Synthesis of Neem-Oil-Infused Niosome and Starch Nanoparticle Coatings for Preserving the Quality of Strawberry Fruit
Abstract
1. Introduction
2. Materials and Methods
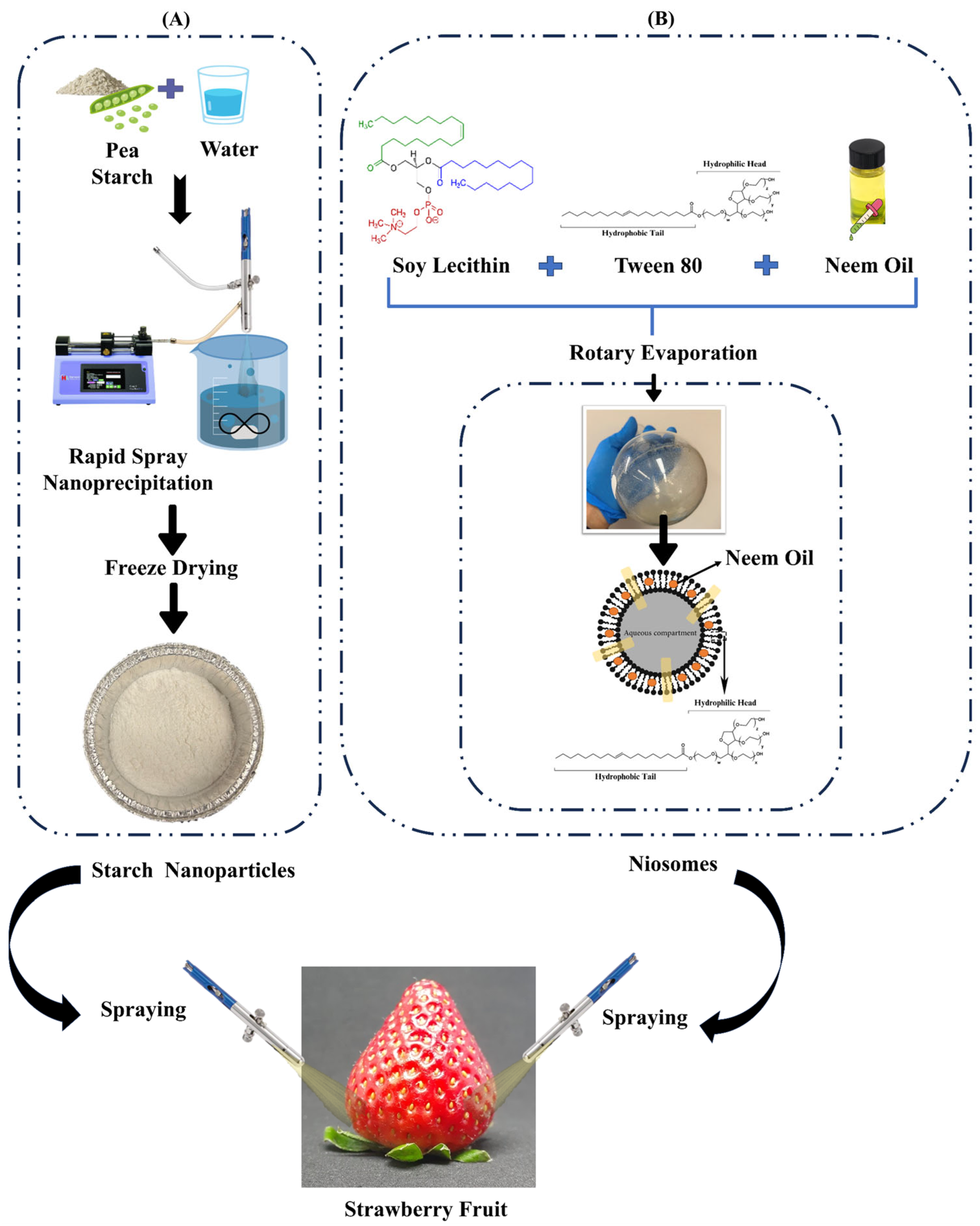
2.1. Preparation of the Neem-Oil-Infused Starch Nanoparticles and Niosomes
2.2. Post-Harvest Treatment
2.3. Weight Loss, Total Soluble Solids (TSS), and pH
2.4. Total Phenolic Content
2.5. Antioxidant Activity
2.6. Colour Measurement
2.7. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. Total Soluble Solids and pH
3.3. Colour Change and Visual Assessment
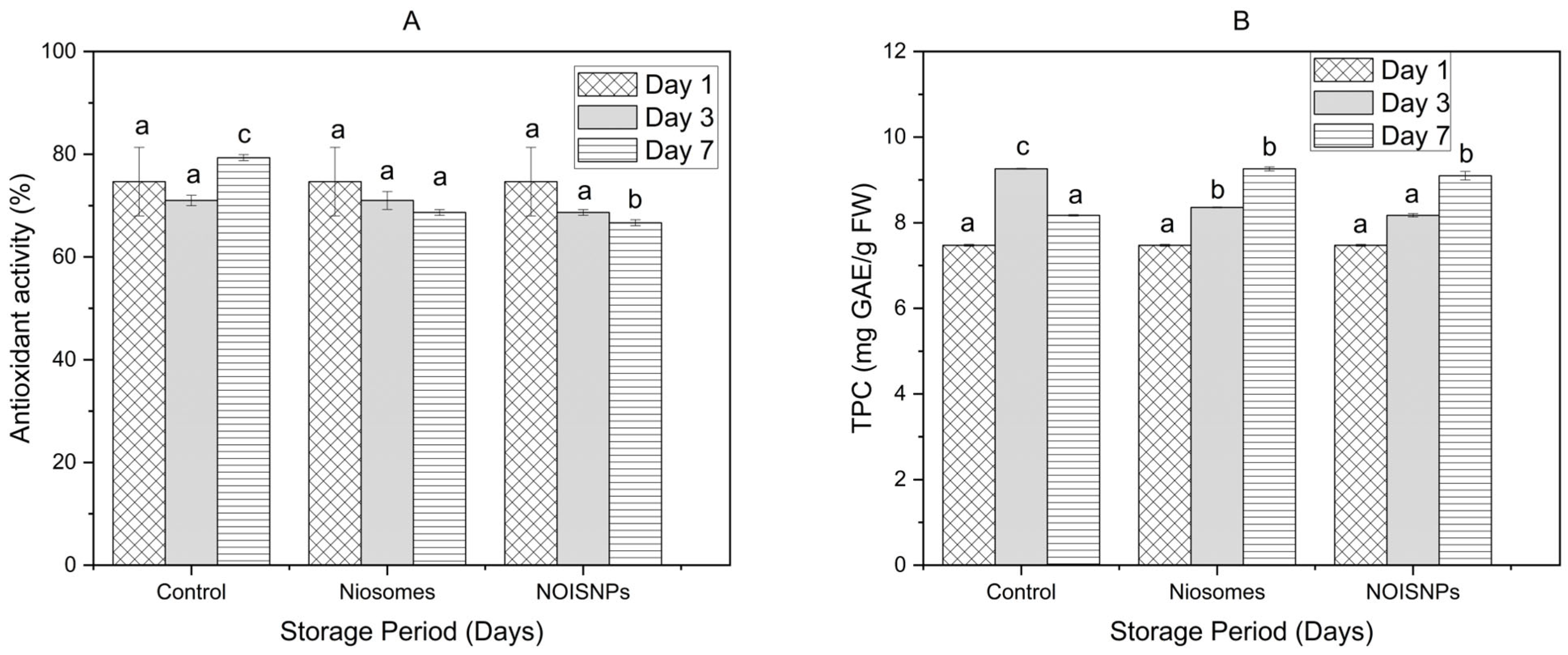
3.4. Antioxidant Activity and Total Phenolic Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansarifar, E.; Moradinezhad, F. Encapsulation of thyme essential oil using electrospun zein fiber for strawberry preservation. Chem. Biol. Technol. Agric. 2022, 9, 2. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Shi, F.; Wang, H.; Chen, K.; Feng, J.; Jia, W. Antifungal activity screening for mint and thyme essential oils against Rhizopus stolonifer and their application in postharvest preservation of strawberry and peach fruits. J. Appl. Microbiol. 2021, 130, 1993–2007. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.; Ahmadzadeh, M. The effect of zinc oxide, copper, and silver nanoparticles synthesized by the green method for controlling strawberry gray mold fungus, B. cinerea pers. J. Plant Sci. Phytopathol. 2023, 7, 050–065. [Google Scholar]
- Oliveira, J.; Parisi, M.; Baggio, J.; Silva, P.; Paviani, B.; Spoto, M.H.F.; Gloria, E.M.d. Control of Rhizopus stolonifer in strawberries by the combination of essential oil with carboxymethylcellulose. Int. J. Food Microbiol. 2019, 292, 150–158. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. Discov. Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Emmanuel, R.S. Use of modified atmosphere packaging combined with essential oils for prolonging post-harvest shelf life of mango (cv. Banganapalli and cv. Totapuri). LWT 2021, 148, 111662. [Google Scholar] [CrossRef]
- Barbhuiya, R.I.; Wroblewski, C.; Elsayed, A.; Subramanian, J.; Kaur, G.; Routray, W.; Singh, A. Development and physicochemical characterization of Azadirachta indica seed oil loaded niosomes nanoparticles: A potential natural pesticide. Chem. Eng. Res. Des. 2024, 203, 197–206. [Google Scholar] [CrossRef]
- Islas, J.F.; Acosta, E.; Zuca, G.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Bratovcic, A.; Suljagic, J. Micro-and nano-encapsulation in food industry. Croat. J. Food Sci. Technol. 2019, 11, 113–121. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Corona-Rangel, M. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. Lwt 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, M.; Arouiee, H.; Golmohammadzadeh, S.; Naseri, M.; Bandian, L. Edible coatings based on solid lipid nanoparticles containing essential oil to improve antimicrobial activity, shelf-life, and quality of strawberries. J. Stored Prod. Res. 2024, 106, 102262. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A review on polymer and lipid-based nanocarriers and its application to nano-pharmaceutical and food-based systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Barbhuiya, R.I.; Kaur, P.; Wroblewski, C.; Elsayed, A.; Subramanian, J.; Nair, G.R.; Singh, A. Role of Nanoparticles in the Suppression of Diseases in Fruits and Vegetables to Improve Agricultural and Environmental Sustainability: A Bibliometric Analysis. In Sustainable Engineering: Concepts and Practices; Springer: Berlin/Heidelberg, Germany, 2024; pp. 349–361. [Google Scholar]
- Barbhuiya, R.I.; Wroblewski, C.; Ravikumar, S.P.; Kaur, G.; Routray, W.; Subramanian, J.; Elsayed, A.; Singh, A. Upcycling of industrial pea starch by rapid spray nanoprecipitation to develop plant-derived oil encapsulated starch nanoparticles for potential agricultural applications. Carbohydr. Polym. 2024, 346, 122618. [Google Scholar] [CrossRef]
- Yu, K.; Newman, M.C.; Archbold, D.D.; Hamilton-Kemp, T.R. Survival of Escherichia coli O157: H7 on strawberry fruit and reduction of the pathogen population by chemical agents. J. Food Prot. 2001, 64, 1334–1340. [Google Scholar] [CrossRef]
- Vardar, C.; Ilhan, K.; Karabulut, O.A. The application of various disinfectants by fogging for decreasing postharvest diseases of strawberry. Postharvest Biol. Technol. 2012, 66, 30–34. [Google Scholar] [CrossRef]
- Kessler, S.J.; Cooksey, K.; Pometto, A.L., III; Hurley, A.; Bridges, W. Shelf-life extension of fresh strawberries packaged in vented clamshells through an in-package widget designed to promote chlorine dioxide gas distribution. ACS Food Sci. Technol. 2023, 3, 394–403. [Google Scholar] [CrossRef]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. Lwt 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Farida, F.; Hamdani, J.; Mubarok, S.; Akutsu, M.; Noviyanti, K.; Nur Rahmat, B. Variability of Strawberry Fruit Quality and Shelf Life with Different Edible Coatings. Horticulturae 2023, 9, 741. [Google Scholar] [CrossRef]
- Kaur, P.; Subramanian, J.; Singh, A. Green extraction of bioactive components from carrot industry waste and evaluation of spent residue as an energy source. Sci. Rep. 2022, 12, 16607. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Peng, X.; Sun, B.; Wang, X.; Tang, H. Characterization of anthocyanin and proanthocyanidin biosynthesis in two strawberry genotypes during fruit development in response to different light qualities. J. Photochem. Photobiol. B Biol. 2018, 186, 225–231. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Garrido-Galand, S.; Taladrid, D.; Moreno-Arribas, M.V.; Villamiel, M.; Montilla, A. Use of natural low-methoxyl pectin from sunflower by-products for the formulation of low-sucrose strawberry jams. J. Sci. Food Agric. 2022, 102, 5957–5964. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Harvest and postharvest factors affecting bruise damage of fresh fruits. Hortic. Plant J. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Rahmati, E. Effect of edible coatings on the shelf-life of fresh strawberries: A comparative study using TOPSIS-Shannon entropy method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of edible coating enriched with natural antioxidant extract and bergamot essential oil on the shelf life of strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef]
- da Silva Bruni, A.R.; da Silva Alves, E.; da Costa, J.C.M.; Friedrichsen, J.s.d.S.A.; Silva, L.G.Z.; de Oliveira Santos Junior, O.; Bonafé, E.G. Extending the Postharvest Shelf Life of Strawberries Through a κ-Carrageenan/Starch-Based Coating Enriched with Zinc Oxide Nanoparticles. ACS Food Sci. Technol. 2024, 4, 2967–2979. [Google Scholar] [CrossRef]
- Basak, J.K.; Madhavi, B.G.K.; Paudel, B.; Kim, N.E.; Kim, H.T. Prediction of total soluble solids and pH of strawberry fruits using RGB, HSV and HSL colour spaces and machine learning models. Foods 2022, 11, 2086. [Google Scholar] [CrossRef]
- Montero, T.M.; Mollá, E.M.; Esteban, R.M.; López-Andréu, F.J. Quality attributes of strawberry during ripening. Sci. Hortic. 1996, 65, 239–250. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Saari, N. Effect of maturity on phenolics (phenolic acids and flavonoids) profile of strawberry cultivars and mulberry species from Pakistan. Int. J. Mol. Sci. 2012, 13, 4591–4607. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.; Luo, Z.; Huang, X.; Li, X. Proteomic response and quality maintenance in postharvest fruit of strawberry (Fragaria × ananassa) to exogenous cytokinin. Sci. Rep. 2016, 6, 27094. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, R.; Razavi, F.; Mortazavi, S.N.; Gohari, G.; Juárez-Maldonado, A. Evaluation of proline-coated chitosan nanoparticles on decay control and quality preservation of strawberry fruit (cv. Camarosa) during cold storage. Horticulturae 2022, 8, 648. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Wang, X.-Y.; Wu, Z.-Y.; Weng, Y.-X. Kinetic analysis of PGA/PBAT plastic films for strawberry fruit preservation quality and enzyme activity. J. Food Compos. Anal. 2022, 108, 104439. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Affes, S.; Boufi, S.; Nasri, M. Optimization of the formulation of chitosan edible coatings supplemented with carotenoproteins and their use for extending strawberries postharvest life. Food Hydrocoll. 2018, 83, 375–392. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.; Hu, Y.; Zhu, Z.; Zhuang, C.; Zhao, Y.; Zhong, Y. The preservation performance of chitosan coating with different molecular weight on strawberry using electrostatic spraying technique. Int. J. Biol. Macromol. 2020, 151, 278–285. [Google Scholar] [CrossRef]
- Alharaty, G.; Ramaswamy, H.S. The effect of sodium alginate-calcium chloride coating on the quality parameters and shelf life of strawberry cut fruits. J. Compos. Sci. 2020, 4, 123. [Google Scholar] [CrossRef]
- Rodas, C.L.; Pereira da Silva, I.; Toledo-Coelho, V.; Guimarães-Ferreira, D.; de Souza, R.J.; Guedes de Carvalho, J. Chemical properties and rates of external color of strawberry fruits grown using nitrogen and potassium fertigation. IDESIA 2013, 31, 53–58. [Google Scholar] [CrossRef]
- Wani, S.M.; Gull, A.; Ahad, T.; Malik, A.; Ganaie, T.A.; Masoodi, F.A.; Gani, A. Effect of gum Arabic, xanthan and carrageenan coatings containing antimicrobial agent on postharvest quality of strawberry: Assessing the physicochemical, enzyme activity and bioactive properties. Int. J. Biol. Macromol. 2021, 183, 2100–2108. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Simkova, K.; Veberic, R.; Grohar, M.C.; Pelacci, M.; Smrke, T.; Ivancic, T.; Medic, A.; Cvelbar Weber, N.; Jakopic, J. Changes in the aroma profile and phenolic compound contents of different strawberry cultivars during ripening. Plants 2024, 13, 1419. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.E. Synthesis, Oxidation, and Distribution of Polyphenols in Strawberry Fruit During Cold Storage; University of South Florida: Tampa, FL, USA, 2018. [Google Scholar]
- Taheri, A.; Behnamian, M.; Dezhsetan, S.; Karimirad, R. Shelf life extension of bell pepper by application of chitosan nanoparticles containing Heracleum persicum fruit essential oil. Postharvest Biol. Technol. 2020, 170, 111313. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Alderson, P.G.; Zahid, N. Effect of gum arabic as an edible coating on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. Postharvest Biol. Technol. 2013, 76, 119–124. [Google Scholar] [CrossRef]
- Niazmand, R.; Yeganehzad, S.; Niazmand, A. Application of laminated and metalized films to prolong the shelf life of dried barberries. J. Stored Prod. Res. 2021, 92, 101809. [Google Scholar] [CrossRef]
- Hara, K.; Someya, T.; Sano, K.; Sagane, Y.; Watanabe, T.; Wijesekara, R. Antioxidant activities of traditional plants in Sri Lanka by DPPH free radical-scavenging assay. Data Brief 2018, 17, 870–875. [Google Scholar] [CrossRef]

| Treatments | Day 1 | Day 3 | Day 7 |
|---|---|---|---|
| Weight loss (%) | |||
| Control | NA | 21.4 ± 2.8 b | 32.45 ± 2.4 b |
| Niosome-treated | NA | 2.17 ± 1.12 a | 5.9 ± 1.50 a |
| Starch-nanoparticle-treated | NA | 3.88 ± 3.2 a | 8.9 ± 3.02 a |
| Total soluble solids (°Brix) | |||
| Control | 7.2 ± 0.1 a | 10 ± 0.2 b | 12.5 ± 0.2 c |
| Niosome-treated | 7.2 ± 0.1 a | 9.2 ± 0.1 a | 9.2 ± 0.1 a |
| Starch-nanoparticle-treated | 7.2 ± 0.1 a | 9.7 ± 0.01 b | 10.1 ± 0.1 b |
| pH | |||
| Control | 3.3 ± 0.02 a | 3.5 ± 0.01 b | 3.6 ± 0.01 b |
| Niosome-treated | 3.3 ± 0.02 a | 3.4 ± 0.01 a | 3.4 ± 0.01 a |
| Starch-nanoparticle-treated | 3.3 ± 0.02 a | 3.4 ± 0.02 a | 3.4 ± 0.03 a |
| Treatment | Days | L* | a* | b* | Chroma | Hue |
|---|---|---|---|---|---|---|
| Control | 1 | 28.33 ± 1.70 b | 29.86 ± 4.75 a | 17.7 ± 3.64 a | 34.76 ± 5.81 a | 30.43 ± 1.46 |
| Niosome-treated | 1 | 32.47 ± 6.40 b | 19.50 ± 8.85 a | 19.26 ± 2.49 a | 33.66 ± 8.5 a | 28.47 ± 1.82 |
| Starch-nanoparticle-treated | 1 | 29.63 ± 7.35 a | 22.77 ± 1.77 a | 15.80 ± 1.73 a | 29.53 ± 5.87 a | 32.23 ± 2.35 |
| Control | 3 | 21.10 ± 1.28 a | 20.77 ± 0.91 a | 9.53 ± 1.46 a | 22.93 ± 0.57 a | 24.60 ± 4.13 a |
| Niosome-treated | 3 | 27.40 ± 4.30 a | 27.73 ± 6.27 a | 14.03 ± 3.25 a | 29.36 ± 6.4 a | 28.46 ± 1.82 a |
| Starch-nanoparticle-treated | 3 | 26.87 ± 2.32 a | 24.37 ± 1.40 a | 13.37 ± 2.08 a | 27.80 ± 2.23 a | 28.67 ± 2.39 a |
| Control | 7 | 20.90 ± 3.53 a | 16.30 ± 0.53 a | 7.57 ± 1.00 a | 18.00 ± 0.61 a | 24.80 ± 3.12 a |
| Niosome-treated | 7 | 26.60 ± 4.76 c | 29.90 ± 4.29 b | 27.50 ± 1.15 c | 44.27 ± 3.30 c | 41.60 ± 1.15 c |
| Starch-nanoparticle-treated | 7 | 19.10 ± 0.40 ab | 25.63 ± 0.31 b | 19.87 ± 0.21 b | 31.73 ± 0.61 b | 36.30 ± 1.51 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbhuiya, R.I.; Wroblewski, C.; Ravikumar, S.P.; Subramanian, J.; Elsayed, A.; Singh, A. Synthesis of Neem-Oil-Infused Niosome and Starch Nanoparticle Coatings for Preserving the Quality of Strawberry Fruit. Foods 2025, 14, 1860. https://doi.org/10.3390/foods14111860
Barbhuiya RI, Wroblewski C, Ravikumar SP, Subramanian J, Elsayed A, Singh A. Synthesis of Neem-Oil-Infused Niosome and Starch Nanoparticle Coatings for Preserving the Quality of Strawberry Fruit. Foods. 2025; 14(11):1860. https://doi.org/10.3390/foods14111860
Chicago/Turabian StyleBarbhuiya, Rahul Islam, Charles Wroblewski, Sivaranjani Palanisamy Ravikumar, Jayasankar Subramanian, Abdallah Elsayed, and Ashutosh Singh. 2025. "Synthesis of Neem-Oil-Infused Niosome and Starch Nanoparticle Coatings for Preserving the Quality of Strawberry Fruit" Foods 14, no. 11: 1860. https://doi.org/10.3390/foods14111860
APA StyleBarbhuiya, R. I., Wroblewski, C., Ravikumar, S. P., Subramanian, J., Elsayed, A., & Singh, A. (2025). Synthesis of Neem-Oil-Infused Niosome and Starch Nanoparticle Coatings for Preserving the Quality of Strawberry Fruit. Foods, 14(11), 1860. https://doi.org/10.3390/foods14111860









