pujari, K.H.; Giri, A.P.; Gupta, V.S. Cultivar Relationships in Mango Based on Fruit Volatile Profiles. Food Chem. 2009, 114, 363–372. [Google Scholar] [CrossRef]
- Zakaria, A.; Shakaff, A.Y.M.; Masnan, M.J.; Saad, F.S.A.; Adom, A.H.; Ahmad, M.N.; Jaafar, M.N.; Abdullah, A.H.; Kamarudin, L.M. Improved Maturity and Ripeness Classifications of Magnifera indica cv. Harumanis Mangoes through Sensor Fusion of an Electronic Nose and Acoustic Sensor. Sensors 2012, 12, 6023–6048. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Falagán, N.; Bohmer, B.; Terry, L.A.; Alamar, M.C. The role of ethylene and 1-MCP in early-season sweet cherry ‘Burlat’ storage life. Sci. Hortic. 2019, 258, 108787. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicka, E.; Sójka, M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef]
- Sharma, M.; Jissy, K.; Subramanian, J.J.; Paliyath, G. Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 239–247. [Google Scholar] [CrossRef]
- Gong, Y.P.; Fan, X.T.; Mattheis, J.P. Responses of ‘Bing’ and ‘Rainier’ sweet cherries to ethylene and 1-methylcyclopropene. J. Am. Soc. Hortic. Sci. 2002, 127, 831–835. [Google Scholar] [CrossRef]
- Mozetič, B.; Simčič, M.; Trebše, P. Anthocyanins and hydroxycinnamic acids of Lambert Compact cherries (Prunus avium L.) after cold storage and 1-methylcyclopropene treatment. Food Chem. 2006, 97, 302–309. [Google Scholar] [CrossRef]
- Zhao, H.; Fu, M.; Du, Y.; Sun, F.; Chen, Q.; Jin, T.; Zhang, Q.; Liu, B. Improvement of fruit quality and pedicel color of cold stored sweet cherry in response to pre-storage 1-methylciclopropene and chlorine dioxide treatments: Combination treatment of 1-MCP plus ClO2 improves post-harvest quality of sweet cherry fruit. Sci. Hortic. 2021, 277, 109806. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Guella, G.; Gasperotti, M.; Pojer, E.; Zancato, M.; Mattivi, F. Clarifying the Identity of the Main Ellagitannin in the Fruit of the Strawberry, Fragaria vesca and Fragaria ananassa Duch. J. Agric. Food Chem. 2012, 60, 2507–2516. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. FisetIn a dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Aaby, K.; Skrede, G.; Wrolstad, R.E. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J. Agric. Food Chem. 2005, 53, 4032–4040. [Google Scholar] [CrossRef]
- Ku, V.V.V.; Wills, R.B.H.; Ben-Yehoshua, S. 1-Methylcyclopropene can differentially affect the postharvest life of strawberries exposed to ethylene. HortScience 1999, 34, 119–120. [Google Scholar] [CrossRef]
- McGlasson, W.B. Ethylene and fruit ripening. HortScience 1985, 20, 51–54. [Google Scholar] [CrossRef]
- Tian, M.S.; Prakash, S.; Elgar, H.J.; Young, H.; Burmeister, D.M.; Ross, G.S. Responses of strawberry fruit to 1-Methylcyclopropene (1-MCP) and ethylene. Plant Growth Regulator 2000, 32, 83–91. [Google Scholar] [CrossRef]
- Jiang, Y.; Daryl, C.J.; Leon, A.T. 1-Methylcyclopropene treatment affects strawberry fruit decay. Postharvest Biol. Technol. 2001, 23, 227–232. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Fan, X.; Blankenship, S.M.; Mattheis, J.P. 1-Methylcyclopropene Inhibits Apple Ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D.C. 1-Methylcyclopropene treatment effects on intact and fresh-cut apple. J. Hortic. Sci. Biotechnol. 2002, 77, 19–21. [Google Scholar] [CrossRef]
- Pre-Aymard, C.; Weksler, A.; Lurie, S. Responses of “Anna”, a rapidly ripening summer apple, to 1-methylcyclopropene. Postharvest Biol. Technol. 2003, 27, 163–170. [Google Scholar] [CrossRef]
- Pre-Aymard, C.; Fallik, E.; Weksler, A.; Lurie, S. Sensory analysis and instrumental measurements of “Anna” apples treated with 1-methylcyclopropene. Postharvest Biol. Technol. 2005, 36, 135–142. [Google Scholar] [CrossRef]
- Watkins, C.B. Principles and practices of postharvest handling and stress. In Apples: Crop physiology, Production and Uses; Feree, D.C., Warrington, I.J.S., Eds.; CABI Publishing: Oxfordshire, UK, 2003; pp. 585–614. [Google Scholar]
- Brackmann, A.; Thewes, F.R.; Anese, R.D.O.; Both, V. Effect of growth regulators on “Brookfield” apple gas diffusion and metabolism under controlled atmosphere storage. Pesqui. Agropecuária Bras. 2014, 49, 323–329. [Google Scholar] [CrossRef]
- Tirgar, A.; Han, D.; Steckl, A.J. Absorption of Ethylene on Membranes Containing Potassium Permanganate Loaded into Alumina-Nanoparticle-Incorporated Alumina/Carbon Nanofibers. J. Agric. Food Chem. 2018, 66, 5635–5643. [Google Scholar] [CrossRef]
- Ramin, A.; Rezaei, A.; Shams, M. Potassium permanganates and short term hypobaric enhances shelf-life of kiwifruits. Acta Hortic. 2010, 877, 849–852. [Google Scholar] [CrossRef]
- Kim, G.-H.; Wills, R.B.H. Interaction of enhanced carbon dioxide and reduced ethylene on the storage life of strawberries. J. Hortic. Sci. Biotechnol. 1998, 73, 181–184. [Google Scholar] [CrossRef]
- Joung, J.; Boonsiriwit, A.; Kim, M.; Lee, Y.S. Application of ethylene scavenging nanocomposite film prepared by loading potassium permanganate-impregnated halloysite nanotubes into low-density polyethylene as active packaging material for fresh produce. LWT 2021, 145, 111309. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- UNECE. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 3rd ed.; United Nations: New York, NY, USA; Geneva, Switzerland, 2009. [Google Scholar]
- WHO. WHO Model List of Essential Medicines, 20th List (April 2017, Amended August 2017). World Health Organization, 2017. Available online: http://www.who.int (accessed on 18 November 2024).
- Zhang, W.; Rhim, J.-W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of health safety aspects of titanium dioxide nanoparticles in food application. NanoImpact 2020, 18, 100224. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, G.H.; Kim, S.-W. Ethylene Gas Decomposition Using ZSM-5/WO3-Pt-Nanorod Composites for Fruit Freshness. ACS Sustain. Chem. Eng. 2019, 7, 11250–11257. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Kang, J.L.; Moon, C.; Lee, H.S.; Lee, H.W.; Park, E.M.; Kim, H.S.; Castranova, V. Comparison of the Biological Activity Between Ultrafine and Fine Titanium Dioxide Particles in RAW 264.7 Cells Associated with Oxidative Stress. J. Toxicol. Environ. Health Part A 2008, 71, 478–485. [Google Scholar] [CrossRef]
- Stone, V.; Miller, M.R.; Clift, M.J.D.; Elder, A.; Mills, N.L.; Møller, P.; Schins, R.P.F.; Vogel, U.; Kreyling, W.G.; Alstrup-Jensen, K.; et al. Nanomaterials Versus Ambient Ultrafine Particles: An Opportunity to Exchange Toxicology Knowledge. Environ. Health Perspect. 2017, 125, 106002. [Google Scholar] [CrossRef]
- López-García, E.; Benítez-Cabello, A.; Rodríguez-Gómez, F.; Martín-Arranz, V.; Garrido-Fernández, A.; Arroyo-López, F.N. Influence of 1-Methylcyclopropene (1-MCP) on the Processing and Microbial Communities of Spanish-Style and Directly Brined Green Table Olive Fermentations. Fermentation 2022, 8, 441. [Google Scholar] [CrossRef]
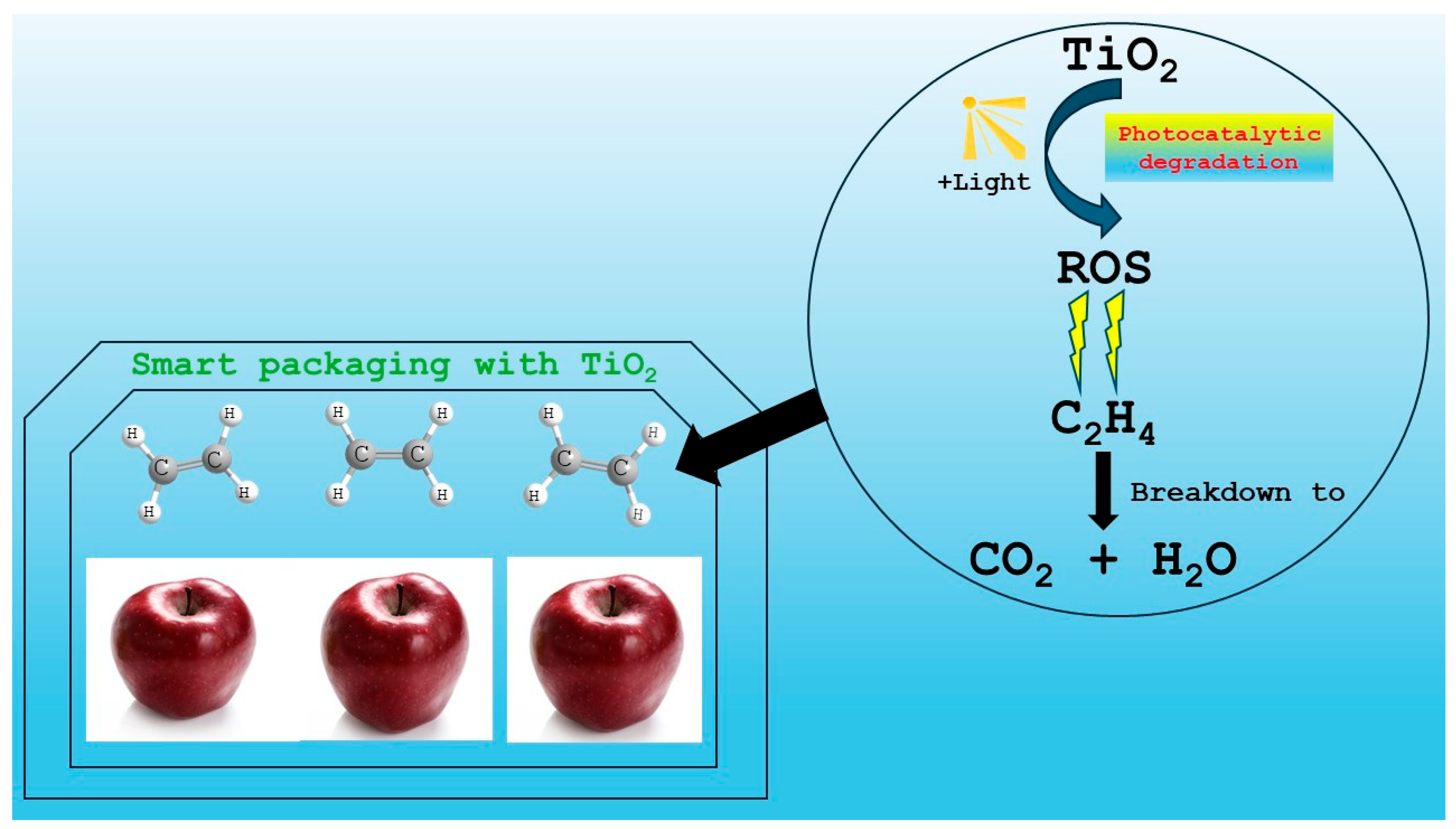
| Fruits | Intelligent Packaging System | Active Packaging System | References |
|---|---|---|---|
| Apple | Colorimetric sensors using ethylene emission, Aldehyde-sensitive colorimetric sensors using pH indicators, Ripe sense ripeness indicator, Color-based ripeness indicator, Label-based colorimetric sensor using methyl red | Ethylene scavengers, Ethylene inhibitors (1-MCP, 1-PCP, 1-OCP) | [4,13,32,33,34,37,38,39,40,41] |
| Avocado | Ethylene absorbers with high sensitivity, Nano zeolite-ammonium molybdate, Ripesense, Ammonium Molybdate | [41,59,61,62] | |
| Banana | TiO2 nanoparticles | Ethylene absorbers, Ethylene inhibitors (1-MCP) | [41,65,66,70,81,82,83,84,85,87,88,89,90,91,92,93,94,95] |
| Sweet cherry | Ethylene inhibitors (1-MCP) | [152,154,155,156,157] | |
| Kiwifruit | Electronic nose, Fluorescence sensor, Colorimetric chemical sensor, Lipase TTI (Time-temperature indicator), | Potassium permanganate, Copper (I) complex and bathophenanthroline- based palladium (Pd) complex | [28,29,30,32,34,48,52,53,54] |
| Mango | Electric nose | Ethylene absorbers | [135,136,137,142,143,144,145,146,147,148] |
| Strawberry | Ripeness indicator based on methyl red | Ethylene inhibitors (1-MCP) | [21,162,165] |
| Tomato | Sodium polyacrylate-cotton mixture, Mixed nanoparticles TiO2/SiO2 | Humidity absorbers (sorbitol, sodium chloride, potassium chloride, bentonite, silica gel, polyacrylate salts, zeolites, and microporous clays), Ethylene scavengers such as potassium permanganate (KMnO4), activated carbon, clay and zeolites, KMnO4-promoted nano zeolite, Granular-activated carbon (GAC), Oxygen absorbers, | [17,113,114,115,116,120,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panou, A.; Lazaridis, D.G.; Karabagias, I.K. Application of Smart Packaging on the Preservation of Different Types of Perishable Fruits. Foods 2025, 14, 1878. https://doi.org/10.3390/foods14111878
Panou A, Lazaridis DG, Karabagias IK. Application of Smart Packaging on the Preservation of Different Types of Perishable Fruits. Foods. 2025; 14(11):1878. https://doi.org/10.3390/foods14111878
Chicago/Turabian StylePanou, Andreas, Dimitrios G. Lazaridis, and Ioannis K. Karabagias. 2025. "Application of Smart Packaging on the Preservation of Different Types of Perishable Fruits" Foods 14, no. 11: 1878. https://doi.org/10.3390/foods14111878
APA StylePanou, A., Lazaridis, D. G., & Karabagias, I. K. (2025). Application of Smart Packaging on the Preservation of Different Types of Perishable Fruits. Foods, 14(11), 1878. https://doi.org/10.3390/foods14111878







