Solid State Fermentation—A Promising Approach to Produce Meat Analogues
Abstract
1. Introduction
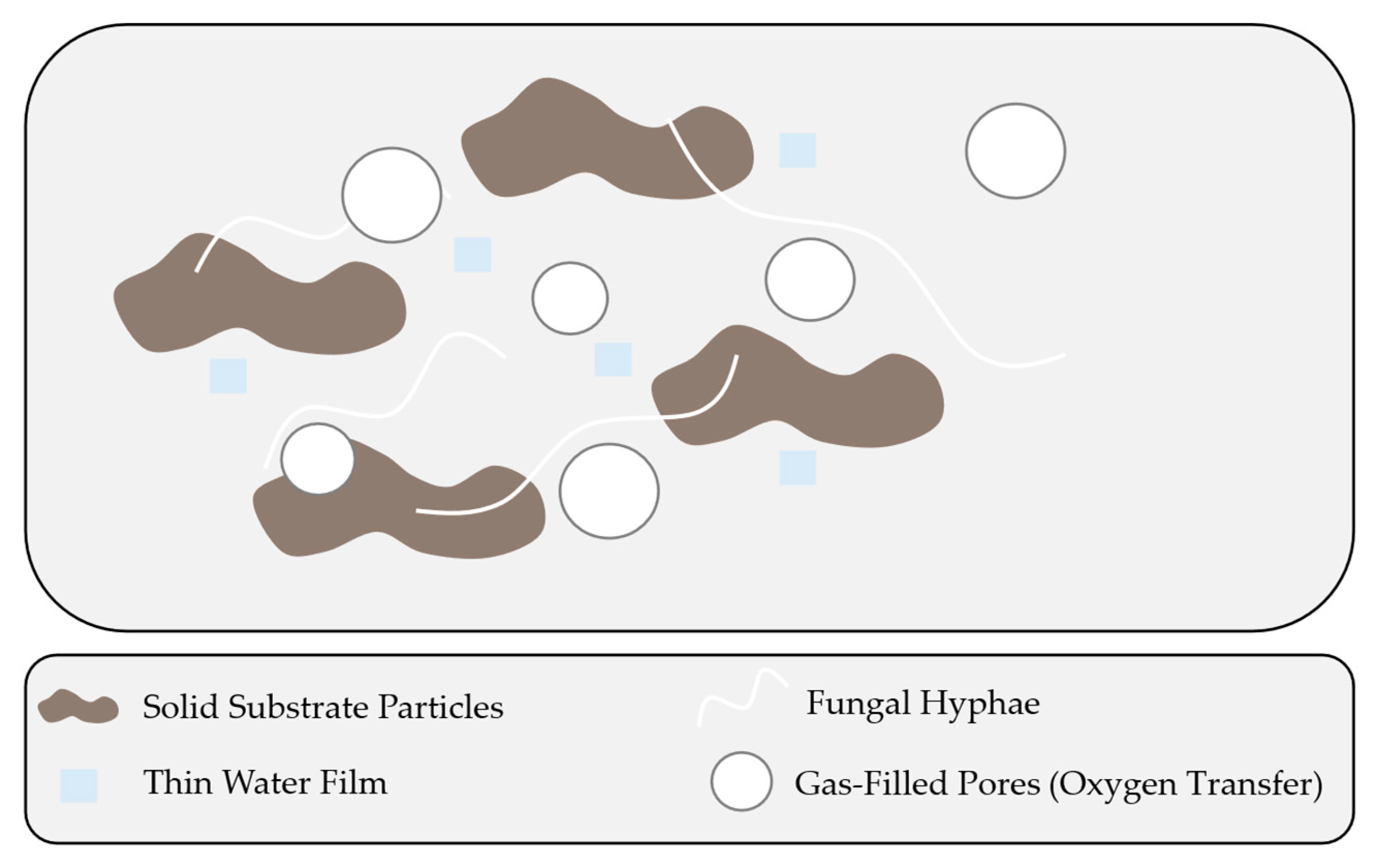
2. Fundamentals of Solid-State Fermentation
| Parameter | Solid-State Fermentation (SSF) | Submerged Fermentation (SmF) | Refs. |
|---|---|---|---|
| Medium Composition | Solid substrates with minimal free water | Liquid media with dissolved nutrients | [10,11] |
| Water Content | Low (40–80% moisture) | High (>95% water) | [13,14] |
| Oxygen Transfer | Through gas-filled pores in substrate matrix | Via mechanical agitation and aeration | [11] |
| Preferred Micro-organisms | Filamentous fungi (hyphal growth mode) | Bacteria, yeasts, unicellular organisms | [15] |
| Growth Kinetics | Often linear growth patterns | Exponential growth patterns | [16] |
| Heat Transfer | Poor (low thermal conductivity) | Efficient (convection in liquid) | [17] |
| pH Control | Difficult (relies on initial adjustment) | Relatively easy (continuous monitoring) | [10] |
| Scale-up Complexity | High (heterogeneous environment) | Moderate (well-established principles) | [18] |
| Capital Costs | Lower | Higher | [16] |
| Product Yield/Concentration | Often higher for certain processes | Variable, depending on application | [12] |
| Common Applications | Enzymes, bioactive compounds, biomass | Antibiotics, organic acids, recombinant proteins | [10,12] |
3. Substrates for SSF in Meat Analogue Production
4. Microorganisms in SSF for Meat Analogues
| Category | Examples | Key Functionalities | Refs. |
|---|---|---|---|
| Filamentous Fungi | Rhizopus (R. oligosporus, R. oryzae) | Rapid growth, enzyme secretion (amylases, proteases), B-vitamin synthesis, meat-like texture formation | [24,25] |
| Aspergillus (A. oryzae) | Hydrolytic enzyme production (amylases, proteases, lipases), deep substrate penetration, enhanced digestibility | [28] | |
| Neurospora (N. intermedia) | Fast growth on diverse substrates, carotenoid production (natural pigmentation) | [29] | |
| Bacteria | Bacillus (B. subtilis) | Protease production, protein hydrolysis, flavour enhancement, improved digestibility | [30] |
| Lactic Acid Bacteria (Lactobacillus, Pediococcus, Lactococcus) | Lactic acid production, pH reduction, microbial inhibition, preservation | [31] | |
| Mixed Cultures | Co-cultures (e.g., Rhizopus + LAB) | Combination of complementary metabolic pathways for enhanced texture, flavour, and safety | [32] |
5. Technological Aspects of Meat Analogue Production via SSF
6. Nutritional and Functional Properties of SSF-Derived Meat Analogues
7. Sensory Attributes and Consumer Acceptance
8. Food Safety Considerations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elhalis, H.; See, X.Y.; Osen, R.; Chin, X.H.; Chow, Y. Significance of Fermentation in Plant-Based Meat Analogs: A Critical Review of Nutrition, and Safety-Related Aspects. Foods 2023, 12, 3222. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; See, X.Y.; Osen, R.; Chin, X.H.; Chow, Y. The Potentials and Challenges of Using Fermentation to Improve the Sensory Quality of Plant-Based Meat Analogs. Front. Microbiol. 2023, 14, 1267227. [Google Scholar] [CrossRef] [PubMed]
- Mandliya, S.; Pratap-Singh, A.; Vishwakarma, S.; Dalbhagat, C.G.; Mishra, H.N. Incorporation of Mycelium (Pleurotus eryngii) in Pea Protein Based Low Moisture Meat Analogue: Effect on Its Physicochemical, Rehydration and Structural Properties. Foods 2022, 11, 2476. [Google Scholar] [CrossRef]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-Based Meat Alternatives: Technological, Nutritional, Environmental, Market, and Social Challenges and Opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef]
- Juhrich, L.C.; Grosse, M.; Mörlein, J.; Bergmann, P.; Zorn, H.; Gand, M. Nutritional and Sensory Properties of Meat Analogues: A Current Overview and Future Considerations. J. Agric. Food Chem. 2025, 73, 2236–2248. [Google Scholar] [CrossRef]
- Betchem, G.; Monto, A.R.; Lu, F.; Billong, L.F.; Ma, H. Prospects and Application of Solid-State Fermentation in Animal Feed Production—A Review. Ann. Anim. Sci. 2024, 24, 1123–1137. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. A Brief Review of the Science behind the Design of Healthy and Sustainable Plant-Based Foods. NPJ Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented Foods, Their Microbiome and Its Potential in Boosting Human Health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Larroche, C. Current Developments in Solid-State Fermentation; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological Advantages of Laboratory-Scale Solid-State Fermentation with Fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef]
- Cerda, A.; Gea, T.; Vargas-García, M.C.; Sánchez, A. Towards a Competitive Solid State Fermentation: Cellulases Production from Coffee Husk by Sequential Batch Operation and Role of Microbial Diversity. Sci. Total Environ. 2017, 589, 56–65. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current Developments in Solid-State Fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring Processes for Meat Analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef]
- Day, L. Proteins from Land Plants—Potential Resources for Human Nutrition and Food Security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Effect of Solid-State Fermentation with Cordyceps Militaris SN-18 on Physicochemical and Functional Properties of Chickpea (Cicer arietinum L.) Flour. LWT 2015, 63, 1317–1324. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Meat Analog as Future Food: A Review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil Cakes and Their Biotechnological Applications—A Review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef]
- Rahardjo, Y.S.P.; Tramper, J.; Rinzema, A. Modeling Conversion and Transport Phenomena in Solid-State Fermentation: A Review and Perspectives. Biotechnol. Adv. 2006, 24, 161–179. [Google Scholar] [CrossRef]
- Gervais, P.; Molin, P. The Role of Water in Solid-State Fermentation; Elsevier: Amsterdam, The Netherlands, 2003; Volume 13. [Google Scholar]
- Zhang, W.; Deng, Z.; Liu, T.; Liang, J.; Liu, J. Fermentation with Edible Mushroom Mycelia Improves Flavor Characteristics and Techno-Functionalities of Soybean Protein. Food Biosci. 2024, 59, 104123. [Google Scholar] [CrossRef]
- Artola, A.; Font, X.; Moral-Vico, J.; Sánchez, A. The Role of Solid-State Fermentation to Transform Existing Waste Treatment Plants Based on Composting and Anaerobic Digestion into Modern Organic Waste-Based Biorefineries, in the Framework of Circular Bioeconomy. Front. Chem. Eng. 2024, 6, 1463785. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Handoyo, T.; Morita, N. Structural and Functional Properties of Fermented Soybean (Tempeh) by Using Rhizopus Oligosporus. Int. J. Food Prop. 2006, 9, 347–355. [Google Scholar] [CrossRef]
- Bohrer, B.M. An Investigation of the Formulation and Nutritional Composition of Modern Meat Analogue Products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Appiani, M.; Cattaneo, C.; Laureati, M. Sensory Properties and Consumer Acceptance of Plant-Based Meat, Dairy, Fish and Eggs Analogs: A Systematic Review. Front. Sustain. Food Syst. 2023, 7, 1268068. [Google Scholar] [CrossRef]
- Chen, H. Modern Solid State Fermentation Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Surono, I.S. Ethnic fermented foods and beverages of indonesia. In Ethnic Fermented Foods and Alcoholic Beverages of Asia; Springer: New Delhi, India, 2016; pp. 341–382. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Industrialization of Indigenous Fermented Foods; Marcel, D., Ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0824747844. [Google Scholar]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of Vegetables and Fruits through Lactic Acid Fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Nout, M.J.R.; Kiers, J.L. Tempe Fermentation, Innovation and Functionality: Update into the Third Millenium. J. Appl. Microbiol. 2005, 98, 789–805. [Google Scholar] [CrossRef]
- Ng, G.C.F.; Choy, M.J.Y.; Tan, V.W.K.; Theng, A.H.P.; Ng, F.S.K.; Ong, D.S.M.; Ong, K.S.; Lim, P.Y.; Madathummal, M.; Chong, P.H.; et al. Comparative Analysis of Sensory, Textural, Microstructural, Amino Acids and Protein Digestibility Properties of Animal and Alternative Meat Products in the Asian Market. Int. J. Food Sci. Technol. 2024, 59, 5837–5858. [Google Scholar] [CrossRef]
- Kim, A.; Öström, Å.; Mihnea, M.; Niimi, J. Consumers’ Attachment to Meat: Association between Sensory Properties and Preferences for Plant-Based Meat Alternatives. Food Qual. Prefer. 2024, 116, 105134. [Google Scholar] [CrossRef]
- Sánchez, A.; Oiza, N.; Artola, A.; Font, X.; Barrena, R.; Moral-Vico, J.; Gea, T. Solid-State Fermentation: A Review of Its Opportunities and Challenges in the Framework of Circular Bioeconomy. Afinidad 2024, 81, 50–56. [Google Scholar] [CrossRef]
- Szenderák, J.; Fróna, D.; Rákos, M. Consumer Acceptance of Plant-Based Meat Substitutes: A Narrative Review. Foods 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Ruttle, D.I.; Hesseltine, C.W. Antibacterial Compound from a Soybean Product Fermented by Rhizopus oligosporus. Proc. Soc. Exp. Biol. Med. 1969, 131, 579–583. [Google Scholar] [CrossRef]
- Machida, M.; Yamada, O.; Gomi, K. Genomics of Aspergillus Oryzae: Learning from the History of Koji Mold and Exploration of Its Future. DNA Res. 2008, 15, 173–183. [Google Scholar] [CrossRef]
- Malila, Y.; Owolabi, I.O.; Chotanaphuti, T.; Sakdibhornssup, N.; Elliott, C.T.; Visessanguan, W.; Karoonuthaisiri, N.; Petchkongkaew, A. Current Challenges of Alternative Proteins as Future Foods. NPJ Sci. Food 2024, 8, 53. [Google Scholar] [CrossRef]
- Keuth, S.; Bisping, B. Vitamin B12 Production by Citrobacter Freundii or Klebsiella Pneumoniae during Tempeh Fermentation and Proof of Enterotoxin Absence by PCR. Appl. Environ. Microbiol. 1994, 60, 1495–1499. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of Soy Protein to Wheat Gluten Ratio on the Physicochemical Properties of Extruded Meat Analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Feng, X.M.; Passoth, V.; Eklund-Jonsson, C.; Alminger, M.L.; Schnürer, J. Rhizopus Oligosporus and Yeast Co-Cultivation during Barley Tempeh Fermentation-Nutritional Impact and Real-Time PCR Quantification of Fungal Growth Dynamics. Food Microbiol. 2007, 24, 393–402. [Google Scholar] [CrossRef]
- Tao, A.; Zhang, H.; Duan, J.; Xiao, Y.; Liu, Y.; Li, J.; Huang, J.; Zhong, T.; Yu, X. Mechanism and Application of Fermentation to Remove Beany Flavor from Plant-Based Meat Analogs: A Mini Review. Front Microbiol. 2022, 13, 1070773. [Google Scholar] [CrossRef]
- Godschalk-Broers, L.; Sala, G.; Scholten, E. Meat Analogues: Relating Structure to Texture and Sensory Perception. Foods 2022, 11, 2227. [Google Scholar] [CrossRef]
- Lin, J.W.X.; Maran, N.; Lim, A.J.; Ng, S.B.; Teo, P.S. Current Challenges, and Potential Solutions to Increase Acceptance and Long-Term Consumption of Cultured Meat and Edible Insects—A Review. Future Foods 2025, 11, 100544. [Google Scholar] [CrossRef]
- Su, T.; Le, B.; Zhang, W.; Bak, K.H.; Soladoye, P.O.; Zhao, Z.; Zhao, Y.; Fu, Y.; Wu, W. Technological Challenges and Future Perspectives of Plant-Based Meat Analogues: From the Viewpoint of Proteins. Food Res. Int. 2024, 186, 114351. [Google Scholar] [CrossRef] [PubMed]
- Han, B.Z.; Beumer, R.R.; Rombouts, F.M.; Nout, M.J.R. Microbiological safety and quality of commercial sufu—A Chinese fermented soybean food. Food Control 2001, 12, 541–547. [Google Scholar] [CrossRef]
- Nout, M.J.R.; de Dreu, M.A.; Zuurbier, A.M.; Bonants-van Laarhoven, T.M.G. Ecology of Controlled Soyabean Acidification for Tempe Manufacture. Food Microbiol. 1987, 4, 165–172. [Google Scholar] [CrossRef]






| Area | Specific Advantages | Impact on Meat Analogues | Refs. |
|---|---|---|---|
| Nutritional Enhancement |
| Better nutritional match to conventional meat | [5,6] |
| Sensory Properties |
| Enhanced consumer acceptance and palatability | [4,5] |
| Sustainability |
| Reduced environmental footprint | [7,8] |
| Functional Properties |
| Improved health benefits and shelf stability | [6,8] |
| Economic Benefits |
| Lower production costs compared to alternatives | [6,7] |
| Substrate Class | Examples | Protein Content (% d.w.) | Key Properties | Ref. |
|---|---|---|---|---|
| Legumes | Soybeans, chickpeas, lentils, fava beans, lupins | 20–45% | High protein content, rich amino acid profile, good fermentability | [17] |
| Cereal Grains | Wheat, rice | 7–14% | Structural properties (glutenin & gliadin in wheat), neutral flavour profile (rice) | [13] |
| Agricultural by-products | Wheat bran | Variable | Structural support for fungal growth, high fibre and micronutrient content | [16] |
| Oilseed Cakes and Meals | Residues from oil extraction (soybean, rapeseed meals) | >45% | High protein content, residual lipids enhancing sensory properties | [19] |
| Optimization Method | Key Characteristics | Application to SSF | Advantages | Limitations | Applicable Conditions and Practical Limitations | Ref. |
|---|---|---|---|---|---|---|
| Response Surface Methodology (RSM) | Statistical technique that explores relationships between variables and responses | Optimizing temperature (30 °C), relative humidity (85%), and fermentation time (36 h) for tempeh production | Provides visual representation of optimal conditions; identifies interactions between variables | Limited to relatively simple systems with few variables | Best for small-scale studies with few variables; less suited for complex systems | [24] |
| Artificial Neural Networks (ANNs) | A machine learning approach that models complex nonlinear relationships | Prediction of glucoamylase production in Aspergillus niger SSF | Superior predictive accuracy for complex systems; handles highly nonlinear responses | Requires substantial data for training; “black box” nature limits interpretability | Ideal with large datasets; “black-box” may limit insight | [33] |
| Genetic Algorithms | The evolutionary computational approach that mimics natural selection | Optimizing multi-variable SSF processes with complex interactions | Can search large solution spaces efficiently; not limited by mathematical constraints | Computationally intensive; may converge to local optima | Best for complex, multidimensional problems | [34] |
| Design of Experiments (DoE) | Structured approach to determine cause-and-effect relationships | Identifying critical process parameters in SSF systems | Reduces experimental burden; systematic approach | May oversimplify complex biological systems | Good for early-phase or screening studies | [13] |
| Process Analytical Technology (PAT) | Framework for designing, analyzing, and controlling manufacturing | Real-time monitoring of moisture, protein concentration, and substrate consumption | Enables real-time process adjustments; improves consistency | Implementation challenges in heterogeneous SSF systems | Suited for well-equipped setups; costly to implement | [10] |
| Hybrid Approaches | Combination of multiple optimization techniques | Integration of empirical models with machine learning for comprehensive process optimization | Leverages strengths of multiple methods; improved robustness | Increased complexity; requires multidisciplinary expertise | Great for advanced settings with computational tools | [19] |
| Nutritional Parameter | Enhancement During SSF | Key Findings from Research | Refs. |
|---|---|---|---|
| Protein Quality | Addition of complementary amino acids | Rhizopus oligosporus biomass contributes high levels of lysine and methionine | [20] |
| Protein Contribution | Increased protein content | Fungal biomass contributes 12–15% of total protein content | [36] |
| Protein Digestibility | 15–25% improvement | Enzymatic hydrolysis, inactivation of protease inhibitors, structural modifications | [37,38] |
| Mineral Bioavailability | Significant increase | 97% reduction in phytate content, increased iron, zinc, and calcium accessibility | [39] |
| Vitamin Content | 2–4-fold increases | 2.5-fold increase in riboflavin, 2–4-fold increase in niacin | [40] |
| Antioxidant Capacity | 2–3-fold increases | Enhanced DPPH radical scavenging activity in Aspergillus oryzae fermented soybeans | [38] |
| Sensory Attribute | Observations | Influencing Factors | Refs. |
|---|---|---|---|
| Flavour Development | Complex profiles with umami notes; over 45 distinct aroma compounds including 2-methylpyrazine, 2,5-dimethylpyrazine, and 2-acetylpyrrole | Microbial strain selection, fermentation duration, proteolytic activities releasing amino acids | [41,42] |
| Texture Formation | Natural fibrous structure from mycelial network; meat-like properties without extensive extrusion | Substrate particle size (2–3 mm optimal), moisture content (55–70%), fermentation time and temperature | [19,24] |
| Colour and Appearance | Typically off-white to greyish appearance; Neurospora strains produce orange-red pigments; Monascus purpureus generates red pigments | Fungal strain selection, fermentation conditions, substrate composition | [2,25] |
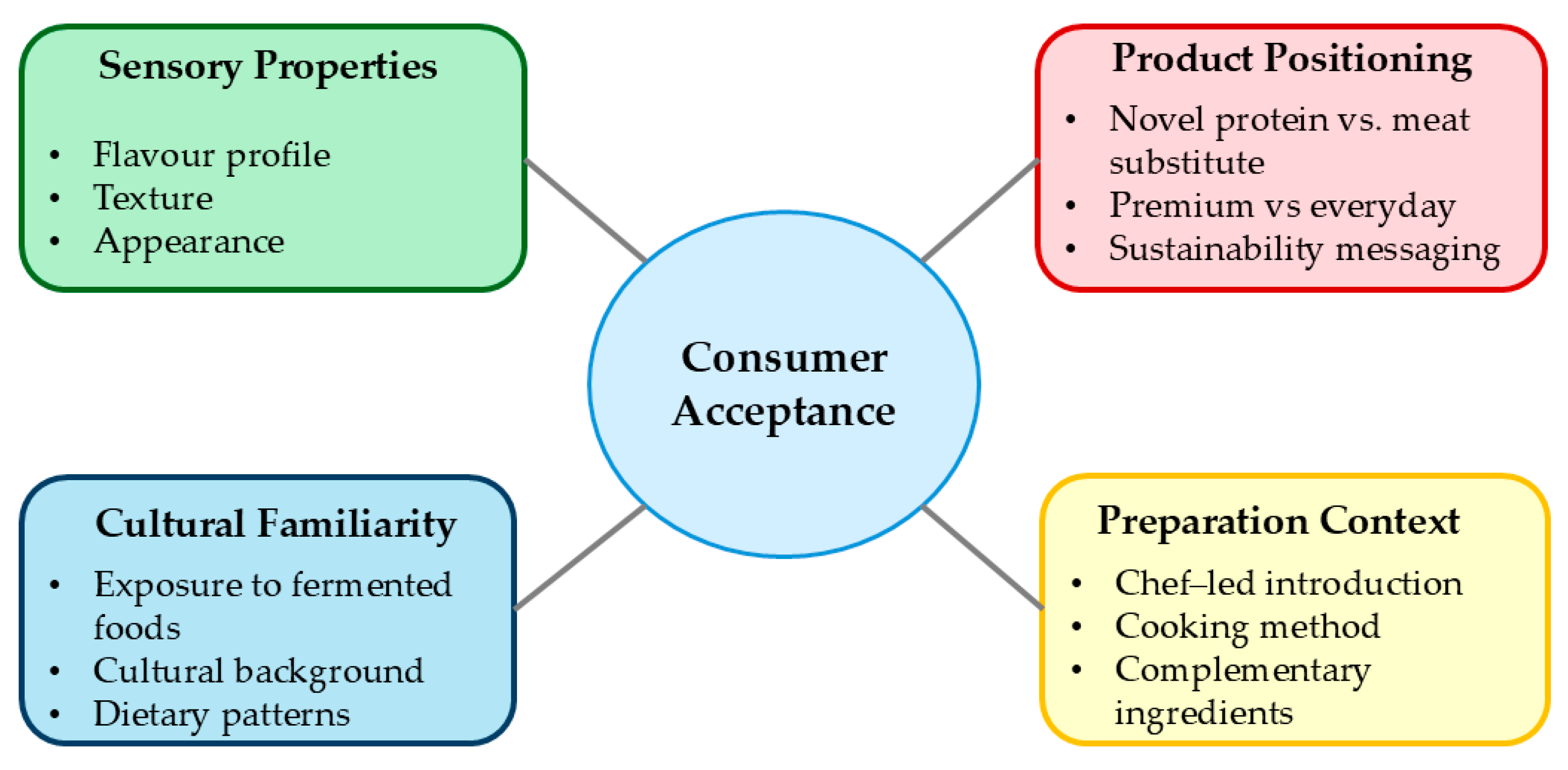
| Consumer Perception | Higher acceptance when positioned as novel protein sources rather than meat substitutes; cultural background influences acceptance | Familiarity with fermented foods, product positioning, chef-led introduction | [43] |
| Enhancement Strategies | Substrate blending, co-culture fermentation, post-fermentation treatments (marination, smoking) | Integration of precision fermentation and metabolomic monitoring | [6,43] |
| Safety Consideration | Control Measures | Observations | Refs. |
|---|---|---|---|
| Starter Culture Safety | Use of defined GRAS cultures; validation of purity | Commercial lyophilized cultures show superior consistency and safety compared to traditional starters | [42,47] |
| Substrate Preparation | Thermal treatments (boiling, steaming, autoclaving); acidification to pH 4.5 | Pre-fermentation acidification effectively inhibits pathogen growth while allowing normal Rhizopus development | [25,48] |
| Process Control | HACCP implementation; temperature control (<35 °C); monitoring of fermentation parameters | Critical control points include substrate thermal treatment, acidification, and fermentation temperature | [44] |
| Mycotoxin Prevention | Selection of non-mycotoxigenic strains; control of fermentation conditions | Rhizopus species do not produce significant mycotoxins; industrial A. oryzae strains lack genetic capacity for aflatoxin production. | [26,37] |
| Allergenicity Management | Careful substrate selection; appropriate thermal processing | Thermal processing reduces allergenic potential of fungal biomass proteins | [1] |
| Regulatory Compliance | Adherence to regional requirements; safety validation protocols | Harmonized regulatory approaches are needed to support global trade of SSF meat analogues | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milcarz, A.; Harasym, J. Solid State Fermentation—A Promising Approach to Produce Meat Analogues. Foods 2025, 14, 1820. https://doi.org/10.3390/foods14101820
Milcarz A, Harasym J. Solid State Fermentation—A Promising Approach to Produce Meat Analogues. Foods. 2025; 14(10):1820. https://doi.org/10.3390/foods14101820
Chicago/Turabian StyleMilcarz, Agata, and Joanna Harasym. 2025. "Solid State Fermentation—A Promising Approach to Produce Meat Analogues" Foods 14, no. 10: 1820. https://doi.org/10.3390/foods14101820
APA StyleMilcarz, A., & Harasym, J. (2025). Solid State Fermentation—A Promising Approach to Produce Meat Analogues. Foods, 14(10), 1820. https://doi.org/10.3390/foods14101820