Classification of Crab-Field Rice and Conventional Rice Based on Multi-Element, Stable Isotope, and Non-Targeted Metabolome Combined with Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Material Reagents and Instruments
2.3. Method for Multi-Element Analysis
2.4. Method for Stable Isotope Analysis
2.5. Method for Metabolomics Analysis
2.6. Statistical and Chemometric Analysis
3. Results and Discussion
3.1. Multielement Analysis
3.1.1. Differential Analysis of Multi-Element Content Between Crab-Field Rice and Ordinary Rice
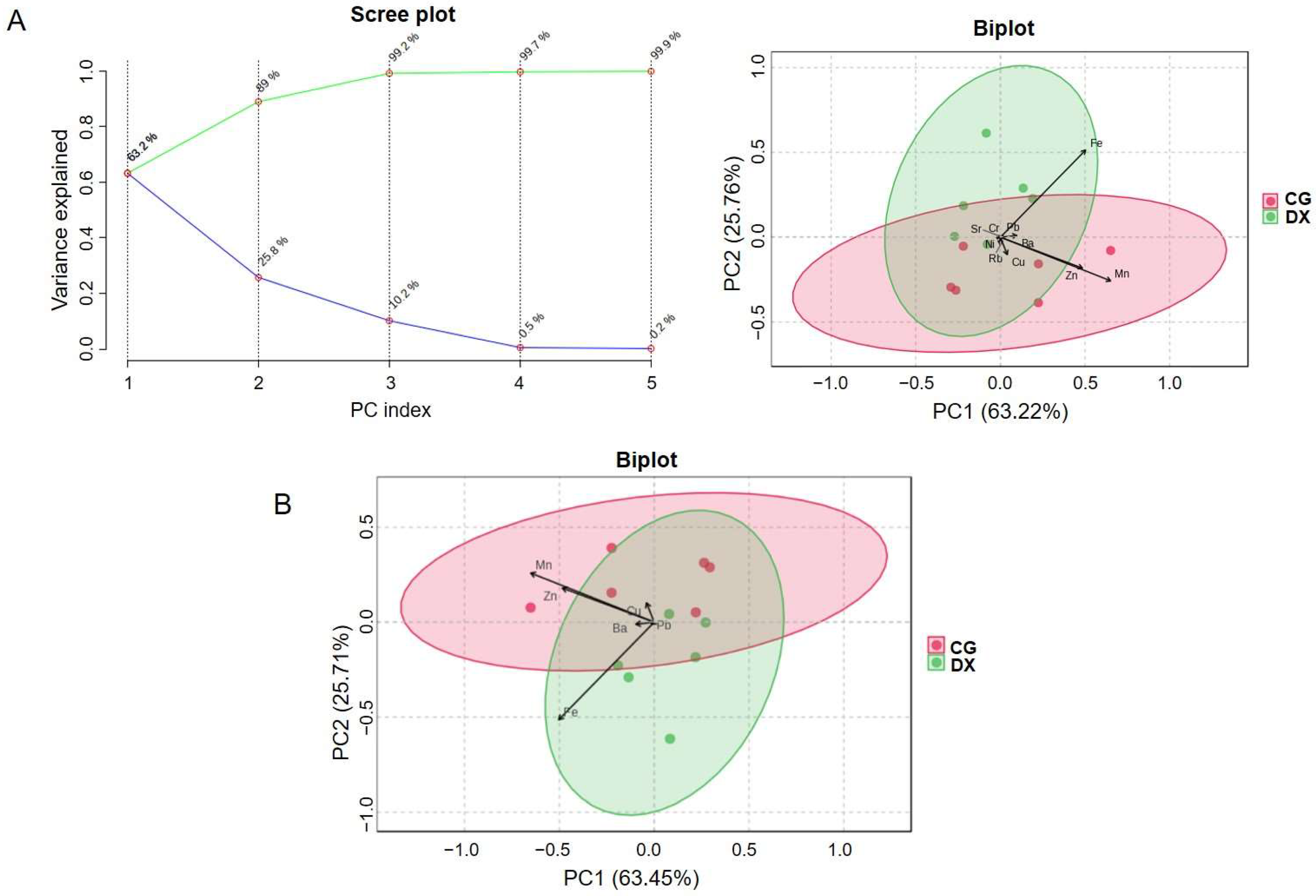
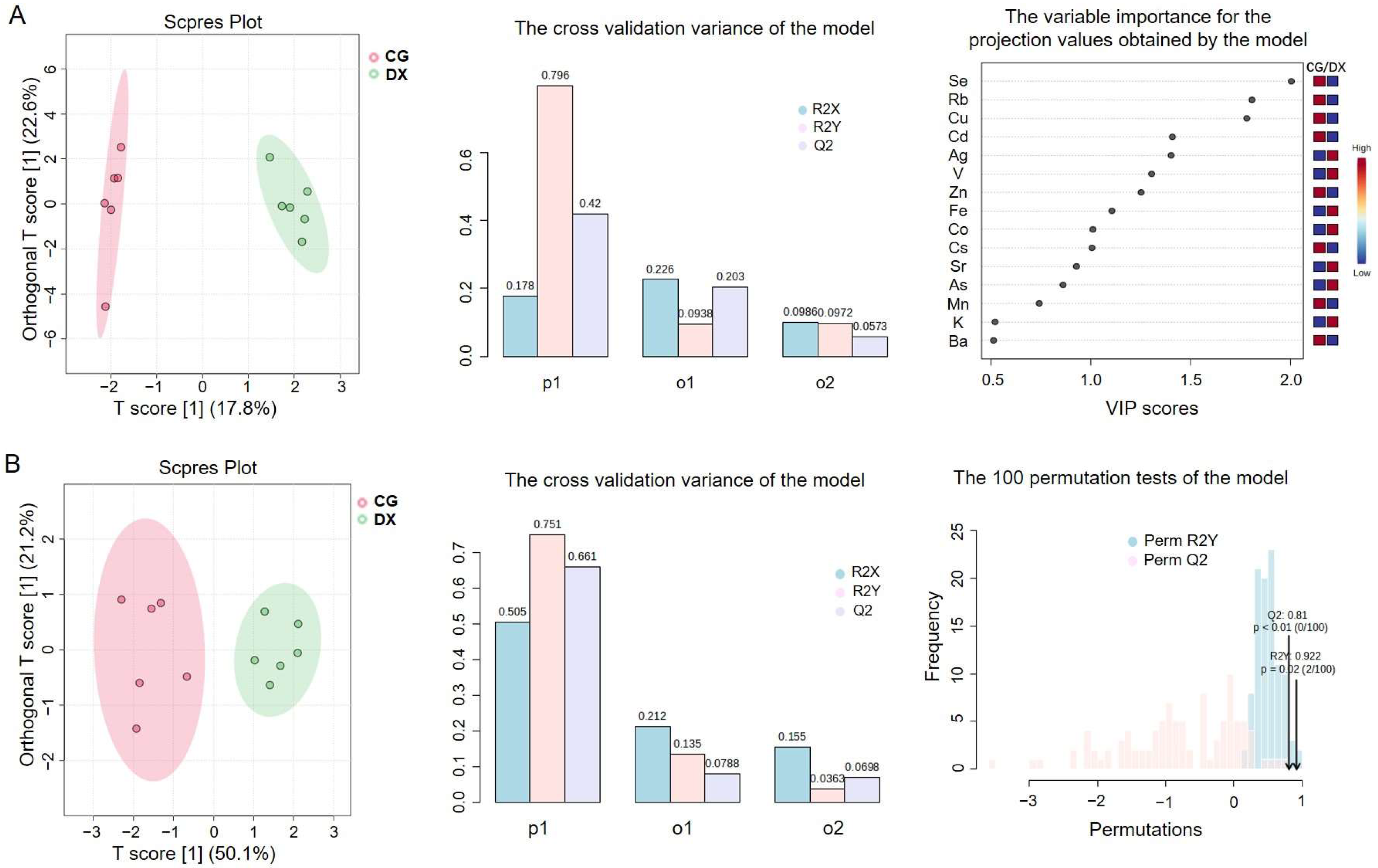
3.1.2. Chemometric Analysis by Elemental Fingerprinting
3.2. Stable Isotope Analysis
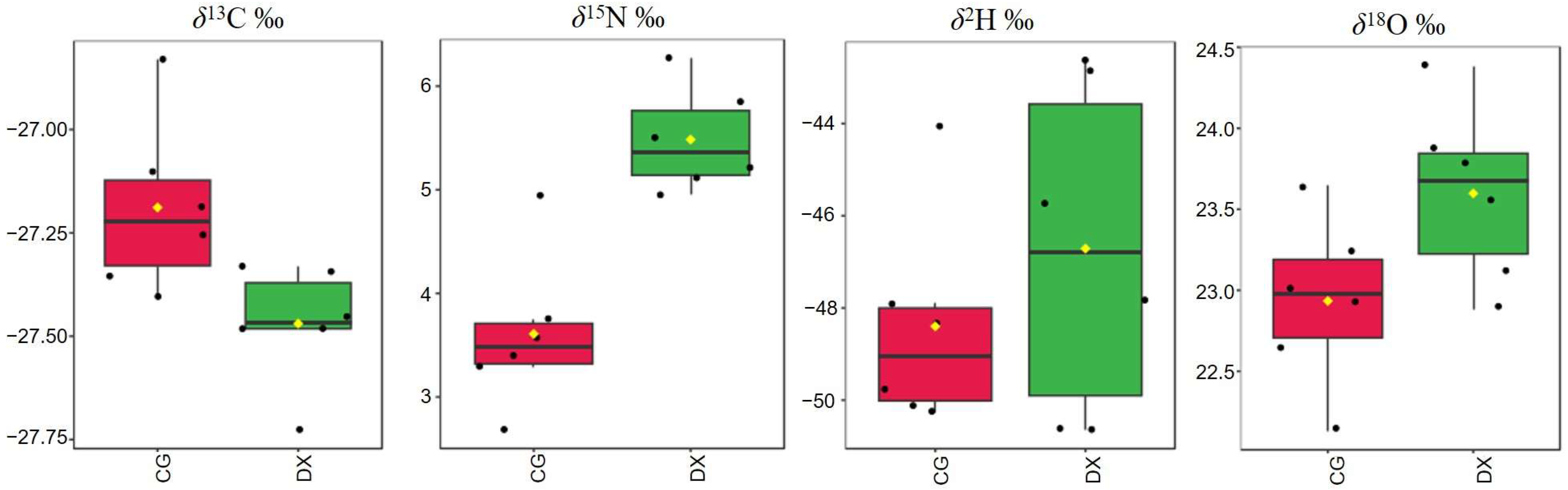
3.2.1. Differential Analysis of Stable Isotopes Between Crab-Field Rice and Ordinary Rice
3.2.2. Chemometric Analysis by Stable Isotope Fingerprinting
3.3. Joint Analysis of Multiple Elements and Stable Isotopes
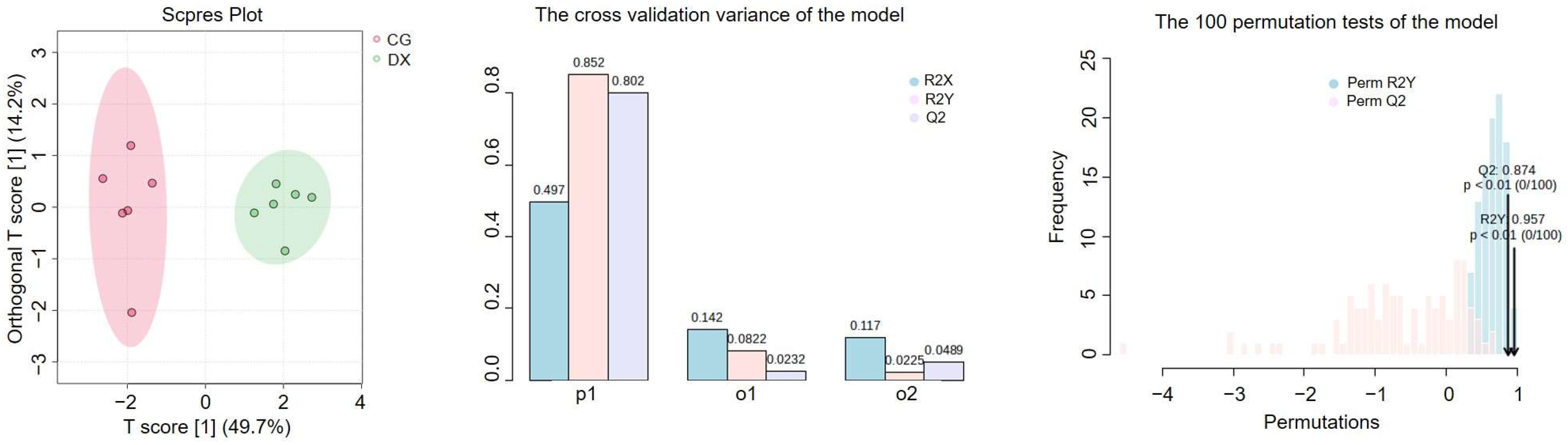
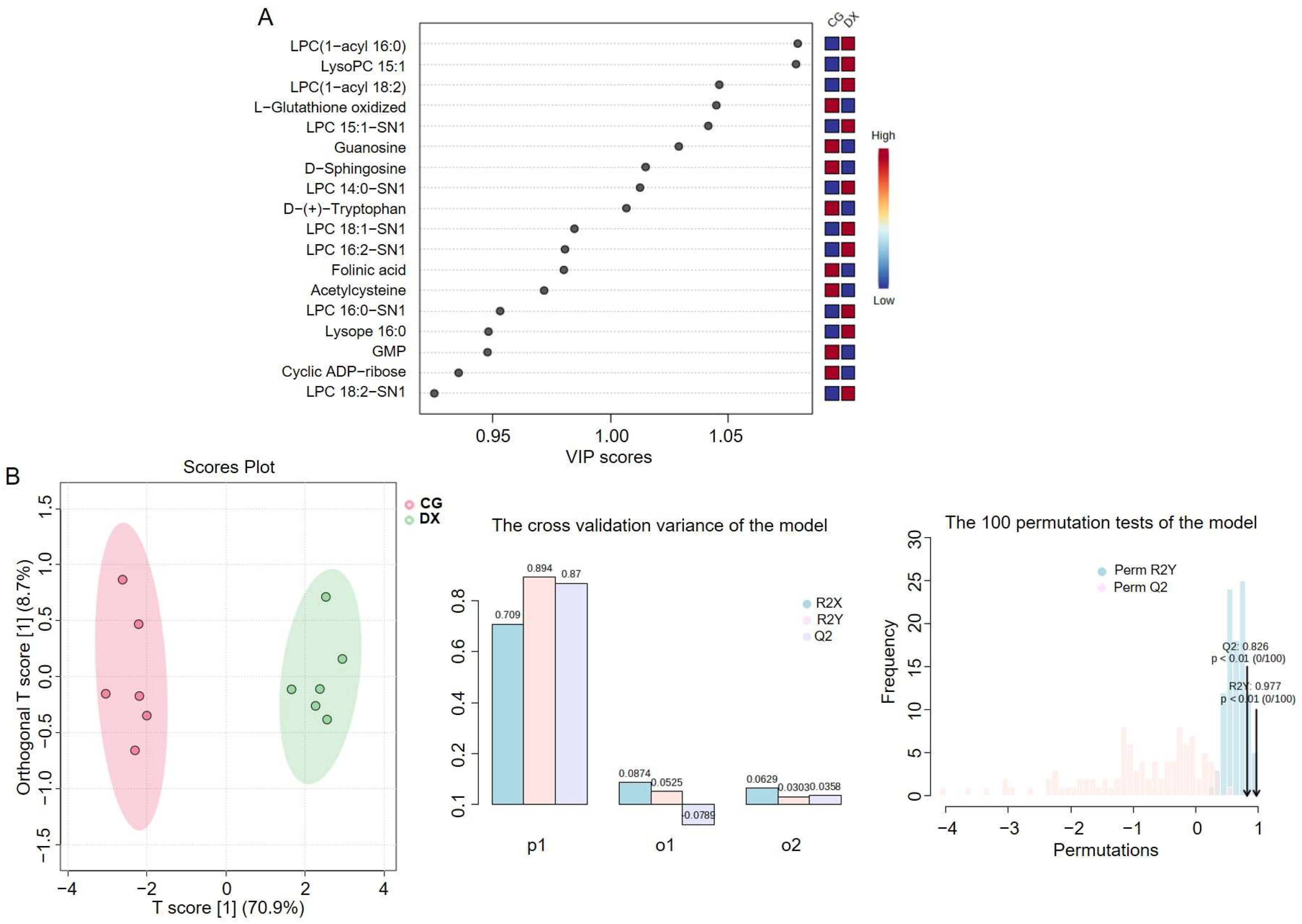
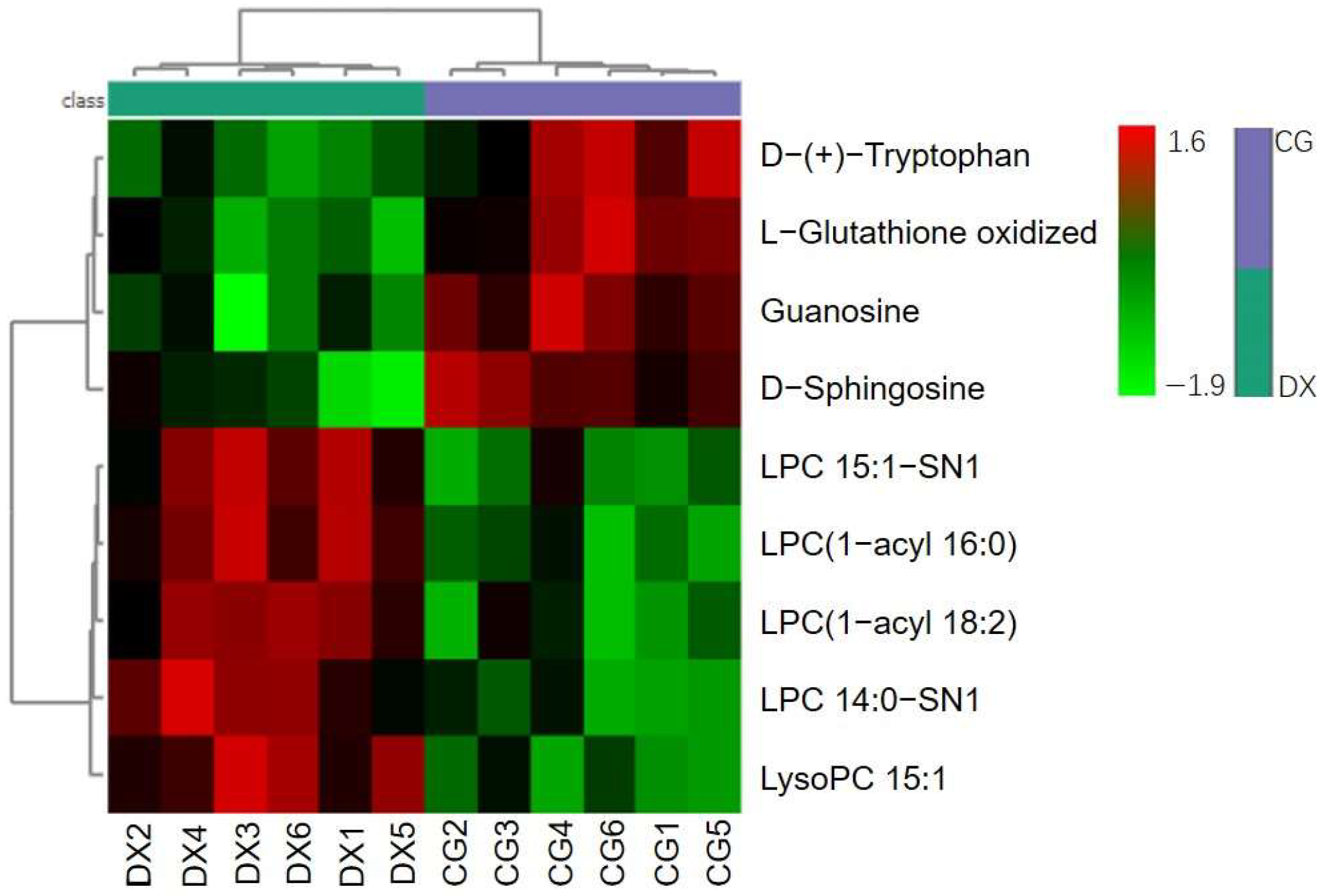
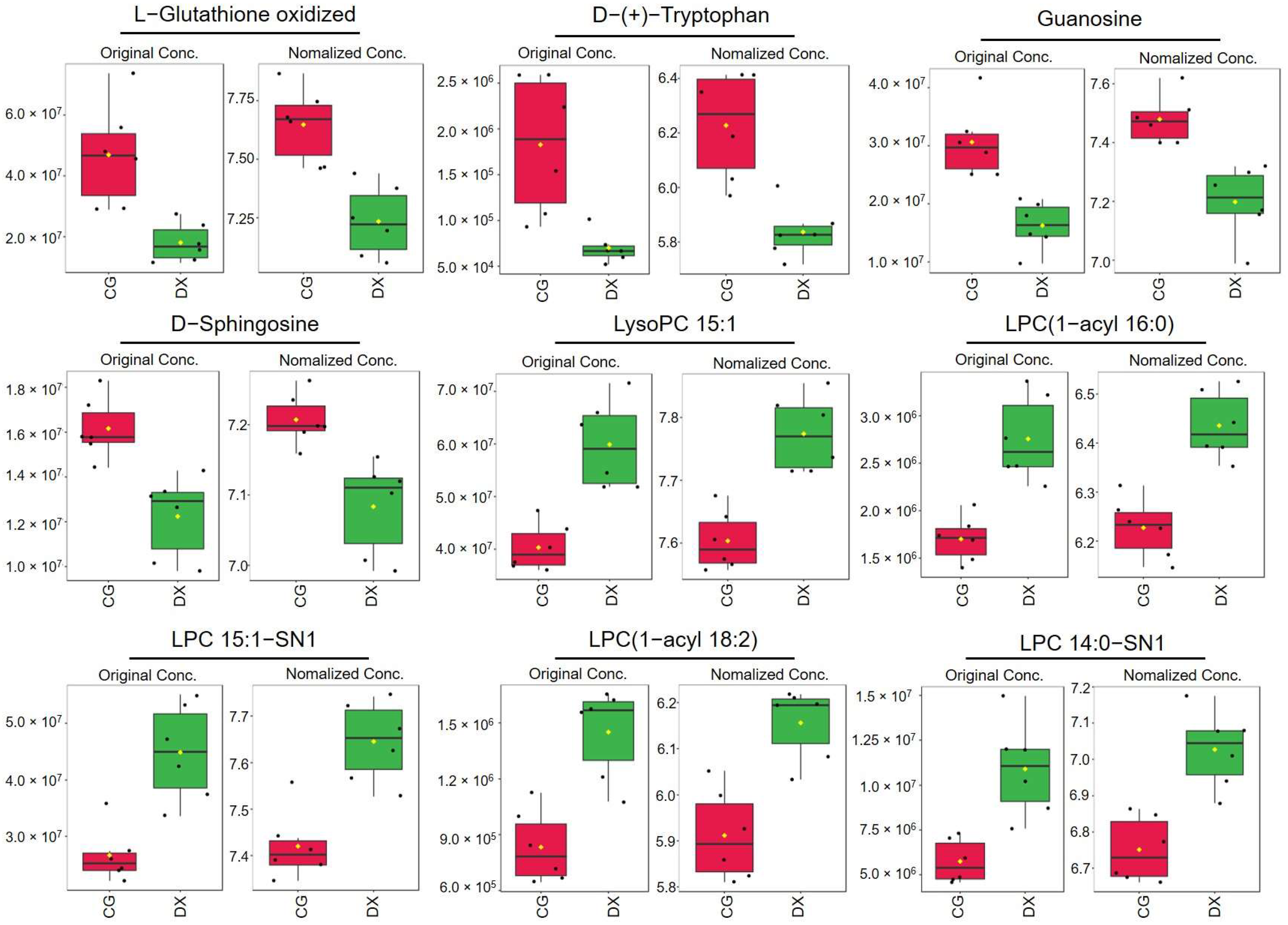
3.4. Metabolomic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liang, H.; Fu, Y.; Liu, Y.; Yang, C.; Zhang, T.; Wang, T.; Rong, L.; Zhang, S.; Wu, Z.; et al. Effects of different fertilization modes on rice yield and quality under a rice-crab culture system. PLoS ONE 2020, 15, e0230600. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, X.; Xiao, B.; Hu, K. Rice-crab coculture to sustain cleaner food production in Liaohe River Basin, China: An economic and environmental assessment. J. Clean. Product. 2019, 208, 188–198. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, J.; Ren, W.; Guo, L.; Cheng, Y.; Li, J.; Li, K.; Zhu, Z.; Zhang, J.; Luo, S.; et al. Can the co-cultivation of rice and fish help sustain rice production? Sci. Rep. 2016, 6, 28728. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Huang, L.; Zhang, Y.; Cao, N.; Guo, X.; Yao, C.; Li, X.; Duan, L.; Pang, S. Potential toxicity and dietary risk of tricyclazole to Chinese mitten crab (Eriocheir sinensis) in the rice-crab co-culture model. Environ. Pollut. 2023, 316 Pt 1, 120514. [Google Scholar] [CrossRef]
- Wei, D.; Xing, C.; Hou, D.; Zeng, S.; Zhou, R.; Yu, L.; Wang, H.; Deng, Z.; Weng, S.; He, J.; et al. Distinct bacterial communities in the environmental water, sediment and intestine between two crayfish-plant coculture ecosystems. Appl. Microbiol. Biot. 2021, 105, 5087–5101. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Y.; Yuan, J.; Zhang, X.; Ventura, T.; Ma, K.Y.; Sun, S.; Song, C.; Zhan, D.; Yang, Y.; et al. The Chinese mitten crab genome provides insights into adaptive plasticity and developmental regulation. Nat. Commu. 2021, 12, 2395. [Google Scholar] [CrossRef]
- Li, C.; Kang, X.; Nie, J.; Li, A.; Farag, M.A.; Liu, C.; Rogers, K.M.; Xiao, J.; Yuan, Y. Recent advances in Chinese food authentication and origin verification using isotope ratio mass spectrometry. Food Chem. 2023, 398, 133896. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Qi, B.; Yu, S.; Yao, Z.; Zhu, G.; Fu, Q.; Li, C. Inhibiting release of phenanthrene from rice-crab coculture sediments to overlying water with rice stalk biochar: Performance and mechanisms. Sci. Total Environ. 2024, 908, 168385. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, G.; Chen, T.; Zhao, M.; Zhang, Y.; Li, Y.; Xu, X.; Shao, S.; Zhu, J.; Wang, Q.; et al. Improved discrimination for brassica vegetables treated with agricultural fertilizers using a combined chemometric approach. J. Agric. Food Chem. 2016, 64, 5633–5643. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Abbaspour-Gilandeh, Y.; Heidarbeigi, K.; Momeny, M. Detection of fraud in ginger powder using an automatic sorting system based on image processing technique and deep learning. Comput. Biol. Med. 2021, 136, 104764. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska-Bartel, M.; Burns, D.T.; Elliott, C. Rice fraud a global problem: A review of analytical tools to detect species, country of origin and adulterations. Trends Food Sci. Technol. 2021, 116, 36–46. [Google Scholar] [CrossRef]
- Sheng, M.; Zhang, W.; Nie, J.; Li, C.; Zhu, A.X.; Hu, H.; Lou, W.; Deng, X.; Lyu, X.; Ren, Z.; et al. Predicting isoscapes based on an environmental similarity model for the geographical origin of Chinese rice. Food Chem. 2022, 397, 133744. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, W.; Zhang, Y.; Liu, Z.; Shao, S.; Zhou, L.; Rogers, K.M. Differentiating organically farmed rice from conventional and green rice harvested from an experimental field trial using stable isotopes and multi-element chemometrics. J. Agri. Food Chem. 2018, 66, 2607–2615. [Google Scholar] [CrossRef]
- Li, A.; Zhao, J.; Xi, J.; Yang, X.; Jin, X.; Chen, Q.; Pan, L. Geographical authentication of peach in China based on stable isotope combined with multielement analysis of peach juice. Food Control 2021, 127, 108126. [Google Scholar] [CrossRef]
- Nie, J.; Weng, R.; Li, C.; Liu, X.; Wang, F.; Rogers, K.M.; Qian, Y.; Zhang, Y.; Yuan, Y. Chemometric origin classification of Chinese garlic using sulfur-containing compounds, assisted by stable isotopes and bioelements. Food Chem. 2022, 394, 133557. [Google Scholar] [CrossRef]
- Peng, C.Y.; Ren, Y.F.; Ye, Z.H.; Zhu, H.Y.; Liu, X.Q.; Chen, X.T.; Hou, R.Y.; Granato, D.; Cai, H.M. A comparative UHPLC-Q/TOF-MS-based metabolomics approach coupled with machine learning algorithms to differentiate Keemun black teas from narrow-geographic origins. Food Res. Int. 2022, 158, 111512. [Google Scholar] [CrossRef]
- Yu, D.X.; Guo, S.; Zhang, X.; Yan, H.; Mao, S.W.; Wang, J.M.; Zhou, J.Q.; Yang, J.; Yuan, Y.W.; Duan, J.A. Combining stable isotope, multielement and untargeted metabolomics with chemometrics to discriminate the geographical origins of ginger (Zingiber officinale Roscoe). Food Chem. 2023, 426, 136577. [Google Scholar] [CrossRef]
- Shi, S.; Ma, Y.; Zhao, D.; Li, L.; Cao, C.; Jiang, Y. The differences in metabolites, starch structure, and physicochemical properties of rice were related to the decrease in taste quality under high nitrogen fertilizer application. Int. J. Biol. Macromo. 2023, 253 Pt 1, 126546. [Google Scholar] [CrossRef]
- Li, Z.; Tan, M.; Deng, H.; Yang, X.; Yu, Y.; Zhou, D.; Dong, H. Geographical origin differentiation of rice by LC-MS-based non-targeted metabolomics. Foods 2022, 11, 3318. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Zhang, D.; Qian, L. Discrimination of conventional and organic rice using untargeted LC-MS-based metabolomics. J. Cereal Sci. 2018, 82, 73–81. [Google Scholar] [CrossRef]
- Wen, H.; Shi, H.; Jiang, N.; Qiu, J.; Lin, F.; Kou, Y. Antifungal mechanisms of silver nanoparticles on mycotoxin producing rice false smut fungus. iScience 2022, 26, 105763. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, S.; Pan, Z.; Zhao, W.; Rui, Y.; Zhao, L. AgNPs-triggered seed metabolic and transcriptional reprogramming enhanced rice salt tolerance and blast resistance. ACS Nano 2023, 17, 492–504. [Google Scholar] [CrossRef]
- Cui, Z.; Ahmed, K.; Zaidi, S.; Muhammad, J. Ins and outs of cadmium-induced carcinogenesis: Mechanism and prevention. Cancer. Treat. Res. Commun. 2021, 27, 100372. [Google Scholar] [CrossRef]
- Chen, H.Q.; Zhang, M.Q.; Wu, G.H.; Chen, L.Z.; Pan, D.D. Accumulation and release characteristics of eriocheir sinensis for Pb and Cd. Acta Hydrobiol. Sinica 2010, 34, 828–836. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Q.; Li, G.; Guo, C.; Li, L.; Wang, J. Evaluating water management efficiency in regulating cadmium and arsenic accumulation in rice in typical japonica paddy soils at varied pH levels. Agriculture 2024, 14, 407. [Google Scholar] [CrossRef]
- Bao, J.; Jiang, H.B.; Li, X.D. Thirty years of rice-crab coculture in China—Research progress and prospects. Rev. Aquac. 2022, 14, 1597–1612. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Liu, Y.; Chen, C.; Tian, J.; Liu, Z.; Li, B.; Mei, X.; Chen, Y.; Zhang, Y.; et al. Geographical origin traceability of sweet cherry (Prunus avium (L.) Moench) in China using stable isotope and multi-element analysis with multivariate modeling. Food Chem. X 2024, 23, 101477. [Google Scholar] [CrossRef]
- Yan, H.; Li, P.H.; Zhou, G.S.; Wang, Y.J.; Bao, B.H.; Wu, Q.N.; Huang, S.L. Rapid and practical qualitative and quantitative evaluation of non-fumigated ginger and sulfur-fumigated ginger via Fourier-transform infrared spectroscopy and chemometric methods. Food Chem. 2021, 341, 128241. [Google Scholar] [CrossRef]
- Zhao, J.; Li, A.; Jin, X.; Liang, G.; Pan, L. Discrimination of geographical origin of agricultural products from small-scale districts by widely targeted metabolomics with a case study on pinggu peach. Front. Nutr. 2022, 9, 891302. [Google Scholar] [CrossRef]
- Rogers, K.M. Nitrogen isotopes as a screening tool to determine the growing regimen of some organic and nonorganic supermarket produce from New Zealand. J. Agric. Food Chem. 2008, 56, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- De Bauw, P.; Bod’e, S.; Perneel, M.; Billard, D.; Boeckx, P. Nitrogen fertilizer classification using multivariate fingerprinting with stable isotopes. Nutr. Cycl. Agroecosyst. 2024, 129, 521–534. [Google Scholar] [CrossRef]
- Xiong, F.; Yuan, Y.; Li, C.; Lyu, C.; Wan, X.; Nie, J.; Li, H.; Yang, J.; Guo, L. Stable isotopic and elemental characteristics with chemometrics for the geographical origin authentication of Dendrobium officinale at two spatial scales. LWT-Food Sci. Technol. 2022, 167, 113871. [Google Scholar] [CrossRef]
- Meng, Y.; Jin, B.; Rogers, K.M.; Zhou, H.; Song, X.; Zhang, Y.; Lin, G.; Wu, H. Hydrogen and oxygen isotope fractionation effects in different organ tissues of grapes under drought conditions. J. Agric. Food Chem. 2023, 71, 13662–13671. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, P.; Sun, J.; Li, X.; Wang, T.; Zhou, Q.; Xie, Z.; Jiang, C.; Wang, J. Metabolomics and molecular networking approaches reveal differential metabolites of Radix Scrophulariae from different geographical origins: Correlations with climatic factors and biochemical compounds in soil. Ind. Crops Prod. 2021, 174, 114169. [Google Scholar] [CrossRef]
- Liang, L.; Li, Y.; Mao, X.; Wang, Y. Metabolomics applications for plant-based foods origin tracing, cultivars identification and processing: Feasibility and future aspects. Food Chem. 2024, 449, 139227. [Google Scholar] [CrossRef]
- Han, Q.; Erasmus, S.W.; Elliott, C.T.; van Ruth, S.M. A sense of ginger fraud: Prevalence and deconstruction of the China-European union supply chain. NPJ Sci. Food 2022, 6, 51. [Google Scholar] [CrossRef]
- Long, N.P.; Lim, D.K.; Mo, C.; Kim, G.; Kwon, S.W. Development and assessment of a lysophospholipid-based deep learning model to discriminate geographical origins of white rice. Sci. Rep. 2017, 7, 8552. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, X.; Shang, B.; Hong, Y.; Sun, H. Analysis of lipidomics profile of rice and changes during storage by UPLC-Q-extractive orbitrap mass spectrometry. Food Res. Int. 2021, 142, 110214. [Google Scholar] [CrossRef]
- Tong, C.; Liu, L.; Waters, D.L.; Rose, T.J.; Bao, J.; King, G.J. Genotypic variation in lysophospholipids of milled rice. J. Agric. Food Chem. 2014, 62, 9353–9361. [Google Scholar] [CrossRef]
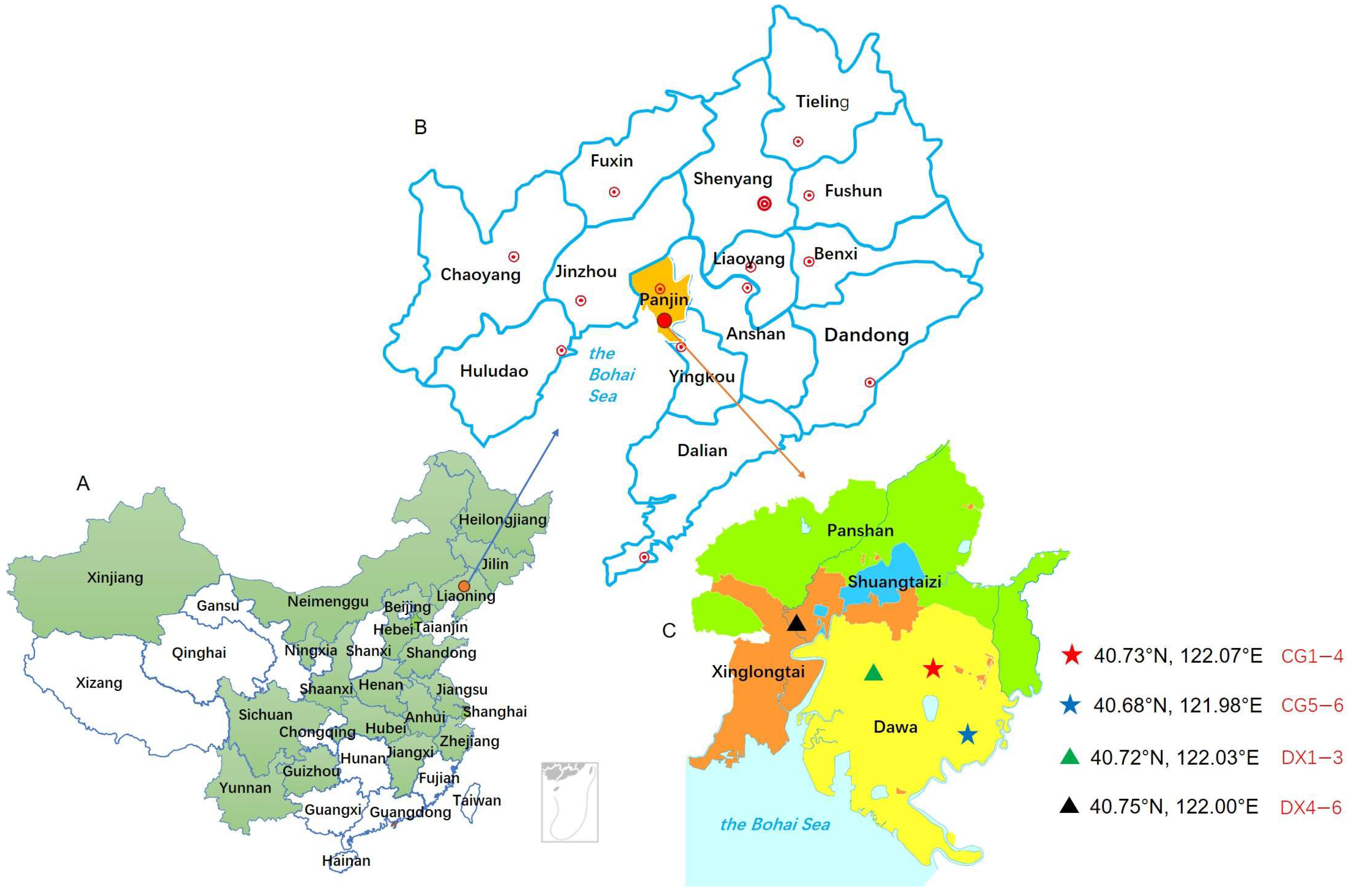


| Sample Group Number | Sampling Sites | Planting Patterns | Rice Variety |
|---|---|---|---|
| CG1 | Liujia Village, Tangjia Town, Dawa District, Panjin City | Traditional planting mode | Yanfeng 47 |
| CG2 | Liujia Village, Tangjia Town, Dawa District, Panjin City | Traditional planting mode | Yanfeng 47 |
| CG3 | Liujia Village, Tangjia Town, Dawa District, Panjin City | Traditional planting mode | Yanfeng 47 |
| CG4 | Liujia Village, Tangjia Town, Dawa District, Panjin City | Traditional planting mode | Yanfeng 47 |
| CG5 | Bianwazi Village, Xi’an Town, Dawa District, Panjin City | Traditional planting mode | Yanfeng 47 |
| CG6 | Bianwazi Village, Xi’an Town, Dawa District, Panjin City | Traditional planting mode | Yanfeng 47 |
| DX1 | Sanjiazi Village, Chengjiao Township, Dawa Street, Panjin City | Ecological planting mode of rice–crab co-cultivation | Yanfeng 47 |
| DX2 | Sanjiazi Village, Chengjiao Township, Dawa Street, Panjin City | Ecological planting mode of rice–crab co-cultivation | Yanfeng 47 |
| DX3 | Sanjiazi Village, Chengjiao Township, Dawa Street, Panjin City | Ecological planting mode of rice–crab co-cultivation | Yanfeng 47 |
| DX4 | Xinsheng Street, Xinglongtai District, Panjin City | Ecological planting mode of rice–crab co-cultivation | Yanfeng 47 |
| DX5 | Xinsheng Street, Xinglongtai District, Panjin City | Ecological planting mode of rice–crab co-cultivation | Yanfeng 47 |
| DX6 | Xinsheng Street, Xinglongtai District, Panjin City | Ecological planting mode of rice–crab co-cultivation | Yanfeng 47 |
| Reagents | Purity | Brand | Origin |
|---|---|---|---|
| Methanol | LC-MS Grade | Thermo Fisher, Waltham, MA, USA | USA |
| H2O | LC-MS Grade | Merck, Darmstadt, Germany | Germany |
| Formic acid | LC-MS Grade | Thermo Fisher | USA |
| Ammonium acetate | LC-MS Grade | Thermo Fisher | USA |
| Nitric acid | Guaranteed reagent | Merck | Germany |
| Standard for multi-element analysis (GBW10043) | — | National Research Center for Certified Reference Materials | China |
| Standards for multi-element analysis (USGS40, USGS90, USGS91,USGS55) | — | United States Geological Survey | USA |
| Name | Model | Brand | Origin |
|---|---|---|---|
| ICP-OES | 5800 | Agilent, Santa Clara, CA, USA | USA |
| ICP-MS | 7900 | Agilent | USA |
| Graphite digestion instrument | ST36-iTOUCH | LabTech, Cambridge, MA, USA | USA |
| Elemental analyzer | Vario PYRO cube | Elementar, Langenselbold, Germany | Germany |
| Isotope ratio mass spectrometer | Isoprime 100 | Elementar | Germany |
| Low-temperature centrifuge | D3024R | Scilogex, Tarrytown, NY, USA | USA |
| Orbitrap liquid chromatography-mass spectrometer | Q Exactive™ HF/Q Exactive™ HF-X | Thermo Fisher | USA |
| Chromatographic column | Hypesil Gold column C18 (100 × 2.1 mm, 1.9 μm) | Thermo Fisher | USA |
| Element/Unit | CG | DX |
|---|---|---|
| Cu (mg/kg) * | 2.274 ± 0.300 | 1.427 ± 0.472 |
| Zn (mg/kg) | 13.368 ± 2.160 | 11.155 ± 1.385 |
| K (g/100 g) | 0.099 ± 0.025 | 0.107 ± 0.014 |
| P (mg/kg) | 0.112 ± 0.025 | 0.112 ± 0.012 |
| Mg (g/100 g) | 0.032 ± 0.007 | 0.033 ± 0.005 |
| Ca (g/100 g) | 0.008 ± 0.002 | 0.007 ± 0.001 |
| Mn (mg/kg) | 10.207 ± 3.375 | 8.528 ± 1.303 |
| Fe (g/100 g) | 4.462 ± 2.662 | 6.450 ± 2.148 |
| As (mg/kg) | 0.167 ± 0.034 | 0.185 ± 0.020 |
| Cd (mg/kg) * | 0.004 ± 0.003 | 0.001 ± 0.001 |
| Pb (mg/kg) | 0.295 ± 0.181 | 0.253 ± 0.156 |
| Al (g/100 g) | 0.003 ± 0.002 | 0.003 ± 0.001 |
| Rb (mg/kg) * | 0.617 ± 0.225 | 0.264 ± 0.094 |
| Se (mg/kg) * | 0.031 ± 0.009 | 0.012 ± 0.004 |
| Sr (mg/kg) | 0.269 ± 0.065 | 0.313 ± 0.038 |
| V (mg/kg) | 0.002 ± 0.001 | 0.003 ± 0.002 |
| Co (mg/kg) | 0.003 ± 0.002 | 0.010 ± 0.013 |
| Ni (mg/kg) | 0.054 ± 0.015 | 0.053 ± 0.029 |
| Ga (mg/kg) | 0.001 ± 0.001 | 0.001 ± 0.001 |
| Cs (mg/kg) | 0.002 ± 0.001 | 0.002 ± 0.001 |
| Ag (mg/kg) * | 0.013 ± 0.005 | 0.022 ± 0.006 |
| Cr (mg/kg) | 0.044 ± 0.024 | 0.055 ± 0.040 |
| Ba (mg/kg) | 0.411 ± 0.558 | 0.212 ± 0.206 |
| Mode | δ13C (‰) | δ15N (‰) | δ2H (‰) | δ18O (‰) |
|---|---|---|---|---|
| CG | −27.188 ± 0.207 | 3.609 ± 0.749 | −48.397 ± 2.332 | 22.934 ± 0.519 |
| DX | −27.469 ± 0.142 * | 5.485 ± 0.500 * | −46.712 ± 3.586 | 23.598 ± 0.542 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Li, L.; Peng, T.; Lin, Q.; Li, G.; Guo, C.; Zou, X.; Wang, J. Classification of Crab-Field Rice and Conventional Rice Based on Multi-Element, Stable Isotope, and Non-Targeted Metabolome Combined with Chemometrics. Foods 2025, 14, 1853. https://doi.org/10.3390/foods14111853
Wu X, Li L, Peng T, Lin Q, Li G, Guo C, Zou X, Wang J. Classification of Crab-Field Rice and Conventional Rice Based on Multi-Element, Stable Isotope, and Non-Targeted Metabolome Combined with Chemometrics. Foods. 2025; 14(11):1853. https://doi.org/10.3390/foods14111853
Chicago/Turabian StyleWu, Xianxin, Lina Li, Tianshu Peng, Qiujun Lin, Guang Li, Chunjing Guo, Xun Zou, and Jianzhong Wang. 2025. "Classification of Crab-Field Rice and Conventional Rice Based on Multi-Element, Stable Isotope, and Non-Targeted Metabolome Combined with Chemometrics" Foods 14, no. 11: 1853. https://doi.org/10.3390/foods14111853
APA StyleWu, X., Li, L., Peng, T., Lin, Q., Li, G., Guo, C., Zou, X., & Wang, J. (2025). Classification of Crab-Field Rice and Conventional Rice Based on Multi-Element, Stable Isotope, and Non-Targeted Metabolome Combined with Chemometrics. Foods, 14(11), 1853. https://doi.org/10.3390/foods14111853






