Restored Intensities from Customized Crops of NMR Experiments (RICC-NMR) to Gain Better Insight on Chemometrics of Sicilian and Sardinian Extra Virgin Olive Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. NMR Sample Preparation
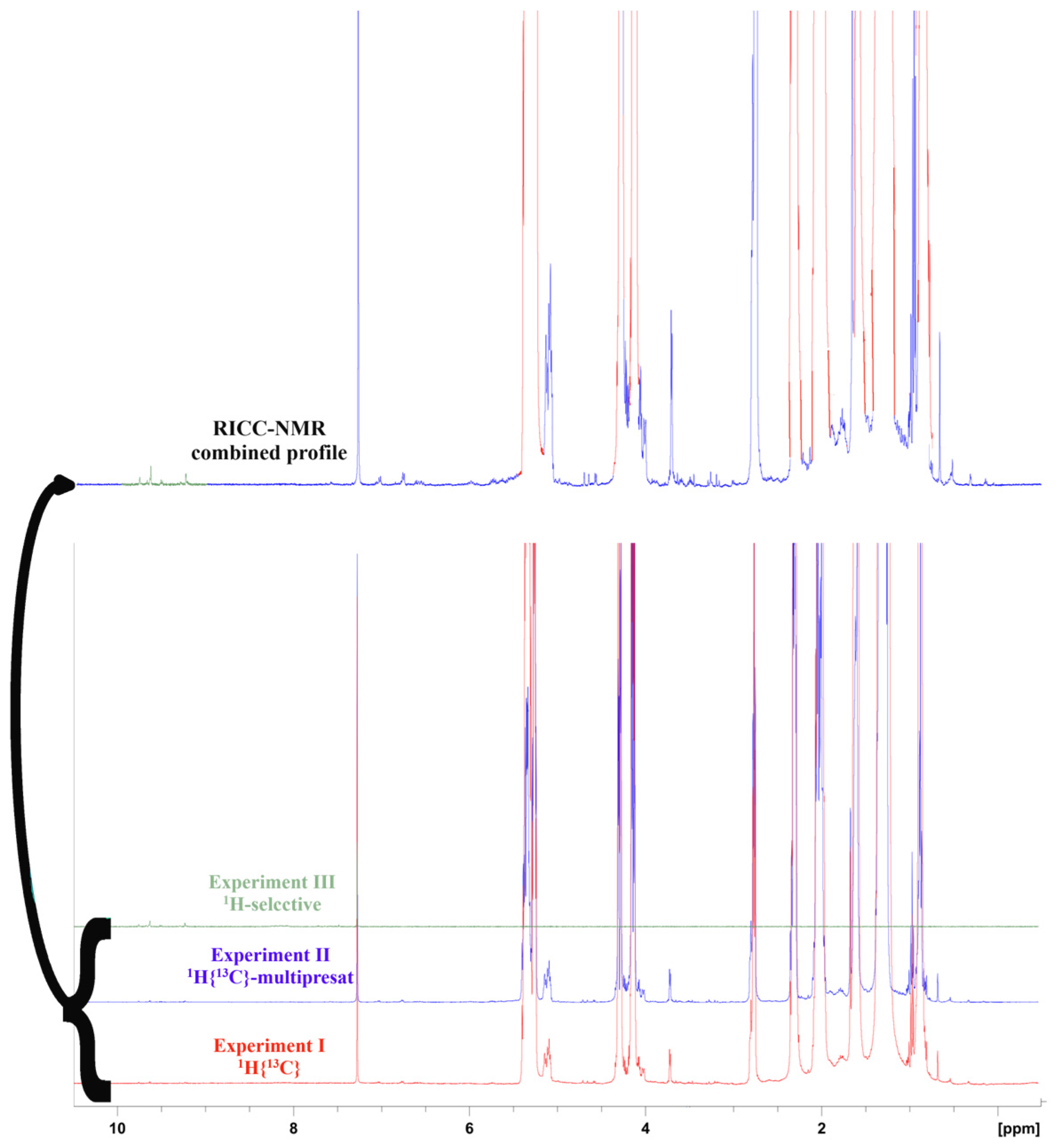
2.4. NMR Experimental Protocol
- Experiment I: A standard 1H spectrum endowed with a 13C decoupling sequence during the acquisition, performed by 16 scans and a 17.2 s recycling delay for quantitative analysis.
- Experiment II: The same experiment I, with a multiple pre-saturated wave able to attenuate the main signals (NOESYGPPS). This experiment was run for 32 scans, with a 17.2 s recycling delay for quantitative analysis.
- Experiment III: The 1H- DPFGSE (selected double-pulsed field gradient spin echo) spectrum [21] was acquired with 40 scans for the determination of aldehydic–phenolic species, namely oleocanthal (TY-EDA, CAS Number: 289030-99-5), oleacein (HTY-EDA, CAS Number: 149183-75-5), ligstroside aglycone (HTY-EA, CAS Number: 174511-64-9), oleuropein aglycone (TY-EA, CAS Number: 31773-95-2), and elenolide (ELNL, CAS Number: 24582-91-0).
- Experiment IV: Full-time 1H-decoupled 13C spectrum with 128 scans, with a suitable recycling delay for quantitative evaluations (more than 18 s per cycle) [20].
2.5. NMR Acquisition and Processing
2.6. NMR Processing Strategies and Quantification
2.7. Statistical Analysis
3. Results
3.1. Data Processing and Treatment
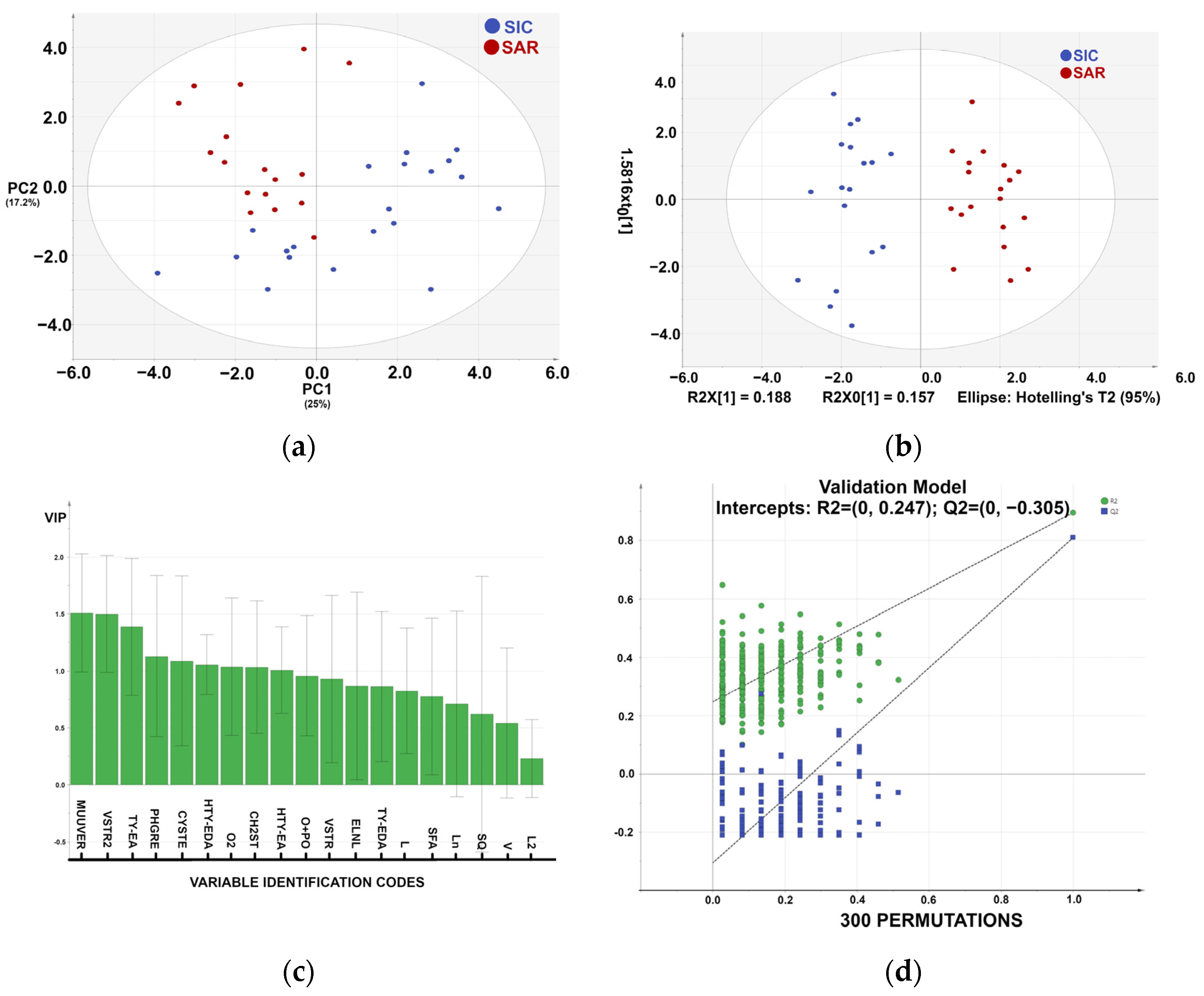
3.2. Statistical Analysis of the Metabolic Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMR | Nuclear magnetic resonance spectroscopy |
| MARA | Least square optimization algorithm called multiple assignment recovered analysis |
| RICC | Restored Intensity through Customized Crops (from NMR experiments), a combined NMR profile |
| EVOO | Extra virgin olive oil |
| SFA | Saturated fatty acids |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| HPLC | High-performance liquid chromatography |
| GC-FID | Gas chromatography with flame ionization detector |
| DAD | Diode array detection |
| LD | Linear dichroism |
| PCA | Principal component analysis |
| PLS-DA | Partial least square-discriminant analysis |
| SIC | Sicilian samples |
| SAR | Sardinian samples |
| NOESYGPPS | Nuclear Overhauser Effect Spectroscopy by Gradient Pulsed for Pure Shift |
| TMS | Tetramethyl silane standard |
| Compound CODE | All the detected compounds are identified with a code reported in Table 1 |
| PO+O | Monounsaturated fatty esters with the cis unsaturation in the ninth position |
| VIP | Variable importance in projection |
| PLS | Projection to latent structures |
References
- Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Effect of Light Exposure on the Quality of Extra Virgin Olive Oils According to Their Chemical Composition. Food Chem. 2017, 229, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Tsimidou, M.; Blekas, G. Olive Oil: Chemistry and Technology, 2nd ed.; AOCS Publishing: Champaign, IL, USA, 2015. [Google Scholar]
- Salvo, A.; Tuttolomondo, A. The Role of Olive Oil in Cardiometabolic Risk. Metabolites 2025, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Covas, M.I.; Fitó, M.; Kušar, A.; Pravst, I. Health Effects of Olive Oil Polyphenols: Recent Advances and Possibilities for the Use of Health Claims. Mol. Nutr. Food Res. 2013, 57, 760–771. [Google Scholar] [CrossRef]
- Xiang, C.; Xu, Z.; Liu, J.; Li, T.; Yang, Z.; Ding, C. Quality, Composition, and Antioxidant Activity of Virgin Olive Oil from Introduced Varieties at Liangshan. LWT 2017, 78, 226–234. [Google Scholar] [CrossRef]
- Kouka, P.; Priftis, A.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xinos, N.; Skaltsounis, A.L.; Mamoulakis, C.; Tsatsakis, A.M.; Spandidos, D.A.; et al. Assessment of the Antioxidant Activity of an Olive Oil Total Polyphenolic Fraction and Hydroxytyrosol from a Greek Olea Europea Variety in Endothelial Cells and Myoblasts. Int. J. Mol. Med. 2017, 40, 703–712. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.H.C.; De Joode, T.; Groenewegen, A.; Alexandre, H. Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-Ligstroside Aglycon as a Key Contributor to Pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- The European Commission. II of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union 2022, L 284, 1–22. [Google Scholar]
- Bella, G.; Rotondo, A. Theoretical Prediction of 13C NMR Spectrum of Mixed Triglycerides by Mean of GIAO Calculations to Improve Vegetable Oils Analysis. Chem. Phys. Lipids 2020, 232, 104973. [Google Scholar] [CrossRef]
- Revelou, P.K.; Pappa, C.; Kakouri, E.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Discrimination of botanical origin of olive oil from selected Greek cultivars by SPME-GC-MS and ATR-FTIR spectroscopy combined with chemometrics. J. Sci. Food Agric. 2021, 101, 2994–3002. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Mendonça, M.A.; Pinho, D.M.M.; Resck, I.S.; Suarez, P.A.Z. Chromatographic Analyses of Fatty Acid Methyl Esters by HPLC-UV and GC-FID. J. Braz. Chem. Soc. 2012, 23, 763–769. [Google Scholar] [CrossRef]
- Ammar, S.; Kelebek, H.; Zribi, A.; Abichou, M.; Selli, S.; Bouaziz, M. LC-DAD/ESI-MS/MS Characterization of Phenolic Constituents in Tunisian Extra-Virgin Olive Oils: Effect of Olive Leaves Addition on Chemical Composition. Food Res. Int. 2017, 100, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct Study of Minor Extra-Virgin Olive Oil Components without Any Sample Modification. 1H NMR Multisupression Experiment: A Powerful Tool. Food Chem. 2017, 228, 301–314. [Google Scholar] [CrossRef]
- Calò, F.; Girelli, C.R.; Angilè, F.; Del Coco, L.; Mazzi, L.; Barbini, D.; Fanizzi, F.P. 1H-NMR Profiling Shows as Specific Constituents Strongly Affect the International EVOO Blends Characteristics: The Case of the Italian Oil. Molecules 2021, 26, 2233. [Google Scholar] [CrossRef] [PubMed]
- Maestrello, V.; Solovyev, P.; Bontempo, L.; Mannina, L.; Camin, F. Nuclear Magnetic Resonance Spectroscopy in Extra Virgin Olive Oil Authentication. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4056–4075. [Google Scholar] [CrossRef]
- Simmler, C.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F. Universal Quantitative NMR Analysis of Complex Natural Samples. Curr. Opin. Biotechnol. 2014, 25, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic Analysis in Food Science: A Review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Belaj, A.; Ninot, A.; Gómez-Gálvez, F.J.; El Riachy, M.; Gurbuz-Veral, M.; Torres, M.; Lazaj, A.; Klepo, T.; Paz, S.; Ugarte, J.; et al. Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba. Plants 2022, 11, 921. [Google Scholar] [CrossRef]
- Rotondo, A.; Bartolomeo, G.; Spanò, I.M.; La Torre, G.L.; Pellicane, G.; Molinu, M.G.; Culeddu, N. Comparison between Traditional and Novel NMR Methods for the Analysis of Sicilian Monovarietal Extra Virgin Olive Oils: Metabolic Profile Is Influenced by Micro-Pedoclimatic Zones. Molecules 2024, 29, 4532. [Google Scholar] [CrossRef]
- Klikarová, J.; Rotondo, A.; Cacciola, F.; Česlová, L.; Dugo, P.; Mondello, L.; Rigano, F. The Phenolic Fraction of Italian Extra Virgin Olive Oils: Elucidation Through Combined Liquid Chromatography and NMR Approaches. Food Anal. Methods 2019, 12, 1759–1770. [Google Scholar] [CrossRef]
- Angilè, F.; Coco, L.D.; Girelli, C.R.; Calò, F.; Mazzi, L.; Fanizzi, F.P.; Vivaldi, G.A.; Camposeo, S. Proton Nuclear Magnetic Resonance (1H NMR) Metabolic Profiles Discriminate Two Monovarietal Extra Virgin Olive Oils, Cultivars Arbequina and Koroneiki, with Different Geographical Origin. Horticulturae 2023, 9, 66. [Google Scholar] [CrossRef]
- Dugo, G.; Rotondo, A.; Mallamace, D.; Cicero, N.; Salvo, A.; Rotondo, E.; Corsaro, C. Enhanced Detection of Aldehydes in Extra-Virgin Olive Oil by Means of Band Selective NMR Spectroscopy. Phys. A Stat. Mech. Appl. 2015, 420, 258–264. [Google Scholar] [CrossRef]
- Rastrelli, F.; Schievano, E.; Bagno, A.; Mammi, S. NMR quantification of trace components in complex matrices by band-selective excitation with adiabatic pulses. Magn. Reson. Chem. 2009, 47, 868–872. [Google Scholar] [CrossRef]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Res. 2010, 202, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, A.; Mannina, L.; Salvo, A. Multiple Assignment Recovered Analysis (MARA) NMR for a Direct Food Labeling: The Case Study of Olive Oils. Food Anal. Methods 2019, 12, 1238–1245. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal Projections to Latent Structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for Significance Testing of PLS and OPLS® Models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Piravi-Vanak, Z.B.; Ghasemi, J.B.; Ghavami, M.; Ezzatpanah, H.; Zolfonoun, E. The influence of growing region on fatty acids and sterol composition of Iranian olive oils by unsupervised clustering methods. J. Am. Oil Chem. Soc. 2012, 89, 371–378. [Google Scholar] [CrossRef]
- Rey-Giménez, R.; Sanchez-Gimeno, A.C. Effect of cultivar and environment on chemical composition and geographical traceability of Spanish olive oil. J. Am. Oil Chem. Soc. 2024, 101, 371–382. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B. Importance of some minor compounds in olive oil authenticity and quality. Trends Food Sci. Technol. 2020, 100, 164–176. [Google Scholar] [CrossRef]
- Willis, C.E.; Perlack, R.D. Multicollinearity: Effects, Symptoms, and Remedies. J. Northeast. Agric. Econ. Counc. 1978, 7, 55–61. [Google Scholar] [CrossRef]
- Pianeta PSR. Available online: https://www.pianetapsr.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/3012 (accessed on 15 January 2025).
- Pierantozzi, P.; Torres, M.; Contreras, C.; Stanzione, V.; Tivani, M.; Gentili, L.; Mastio, V.; Searles, P.; Brizuela, M.; Fernández, F.; et al. Phenolic content and profile of olive fruits: Impact of contrasting thermal regimes in non-Mediterranean growing environments. Eur. J. Agron. 2025, 164, 127506. [Google Scholar] [CrossRef]
- Lukić, M.; Lukić, I.; Moslavac, T. Sterols and Triterpene Diols in Virgin Olive Oil: A Comprehensive Review on Their Properties and Significance, with a Special Emphasis on the Influence of Variety and Ripening Degree. Horticulturae 2021, 7, 493. [Google Scholar] [CrossRef]
- Baldoni, L.; Cultrera, N.G.; Mariotti, R.; Ricciolini, C.; Arcioni, S.; Vendramin, G.G.; Buonamici, A.; Porceddu, A.; Sarri, V.; Ojeda, M.A.; et al. A consensus list of microsatellite markers for olive genotyping. Mol. Breed. 2009, 24, 213–231. [Google Scholar] [CrossRef]
- Marchese, A.; Bonanno, F.; Marra, F.P.; Trippa, D.A.; Zelasco, S.; Rizzo, S.; Giovino, A.; Imperiale, V.; Ioppolo, A.; Sala, G.; et al. Recovery and genotyping ancient Sicilian monumental olive trees. Front. Conserv. Sci. 2023, 4, 1206832. [Google Scholar] [CrossRef]
| Quantified Compound(s) (Units *) | Code | Average Values | SD | |
|---|---|---|---|---|
| 1 | Squalene (mol ppm) | SQ | 1514.994 | 398.483 |
| 2 | Linolenate esters (%) | Ln | 0.745 | 0.098 |
| 3 | Linoleate esters (%) | L | 9.856 | 1.245 |
| 4 | Oleate and Palmitoleate esters (%) | O+PO | 66.291 | 3.089 |
| 5 | cis-Vaccenate esters (%) | V | 3.765 | 0.546 |
| 6 | Palmitate and Stearate esters (%) | SFA | 19.343 | 2.344 |
| 7 | 2-Glyceryl linoleate esters (%) | L2 | 4.743 | 0.588 |
| 8 | 2-Glyceryl oleate esters (%) | O2 | 26.111 | 1.190 |
| 9 | β-Sitosterol, Δ5-avenasterol, Δ5-Campesterol (mol ppm) | VSTR | 2503.748 | 335.051 |
| 10 | Cycloeucalenol, 24-methylene cycloartanol, gramisterol (mol ppm) | CH2ST | 842.772 | 390.310 |
| 11 | Esters of cycloartenol, 24-methylene cycloartanol, and cyclobranol (mol ppm) | CYSTE | 618.625 | 235.650 |
| 12 | Citrostadienol, Δ7-avenasterol, Δ7-campesterol (mol ppm) | VSTR2 | 479.817 | 115.195 |
| 13 | Maslinic and ursolic acid, uvaol, and erythrodiol (mol ppm) | MUUVER | 1664.113 | 415.099 |
| 14 | Phytol and geranylgeraniol esters (mol ppm) | PHGRE | 410.641 | 159.199 |
| 15 | Oleocanthal (mol ppm) | TY-EDA | 397.855 | 201.281 |
| 16 | Oleaceine (mol ppm) | HTY-EDA | 252.839 | 166.735 |
| 17 | Ligustroside aglycone (all the derivatives) (mol ppm) | TY-EA | 163.956 | 150.862 |
| 18 | Oleuropein aglycone (all the derivatives) (mol ppm) | HTY-EA | 116.228 | 105.437 |
| 19 | Elenolide (mol ppm) | ELNL | 54.802 | 170.958 |
| Members | Correct | SIC | SAR | |
|---|---|---|---|---|
| SIC | 18 | 100% | 18 | 0 |
| SAR | 19 | 100% | 0 | 19 |
| Total | 37 | 100% | 18 | 19 |
| Fisher’s prob. | 5.7 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culeddu, N.; Rotondo, A.; Nastasi, F.; Bartolomeo, G.; Deiana, P.; Santona, M.; Tarantilis, P.A.; Pellicane, G.; La Torre, G.L. Restored Intensities from Customized Crops of NMR Experiments (RICC-NMR) to Gain Better Insight on Chemometrics of Sicilian and Sardinian Extra Virgin Olive Oils. Foods 2025, 14, 1807. https://doi.org/10.3390/foods14101807
Culeddu N, Rotondo A, Nastasi F, Bartolomeo G, Deiana P, Santona M, Tarantilis PA, Pellicane G, La Torre GL. Restored Intensities from Customized Crops of NMR Experiments (RICC-NMR) to Gain Better Insight on Chemometrics of Sicilian and Sardinian Extra Virgin Olive Oils. Foods. 2025; 14(10):1807. https://doi.org/10.3390/foods14101807
Chicago/Turabian StyleCuleddu, Nicola, Archimede Rotondo, Federico Nastasi, Giovanni Bartolomeo, Pierfrancesco Deiana, Mario Santona, Petros A. Tarantilis, Giuseppe Pellicane, and Giovanna Loredana La Torre. 2025. "Restored Intensities from Customized Crops of NMR Experiments (RICC-NMR) to Gain Better Insight on Chemometrics of Sicilian and Sardinian Extra Virgin Olive Oils" Foods 14, no. 10: 1807. https://doi.org/10.3390/foods14101807
APA StyleCuleddu, N., Rotondo, A., Nastasi, F., Bartolomeo, G., Deiana, P., Santona, M., Tarantilis, P. A., Pellicane, G., & La Torre, G. L. (2025). Restored Intensities from Customized Crops of NMR Experiments (RICC-NMR) to Gain Better Insight on Chemometrics of Sicilian and Sardinian Extra Virgin Olive Oils. Foods, 14(10), 1807. https://doi.org/10.3390/foods14101807











