Ecological Packaging: Reuse and Recycling of Rosehip Waste to Obtain Biobased Multilayer Starch-Based Material and PLA for Food Trays
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Rosehip Fluff Residue
2.3. Elaboration of Sustainable Materials
2.4. Design of Multilayer Trays
2.5. Physicochemical Properties of the Materials
2.5.1. Color
2.5.2. Surface Roughness
2.5.3. Thickness
2.5.4. Density
2.5.5. Water Content and Surface Wettability
2.5.6. Water Absorption Capacity and Solubility
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Scanning Electron Microscopy (SEM)
2.8. Mechanical Properties
2.9. Statistical Analysis
3. Results and Discussion
3.1. Composition of Rosehip Oil Extraction Residue
3.2. Physicochemical Characterization of the Laminates
3.3. Characteristics of the Developed Multilayer Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric sustainable materials and their emerging applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Sivertsvik, M.; Rosnes, J.T.; Popa, E.E.; Dumitriu, R.P.; Vasile, C. PLA-based materials containing bio-plasticizers and chitosan modified with rosehip seed oil for ecological packaging. Polymers 2021, 13, 1610. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Sreedevi, K.; Sharma, S.S.; Anjana, V.N. Polylactic Acid (PLA) Applications. In Handbook of Biopolymers; Springer Nature: Berlin, Germany, 2023; pp. 1–33. [Google Scholar] [CrossRef]
- Hosen, M.D.; Hossain, M.S.; Islam, M.A.; Haque, A.N.M.A.; Naebe, M. Utilisation of natural wastes: Water-resistant semi-transparent paper for food packaging. J. Clean. Prod. 2022, 364, 132665. [Google Scholar] [CrossRef]
- Ortega, F.; Versino, F.; López, O.V.; García, M.A. Biobased composites from agro-industrial wastes and by-products. Emergent Mater. 2022, 5, 873–921. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, E.; Minichiello, C.; Sisti, L.; Totaro, G.; Baffoni, L.; Di Gioia, D.; Saccani, A. From food waste to eco-friendly functionalized polymer composites: Investigation of orange peels as active filler. New Biotechnol. 2024, 80, 37–45. [Google Scholar] [CrossRef]
- Sethulakshmi, A.G.; Saravanakumar, M.P. Sustainable papaya plant waste and green tea residue composite films integrated with starch and gelatin for active food packaging applications. Int. J. Biol. Macromol. 2024, 260, 129153. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Molina, G.; Pelissari, F.M. Biodegradable trays based on cassava starch blended with agroindustrial residues. Compos. Part B Eng. 2020, 183, 107682. [Google Scholar] [CrossRef]
- Rodríguez, L.J.; Fabbri, S.; Orrego, C.E.; Owsianiak, M. Comparative life cycle assessment of coffee jar lids made from biocomposites containing poly (lactic acid) and banana fiber. J. Environ. Manag. 2020, 266, 110493. [Google Scholar] [CrossRef]
- Versino, F.; López, O.V.; García, M.A. Sunflower Oil Industry By-product as Natural Filler of Biocomposite Foams for Packaging Applications. J. Polym. Environ. 2021, 29, 1869–1879. [Google Scholar] [CrossRef]
- Mohammed, A.; Gaduan, A.; Chaitram, P.; Pooran, A.; Lee, K.Y.; Ward, K. Sargassum inspired, optimized calcium alginate bioplastic composites for food packaging. Food Hydrocoll. 2023, 135, 108192. [Google Scholar] [CrossRef]
- Silva, J.M.; Vilela, C.; Girão, A.V.; Branco, P.C.; Martins, J.; Freire, M.G.; Freire, C.S. Wood inspired biobased nanocomposite films composed of xylans, lignosulfonates and cellulose nanofibers for active food packaging. Carbohydr. Polym. 2024, 337, 122112. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Cheng, C.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Active gellan gum/purple sweet potato composite films capable of monitoring pH variations. Food Hydrocoll. 2017, 69, 491–502. [Google Scholar] [CrossRef]
- Kanatt, R.S. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Doğan, C.; Doğan, N.; Gungor, M.; Eticha, A.K.; Akgul, Y. Novel active food packaging based on centrifugally spun nanofibers containing lavender essential oil: Rapid fabrication, characterization, and application to preserve of minced lamb meat. Food Packag. Shelf Life 2022, 34, 100942. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Mayandi, V.; Patel, M.; Swathi, B.; Vishwakarma, J.; Dhand, C. Mussel-inspired chemistry to design biodegradable food packaging films with antimicrobial properties. Process Saf. Environ. Prot. 2022, 162, 17–29. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Oxygen absorbing food packaging made by extrusion compounding of thermoplastic cassava starch with gallic acid. Food Control 2022, 142, 109273. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, K.; Qin, Z.; Zou, Y.; Zhong, H.; Zhang, H. Cross-linked gluten/zein nanofibers via Maillard reaction with the loading of star anise essential oil/β-cyclodextrin inclusions for food-active packaging. Food Packag. Shelf Life 2022, 34, 100950. [Google Scholar] [CrossRef]
- Almeida, T.; Karamysheva, A.; Valente, B.F.; Silva, J.M.; Braz, M.; Almeida, A.; Freire, C.S. Biobased ternary films of thermoplastic starch, bacterial nanocellulose and gallic acid for active food packaging. Food Hydrocoll. 2023, 144, 108934. [Google Scholar] [CrossRef]
- Alves, Z.; Brites, P.; Ferreira, N.M.; Figueiredo, G.; Otero-Irurueta, G.; Gonçalves, I.; Nunes, C. Thermoplastic starch-based films loaded with biochar-ZnO particles for active food packaging. J. Food Eng. 2024, 361, 111741. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and bio-based food packaging: A review on past and current design innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of polymer-based multilayer packaging: A review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- Reis, M.O.; Olivato, J.B.; Bilck, A.P.; Zanela, J.; Grossmann, M.V.E.; Yamashita, F. Biodegradable trays of thermoplastic starch/poly (lactic acid) coated with beeswax. Ind. Crops Prod. 2018, 112, 481–487. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Sustainable panels design based on modified cassava starch bioadhesives and wood processing byproducts. Ind. Crops Prod. 2019, 137, 171–179. [Google Scholar] [CrossRef]
- Monroy, Y.; García, M.A.; Deladino, L.; Rivero, S. Valorization of a by-product of the yerba mate industry by assembling with cassava starch adhesive for packaging material production. Int. J. Biol. Macromol. 2024, 266, 131271. [Google Scholar] [CrossRef]
- Mireles, B.J.H.; Díaz, E.D.R.C.; Medina, R.S.C. Ecological trays based on banana (Musa paradisiaca) and achira (Canna indica) leaf blades: Physical, mechanical and chemical characteristics. Agroindustrial Sci. 2021, 11, 87–96. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Svriz, M.; Rouco, C.; Ostrowska, A.; Gadzinowska, J.; Urban, K.; Pawłowska, B. Physiological and molecular features predispose native and invasive populations of sweet briar (Rosa rubiginosa L.) to colonization and restoration of drought degraded environments. Perspect. Plant Ecol. Evol. Syst. 2022, 56, 125690. [Google Scholar] [CrossRef]
- Torres-Sciancalepore, R.; Riveros-Gomez, M.; Zalazar-García, D.; Asensio, D.; Rodriguez, R.; Mazza, G. Two-step valorization of invasive species Rosa rubiginosa L. husk waste through eco-friendly optimized pectin extraction and subsequent pyrolysis. J. Environ. Chem. Eng. 2023, 11, 110802. [Google Scholar] [CrossRef]
- Resolution No. 109/21. Ministerio de Ambiente y Desarrollo Sostenible, República Argentina. Available online: https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-109-2021-348718/texto (accessed on 18 April 2024).
- Quiroga, J.M. Análisis preliminar de la cadena de valor de la rosa mosqueta en Bariloche y zona de influencia, Argentina. SaberEs 2019, 11, 65–80. [Google Scholar] [CrossRef]
- Roggero-Luque, J.M.; Rajchenberg, M.; Barroetaveña, C. Assessment of lignocellulosic residues from Northern Patagonian Andes (Argentina) for cultivation of Pleurotus ostreatus. Univ. Sci. 2021, 26, 159–177. [Google Scholar]
- Torres-Sciancalepore, R.; Asensio, D.; Nassini, D.; Fernandez, A.; Rodriguez, R.; Fouga, G.; Mazza, G. Assessment of the behavior of Rosa rubiginosa seed waste during slow pyrolysis process towards complete recovery: Kinetic modeling and product analysis. Energy Convers. Manag. 2022, 272, 116340. [Google Scholar] [CrossRef]
- Widyorini, R.; Umemura, K.; Kusumaningtyas, A.R.; Prayitno, T.A. Effect of starch addition on properties of citric acid-bonded particleboard made from bamboo. BioResources 2017, 12, 8068–8077. [Google Scholar] [CrossRef]
- Gebresas, G.A.; Szabó, T.; Marossy, K. A comparative study of carboxylic acids on the cross-linking potential of corn starch films. J. Mol. Struct. 2023, 1277, 134886. [Google Scholar] [CrossRef]
- Monroy, Y.; Seré, P.; Rivero, S.; García, M.A. Sustainable panels based on starch bioadhesives: An insight into structural and tribological performance. Int. J. Biol. Macromol. 2020, 148, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Park, J.Y.; Park, E.Y. Effect of ethanol, phytic acid and citric acid treatment on the physicochemical and heavy metal adsorption properties of corn starch. Food Chem. 2024, 431, 137167. [Google Scholar] [CrossRef]
- Cai, L.; Chen, Y.; Lu, Z.; Wei, M.; Zhao, X.; Xie, Y.; Li, J.; Xiao, S. Citric acid/chitosan adhesive with viscosity-controlled for wood bonding through supramolecular self-assembly. Carbohydr. Polym. 2024, 329, 121765. [Google Scholar] [CrossRef]
- Monteiro, S.; Martins, J.; Magalhães, F.D.; Carvalho, L. Low density wood particleboards bonded with starch foam-study of production process conditions. Materials 2019, 12, 1975. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Monroy, Y. Desarrollo de Bioadhesivos a Base de Almidones Modificados con Aplicaciones Potenciales en el Área De Envases. Doctoral Thesis, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, La Plata, Argentina, 2021. [Google Scholar] [CrossRef]
- Rhim, J.W.; Mohanty, A.K.; Singh, S.P. Effect of the processing methods on the performance of polylactide films: Thermocompression versus solvent casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742. [Google Scholar] [CrossRef]
- Salgado, P.R.; Schmidt, V.C.; Ortiz, S.E.M.; Mauri, A.N.; Laurindo, J.B. Biodegradable foams based on cassava starch, sunflower proteins and cellulose fibers obtained by a baking process. J. Food Eng. 2008, 85, 435–443. [Google Scholar] [CrossRef]
- Rivero, S.; Garcia, M.A.; Pinotti, A. Composite and bi-layer films based on gelatin and chitosan. J. Food Eng. 2009, 90, 531–539. [Google Scholar] [CrossRef]
- Versino, F.; López, O.V.; García, M.A. Exploitation of by-products from cassava and ahipa starch extraction as filler of thermoplastic corn starch. Compos. Part B Eng. 2020, 182, 107653. [Google Scholar] [CrossRef]
- Serena, A.; Knudsen, K.B. Chemical and physicochemical characterisation of co-products from the vegetable food and agro industries. Anim. Feed. Sci. Technol. 2007, 139, 109–124. [Google Scholar] [CrossRef]
- Osman, N.S.; Sapawe, N.; Sapuan, M.A.U.; Fozi, M.F.M.; Azman, M.H.I.F.; Fazry, A.H.Z.; Hanafi, M.F. Sunflower shell waste as an alternative animal feed. Mater. Today Proc. 2018, 5, 21905–21910. [Google Scholar] [CrossRef]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Mardones, C.; Von Baer, D.; Bautista-Baños, S.; Dublan Garcia, O. Physical-chemical evaluation of active food packaging material based on thermoplastic starch loaded with grape cane extract. Molecules 2020, 25, 1306. [Google Scholar] [CrossRef]
- Jimenez, P.V. Uso de Residuos de Garlopa y Cepilladora de las Especies Prosopis Alba y Pinus sp. en Aglomerados. Trabajo Final de Grado; Instituto de Tecnología de la Madera, Facultad de Ciencias Forestales, Universidad Nacional de Santiago del Estero: Santiago del Estero, Argentina, 2013; Available online: https://fcf.unse.edu.ar/archivos/biblioteca/ (accessed on 12 December 2024).
- Duarte, G.A.; Bezerra, M.C.; Bettini, S.H.; Lucas, A.A. Real-time monitoring of the starch cross-linking with citric acid by chemorheological analysis. Carbohydr. Polym. 2023, 311, 120733. [Google Scholar] [CrossRef]
- Hassani, F.Z.S.A.; Salim, M.H.; Kassab, Z.; Sehaqui, H.; Ablouh, E.H.; Bouhfid, R. Crosslinked starch-coated cellulosic papers as alternative food-packaging materials. RSC Adv. 2022, 12, 8536–8546. [Google Scholar] [CrossRef]
- Ramos, P.M.; Gil, J.M.; Ramos Sánchez, M.C.; Navas Gracia, L.M.; Hernández Navarro, S.; Martín Gil, F.J. Vibrational and thermal characterization of seeds, pulp, leaves and seed oil of Rosa rubiginosa. Boletín de la Sociedad Argentina de Botánica 2016, 51, 429–439. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, A.; Wang, S.; Zhang, Q. Effect of different heat treatments temperatures on the chemical composition and structure of chinese fir wood. BioResources 2016, 11, 4006–4016. [Google Scholar] [CrossRef]
- Ulker, O. Surface roughness of composite panels as a quality control tool. Materials 2018, 11, 407. [Google Scholar] [CrossRef]
- Ahmad, D.; Van Den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Uranga, J.; Nguyen, B.T.; Si, T.T.; Guerrero, P.; de la Caba, K. The Effect of Cross-Linking with Citric Acid on the Properties of Agar/Fish Gelatin Films. Polymers 2020, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; You, X.; Rissanen, M.; Schlapp-Hackl, I.; Sixta, H. Sustainable cross-linking of man-made cellulosic fibers with poly (carboxylic acids) for fibrillation control. ACS Sustain. Chem. Eng. 2021, 9, 16749–16756. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Assadpour, E.; Cong, X.; Jafari, S.M. Cross-linked biopolymeric films by citric acid for food packaging and preservation. Adv. Colloid Interface Sci. 2023, 314, 102886. [Google Scholar] [CrossRef] [PubMed]
- Pornsuksomboon, K.; Holló, B.B.; Szécsényi, K.M.; Kaewtatip, K. Properties of baked foams from citric acid modified cassava starch and native cassava starch blends. Carbohydr. Polym. 2016, 136, 107–112. [Google Scholar] [CrossRef]
- Kaisangsri, N.; Kerdchoechuen, O.; Laohakunjit, N. Characterization of cassava starch based foam blended with plant proteins, kraft fiber, and palm oil. Carbohydr. Polym. 2014, 110, 70–77. [Google Scholar] [CrossRef]
- Ingrao, C.; Giudice, A.L.; Bacenetti, J.; Khaneghah, A.M.; Sant’Ana, A.S.; Rana, R.; Siracusa, V. Foamy polystyrene trays for fresh-meat packaging: Life-cycle inventory data collection and environmental impact assessment. Food Res. Int. 2015, 76, 418–426. [Google Scholar] [CrossRef]
- Razza, F.; Degli Innocenti, F.; Dobon, A.; Aliaga, C.; Sanchez, C.; Hortal, M. Environmental profile of a bio-based and biodegradable foamed packaging prototype in comparison with the current benchmark. J. Clean. Prod. 2015, 102, 493–500. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Chen, M.; Zhao, P.; Wang, Y.; Wang, X.; Han, X.; Wang, J. Development and characterization of biodegradable bilayer packaging films based on corn starch-polylactic acid as raw material. J. Food Meas. Charact. 2024, 18, 625–639. [Google Scholar] [CrossRef]

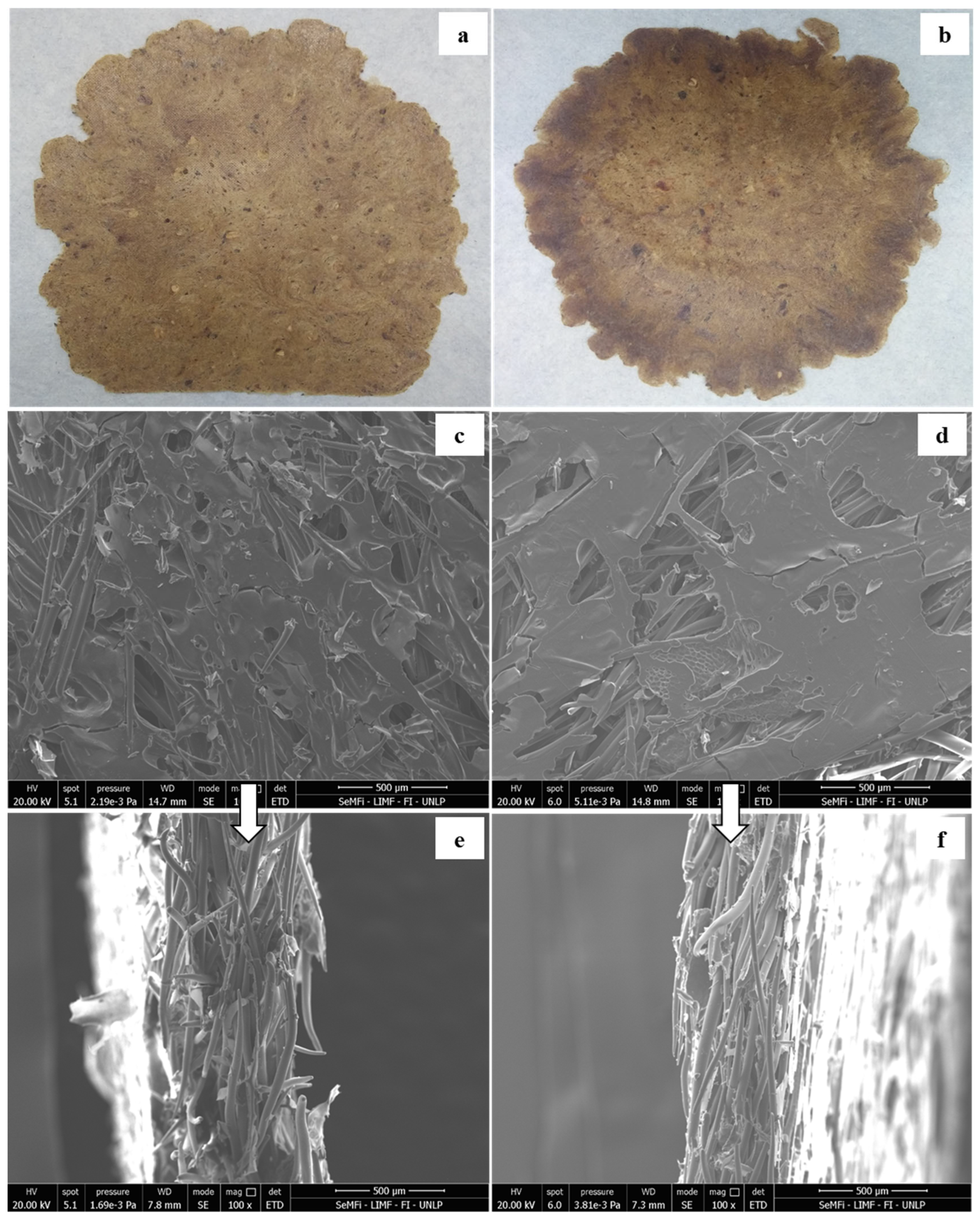
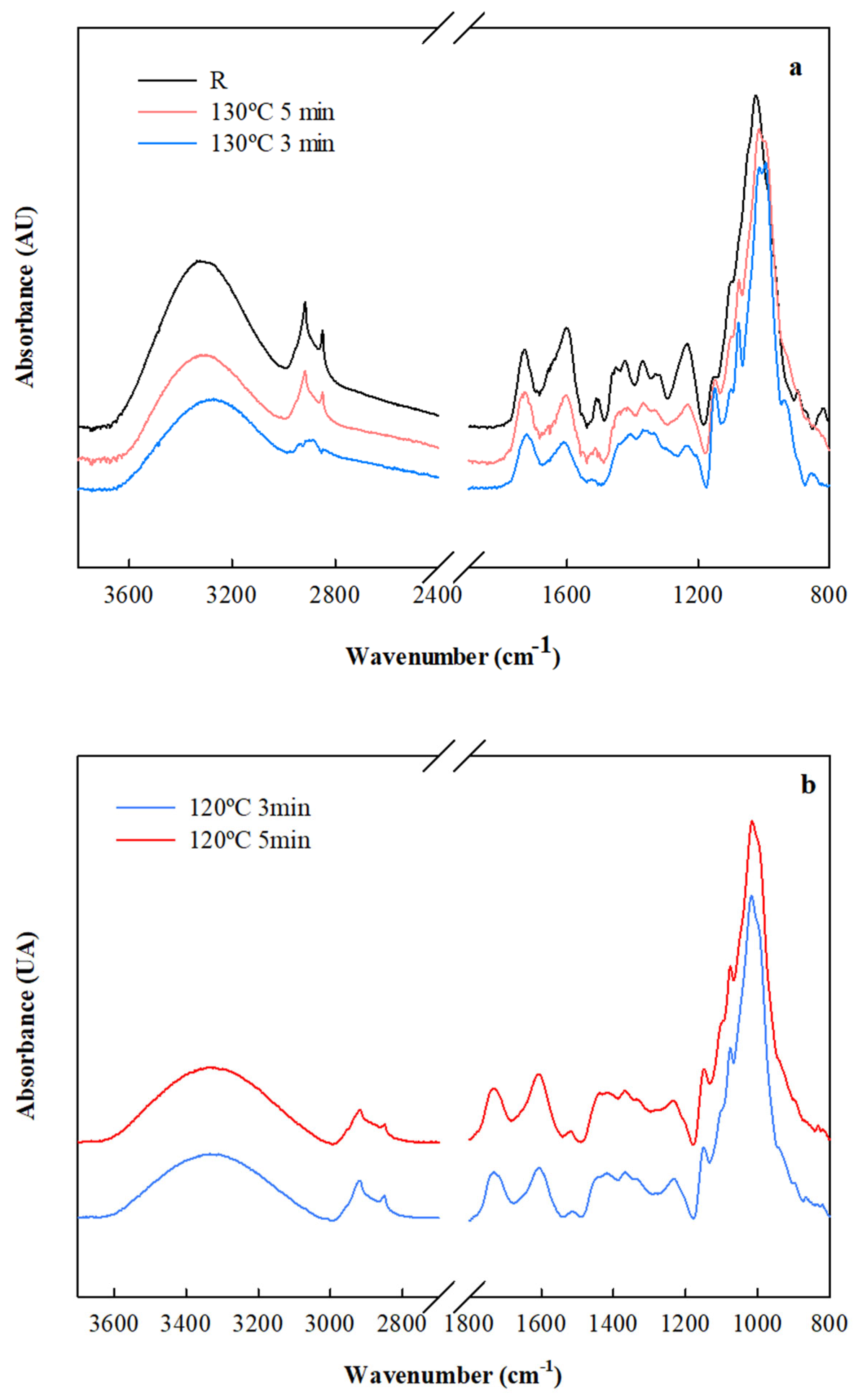


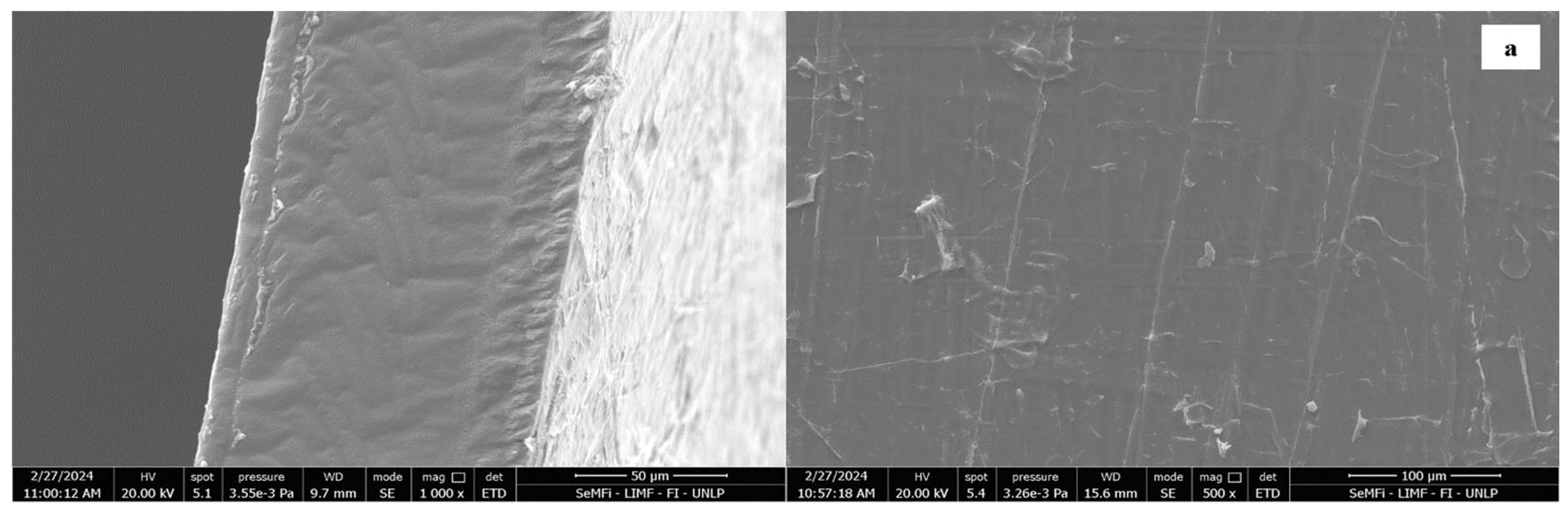

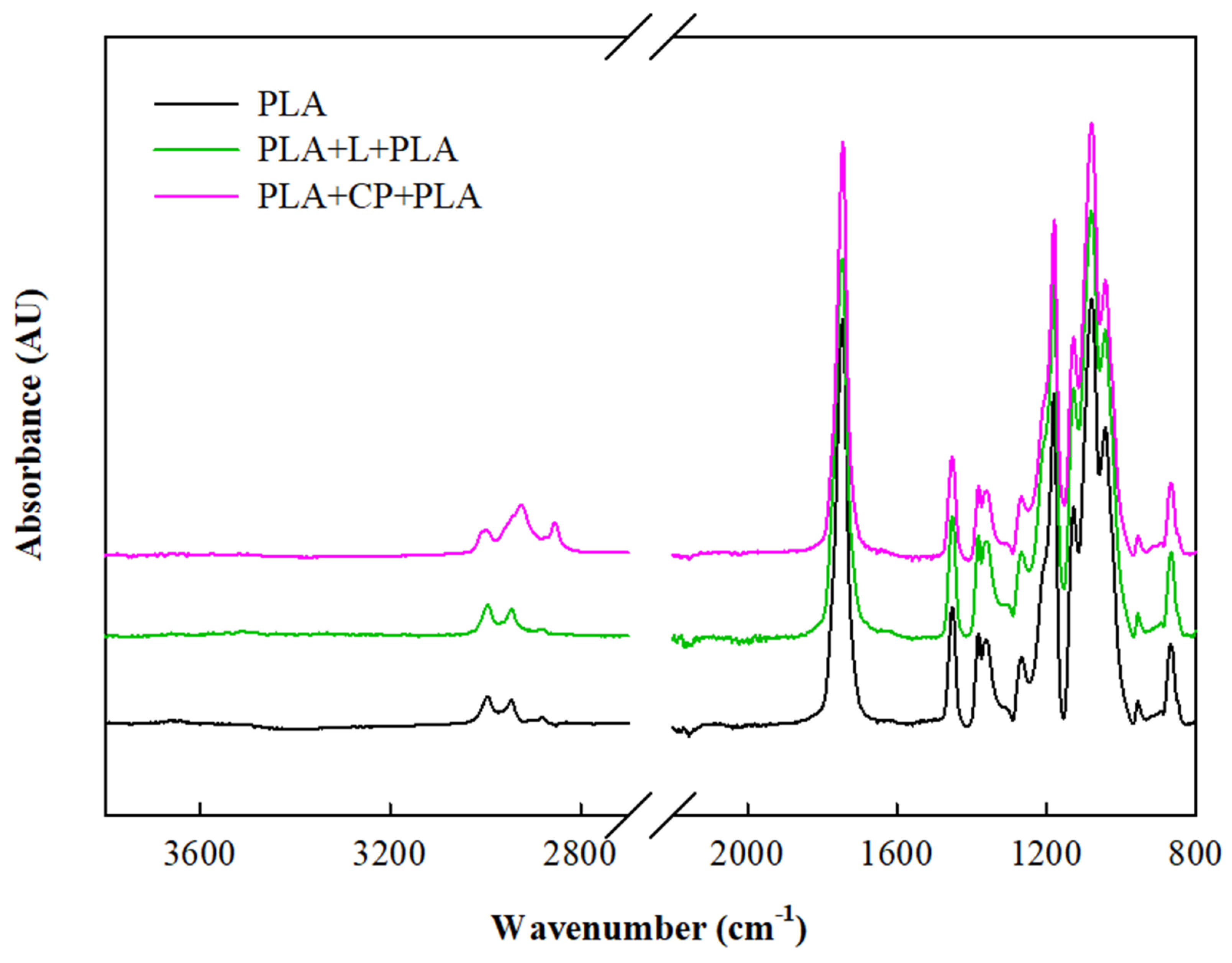

| Moisture | Total Fiber | Ashes | Proteins | Lipid | ||
|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||||
| 9.91 ± 1.3 | 23.84 ± 1.3 | 11.63 ± 2.3 | 28.21 ± 0.6 | 2.63 ± 0.1 | 1.69 ± 0.2 | 0.79 ± 0.1 |
| Sample | ΔE | Thickness (µm) | Moisture (%) | Roughness Parameters (μm) | |
|---|---|---|---|---|---|
| Ra | Rz | ||||
| 120 °C, 3 min | 48.2 ± 1.9 a | 819 ± 43 c | 3.48 ± 0.08 a | 10.9 ± 0.9 a | 30.9 ± 2.6 a |
| 120 °C, 5 min | 52.8 ± 1.9 c | 723 ± 40 a | 4.23 ± 0.01 b | 15.9 ± 1.9 b | 47.9 ± 5.0 b |
| 130 °C, 3 min | 52.5 ± 0.9 c | 761 ± 17 b | 3.54 ± 0.05 a | 22.5 ± 2.3 c | 63.6 ± 6.5 c |
| 130 °C, 5 min | 50.4 ± 0.7 b | 707 ± 30 a | 4.68 ± 0.44 b | 20.6 ± 2.5 c | 60.1 ± 4.5 c |
| Properties/Samples | L | PLA+CP+PLA | PLA+L+PLA | |
|---|---|---|---|---|
| ΔE | 52.5 ± 0.9 b | 49.3 ± 0.7 a | 55.4 ± 0.8 c | |
| Ra (μm) | 22 ± 3 a | 17 ± 6 a | 16 ± 6 a | |
| Rz (μm) | 64 ± 6 a | 48 ± 17 a | 45 ± 17 a | |
| Density (g/cm3) | 0.48 ± 0.005 a | 0.93 ± 0.02 b | 1.05 ± 0.003 c | |
| WAC (%) | 1 min | 39.7 ± 1.3 c | 5.8 ± 0.08 b | 3.5 ± 0.1 a |
| 60 min | 104 ± 13 c | 19 ± 1 a | 22 ± 1 b | |
| Moisture (%) | 6.2 ± 0.1 c | 3.3 ± 0.1 b | 2.5 ± 0.1 a | |
| Solubility (%) | 20.6 ± 1 c | 11.9 ± 0.9 b | 5.9 ± 0.4 a | |
| Contact angle (°) | 94 ± 1 b | 82 ± 3 a | 92 ± 3 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy, Y.; Versino, F.; García, M.A.; Rivero, S. Ecological Packaging: Reuse and Recycling of Rosehip Waste to Obtain Biobased Multilayer Starch-Based Material and PLA for Food Trays. Foods 2025, 14, 1843. https://doi.org/10.3390/foods14111843
Monroy Y, Versino F, García MA, Rivero S. Ecological Packaging: Reuse and Recycling of Rosehip Waste to Obtain Biobased Multilayer Starch-Based Material and PLA for Food Trays. Foods. 2025; 14(11):1843. https://doi.org/10.3390/foods14111843
Chicago/Turabian StyleMonroy, Yuliana, Florencia Versino, Maria Alejandra García, and Sandra Rivero. 2025. "Ecological Packaging: Reuse and Recycling of Rosehip Waste to Obtain Biobased Multilayer Starch-Based Material and PLA for Food Trays" Foods 14, no. 11: 1843. https://doi.org/10.3390/foods14111843
APA StyleMonroy, Y., Versino, F., García, M. A., & Rivero, S. (2025). Ecological Packaging: Reuse and Recycling of Rosehip Waste to Obtain Biobased Multilayer Starch-Based Material and PLA for Food Trays. Foods, 14(11), 1843. https://doi.org/10.3390/foods14111843








