Physicochemical and Rheological Characteristics of Monofloral Honeys—Kinetics of Creaming–Crystallization
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Samples
2.3. Physicochemical Parameters of Honey
2.3.1. Moisture Content
2.3.2. Water Activity
2.3.3. Electrical Conductivity
2.3.4. Color
2.3.5. Hydroxymethylfurfural Analysis (HMF)
- Sample preparation:
- Spectrophotometric analysis:
2.3.6. Diastase Activity
- Starch solution titration procedure:
- Honey analysis:
2.3.7. Sugar Composition
2.4. Rheological Properties
2.5. Thermal Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters of Honeys
3.1.1. Moisture Content
3.1.2. Water Activity
3.1.3. Electrical Conductivity
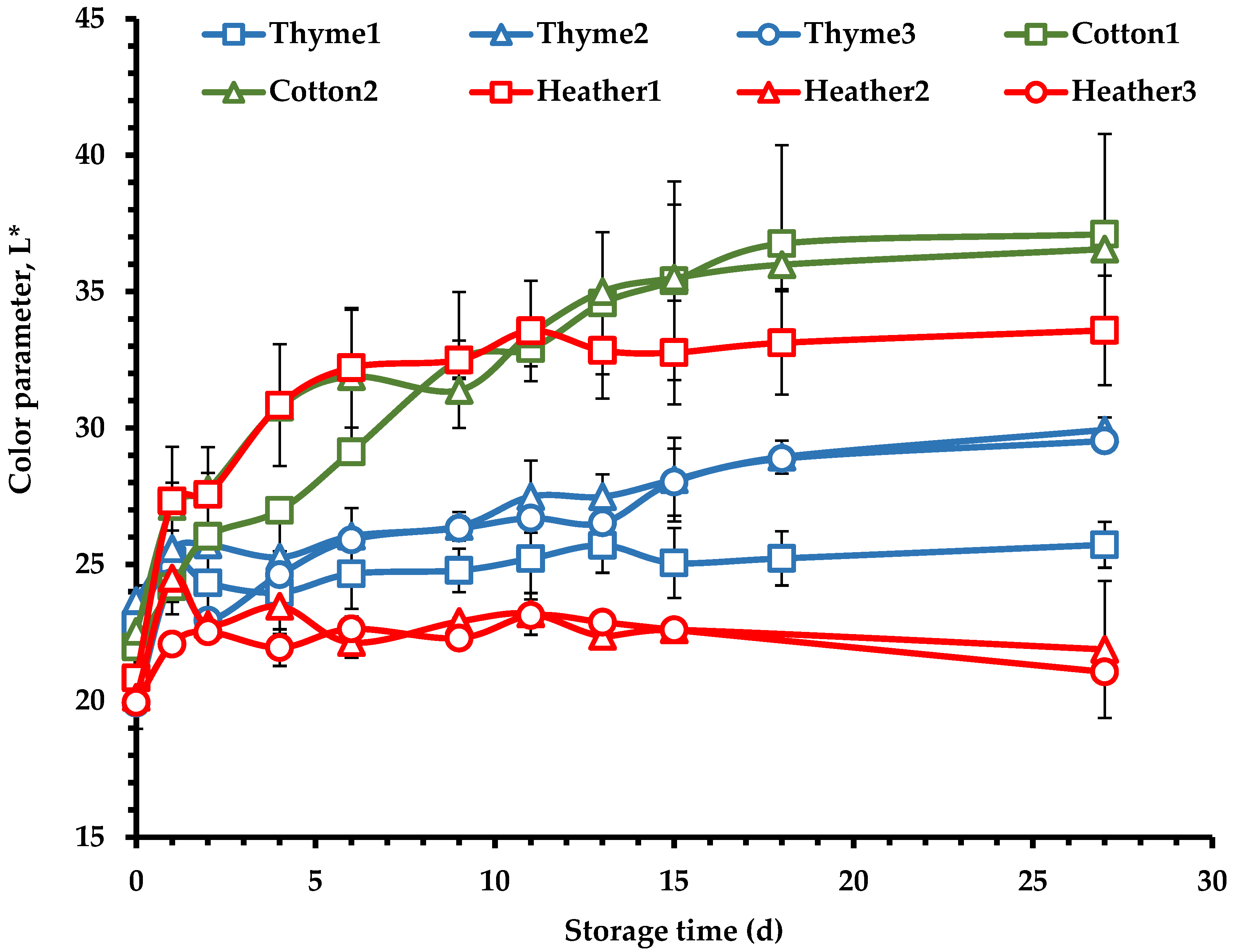
3.1.4. Color
3.1.5. Hydroxymethylfurfural Content (HMF)
3.1.6. Diastase Activity
3.1.7. Sugar Composition
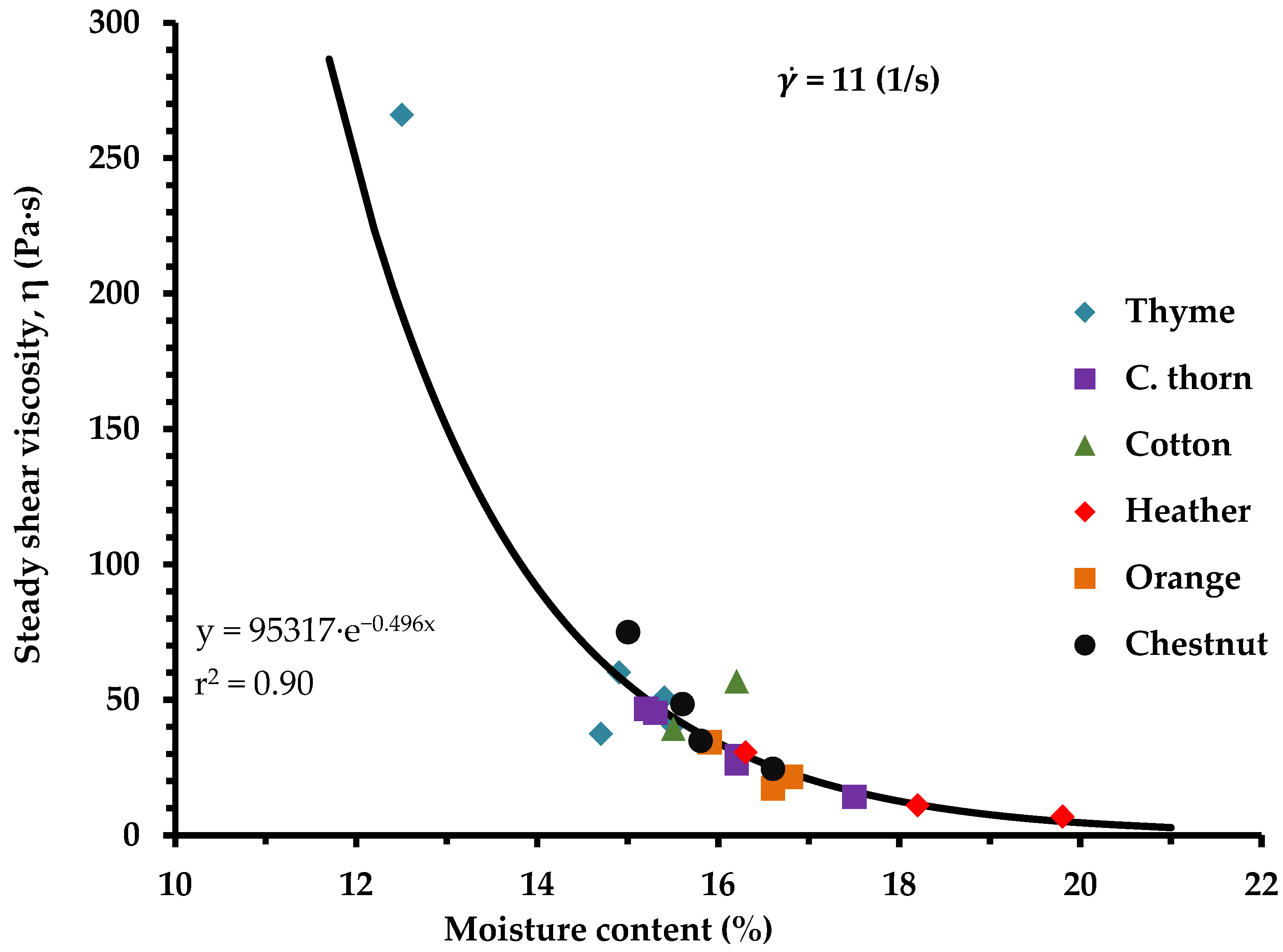
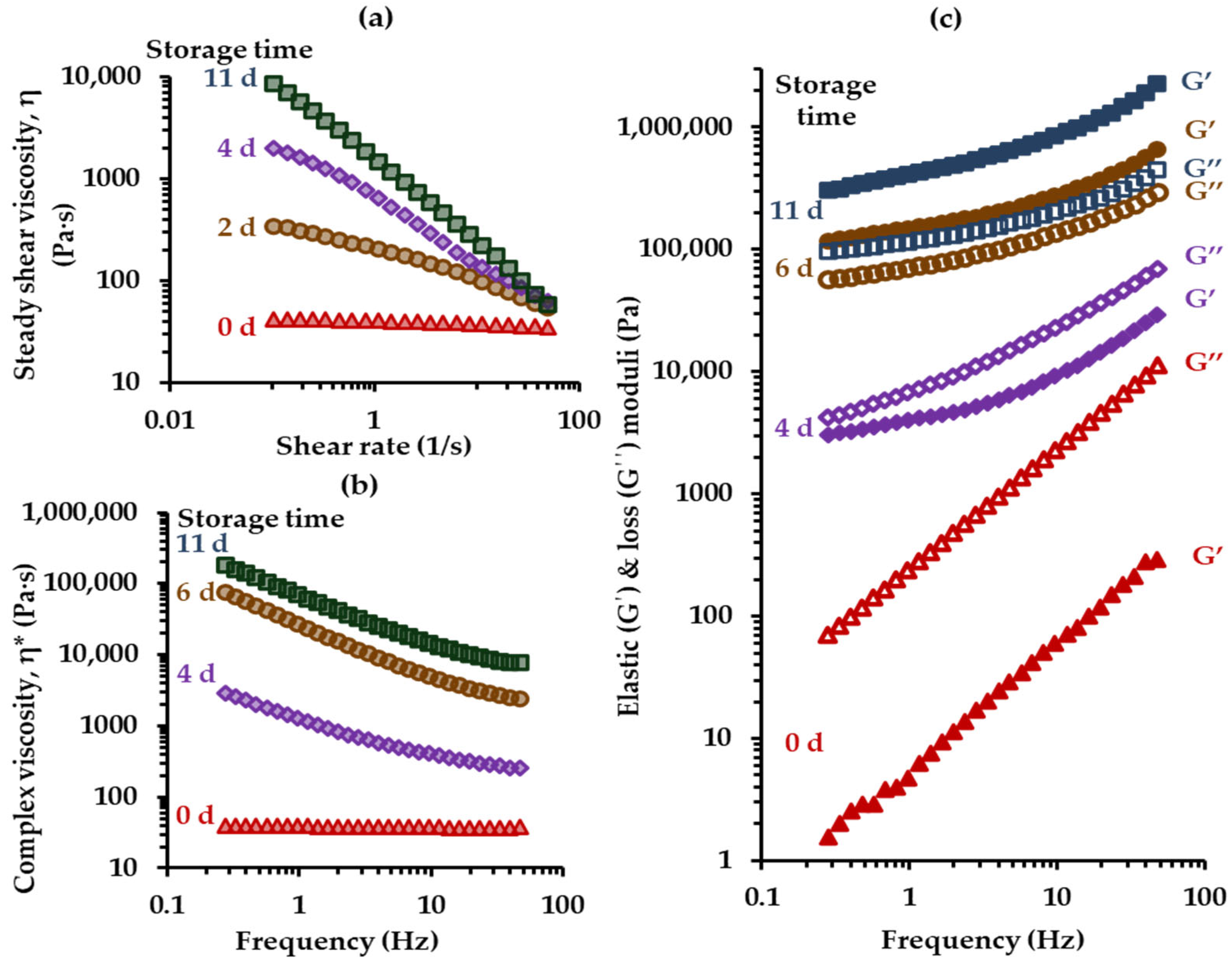
3.2. Rheological Behavior
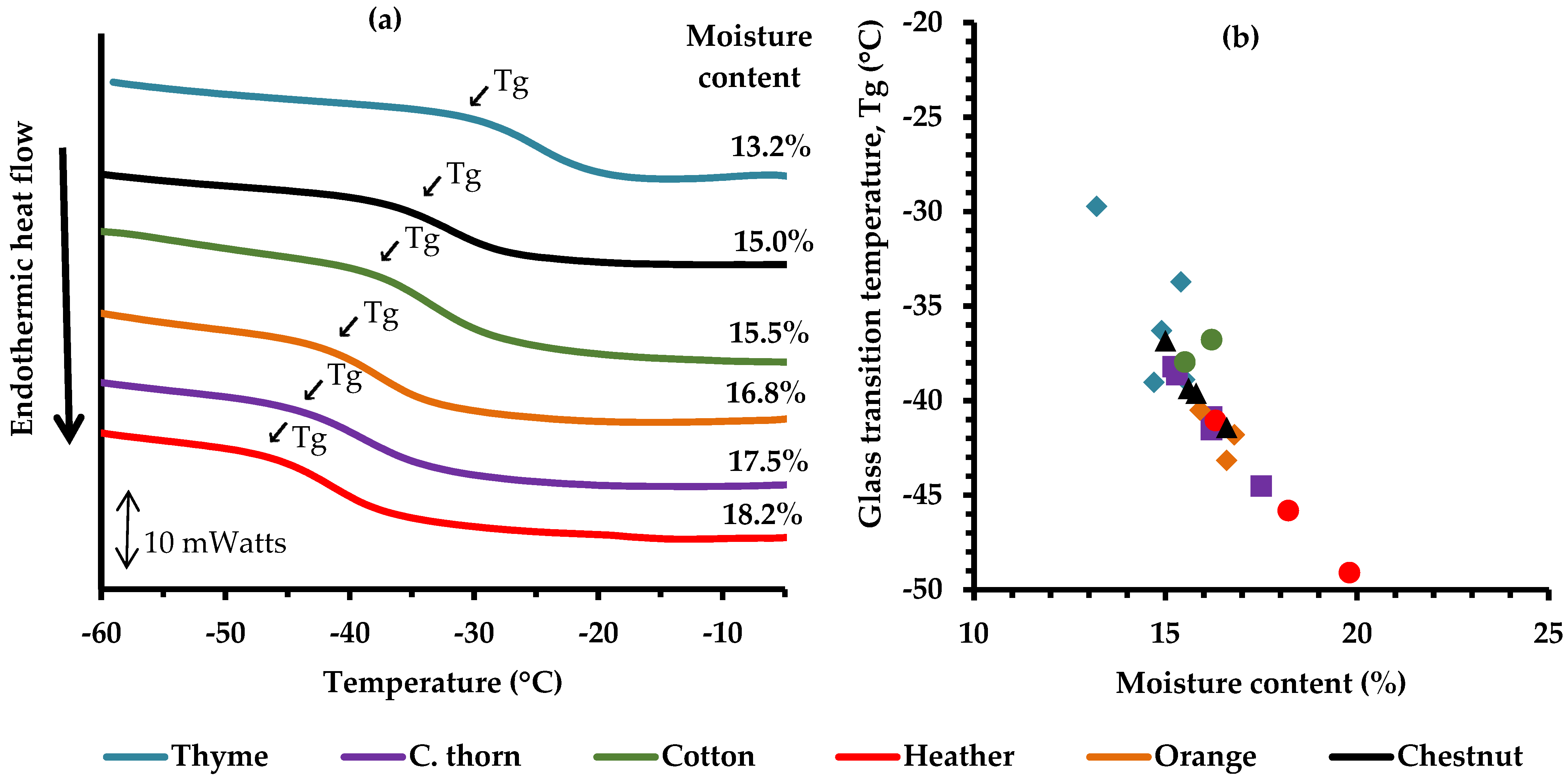
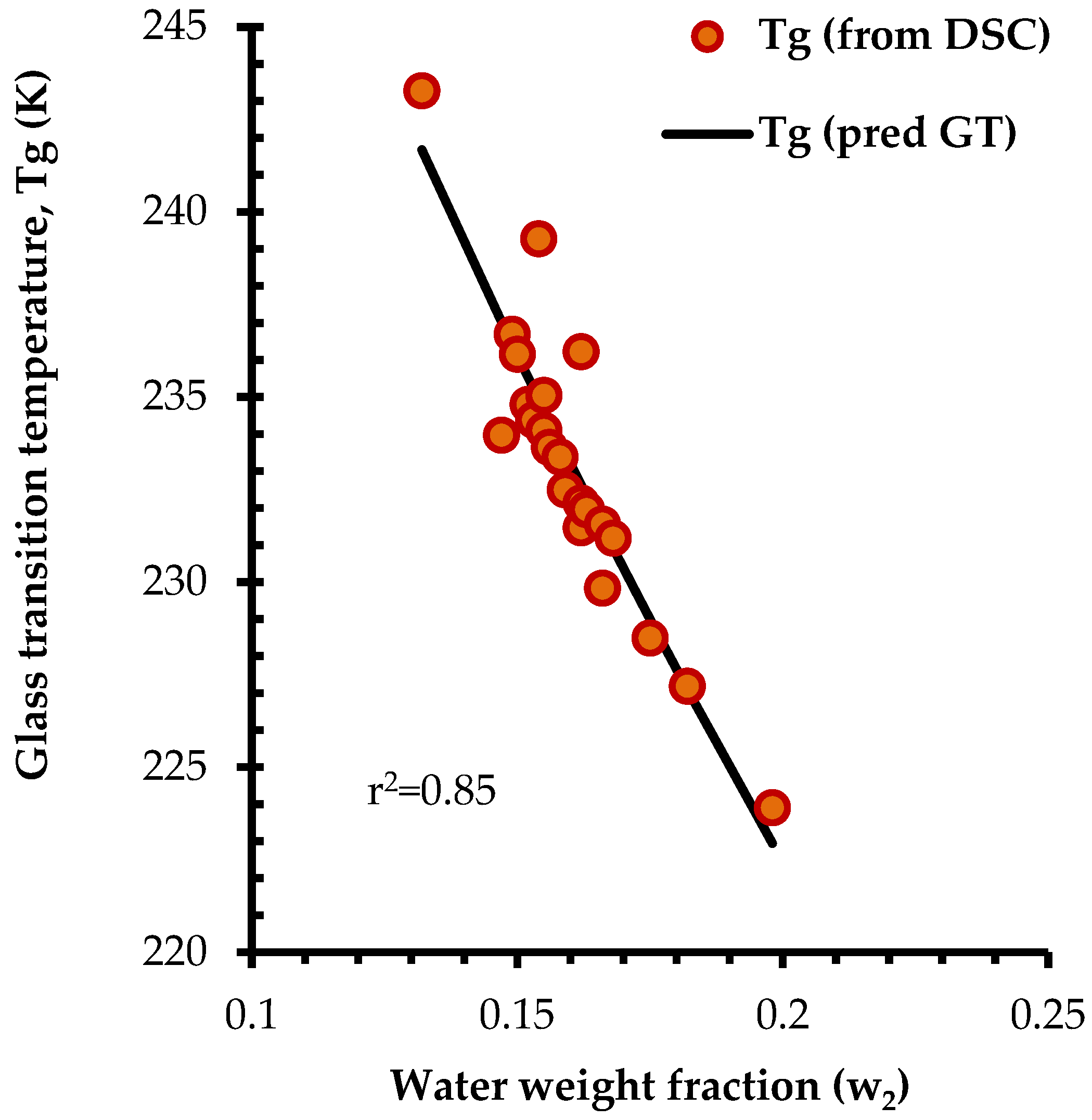
3.3. Thermal Behavior
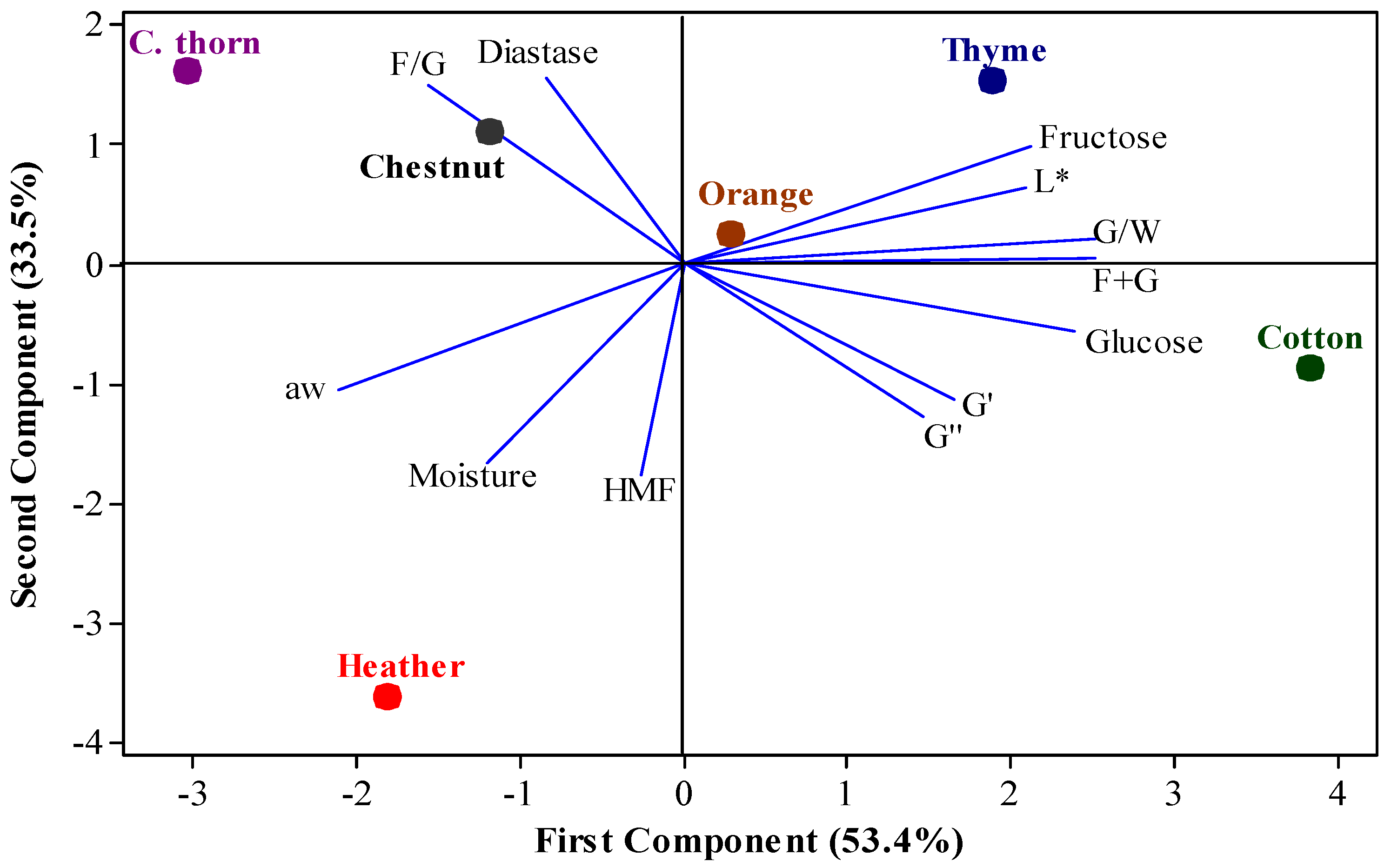
3.4. Principal Component Analysis
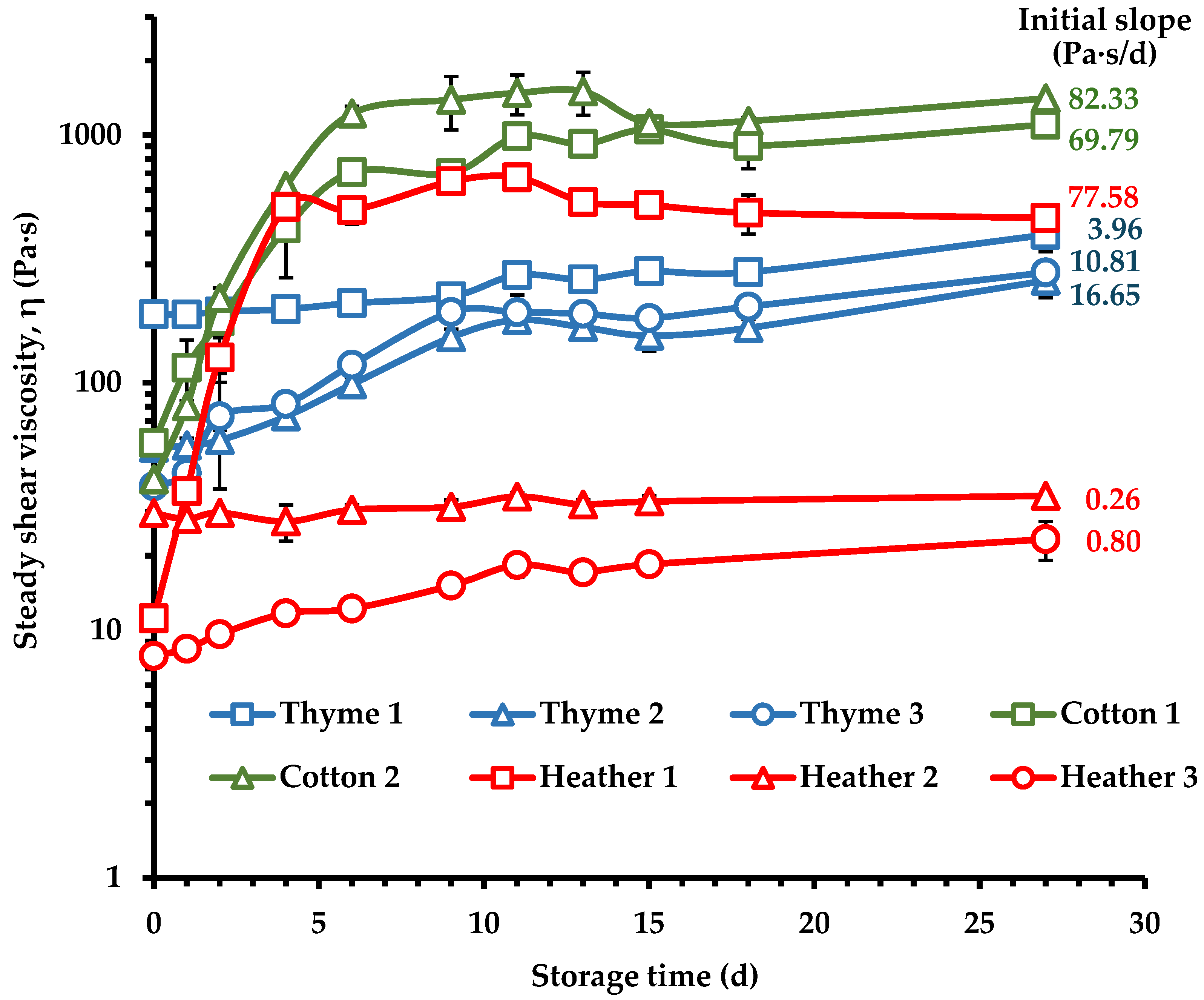
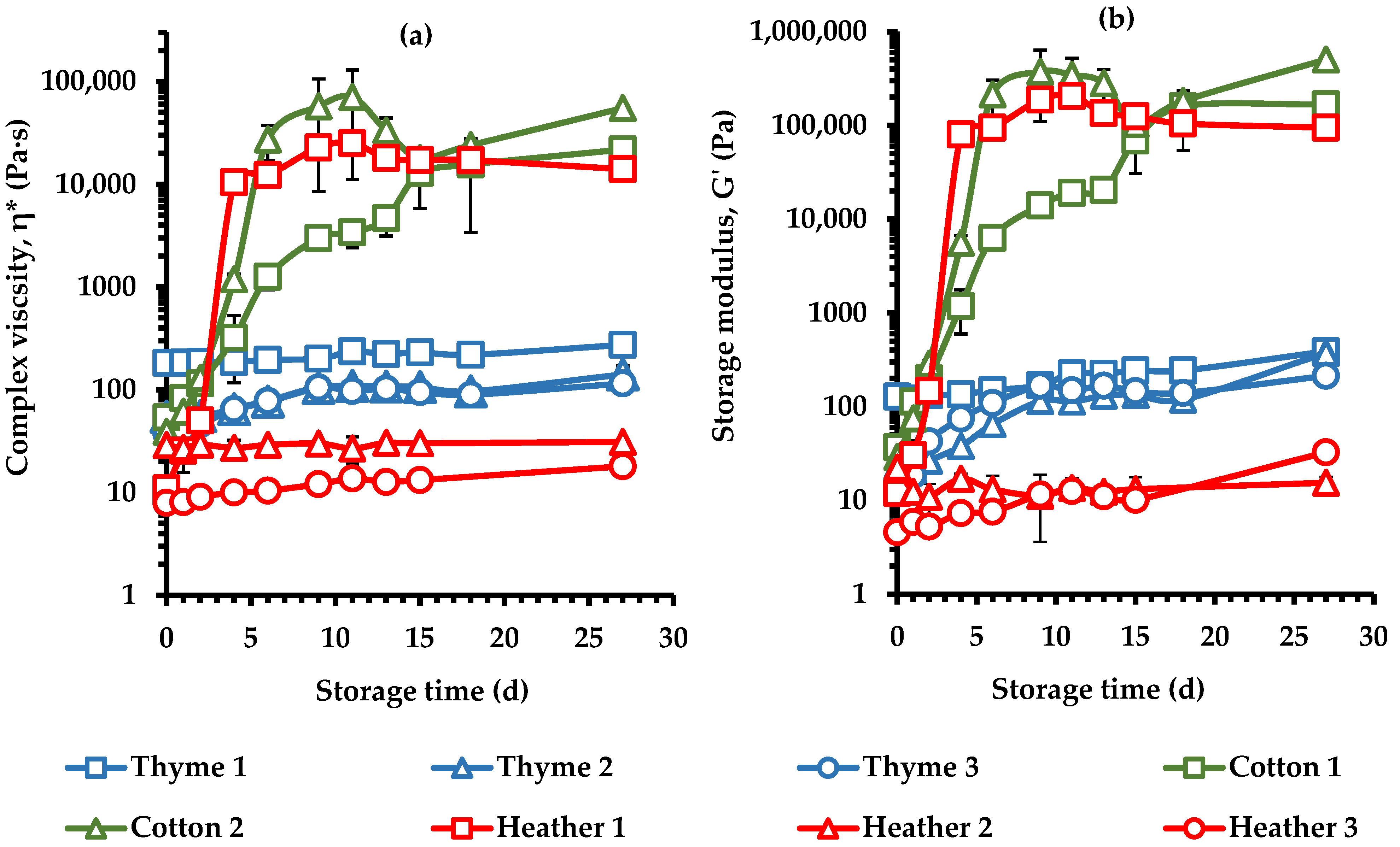
3.5. Crystallization Kinetics of Creaming Process of Monofloral Honeys
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Nurul Zaizuliana, R.A.; Anis Mastura, A.F.; Abd Jamil, Z.; Norshazila, S.; Zarinah, Z. Effect of Storage Conditions on the Crystallisation Behaviour of Selected Malaysian Honeys. Int. Food Res. J. 2017, 24, 475–480. [Google Scholar]
- Oddo, L.P.; Piro, R.; Bruneau, É.; Guyot-Declerck, C.; Ivanov, T.; Piskulová, J.; Flamini, C.; Lheritier, J.; Morlot, M.; Russmann, H.; et al. Main European Unifloral Honeys: Descriptive Sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Zamora, M.C.; Chirife, J. Determination of Water Activity Change Due to Crystallization in Honeys from Argentina. Food Control 2006, 17, 59–64. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Quiles, J.L.; Battino, M. The Inhibitory Effect of Manuka Honey on Human Colon Cancer HCT-116 and LoVo Cell Growth. Part 2: Induction of Oxidative Stress, Alteration of Mitochondrial Respiration and Glycolysis, and Suppres-sion of Metastatic Ability. Food Funct. 2018, 9, 2158–2170. [Google Scholar] [CrossRef]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A.; et al. Antibacterial and Antioxidant Activity of Different Types of Honey Derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physico-chemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Escuredo, O.; Seijo, M.C.; Fernández-González, M. Descriptive Analysis of Rubus Honey from the North-West of Spain. Int. J. Food Sci. Technol. 2011, 46, 2329–2336. [Google Scholar] [CrossRef]
- Mohammed, M.E.A. Factors Affecting the Physicochemical Properties and Chemical Composition of Bee’s Honey. Food Rev. Int. 2022, 38, 1330–1341. [Google Scholar] [CrossRef]
- Cavia, M.M.; Fernández-Muiño, M.A.; Gömez-Alonso, E.; Montes-Pérez, M.J.; Huidobro, J.F.; Sancho, M.T. Evolution of Fructose and Glucose in Honey over One Year: Influence of Induced Granulation. Food Chem. 2002, 78, 157–161. [Google Scholar] [CrossRef]
- Conforti, P.A.; Lupano, C.E.; Malacalza, N.H.; Arias, V.; Castells, C.B. Crystallization of Honey at −20 °C. Int. J. Food Prop. 2006, 9, 99–107. [Google Scholar] [CrossRef]
- Naik, R.R.; Gandhi, N.S.; Thakur, M.; Nanda, V. Analysis of Crystallization Phenomenon in Indian Honey Using Molec-ular Dynamics Simulations and Artificial Neural Network. Food Chem. 2019, 300, 125182. [Google Scholar] [CrossRef]
- Dettori, A.; Tappi, S.; Piana, L.; Dalla Rosa, M.; Rocculi, P. Kinetic of Induced Honey Crystallization and Related Evolution of Structural and Physical Properties. LWT 2018, 95, 333–338. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H.; Reid, D.S. Beyond Water Activity: Recent Advances Based on an Alternative Approach to the Assessment of Food Quality and Safety. Crit. Rev. Food Sci. Nutr. 1991, 30, 115–360. [Google Scholar] [CrossRef] [PubMed]
- Sopade, P.A.; Halley, P.; Bhandari, B.; D’Arcy, B.; Doebler, C.; Caffin, N. Application of the Williams–Landel–Ferry Model to the Viscosity–Temperature Relationship of Australian Honeys. J. Food Eng. 2003, 56, 67–75. [Google Scholar] [CrossRef]
- Tappi, S.; Glicerina, V.; Ragni, L.; Dettori, A.; Romani, S.; Rocculi, P. Physical and Structural Properties of Honey Crys-tallized by Static and Dynamic Processes. J. Food Eng. 2021, 292, 110316. [Google Scholar] [CrossRef]
- Dobre, I.; Georgescu, L.A.; Alexe, P.; Escuredo, O.; Seijo, M.C. Rheological Behavior of Different Honey Types from Romania. Food Res. Int. 2012, 49, 126–132. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of Botanical Origin and Sugar Composition of Honeys on the Crystallization Phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Amariei, S.; Norocel, L.; Scripcă, L.A. An Innovative Method for Preventing Honey Crystallization. Innov. Food Sci. Emerg. Technol. 2020, 66, 102481. [Google Scholar] [CrossRef]
- Costa, L.C.V.; Kaspchak, E.; Queiroz, M.B.; de Almeida, M.M.; Quast, E.; Quast, L.B. Influence of Temperature and Homogenization on Honey Crystallization. Braz. J. Food Technol. 2015, 18, 155–161. [Google Scholar] [CrossRef]
- Krishnan, R.; Mohammed, T.; Kumar, G.S.; Sh, A. Honey Crystallization: Mechanism, Evaluation and Application. Pharma Innov. J. 2021, 10, 222–231. [Google Scholar] [CrossRef]
- Hartel, R.W. Advances in Food Crystallization. Annu. Rev. Food Sci. Technol. 2013, 4, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Sereti, V.; Lazaridou, A.; Tananaki, C.; Biliaderis, C.G. Development of a Cotton Honey-Based Spread by Controlling Compositional and Processing Parameters. Food Biophys. 2021, 16, 365–380. [Google Scholar] [CrossRef]
- Tosi, E.A.; Ré, E.; Lucero, H.; Bulacio, L. Effect of Honey High-Temperature Short-Time Heating on Parameters Related to Quality, Crystallisation Phenomena and Fungal Inhibition. LWT—Food Sci. Technol. 2004, 37, 669–678. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, Microbiological and Antimicrobial Properties of Commercial Honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef]
- Belay, A.; Solomon, W.K.; Bultossa, G.; Adgaba, N.; Melaku, S. Physicochemical Properties of the Harenna Forest Honey, Bale, Ethiopia. Food Chem. 2013, 141, 3386–3392. [Google Scholar] [CrossRef]
- IHC. Harmonized Methods of the International Honey Commission. 2009. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 3 March 2025).
- White, J.W., Jr. Spectrophotometric Method for Hydroxymethylfurfural in Honey. J. Assoc. Off. Anal. Chem. 1979, 62, 509–514. [Google Scholar] [CrossRef]
- Schade, J.E.; Marsh, G.L.; Eckert, J.E. Diastase Activity and Hydroxy-Methyl-Furfural in Honey and Their Usefulness in Detecting Heat Alteration. J. Food Sci. 1958, 23, 446–463. [Google Scholar] [CrossRef]
- Bakier, S.; Miastkowski, K.; Bakoniuk, J.R. Rheological Properties of Some Honeys in Liquefied and Crystallised States. J. Apic. Sci. 2016, 60, 153–166. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal Copolymers and the Second-Order Transitions of Synthetic Rubbers. i. Non-Crystalline Copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- The Council Of The European Union. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. Off. J. Eur. Communities 2002, L, L10/47–L10/52. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:010:0047:0052:EN:PDF (accessed on 3 March 2025).
- Chirsanova, A.; Capcanari, T.; Boistean, A.; Siminiuc, R. Physico-Chemical Profile of Four Types of Honey from the South of the Republic of Moldova. Food Nutr. Sci. 2021, 12, 874–888. [Google Scholar] [CrossRef]
- Escriche, I.; Tanleque-Alberto, F.; Visquert, M.; Oroian, M. Physicochemical and Rheological Characterization of Honey from Mozambique. LWT 2017, 86, 108–115. [Google Scholar] [CrossRef]
- Al-Kafaween, M.A.; Alwahsh, M.; Mohd Hilmi, A.B.; Abulebdah, D.H. Physicochemical Characteristics and Bioactive Compounds of Different Types of Honey and Their Biological and Therapeutic Properties: A Comprehensive Review. Antibiotics 2023, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Baloš, M.Ž.; Popov, N.; Vidaković, S.; Pelić, D.L.; Pelić, M.; Mihaljev, Ž.; Jakšić, S. Electrical Conductivity and Acidity of Honey. Arch. Vet. Med. 2018, 11, 91–101. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R.; Čeksterytė, V. Carbohydrate Composition and Electrical Conductivity of Different Origin Honeys from Lithuania. LWT—Food Sci. Technol. 2010, 43, 801–807. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission Standards Codex Standards for Honey; 1981/Revised 1987/Revised 2001; FAO: Rome, Italy, 2001; pp. 1–7.
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Rodopoulou, M.A.; Tananaki, C.; Kanelis, D.; Liolios, V.; Dimou, M.; Thrasyvoulou, A. A chemometric approach for the differentiation of 15 monofloral honeys based on physicochemical parameters. J. Sci. Food Agric. 2022, 102, 139–146. [Google Scholar] [CrossRef]
- Kropf, U.; Jamnik, M.; Bertoncelj, J.; Golob, T. Linear Regression Model of the Ash Mass Fraction and Electrical Conduc-tivity for Slovenian Honey. Food Technol. Biotechnol. 2008, 46, 335–340. [Google Scholar]
- Nguyen, T.Q.N.; Kružík, V.; Škorpilová, T.; Javůrková, Z.; Pospiech, M.; Anh, L.T.H.; Nguyen, T.K.T.; Nguyen Tran, V.; Čížková, H. Physicochemical, Sugar, and Volatile Profile Characterization of Blong Song, Bidens, Coffee, and Mint Honeys Originating from Vietnam. J. Apic. Res. 2024, 63, 946–958. [Google Scholar] [CrossRef]
- Harris, E.D. Minerals in Food: Nutrition, Metabolism, Bioactivity; DEStech Publications, Inc.: Lancaster, PA, USA, 2014. [Google Scholar]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Smetanska, I.; Alharthi, S.S.; Selim, K.A. Physicochemical, Antioxidant Capacity and Color Analysis of Six Honeys from Different Origin. J. King Saud Univ.—Sci. 2021, 33, 101447. [Google Scholar] [CrossRef]
- Baloš, M.M.Ž.; Popov, N.S.; Radulović, J.Z.P.; Stojanov, I.M.; Jakšić, S.M. Sugar Profile of Different Floral Origin Honeys from Serbia. J. Apic. Res. 2020, 59, 398–405. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Sulaiman, S.A.; Azlan, S.A.M.; Gan, S.H. Two-Year Variations of Phenolics, Flavonoids and Anti-oxidant Contents in Acacia Honey. Molecules 2013, 18, 14694–14710. [Google Scholar] [CrossRef]
- White, J.W. The Role of HMF and Diastase Assays in Honey Quality Evaluation. Bee World 1994, 75, 104–117. [Google Scholar] [CrossRef]
- Pasias, I.N.; Kiriakou, I.K.; Kaitatzis, A.; Koutelidakis, A.E.; Proestos, C. Effect of Late Harvest and Floral Origin on Honey Antibacterial Properties and Quality Parameters. Food Chem. 2018, 242, 513–518. [Google Scholar] [CrossRef]
- Rosyidah, R.A.; Hasan, A.E.Z.; Andrianto, D. Antibacterial and diastase enzyme activities of honey Apis Mellifera from Indonesia. Biotropia 2024, 31, 23–33. [Google Scholar] [CrossRef]
- Mongi, R.J.; Ruhembe, C.C. Sugar Profile and Sensory Properties of Honey from Different Geographical Zones and Bo-tanical Origins in Tanzania. Heliyon 2024, 10, e38094. [Google Scholar] [CrossRef]
- Valverde, S.; Ares, A.M.; Stephen Elmore, J.; Bernal, J. Recent Trends in the Analysis of Honey Constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef]
- Madas, M.N.; Francis, F.; Marghitas, L.A.; Haubruge, E.; Fauconnier, M.L.; Nguyen, B.K. Physico-Chemical Properties and Aroma Profile of Acacia Honey Produced in Romania. Commun. Agric. Appl. Biol. Sci. 2014, 79, 133–135. [Google Scholar]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.-G.; Mar-cazzan, G.L.; Piro, R.; et al. Honey Quality and International Regulatory Standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Laos, K.; Kirs, E.; Pall, R.; Martverk, K. The Crystallization Behaviour of Estonian Honeys. Agron. Res. 2011, 9, 427–432. [Google Scholar]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical Characteristics of Honey from Different Origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Nakib, R.; Ghorab, A.; Harbane, S.; Saker, Y.; Ouelhadj, A.; Rodríguez-Flores, M.S.; Seijo, M.C.; Escuredo, O. Sensory Attributes and Chemical Composition: The Case of Three Monofloral Honey Types from Algeria. Foods 2024, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, F.; Kongoli, R.; Malollari, I. Using ratio of the main sugars and some oligosaccharides content to indicate market’s honey authenticity. EJ-ENG 2019, 7, 1–8. [Google Scholar]
- Hossain, M.M.; Nath Barman, D.; Rahman, M.A.; Khandker, S.S. Carbohydrates in Honey. In Honey; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 32–45. ISBN 978-1-119-11332-4. [Google Scholar]
- Voelker, A.L.; Verbeek, G.; Taylor, L.S.; Mauer, L.J. Effects of Emulsifiers on the Moisture Sorption and Crystallization of Amorphous Sucrose Lyophiles. Food Chem. X 2019, 3, 100050. [Google Scholar] [CrossRef]
- Gleiter, R.A.; Horn, H.; Isengard, H.-D. Influence of Type and State of Crystallisation on the Water Activity of Honey. Food Chem. 2006, 96, 441–445. [Google Scholar] [CrossRef]
- Smanalieva, J.; Senge, B. Analytical and rheological investigations into selected unifloral German honey. Eur. Food Res. Technol. 2009, 229, 107–113. [Google Scholar] [CrossRef]
- Witczak, M.; Juszczak, L.; Gałkowska, D. Non-Newtonian behaviour of heather honey. J. Food Eng. 2011, 104, 532–537. [Google Scholar] [CrossRef]
- Gobessa, S.; Seifu, E.; Bezabih, A. Physicochemical Properties of Honey Produced in the Homesha District of Western Ethiopia. J. Apic. Sci. 2012, 56, 33–40. [Google Scholar] [CrossRef]
- Cui, Z.-W.; Sun, L.-J.; Chen, W.; Sun, D.-W. Preparation of Dry Honey by Microwave–Vacuum Drying. J. Food Eng. 2008, 84, 582–590. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G.; Bacandritsos, N.; Sabatini, A.G. Composition, Thermal and Rheological Behaviour of Selected Greek Honeys. J. Food Eng. 2004, 64, 9–21. [Google Scholar] [CrossRef]
- Yap, S.K.; Chin, N.L.; Yusof, Y.A.; Chong, K.Y. Quality Characteristics of Dehydrated Raw Kelulut Honey. Int. J. Food Prop. 2019, 22, 556–571. [Google Scholar] [CrossRef]
- Yanniotis, S.; Skaltsi, S.; Karaburnioti, S. Effect of Moisture Content on the Viscosity of Honey at Different Temperatures. J. Food Eng. 2006, 72, 372–377. [Google Scholar] [CrossRef]
- Umesh Hebbar, H.; Rastogi, N.K.; Subramanian, R. Properties of Dried and Intermediate Moisture Honey Products: A Review. Int. J. Food Prop. 2008, 11, 804–819. [Google Scholar] [CrossRef]
- Dekeba Tafa, K.; Sundramurthy, V.P.; Subramanian, N. Rheological and Thermal Properties of Honey Produced in Algeria and Ethiopia: A Review. Int. J. Food Prop. 2021, 24, 1117–1131. [Google Scholar] [CrossRef]
- Dranca, F.; Ropciuc, S.; Pauliuc, D.; Oroian, M. Honey Adulteration Detection Based on Composition and Differential Scanning Calorimetry (DSC) Parameters. LWT 2022, 168, 113910. [Google Scholar] [CrossRef]
- Tomaszewska-Gras, J.; Bakier, S.; Goderska, K.; Mansfeld, K. Differential Scanning Calorimetry for Determining the Thermodynamic Properties of Selected Honeys. J. Apic. Sci. 2015, 59, 109–118. [Google Scholar] [CrossRef]
- Carter, B.P.; Schmidt, S.J. Developments in Glass Transition Determination in Foods Using Moisture Sorption Isotherms. Food Chem. 2012, 132, 1693–1698. [Google Scholar] [CrossRef]
- Mahani; Ferdian, P.R.; Ghibran, H.M.; Herlina, A.F.; Nurhasanah, S.; Nurjanah, N.; Elfirta, R.R.; Pribadi, A.; Amalia, R.L.R.; Samudra, I.M. A Report on the Physicochemical and Antioxidant Properties of Three Indonesian Forest Honeys Produced by Apis Dorsata. Food Chem. X 2025, 25, 102156. [Google Scholar] [CrossRef]
- Scripcă, L.A.; Amariei, S. The Use of Ultrasound for Preventing Honey Crystallization. Foods 2021, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Ciursa, P.; Oroian, M. Rheological Behavior of Honey Adulterated with Agave, Maple, Corn, Rice and Inverted Sugar Syrups. Sci. Rep. 2021, 11, 23408. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Pinheiro, L. Analytical Rheology of Honey: A State-of-the-Art Review. Foods 2021, 10, 1709. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Liu, X.; Yang, C.; Wu, F.; Sun, J.; Cao, W.; Zhao, H. Natural Crystallization Properties of Honey and Seed Crys-tals-Induced Crystallization Process for Honey Performance Enhancing. Food Chem. 2023, 405, 134972. [Google Scholar] [CrossRef]
- Piotraszewska-Pająk, A.; Gliszczyńska-Świgło, A. Directions of Colour Changes of Nectar Honeys Depending on Honey Type and Storage Conditions. J. Apic. Sci. 2015, 59, 51–61. [Google Scholar] [CrossRef]
- Negueruela, A.I.; Perez-Arquillue, C. Color Measurement of Rosemary Honey in the Solid State by Reflectance Spectroscopy with Black Background. J. AOAC Int. 2000, 83, 669–674. [Google Scholar] [CrossRef]

| Plant Origin | Moisture Content (%) | aw | Electrical Conductivity (mS/cm) | Color Parameters | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| Thyme (n = 5) | 14.60 ± 1.09 a 1 | 0.53 ± 0.02 a | 0.36 ± 0.09 a | 33.17 ± 0.64 cd | 1.52 ± 0.62 b | 6.03 ± 0.34 bc |
| C. thorn (n = 5) | 16.08 ± 0.83 a | 0.57 ± 0.02 bc | 0.89 ± 0.15 b | 31.88 ± 0.72 b | 2.74 ± 0.38 cd | 5.20 ± 1.03 b |
| Chestnut (n = 4) | 15.75 ± 0.57 a | 0.56 ± 0.01 abc | 1.32 ± 0.10 c | 30.44 ± 0.16 a | 2.91 ± 0.21 d | 2.81 ± 0.34 a |
| Heather (n = 3) | 18.10 ± 1.43 a | 0.60 ± 0.02 c | 1.25 ± 0.04 c | 29.50 ± 0.09 a | 2.34 ± 0.37 bcd | 1.26 ± 0.18 a |
| Orange (n = 3) | 16.43 ± 0.39 a | 0.57 ± 0.01 abc | 0.28 ± 0.01 a | 34.40 ± 0.58 cd | 0.21 ± 0.29 a | 5.43 ± 0.97 b |
| Cotton (n = 2) | 15.85 ± 0.35 a | 0.53 ± 0.00 bc | 0.75 ± 0.16 b | 35.71 ± 0.69 d | 1.36 ± 0.78 abc | 7.85 ± 0.45 c |
| HΜF (mg/kg) | Diastase Activity (DN) | |||
|---|---|---|---|---|
| Plant Origin | Range 1 | Average Value 2 | Range 1 | Average Value 2 |
| Thyme (n = 5) | 6.0–27.7 | 13.6 ± 8.1 a 3 | 7.0–17.0 | 14.00 ± 3.8 a,b |
| C. thorn (n = 5) | 0.0–10.6 | 4.1 ± 3.8 a | 18.5–32.0 | 25.7 ± 5.1 c |
| Chestnut (n = 4) | 0.0–1.6 | 0.6 ± 0.7 a | 14.3–27.9 | 23.6 ± 5.5 b,c |
| Heather (n = 3) | 5.2–37.1 | 16.8 ± 14.4 a | 4.7–14.7 | 8.2 ± 4.6 a |
| Orange (n = 3) | 0.2–6.4 | 3.4 ± 2.6 a | 6.4–15.4 | 11.2 ± 3.7 a,b |
| Cotton (n = 2) | 3.1–9.4 | 6.3 ± 3.1 a | 14.8–14.8 | 14.8 ± 0.0 a,b,c |
| Plant Origin | Sugars (g/100 g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fructose | Glucose | Fructose + Glucose | Fructose/ Glucose | Glucose/ Water | Sucrose | Maltose | Trehalose | Melezitose | Melibiose | Raffinose | |
| Thyme (n = 5) | 40.75 ± 5.18 b 1 | 31.96 ± 4.38 ab | 72.72 ± 4.39 b | 1.28 ± 0.11 abc | 2.30 ± 0.30 bc | 1.52 ± 0.45 a | 3.13 ± 0.77 a | 0.48 ± 0.18 ab | n.d. | 2.24 ± 0.29 b | 0.83 ± 0.22 |
| C. thorn (n = 5) | 34.04 ± 1.66 ab | 24.72 ± 2.13 a | 58.79 ± 4.66 a | 1.38 ± 0.06 c | 1.54 ± 0.14 a | n.d. 2 | 3.23 ± 1.68 a | 0.71 ± 0.23 b | n.d. | 0.08 ± 0.01 a | n.d. |
| Chestnut (n = 4) | 36.36 ± 0.90 ab | 27.73 ± 2.94 a | 64.09 ± 4.31 ab | 1.32 ± 0.15 bc | 1.76 ± 0.15 ab | n.d. | 1.54 ± 0.52 a | 0.08 ± 0.01 a | n.d. | 0.37 ± 0.07 a | n.d. |
| Heather (n = 4) | 32.46 ± 5.12 a | 29.61 ± 6.61 ab | 62.07 ± 1.43 ab | 1.11 ± 0.07 ab | 1.64 ± 0.29 a | n.d. | 2.11 ± 0.17 a | 0.45 ± 0.21 ab | n.d. | n.d. | n.d. |
| Orange (n = 3) | 37.67 ± 0.79 ab | 32.46 ± 0.44 ab | 70.12 ± 2.61 ab | 1.16 ± 0.03 abc | 1.98 ± 0.07 abc | 0.41 ± 0.12 a | 1.66 ± 1.20 a | 0.09 ± 0.02 ab | n.d. | n.d. | n.d. |
| Cotton (n = 2) | 39.01 ± 1.20 ab | 38.42 ± 2.33 b | 77.43 ± 0.30 b | 1.01 ± 0.02 a | 2.43 ± 0.16 c | n.d. | 1.73 ± 0.33 a | 0.39 ± 0.09 ab | n.d. | 0.15 ± 0.02 a | n.d. |
| Storage Time (d) | Cotton1 | Cotton2 | Heather1 | Heather2 | Heather3 | Thyme1 | Thyme2 | Thyme3 |
|---|---|---|---|---|---|---|---|---|
| Consistency Factor (K, Pa∙sn) | ||||||||
| 0 | 55.636 a,D 1 | 39.663 a,C | 11.429 a,A | 30.023 abc,B | 7.830 a,A | 187.270 a,E | 54.360 a,D | 38.103 a,C |
| 1 | 96.710 a,E | 69.670 a,D | 29.614 b,B | 28.326 a,B | 7.767 a,A | 185.980 a,F | 54.776 a,CD | 41.716 a,BC |
| 2 | 148.815 a,C | 175.795 ab,C | 112.925 c,B | 28.361 ab,AB | 9.458 ab,A | 191.775 a,C | 57.937 a,AB | 54.567 a,AB |
| 4 | 345.190 b,C | 542.195 b,D | 544.409 d,D | 27.888 a,A | 10.916 b,A | 194.39 a,B | 68.908 ab,A | 78.597 b,A |
| 6 | 611.095 c,C | 1144.800 c,D | 528.585 d,C | 29.424 abc,A | 11.222 bc,A | 204.285 a,B | 86.107 b,A | 97.227 c,AB |
| 9 | 732.110 cd,C | 1420.850 cd,D | 692.175 d,C | 31.293 bcd,A | 14.172 cd,A | 212.215 ab,B | 124.710 c,B | 147.535 d,B |
| 11 | 910.815 de,C | 1477.150 d,D | 724.100 c,C | 33.668 a,A | 16.647 cd,A | 248.140 c,B | 146.505 c,B | 151.835 d,B |
| 13 | 752.085 cd,D | 1287.550 cd,E | 566.300 b,C | 31.599 cd,A | 15.512 c,A | 242.075 bc,B | 145.630 c,B | 150.625 d,B |
| 15 | 1000.555 e,D | 1021.600 c,D | 552.140 b,C | 32.027 cd,A | 17.020 de,A | 257.275 c,B | 134.250 c,B | 148.531 d,B |
| 18 | 844.890 cde,D | 1053.600 cd,E | 501.730 c,C | 33.077 d,A | 19.138 ef,A | 253.750 c,B | 139.080 c,B | 155.82 d,B |
| 27 | 1014.915 e,E | 1336.700 cd,F | 466.905 b,D | 34.011 d,A | 21.103 f,A | 338.140 d,C | 205.300 d,B | 209.260 e,B |
| Flow behavior index (n) | ||||||||
| 0 | 0.983 d,B | 0.986 h,B | 0.964 f,A | 0.989 ab,B | 0.996 bcd,B | 0.991 d,B | 0.996 d,B | 0.992 f,B |
| 1 | 0.931 d,A | 0.943 h,A | 0.908 f,AB | 0.988 ab,C | 1.000 d,C | 0.993 d,C | 0.997 d,C | 0.988 ef,BC |
| 2 | 0.878 d,B | 0.809 g,A | 0.775 e,A | 0.983 ab,C | 0.986 bc,C | 0.991 d,C | 0.990 d,C | 0.938 de,BC |
| 4 | 0.698 c,C | 0.505 f,B | 0.225 ab,A | 0.979 a,E | 0.9886 bc,E | 0.991 d,E | 0.988 d,E | 0.874 ab,D |
| 6 | 0.537 b,C | 0.275 bc,B | 0.224 ab,A | 0.999 b,E | 0.977 abc,E | 0.988 d,E | 0.972 cd,E | 0.922 cd,D |
| 9 | 0.477 ab,B | 0.217 a,A | 0.192 a,A | 0.982 ab,D | 0.980 abc,D | 0.982 cd,D | 0.942 bc,D | 0.877 bc,C |
| 11 | 0.391 ab,B | 0.276 bc,A | 0.224 ab,A | 0.993 ab,D | 0.993 bcd,D | 0.979 cd,D | 0.930 b,CD | 0.879 bc,C |
| 13 | 0.474 a,C | 0.313 cd,B | 0.260 bc,A | 0.990 ab,F | 1.000 cd,F | 0.971 c,EF | 0.932 b,DE | 0.899 bcd,D |
| 15 | 0.384 a,B | 0.375 e,B | 0.293 cd,A | 0.988 ab,E | 0.982 abc,E | 0.970 c,E | 0.929 b,D | 0.894 bcd,C |
| 18 | 0.376 a,A | 0.340 de,A | 0.313 cd,A | 0.990 ab,C | 0.972 ab,C | 0.947 a,BC | 0.930 b,BC | 0.886 bcd,B |
| 27 | 0.365 a,B | 0.246 ab,A | 0.328 d,B | 0.995 ab,F | 0.955 a,EF | 0.906 a,DE | 0.857 a,CD | 0.821 a,C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polatidou, K.; Nouska, C.; Tananaki, C.; Biliaderis, C.G.; Lazaridou, A. Physicochemical and Rheological Characteristics of Monofloral Honeys—Kinetics of Creaming–Crystallization. Foods 2025, 14, 1835. https://doi.org/10.3390/foods14101835
Polatidou K, Nouska C, Tananaki C, Biliaderis CG, Lazaridou A. Physicochemical and Rheological Characteristics of Monofloral Honeys—Kinetics of Creaming–Crystallization. Foods. 2025; 14(10):1835. https://doi.org/10.3390/foods14101835
Chicago/Turabian StylePolatidou, Kerasia, Chrysanthi Nouska, Chrysoula Tananaki, Costas G. Biliaderis, and Athina Lazaridou. 2025. "Physicochemical and Rheological Characteristics of Monofloral Honeys—Kinetics of Creaming–Crystallization" Foods 14, no. 10: 1835. https://doi.org/10.3390/foods14101835
APA StylePolatidou, K., Nouska, C., Tananaki, C., Biliaderis, C. G., & Lazaridou, A. (2025). Physicochemical and Rheological Characteristics of Monofloral Honeys—Kinetics of Creaming–Crystallization. Foods, 14(10), 1835. https://doi.org/10.3390/foods14101835