Extraction, Purification, Component Analysis and Bioactivity of Polyphenols from Artemisia dracunculus L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Polyphenol Extraction Methods
2.3. Static Adsorption and Desorption Experiments on CPA
2.4. Determination of Polyphenol Purification Process
2.5. Dynamic Adsorption and Desorption of D101 Macroporous Resin for CPA
2.6. Component Analysis of PPA
2.6.1. Sample Preparation and Extraction
2.6.2. UPLC Conditions
2.6.3. MS Analysis
2.7. Infrared Spectroscopy (IR) Analysis
2.8. In Vitro Antioxidant Activity
2.8.1. DPPH Radical Scavenging Activity
2.8.2. Assay of ABTS+ Radical Scavenging Activity
2.8.3. The Scavenging Ability of Hydroxyl Radicals
2.9. In Vitro Antibacterial and Antifungal Activity
2.9.1. Determination of Antibacterial and Antifungal Activity
2.9.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.10. Statistical Analysis
3. Results
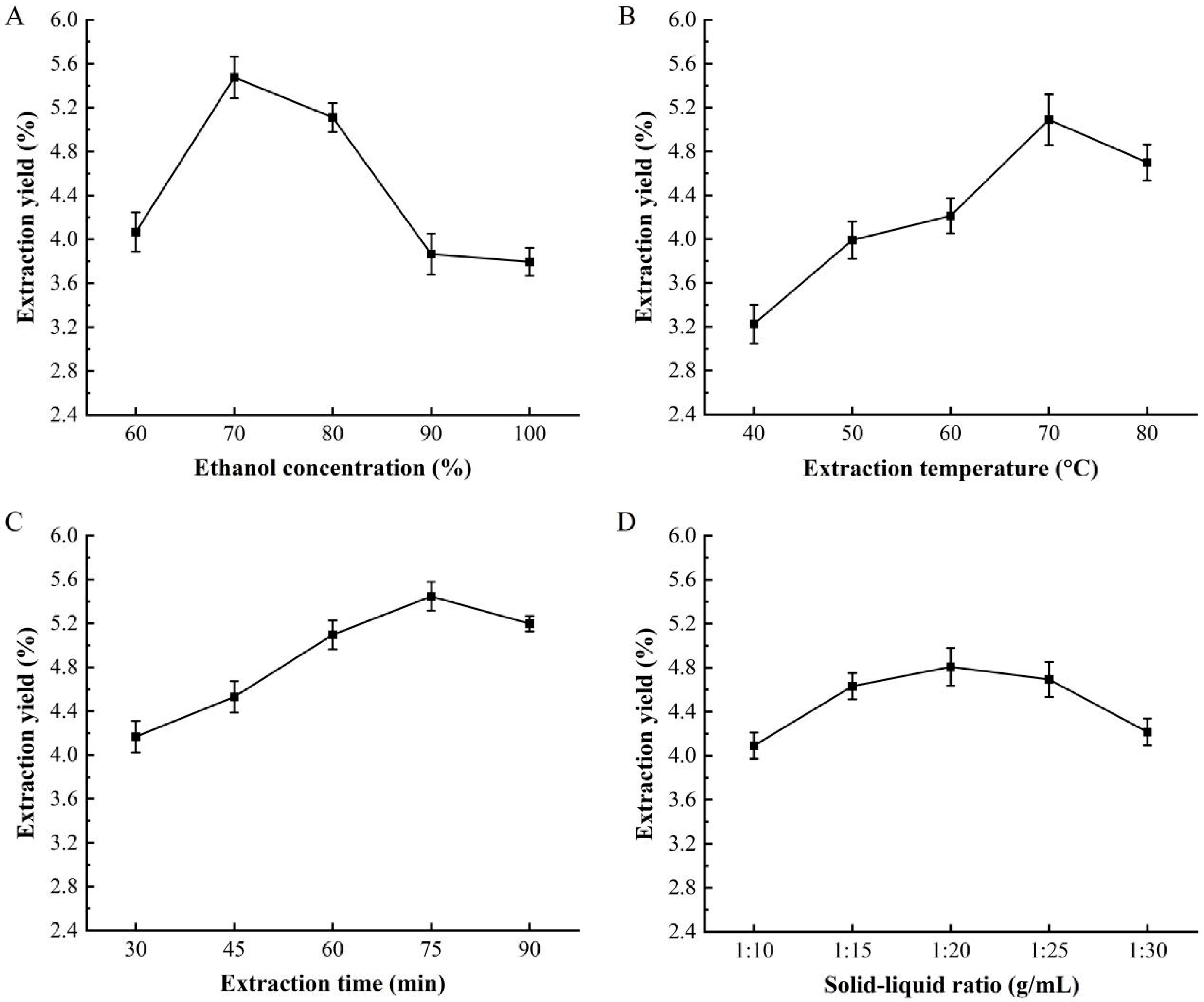
3.1. The Effect of Extraction Condition to the CPA Yield
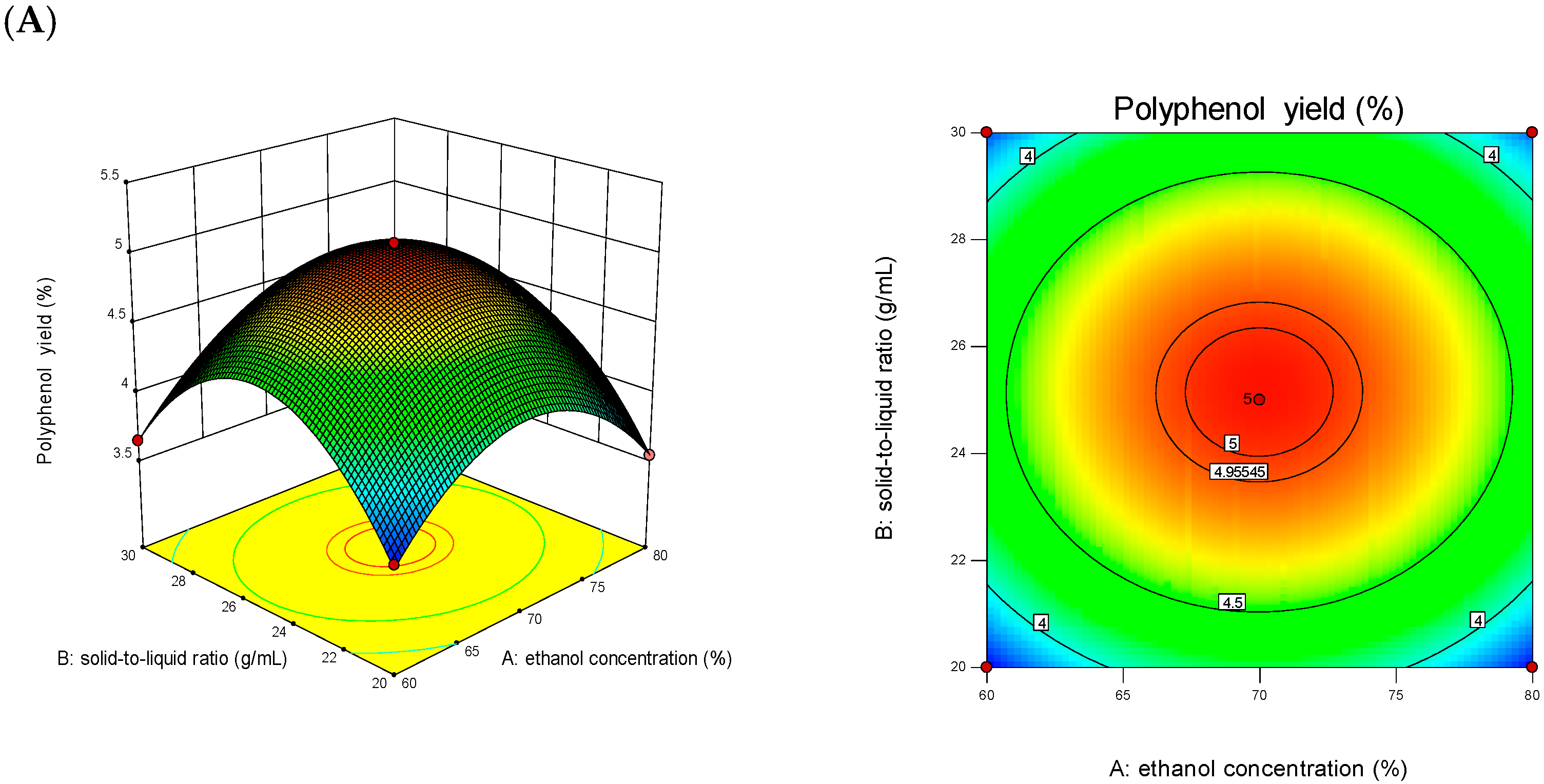
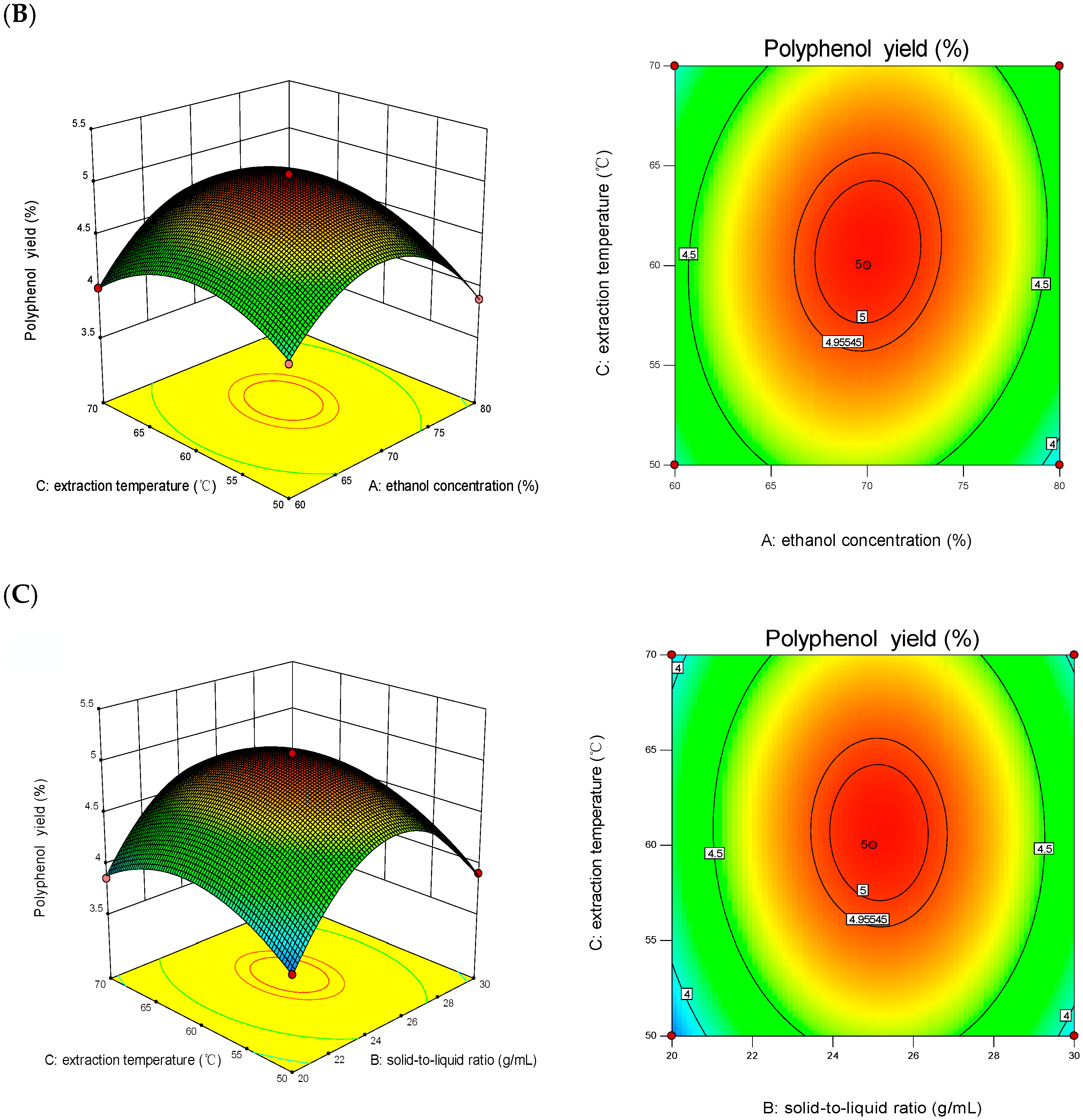
3.2. Results of Response Surface Test
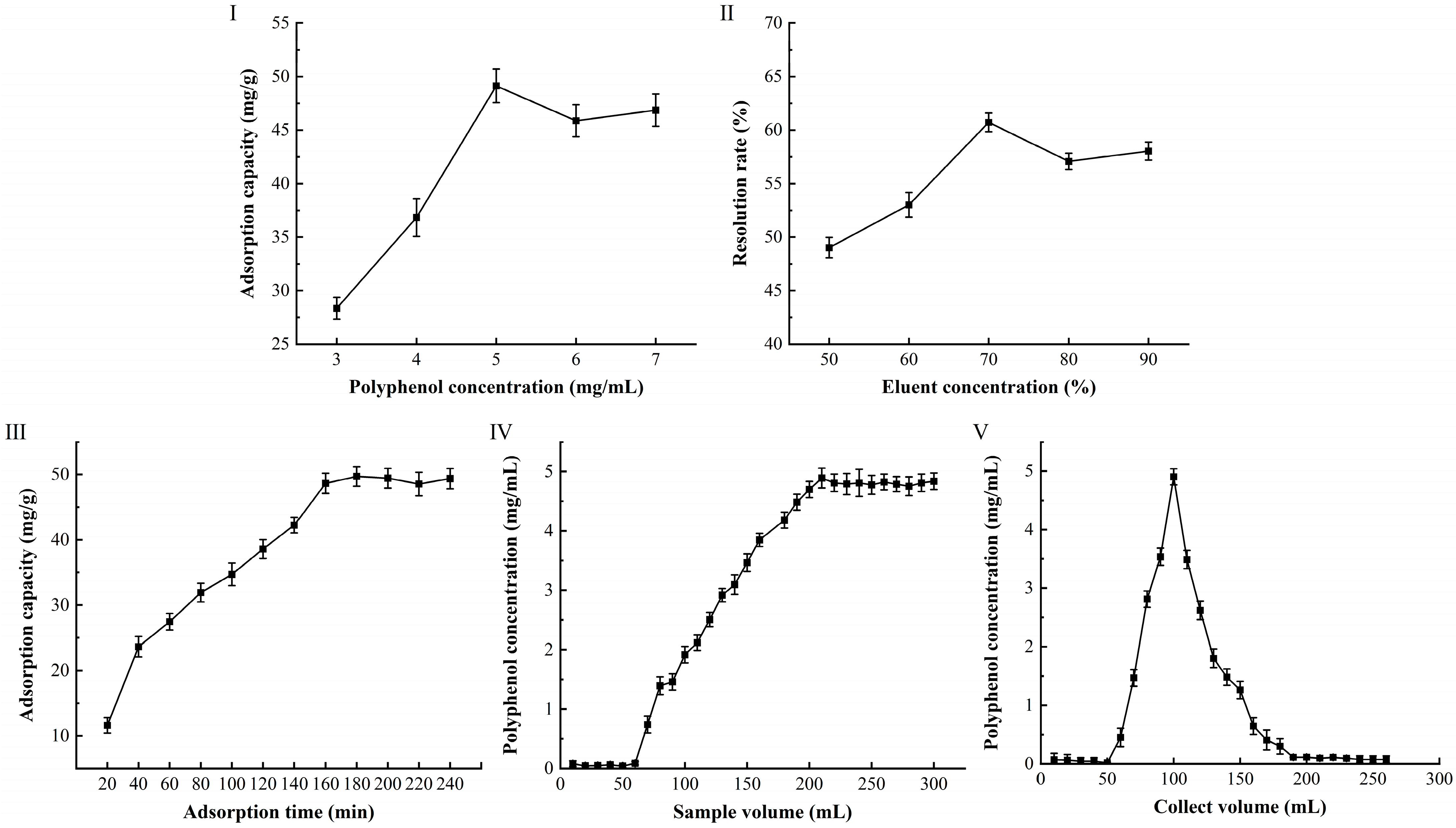
3.3. Purification of CPA by Macroporous Resin
3.4. Dynamic Adsorption and Desorption
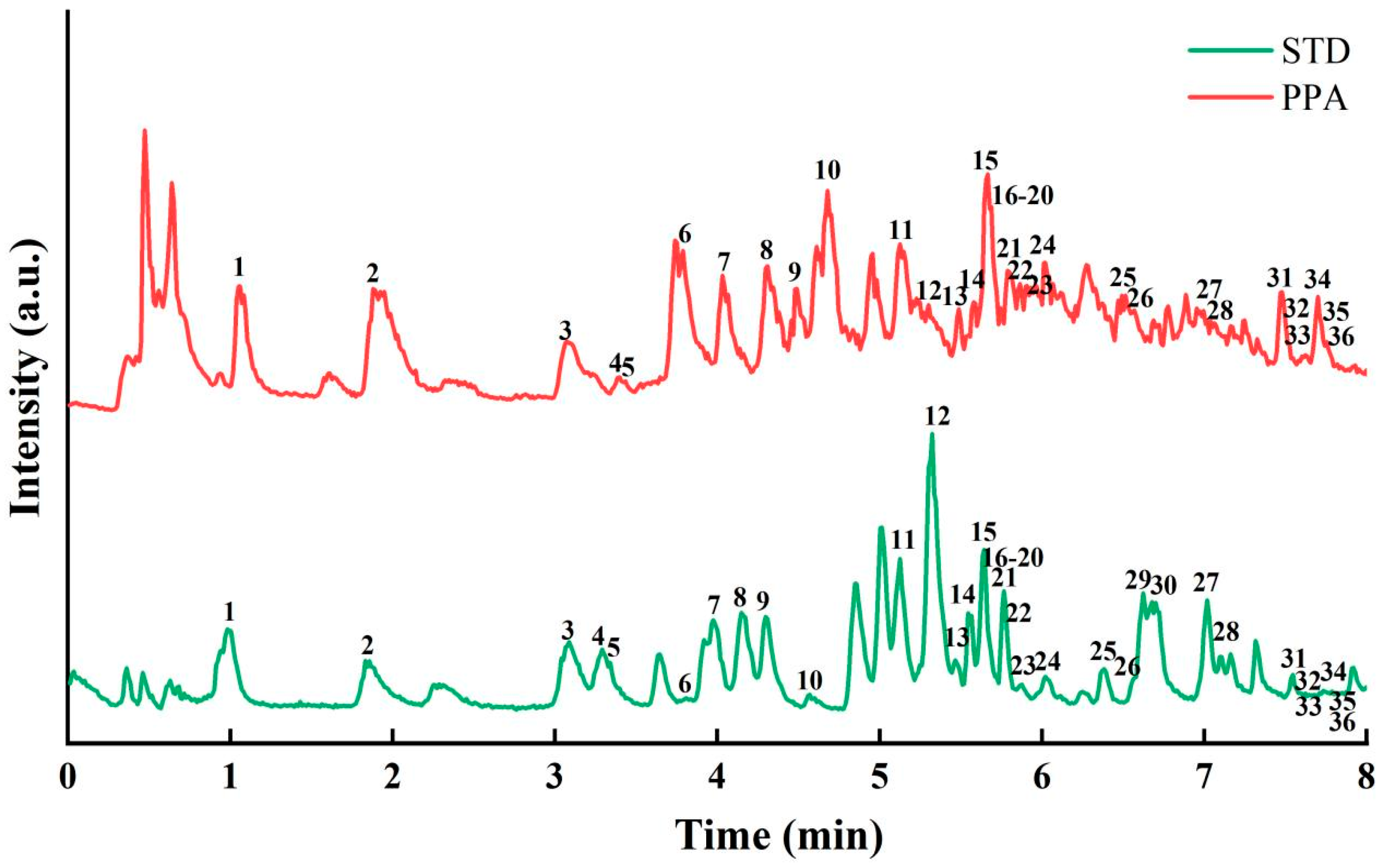
3.5. Component Analysis of PPA by UPLC-QE
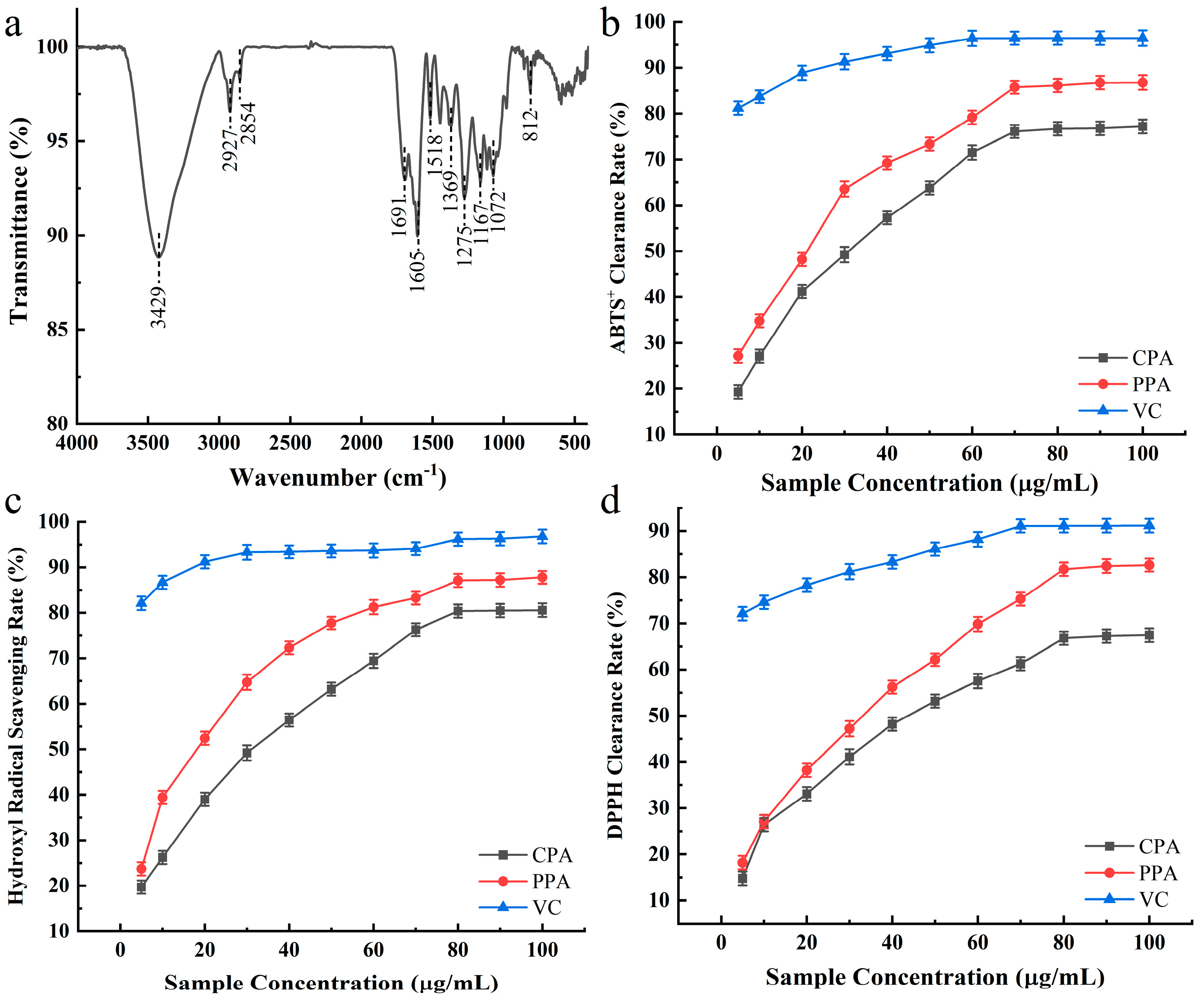
3.6. Infrared Spectroscopy (IR) Analysis
3.7. In Vitro Determination of Antioxidant Properties
3.8. Determination of Antibacterial and Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPA | Crude polyphenols of A. dracunculus |
| PPA | Purified polyphenols of A. dracunculus |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| UPLC-QE | Ultra-Performance Liquid Chromatography-Q Exactive |
References
- Mumivand, H.; Babalar, M.; Tabrizi, L.; Craker, L.E.; Shokrpour, M.; Hadian, J. Antioxidant properties and principal phenolic phytochemicals of Iranian tarragon (Artemisia dracunculus L.) accessions. Hortic. Environ. Biotechnol. 2017, 58, 414–422. [Google Scholar] [CrossRef]
- Sahakyan, N.; Andreoletti, P.; Cherkaoui-Malki, M.; Petrosyan, M.; Trchounian, A. Artemisia dracunculus L. essential oil phytochemical components trigger the activity of cellular antioxidant enzymes. J. Food Biochem. 2021, 45, e13691. [Google Scholar] [CrossRef] [PubMed]
- Zarezade, V.; Moludi, J.; Mostafazadeh, M.; Mohammadi, M.; Veisi, A. Antioxidant and hepatoprotective effects of Artemisia dracunculus against CCl4-induced hepatotoxicity in rats. Avicenna J. Phytomed. 2018, 8, 51. [Google Scholar] [PubMed Central]
- Jaradat, N.; Dwikat, M.; Amer, J.; Ghanim, M.; Hawash, M.; Hussein, F.; Issa, L.; Ishtawe, S.; Salah, S.; Nasser, S. Total phenolic contents, cytotoxic, free radicals, porcine pancreatic α-amylase, and lipase suppressant activities of Artemisia dracunculus plant from Palestine. Front. Pharmacol. 2024, 15, 1351743. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Świątkowska, J.; Knut, E.; Klin, P.; Rzepiela, A.; Tomczyk, M.; Szopa, A. Artemisia dracunculus (Tarragon): A review of its traditional uses, phytochemistry and pharmacology. Front. Pharmacol. 2021, 12, 653993. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Dertli, E. A green nano-biosynthesis of selenium nanoparticles with Tarragon extract: Structural, thermal, and antimicrobial characterization. LWT 2021, 141, 110969. [Google Scholar] [CrossRef]
- Duric, K.; Kovac-Besovic, E.E.; Niksic, H.; Muratovic, S.; Sofic, E. Anticoagulant activity of some Artemisia dracunculus leaf extracts. Bosn. J. Basic Med. Sci. 2015, 15, 9. [Google Scholar] [CrossRef]
- Azizkhani, M.; Jafari Kiasari, F.; Tooryan, F.; Shahavi, M.H.; Partovi, R. Preparation and evaluation of food-grade nanoemulsion of tarragon (Artemisia dracunculus L.) essential oil: Antioxidant and antibacterial properties. J. Food Sci. Technol. 2021, 58, 1341–1348. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Raal, A.; Vahtra, A.; Koshovyi, O.; Ilina, T.; Kovalyova, A.; Püssa, T. Polyphenolic Compounds in the Stems of Raspberry (Rubus idaeus) Growing Wild and Cultivated. Molecules 2024, 29, 5016. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions or future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Ahmad, Z.; Formanowicz, D.; Ribaudo, G.; Alomar, T.S. Antioxidant potential of polyphenolic and flavonoid compounds. Front. Chem. 2024, 12, 1463755. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Du, L.; Wang, J.; Liu, H.; Zhao, H.; Lyu, L.; Wang, W.; Wu, W.; Li, W. Polyphenols from Prunus mume: Extraction, purification, and anticancer activity. Food Funct. 2023, 14, 4380–4391. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, K.; Hu, L. Advancements in delivery systems for dietary polyphenols in enhancing radioprotection effects: Challenges and opportunities. Npj Sci. Food 2025, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.K.; Kwak, B.M.; Jang, H.W. Optimization and comparative analysis of quality characteristics and volatile profiles in edible insect oils extracted using supercritical fluid extraction and ultrasound-assisted extraction methods. Food Chem. 2025, 474, 143237. [Google Scholar] [CrossRef]
- Hiranpradith, V.; Therdthai, N.; Soontrunnarudrungsri, A.; Rungsuriyawiboon, O. Optimisation of Ultrasound-Assisted Extraction of Total Phenolics and Flavonoids Content from Centella asiatica. Foods 2025, 14, 291. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, Y.; Miao, N.; Xing, W.; Yun, C.; Wang, S.; Wang, W.; Wang, H. A green ultrasound-assisted enzymatic extraction method for efficient extraction of total polyphenols from Empetrum nigrum and determination of its bioactivities. J. Ind. Eng. Chem. 2022, 109, 559–567. [Google Scholar] [CrossRef]
- Cao, S.; Liang, J.; Chen, M.; Xu, C.; Wang, X.; Qiu, L.; Zhao, X.; Hu, W. Comparative analysis of extraction technologies for plant extracts and absolutes. Front. Chem. 2025, 13, 1536590. [Google Scholar] [CrossRef]
- Ma, S.; Cai, C.; Lu, Q.; Tan, Z. A review of green solvents for the extraction and separation of bioactive ingredients from natural products. Food Chem. 2025, 478, 143703. [Google Scholar] [CrossRef]
- Yang, L.F.; Nie, W.; Cui, Y.P.; Yue, F.; Fan, X.; Sun, R. Purification with macroporous resin and antioxidant activity of polyphenols from sweet potato leaves. Chem. Pap. 2024, 78, 181–188. [Google Scholar] [CrossRef]
- Tang, W.; Yao, W.; Lei, X.; Zhou, Y.; Liu, M.; Zeng, Z.; Zhao, C. Efficient enrichment and purification of anti-inflammatory nuezhenoside G13 from Osmanthus fragrans fruit by macroporous resin adsorption. Ind. Crops Prod. 2023, 192, 116092. [Google Scholar] [CrossRef]
- Aljawarneh, R.Y.A.; Che Zain, M.S.; Zakaria, F. Macroporous polymeric resin for the purification of flavonoids from medicinal plants: A review. J. Sep. Sci. 2024, 47, 2400372. [Google Scholar] [CrossRef]
- Scott, M.C.; Bourgeois, A.; Yu, Y.; Burk, D.H.; Smith, B.J.; Floyd, Z.E. Extract of Artemisia dracunculus L. modulates osteoblast proliferation and mineralization. Int. J. Mol. Sci. 2023, 24, 13423. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.B.; Chen, Z.Y.; Wang, G.; Su, Q.; Liu, Y.J. Preparation, structural characterization and neuroprotective effects of polysaccharides from the pericarp of Zanthoxylum bungeanum Maxim against H2O2-induced oxidative damage in PC12 cells. S. Afr. J. Bot. 2021, 142, 165–174. [Google Scholar] [CrossRef]
- Ren, Y.; Makhele, M.; Zhou, J.; Sun, P. Extraction, purification, component analysis and bioactivity of polyphenols from wampee. Process Biochem. 2025, 150, 318–327. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Q.; Zhang, B.; Zhang, J.; Fan, L.; Kang, J.; Lin, Y.; Huang, Y.; Tan, T.; Ho, L. Adsorption and desorption characteristics of flavonoids from white tea using macroporous adsorption resin. J. Chromatogr. A 2024, 1715, 464621. [Google Scholar] [CrossRef]
- Che Zain, M.S.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and desorption properties of total flavonoids from oil palm (Elaeis guineensis Jacq.) mature leaf on macroporous adsorption resins. Molecules 2020, 25, 778. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, F.; Alam, M.B.; Naznin, M.; Kim, S.; Lee, S.H. Optimization, metabolomic analysis, antioxidant potential and depigmenting activity of polyphenolic compounds from unmature ajwa date seeds (Phoenix dactylifera L.) using ultrasonic-assisted extraction. Antioxidants 2024, 13, 238. [Google Scholar] [CrossRef]
- Żurek, N.; Świeca, M.; Kapusta, I. UPLC-ESI-TQD-MS/MS Identification and Antioxidant, Anti-Inflammatory, Anti-Diabetic, Anti-Obesity and Anticancer Properties of Polyphenolic Compounds of Hawthorn Seeds. Plant Foods Hum. Nutr. 2024, 79, 594–600. [Google Scholar] [CrossRef]
- Lekshmi, S.G.; Sethi, S.; Kundu, A.; Singh, A.; Singh, K.P.; Namita; Kumar, R.; Asrey, R.; Sindhu, P.M. Extraction of polyphenolic compounds from rose and marigold, UPLC-ESI-QToF-MS/MS, FTIR characterization and assessment of antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2025, 44, 100608. [Google Scholar] [CrossRef]
- Lukhmana, N.; Kong, F.; Singh, R.K. Micronization enhanced extractability of polyphenols and anthocyanins in tart cherry puree. Food Biosci. 2022, 50, 102063. [Google Scholar] [CrossRef]
- Abudureheman, B.; Zhou, X.; Shu, X.; Chai, Z.; Xu, Y.; Li, S.; Hu, J.; Pan, H.; Ye, X. Evaluation of biochemical properties, antioxidant activities and phenolic content of two wild-grown berberis fruits: Berberis nummularia and Berberis atrocarpa. Foods 2022, 11, 2569. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, F.; Zhao, Z.; Hu, K.; Zhou, C. Optimization, purification and antioxidant potential of polyphenol ultrasonic-assisted extraction from pecan ‘Shaoxing’ green husk. Food Prod. Process. Nutr. 2025, 7, 20. [Google Scholar] [CrossRef]
- Ngamsurach, P.; Praipipat, P. Antibacterial activities against Staphylococcus aureus and Escherichia coli of extracted Piper betle leaf materials by disc diffusion assay and batch experiments. RSC Adv. 2022, 12, 26435–26454. [Google Scholar] [CrossRef]
- Sfaxi, A.; Tavaszi-Sárosi, S.; Flórián, K.; Patonay, K.; Radácsi, P.; Juhász, Á. Comparative Evaluation of Different Mint Species Based on Their In Vitro Antioxidant and Antibacterial Effect. Plants 2025, 14, 105. [Google Scholar] [CrossRef]
- Ayil-Gutiérrez, B.A.; Amaya-Guardia, K.C.; Alvarado-Segura, A.A.; Polanco-Hernández, G.; Uc-Chuc, M.A.; Acosta-Viana, K.Y.; Guzmán-Marin, E.; Samaniego-Gamez, B.Y.; Poot-Poot, W.A.; Lizama-Uc, G.; et al. Compound identification from Bromelia karatas fruit juice using gas chromatography–mass spectrometry and evaluation of the bactericidal activity of the extract. Appl. Sci. 2022, 12, 7275. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Bhosale, S.S.; Granato, D.; Mohammadi, N. Purification and characterization of anthocyanin from Irish wild blackberry: Impact on color, composition, and antioxidant capacity. J. Food Meas. Charact. 2025, 19, 2730–2738. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Effect of extraction temperature on composition, structure and functional properties of flaxseed gum. Food Chem. 2017, 215, 333–340. [Google Scholar] [CrossRef]
- Khadaroo, S.N.B.A.; Grassia, P.; Gouwanda, D.; Poh, P.E. The influence of different solid-liquid ratios on the thermophilic anaerobic digestion performance of palm oil mill effluent. J. Environ. Manag. 2020, 257, 109996. [Google Scholar] [CrossRef]
- Khan, M.S.; Evans, G.M.; Peng, Z.; Doroodchi, E.; Moghtaderi, B.; Joshi, J.B.; Mitra, S. Expansion behaviour of a binary solid-liquid fluidised bed with different solid mass ratio. Adv. Powder Technol. 2017, 28, 3111–3129. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yamada, S.; Sakuma, S.; Kanba, S.; Youda, C.; Ono, M.; Ito, R.; Kamei, J.; Akiyama, H. Optimization of QuEChERS Extraction for Determination of Carotenoids, Polyphenols, and Sterols in Orange Juice Using Design of Experiments and Response Surface Methodology. Foods 2023, 12, 3064. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Gong, G.; Li, F.; Ren, H.; Liu, Y. Adsorption and desorption properties of macroporous resins for flavonoids from the extract of Chinese wolfberry (Lycium barbarum L.). Food Bioprod. Process. 2015, 93, 148–155. [Google Scholar] [CrossRef]
- Aliya, K.; Cho, H.S.; Olawuyi, I.F.; Park, J.H.; Nam, J.O.; Lee, W.Y. Macroporous Resin Recovery of Antioxidant Polyphenol Compounds from Red Onion (Allium cepa L.). Antioxidants 2025, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Lin, C.; Ma, Y.; Shi, J.; Liu, J.; Zhu, L.; Bian, Z. One-step enrichment of phenolics from Chaenomeles speciosa (Sweet) Nakai fruit using macroporous resin: Adsorption/desorption characteristics, process optimization and UPLC-QqQ-MS/MS-based quantification. Food Chem. 2024, 439, 138085. [Google Scholar] [CrossRef]
- Xu, S.; Ning, F.; Jiang, B.; Xu, H.; Liu, Z. Refining androstenedione and bisnorcholenaldehyde from mother liquor of phytosterol fermentation using macroporous resin column chromatography followed by crystallization. J. Chromatogr. B 2018, 1079, 9–14. [Google Scholar] [CrossRef]
- Hou, M.; Zhang, L. Adsorption/desorption characteristics and chromatographic purification of polyphenols from Vernonia patula (Dryand.) Merr. using macroporous adsorption resin. Ind. Crops Prod. 2021, 170, 113729. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Luo, Q.; Wu, D.; Zou, L.; Liu, Y.; Li, H.; Gan, R. Current extraction, purification, and identification techniques of tea polyphenols: An updated review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3912–3930. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, B.; Ganapathy, D.; Bharathi, S. Exploration of Sargassum wightii: Extraction, phytochemical analysis, and antioxidant potential of polyphenol. Cureus 2024, 16, e63706. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Huang, S.; Zhang, L.; Ge, Z.; Sun, L.; Zong, W. Purification of polyphenols from distiller’s grains by macroporous resin and analysis of the polyphenolic components. Molecules 2019, 24, 1284. [Google Scholar] [CrossRef]
- Xu, L.; Ma, T.; Wang, X.; Liu, W.; Li, J.; Wang, X. Preparative separation of seven polyphenols from Perillae Folium via pH-zone-refining counter-current chromatography combined with high-speed counter-current chromatography. Anal. Methods 2021, 13, 1232–1237. [Google Scholar] [CrossRef]
- Genovese, C.; Cambria, M.T.; D’angeli, F.; Addamo, A.P.; Malfa, G.A.; Siracusa, L.; Pulvirenti, L.; Anfuso, C.D.; Lupo, G.; Salmeri, M. The double effect of walnut septum extract (Juglans regia L.) counteracts A172 glioblastoma cell survival and bacterial growth. Int. J. Oncol. 2020, 57, 1129–1144. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Djordjević, V.B.; Petrović, P.M.; Pljevljakušić, D.S.; Zdunić, G.M.; Šavikin, K.P.; Bugarski, B.M. The influence of different extraction conditions on polyphenol content, antioxidant and antimicrobial activities of wild thyme. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100328. [Google Scholar] [CrossRef]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-resistant enterococci: A review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef] [PubMed]
| Resins | D101 | D201 | D301 | AB-8 | NKA-9 |
|---|---|---|---|---|---|
| Physicochemical properties | |||||
| Color | Milky white | Light yellow | Milky white | Milky white | Milky white |
| Particle size range (mm) | 0.9–1.0 | 0.5–0.7 | 0.4–0.6 | 1.3–1.4 | 1.5–1.65 |
| Polarity | Non-polarity | Strong-polarity | Weakly-polarity | Middle-polarity | Non-polarity |
| Water content (%) | 65–75 | 65–75 | 65–75 | 60–70 | 65–75 |
| Specific surface area (m2/g) | 500–550 | 650–700 | 450–480 | 480–520 | 250–290 |
| Independent Variables | Coded Levels of Variables | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Ethanol concentration (A) (%) | 60 | 70 | 80 |
| Solid-to-liquid ratio (B) (g/mL) | 1:20 | 1:25 | 1:30 |
| Extraction temperature (C) (°C) | 50 | 60 | 70 |
| No. | A | B | C | Polyphenol Yield (%) | |
|---|---|---|---|---|---|
| Ethanol Concentration (%) | Solid-to-Liquid Ratio (g/mL) | Extraction Temperature (°C) | Actual Value | Predicted Value | |
| 1 | 60 | 20 | 60 | 3.61 | 3.59 |
| 2 | 80 | 20 | 60 | 3.57 | 3.54 |
| 3 | 60 | 30 | 60 | 3.62 | 3.65 |
| 4 | 80 | 30 | 60 | 3.61 | 3.63 |
| 5 | 60 | 25 | 50 | 4.07 | 4.05 |
| 6 | 80 | 25 | 50 | 3.84 | 3.88 |
| 7 | 60 | 25 | 70 | 4.02 | 3.99 |
| 8 | 80 | 25 | 70 | 4.26 | 4.21 |
| 9 | 70 | 20 | 50 | 3.79 | 3.74 |
| 10 | 70 | 30 | 50 | 3.88 | 3.91 |
| 11 | 70 | 20 | 70 | 3.91 | 3.86 |
| 12 | 70 | 30 | 70 | 3.87 | 3.93 |
| 13 | 70 | 25 | 60 | 4.94 | 5.00 |
| 14 | 70 | 25 | 60 | 5.06 | 5.05 |
| 15 | 70 | 25 | 60 | 5.09 | 5.07 |
| 16 | 70 | 25 | 60 | 5.13 | 5.08 |
| 17 | 70 | 25 | 60 | 5.00 | 5.03 |
| Source | Square Sum | Degrees of Freedom | Mean Square Sum | F-Value | p-Value |
|---|---|---|---|---|---|
| model | 5.66 | 9 | 0.6289 | 486.70 | 0.0001 ** |
| A-Ethanol concentration | 0.0000 | 1 | 0.0000 | 0.0387 | 0.8496 |
| B-Solid-to-liquid ratio | 0.0190 | 1 | 0.0190 | 14.71 | 0.0064 ** |
| C-Extraction temperature | 0.0210 | 1 | 0.0210 | 16.26 | 0.0050 ** |
| AB | 0.0002 | 1 | 0.0002 | 0.1741 | 0.6890 |
| AC | 0.0380 | 1 | 0.0380 | 29.43 | 0.0010 ** |
| BC | 0.0025 | 1 | 0.0025 | 1.93 | 0.2068 |
| A2 | 1.70 | 1 | 1.70 | 1316.00 | 0.0001 ** |
| B2 | 2.75 | 1 | 2.75 | 2127.40 | 0.0001 ** |
| C2 | 0.6016 | 1 | 0.6016 | 465.60 | 0.0001 ** |
| residual | 0.0090 | 7 | 0.0013 | ||
| lost proposal | 0.0049 | 3 | 0.0016 | 1.59 | 0.3237 |
| pure error | 0.0041 | 4 | 0.0010 | ||
| aggregate | 5.67 | 16 | |||
| R2 = 0.9984 | Adj R2 = 0.9964 | C.V% = 0.8581 | |||
| D101 | D201 | D301 | AB-8 | NKA-9 | |
|---|---|---|---|---|---|
| Adsorption Rate (%) | 58.7 ± 2.06 b | 43.3 ± 1.89 c | 64.3 ± 3.41 a | 38.7 ± 1.88 d | 36.7 ± 1.94 d |
| Desorption Rate (%) | 66.8 ± 3.17 a | 40.4 ± 2.13 c | 42.9 ± 1.77 c | 63.4 ± 3.42 b | 63.9 ± 3.57 b |
| Classification | No. | Name | RT (min) | MM | [M-H]- | MS/MS | Formula | Chemical Structure | Content ng/mg | |
|---|---|---|---|---|---|---|---|---|---|---|
| phenolic acids | benzoic acid derivatives | 1 | Gallic acid | 0.98 | 170.13 | 169.01 | 69.03/79.01/107.01/125.02 | C7H6O5 | 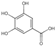 | 2.07 ± 0.11 |
| 2 | Protocatechuic acid | 1.86 | 154.13 | 153.02 | 81.03/91.02/108.02/109.03 | C7H6O4 | 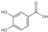 | 1338.05 ± 1.83 | ||
| 4 | 4-Hydroxybenzoic acid | 3.41 | 138.13 | 137.02 | 65.04/92.03/93.03 | C7H6O3 | 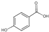 | 82.95 ± 0.15 | ||
| 7 | Vanillic acid | 4.16 | 168.16 | 167.03 | 95.01/108.02/123.01152.01 | C8H8O4 | 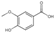 | 33.48 ± 0.07 | ||
| 9 | Syringic acid | 4.49 | 198.19 | 197.05 | 125.02/138.03/153.02/182.02 | C9H10O5 | 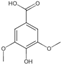 | 13.28 ± 0.16 | ||
| 19 | Salicylic acid | 5.69 | 138.13 | 137.02 | 65.04/92.02/93.03 | C7H6O3 |  | 0.95 ± 0.01 | ||
| 23 | Benzoic acid | 5.99 | 122.13 | 121.03 | 65.04/77.04/93.03 | C7H6O2 | 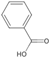 | 5.85 ± 0.03 | ||
| cinnamic acid derivatives | 8 | Caffeic acid | 4.34 | 180.17 | 179.03 | 93.03/107.01/134.04/135.05 | C9H8O4 | 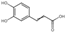 | 308.12 ± 0.18 | |
| 12 | Hydroxycinnamic Acid | 5.30 | 164.17 | 163.04 | 65.04/93.03/19.05 | C9H8O3 |  | 20.09 ± 0.05 | ||
| 16 | Trans-Ferulic acid | 5.63 | 194.20 | 193.05 | 117.03/134.04/149.02/178.03 | C10H10O4 | 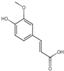 | 5.45 ± 0.04 | ||
| 17 | Sinapic Acid | 5.65 | 224.23 | 223.06 | 149.02/164.01/179.04/208.04 | C11H12O5 |  | 1.14 ± 0.02 | ||
| 29 | Hydrocinnamic acid | 7.10 | 150.19 | 149.06 | 77.04//91.05/105.03 | C9H10O2 | 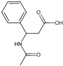 | 0.07 ± 0.00 | ||
| 30 | Trans-Cinnamic acid | 7.19 | 148.17 | 147.04 | 77.04/102.05/103.05 | C9H8O2 | 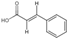 | 0.47 ± 0.01 | ||
| flavonoids | flavonoids | 6 | Catechin | 3.98 | 290.29 | 289.07 | 125.02/179.03/203.07/245.08 | C15H14O6 | 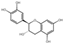 | 524.33 ± 0.15 |
| 10 | Epicatechin | 4.80 | 290.29 | 29.07 | 125.02/ | C15H14O6 | 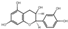 | 70.13 ± 0.15 | ||
| 22 | (+)-Dihydroquercetin | 5.89 | 304.27 | 303.05 | 125.02/217.05/257.05/285.04 | C15H12O7 | 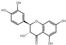 | 269.94 ± 0.37 | ||
| 25 | (+)-Dihydrokaempferol | 6.41 | 288.27 | 287.06 | 151.00/213.06/241.05/269.05 | C15H12O6 | 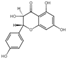 | 14.69 ± 0.09 | ||
| flavonols | 18 | Quercetin 3-β-D-glucoside | 5.69 | 464.41 | 463.09 | 255.03/271.04/301.04/300.03 | C21H20O12 |  | 868.29 ± 1.33 | |
| 24 | Kaempferol- 3-O-glucoside | 6.01 | 448.41 | 447.09 | 227.04/255.03/284.03/285.04 | C21H20O11 | 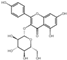 | 759.22 ± 0.27 | ||
| 28 | Quercetin | 7.05 | 302.25 | 313.03 | 107.01/151.00/229.05/257.05/273.04 | C15H10O7 | 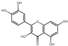 | 64.62 ± 0.19 | ||
| 35 | Kaempferol | 7.63 | 286.25 | 285.04 | 107.01/151.00/227.04/255.03 | C15H10O6 | 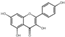 | 68.05 ± 0.67 | ||
| 36 | Isorhamnetin | 7.68 | 316.28 | 315.05 | 151.00/255.03/271.03/300.03 | C16H12O7 |  | 29.37 ± 0.48 | ||
| flavones | 15 | Vitexin | 5.61 | 432.41 | 431.10 | 161.02/269.05/283.06/311.06 | C21H20O10 | 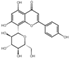 | 1.31 ± 0.06 | |
| 20 | Luteoloside | 5.72 | 448.41 | 447.09 | 151.00/257.05/284.03/285.04 | C21H20O11 |  | 31.73 ± 0.26 | ||
| 27 | Luteolin | 7.01 | 286.25 | 285.04 | 151.00/175.04/199.04/217.05 | C15H10O6 | 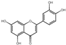 | 125.49 ± 0.43 | ||
| 33 | Apigenin | 7.53 | 270.25 | 269.05 | 117.03/151.00/201.06/225.06 | C15H10O5 | 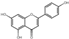 | 36.10 ± 0.10 | ||
| flavanones | 34 | Naringenin | 7.54 | 272.27 | 271.06 | 107.01/119.05/177.06/151.00 | C15H12O5 | 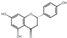 | 6.50 ± 0.09 | |
| 31 | Naringenin Chalcone | 7.49 | 272.27 | 271.06 | 107.01/151.04/177.06/227.07 | C15H12O5 |  | 5.16 ± 0.17 | ||
| flavonoid Glycosides | 14 | Rutin | 5.55 | 610.57 | 609.15 | 255.03/271.03/300.03/301.04 | C27H30O16 | 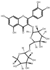 | 884.39 ± 2.21 | |
| 21 | Genistin | 5.78 | 432.41 | 431.10 | 133.03/225.06/268.04/269.05 | C21H20O10 | 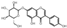 | 5.10 ± 0.19 | ||
| dihydrochalcone | 32 | Phloretin | 7.51 | 274.29 | 273.08 | 93.03/125.02/167.04 | C15H14O5 | 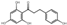 | 1.08 ± 0.04 | |
| benzaldehyde derivatives | 3 | Protocatechualdehyde | 3.16 | 138.13 | 137.02 | 64.02/81.03/92.03/108.02 | C7H6O3 |  | 80.97 ± 0.03 | |
| 11 | Vanillin | 5.12 | 152.16 | 151.04 | 81.03/92.03/108.02/136.02 | C8H8O3 | 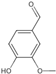 | 6.25 ± 0.12 | ||
| 13 | Syringaldehyde | 5.44 | 182.19 | 181.05 | 108.02/137.02/151.04/166.03 | C9H10O4 | 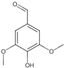 | 3.45 ± 0.10 | ||
| stilbenes | 26 | Resveratrol | 6.64 | 228.26 | 227.07 | 93.04/143.05/185.06 | C14H12O3 |  | 0.46 ± 0.01 | |
| other aromatic acid | 5 | Phthalic acid | 3.49 | 166.14 | 165.02 | 76.02/93.03/121.03 | C8H6O4 | 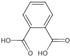 | 11.81 ± 0.31 | |
| Strain Name | 25 mg/mL | 50 mg/mL | 75 mg/mL | 100 mg/mL | 125 mg/mL | 150 mg/mL | 200 mg/mL | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| Siameter of the Inhibition Zone (mm) | |||||||||
| Salmonella enterica | - | 7.41 ± 0.21 | 8.63 ± 0.17 | 9.41 ± 0.19 | 10.12 ± 0.23 | 11.31 ± 0.16 | 11.17 ± 0.14 | 25 | 50 |
| Staphylococcus aureus | 10.4 ± 0.17 | 12.3 ± 0.16 | 13.7 ± 0.23 | 14.4 ± 0.11 | 15.67 ± 0.14 | 17.23 ± 0.21 | 17.35 ± 0.17 | 3.125 | 6.25 |
| Bacillus subtilis | 10.6 ± 0.13 | 12.5 ± 0.13 | 13.9 ± 0.07 | 15.2 ± 0.12 | 16.31 ± 0.26 | 18.47 ± 0.34 | 18.36 ± 0.13 | 3.125 | 3.125 |
| Shigella flexneri | 7.12 ± 0.07 | 8.23 ± 0.12 | 9.7 ± 0.13 | 10.3 ± 0.06 | 11.76 ± 0.15 | 11.64 ± 0.16 | 11.81 ± 0.14 | 6.25 | 12.5 |
| Enterococcus faecalis | 7.89 ± 0.09 | 9.31 ± 0.11 | 12.2 ± 0.16 | 13.4 ± 0.14 | 14.38 ± 0.17 | 15.97 ± 0.13 | 15.37 ± 0.12 | 25 | 25 |
| Escherichia coli | - | - | - | - | - | - | - | - | - |
| Listeria monocytogenes | 7.17 ± 0.04 | 8.36 ± 0.06 | 9.39 ± 0.13 | 9.42 ± 0.21 | 9.57 ± 0.16 | 9.95 ± 0.24 | 9.88 ± 0.10 | 12.5 | 25 |
| Acinetobacter baumannii | 7.23 ± 0.06 | 8.47 ± 0.09 | 9.23 ± 0.11 | 9.36 ± 0.14 | 9.48 ± 0.13 | 10.27 ± 0.16 | 10.29 ± 0.13 | 12.5 | 25 |
| Pseudomonas aeruginosa | 7.87 ± 0.15 | 8.68 ± 0.14 | 9.42 ± 0.13 | 10.2 ± 0.11 | 11.34 ± 0.16 | 12.37 ± 0.27 | 12.88 ± 0.23 | 6.25 | 12.5 |
| Rhizopus stolonifer | 9.52 ± 0.12 | 12.3 ± 0.11 | 13.8 ± 0.16 | 15.6 ± 0.15 | 17.63 ± 0.13 | 18.61 ± 0.25 | 18.23 ± 0.32 | 6.25 | 6.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Abudureheman, B.; Anwar, O.; Abdugini, E.; Zhang, J.; Tang, R.; Gao, Z.; Pan, H.; Ye, X. Extraction, Purification, Component Analysis and Bioactivity of Polyphenols from Artemisia dracunculus L. Foods 2025, 14, 1823. https://doi.org/10.3390/foods14101823
Chen L, Abudureheman B, Anwar O, Abdugini E, Zhang J, Tang R, Gao Z, Pan H, Ye X. Extraction, Purification, Component Analysis and Bioactivity of Polyphenols from Artemisia dracunculus L. Foods. 2025; 14(10):1823. https://doi.org/10.3390/foods14101823
Chicago/Turabian StyleChen, Lin, Buhailiqiemu Abudureheman, Omar Anwar, Emran Abdugini, Jianlin Zhang, Rui Tang, Zhihui Gao, Haibo Pan, and Xingqian Ye. 2025. "Extraction, Purification, Component Analysis and Bioactivity of Polyphenols from Artemisia dracunculus L." Foods 14, no. 10: 1823. https://doi.org/10.3390/foods14101823
APA StyleChen, L., Abudureheman, B., Anwar, O., Abdugini, E., Zhang, J., Tang, R., Gao, Z., Pan, H., & Ye, X. (2025). Extraction, Purification, Component Analysis and Bioactivity of Polyphenols from Artemisia dracunculus L. Foods, 14(10), 1823. https://doi.org/10.3390/foods14101823





