Surface Display Technologies for Whole-Cell Biocatalysts: Advances in Optimization Strategies, Food Applications, and Future Perspectives
Abstract
1. Introduction
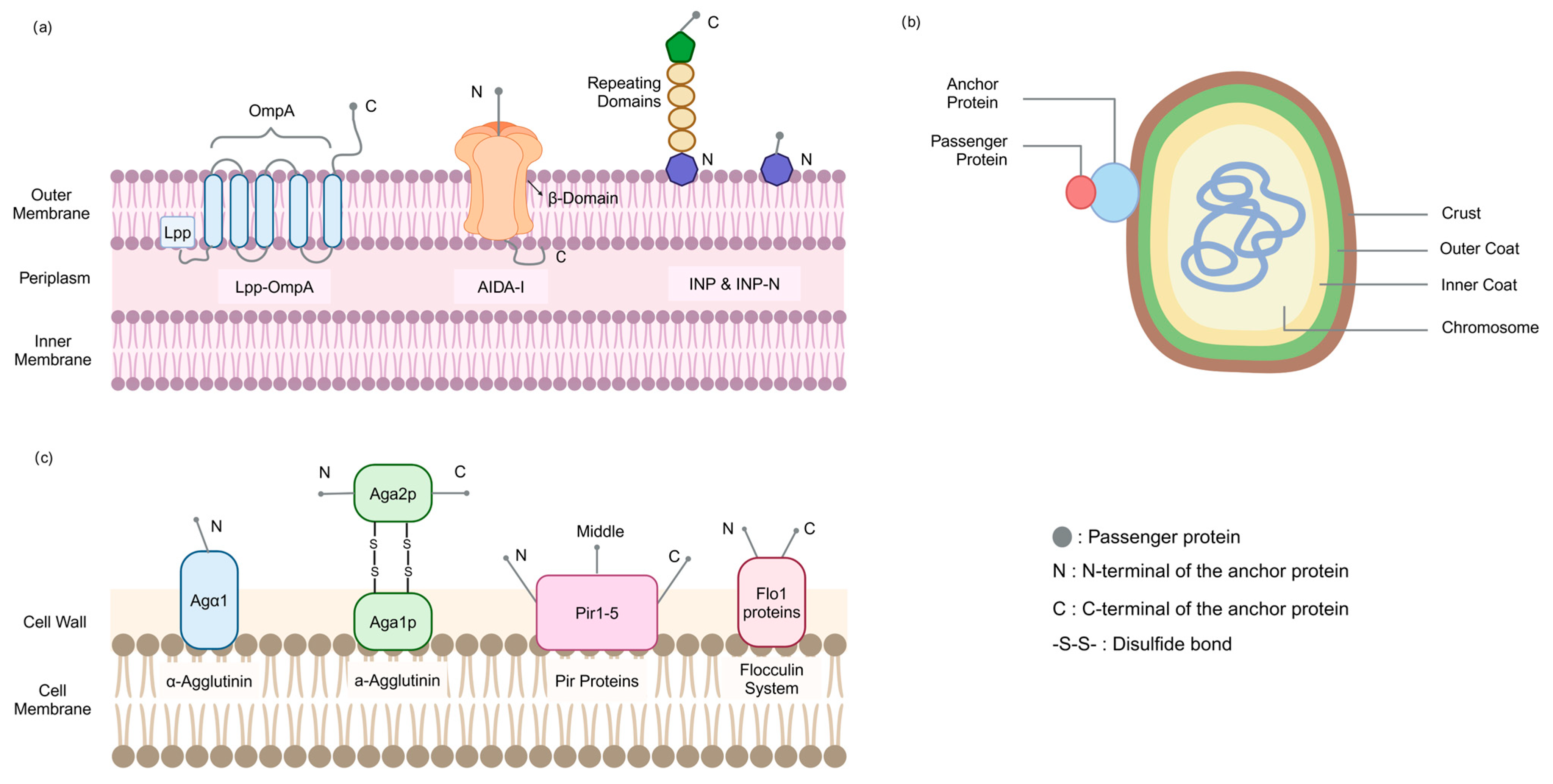
2. Commonly Used Strains for Surface Display
2.1. E. coli
2.1.1. Membrane Structure
2.1.2. Commonly Used Anchor Proteins
2.2. B. subtilis
2.2.1. Membrane Structure (Spore)
2.2.2. Commonly Used Anchor Proteins
2.3. Yeast Cells
2.3.1. Membrane Structure
2.3.2. Commonly Used Anchor Proteins
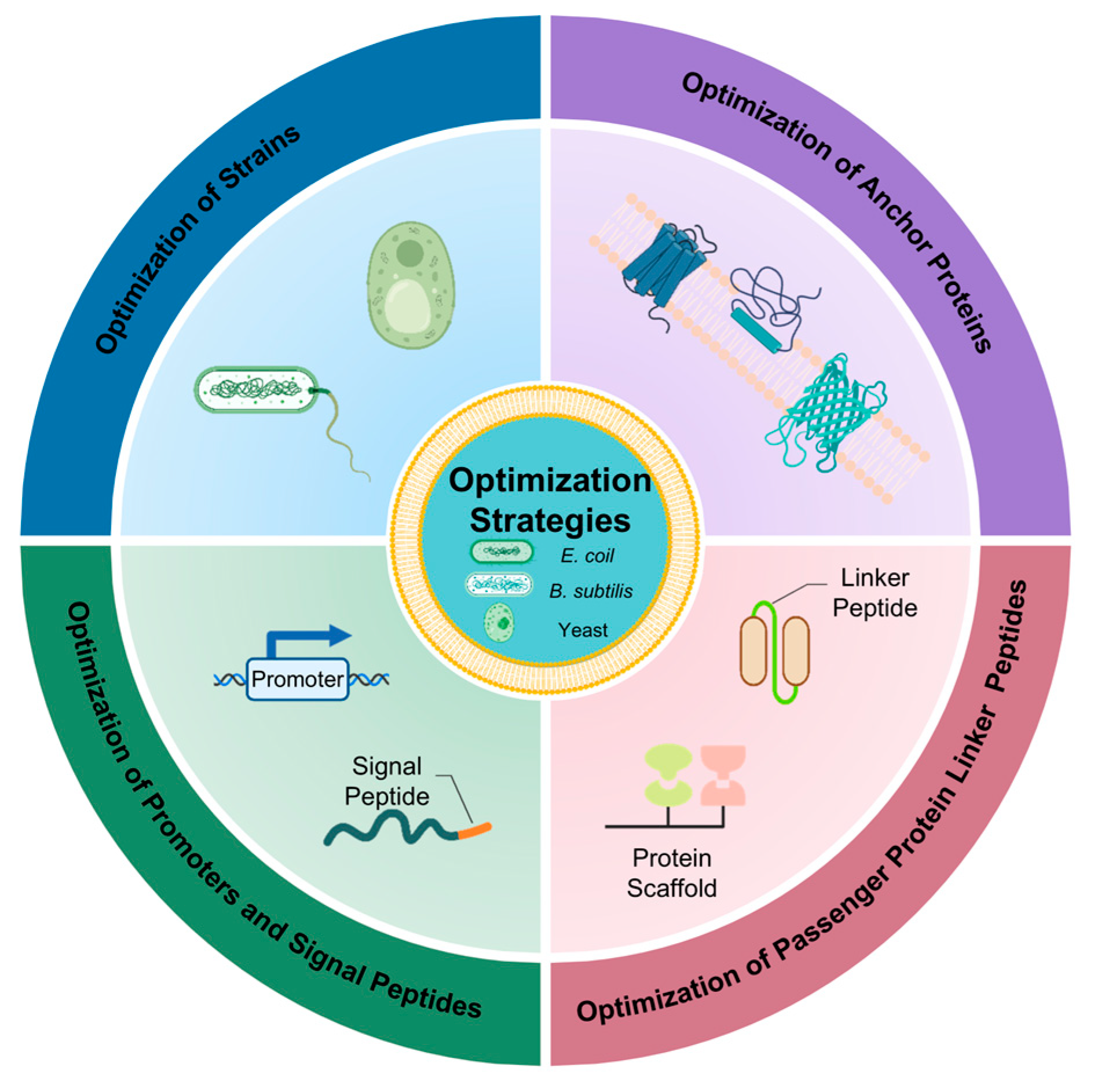
3. Strategies to Enhance the Catalytic Effect of Whole Cells in Surface Display
3.1. Optimization of Strains
3.2. Optimization of Anchor Proteins
3.3. Optimization of Passenger Protein Linker Peptides
3.4. Optimization of Promoters and Signal Peptides
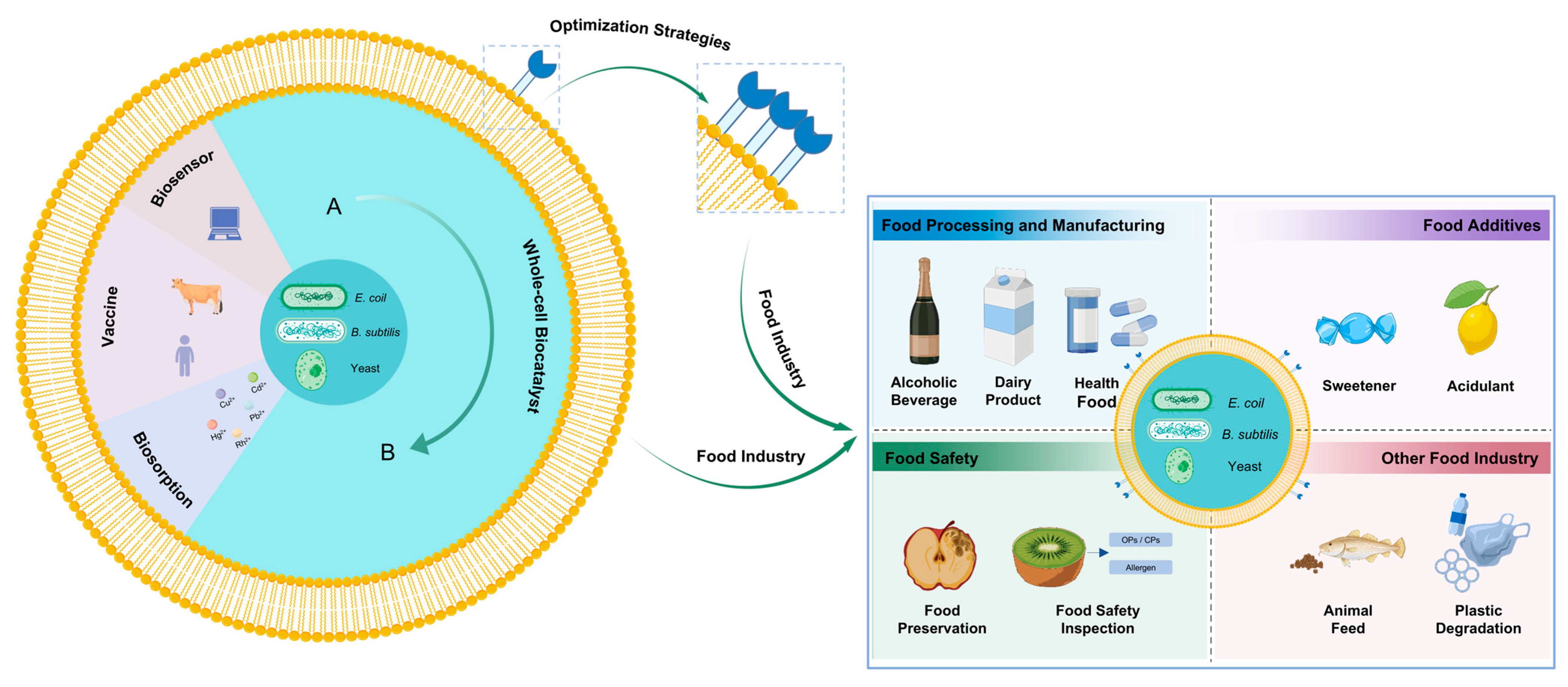
4. Applications in the Food Industry
4.1. Applications in Food Processing and Manufacturing
4.1.1. Alcoholic Beverages
4.1.2. Dairy Products
4.1.3. Health Food
4.2. Applications in Food Additives
4.2.1. Sweeteners
4.2.2. Acidulants
4.2.3. Other Food Additives
4.3. Applications in Food Safety
4.3.1. Food Preservation
4.3.2. Food Safety Inspection
4.4. Applications in Other Food Industry
4.4.1. Animal Feed
4.4.2. Plastic Degradation
5. Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakatani, H.; Hori, K. Cell surface protein engineering for high-performance whole-cell catalysts. Front. Chem. Sci. Eng. 2017, 11, 46–57. [Google Scholar] [CrossRef]
- Miri, S.; Perez, J.A.E.; Brar, S.K.; Rouissi, T.; Martel, R. Sustainable production and co-immobilization of cold-active enzymes from Pseudomonas sp. for BTEX biodegradation. Environ. Pollut. 2021, 285, 117678. [Google Scholar] [CrossRef] [PubMed]
- Zeidman, A.B.; Rodriguez-Narvaez, O.M.; Moon, J.; Bandala, E.R. Removal of antibiotics in aqueous phase using silica-based immobilized nanomaterials: A review. Environ. Technol. Innov. 2020, 20, 101030. [Google Scholar] [CrossRef]
- Xie, W.; Xiong, J.; Xiang, G. Enzyme immobilization on functionalized monolithic CNTs-Ni foam composite for highly active and stable biocatalysis in organic solvent. Mol. Catal. 2020, 483, 110714. [Google Scholar] [CrossRef]
- Miao, C.; Yang, L.; Wang, Z.; Luo, W.; Li, H.; Lv, P.; Yuan, Z. Lipase immobilization on amino-silane modified superparamagnetic Fe3O4 nanoparticles as biocatalyst for biodiesel production. Fuel 2018, 224, 774–782. [Google Scholar] [CrossRef]
- van Bloois, E.; Winter, R.T.; Kolmar, H.; Fraaije, M.W. Decorating microbes: Surface display of proteins on Escherichia coli. Trends Biotechnol. 2011, 29, 79–86. [Google Scholar] [CrossRef]
- Li, T.; Menegatti, S.; Crook, N. Breakdown of polyethylene therepthalate microplastics under saltwater conditions using engineered Vibrio natriegens. AIChE J. 2023, 69, e18228. [Google Scholar] [CrossRef]
- Wang, T.; Yang, W.-T.; Gong, Y.-M.; Zhang, Y.-K.; Fan, X.-X.; Wang, G.-C.; Lu, Z.-H.; Liu, F.; Liu, X.-H.; Zhu, Y.-S. Molecular engineering of PETase for efficient PET biodegradation. Ecotoxicol. Environ. Saf. 2024, 280, 116540. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yuan, H.; Du, J.; Liu, K.; Liu, H.; Wang, T. Progress in research and application development of surface display technology using Bacillus subtilis spores. Appl. Microbiol. Biotechnol. 2020, 104, 2319–2331. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Cheng, Y.; Wang, X.; Tong, S.; Yang, H.; Wang, Z. Efficient biodegradation of highly crystallized polyethylene terephthalate through cell surface display of bacterial PETase. Sci. Total Environ. 2020, 709, 136138. [Google Scholar] [CrossRef]
- Wang, H.; Yang, R.; Hua, X.; Zhang, W.; Zhao, W. An Approach for Lactulose Production Using the CotX-Mediated Spore-Displayed β-Galactosidase as a Biocatalyst. J. Microbiol. Biotechnol. 2016, 26, 1267–1277. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y. Constructing Escherichia coli co-display systems for biodegradation of polyethylene terephthalate. Bioresour. Bioprocess. 2023, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zhou, N.-Y.; Ding, J. Yeast surface display technology: Mechanisms, applications, and perspectives. Biotechnol. Adv. 2024, 76, 108422. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Pan, Z.; Jin, S.; Zhang, X.; Zheng, S.; Han, S.; Pan, L.; Lin, Y. Biocatalytic behavior of a new Aspergillus niger whole-cell biocatalyst with high operational stability during the synthesis of green biosolvent isopropyl esters. J. Mol. Catal. B Enzym. 2016, 131, 10–17. [Google Scholar] [CrossRef]
- Ullah, M.; Xia, Y.; Alshaya, D.S.; Han, J.; Attia, K.A.; Shah, T.A.; Chen, H. Display of Bacterial Exochitanase on Bacillus subtilis Spores Improved Enzyme Stability and Recyclability. Molecules 2024, 29, 4302. [Google Scholar] [CrossRef]
- Liu, H.; Yang, S.; Wang, X.; Wang, T. Production of trehalose with trehalose synthase expressed and displayed on the surface of Bacillus subtilis spores. Microb. Cell Factories 2019, 18, 100. [Google Scholar] [CrossRef]
- Chen, T.; Wang, S.; Niu, H.; Yang, G.; Wang, S.; Wang, Y.; Zhou, C.; Yu, B.; Yang, P.; Sun, W.; et al. Biofilm-Based Biocatalysis for Galactooligosaccharides Production by the Surface Display of β-Galactosidase in Pichia pastoris. Int. J. Mol. Sci. 2023, 24, 6507. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Liu, X.; Zhou, R.; Liao, X.; Min, Y.; Ma, L.; Wang, Y.; Rao, B. Expression and Surface Display of an Acidic Cold-Active Chitosanase in Pichia pastoris Using Multi-Copy Expression and High-Density Cultivation. Molecules 2022, 27, 800. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.L.; Lv, F.L.; Xu, L.D.; Huang, Y.W. Surface Display of Peptides Corresponding to the Heptad Repeat 2 Domain of the Feline Enteric Coronavirus Spike Protein on Bacillus subtilis Spores Elicits Protective Immune Responses Against Homologous Infection in a Feline Aminopeptidase-N-Transduced Mouse Model. Front. Immunol. 2022, 13, 925922. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Chen, B.; Wang, Z.; Cao, Y.; Yang, Y.; Zhang, M.; Zhang, D.; Ni, X.; Zeng, Y.; et al. Evaluation of the Immunity Responses in Mice to Recombinant Bacillus subtilis Displaying Newcastle Disease Virus HN Protein Truncations. Microorganisms 2024, 12, 439. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Sun, J.; Gu, J.; Xu, X.; Cai, X. Surface display of the COE antigen of porcine epidemic diarrhoea virus on Bacillus subtilis spores. Microb. Biotechnol. 2024, 17, e14518. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Ning, Y.; Liu, S.; Liang, Z.; Zhang, Z.; Chen, Y.; Cao, J.; Wang, F.; Lan, L.; et al. Surface display of major capsid protein on Bacillus subtilis spores against largemouth bass virus (LMBV) for oral administration. Fish Shellfish Immunol. 2023, 135, 108627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, S.; Ling, Z.; Zhou, T.; Liu, P.; Li, X. Enhanced removal of trivalent chromium from leather wastewater using engineered bacteria immobilized on magnetic pellets. Sci. Total Environ. 2021, 775, 145647. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhou, Y.; Yan, J.; Zhu, L.; Li, Z.; Hu, X.; Zhan, X. Efficient precious metal Rh(III) adsorption by waste P. pastoris and P. pastoris surface display from high-density culture. J. Hazard. Mater. 2022, 427, 128140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guo, D.; Zhu, Q.; Dou, W.; Guan, W. Display of Microbial Glucose Dehydrogenase and Cholesterol Oxidase on the Yeast Cell Surface for the Detection of Blood Biochemical Parameters. Biosensors 2020, 11, 13. [Google Scholar] [CrossRef]
- Freudl, R.; MacIntyre, S.; Degen, M.; Henning, U. Cell surface exposure of the outer membrane protein OmpA of Escherichia coli K-12. J. Mol. Biol. 1986, 188, 491–494. [Google Scholar] [CrossRef]
- Maurer, J.; Jose, J.; Meyer, T.F. Autodisplay: One-component system for efficient surface display and release of soluble recombinant proteins from Escherichia coli. J. Bacteriol. 1997, 179, 794–804. [Google Scholar] [CrossRef]
- Jose, J.; Jähnig, F.; Meyer, T.F. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol. Microbiol. 1995, 18, 378–380. [Google Scholar] [CrossRef]
- Jung, H.C.; Lebeault, J.M.; Pan, J.G. Surface display of Zymomonas mobilis levansucrase by using the ice-nucleation protein of Pseudomonas syringae. Nat. Biotechnol. 1998, 16, 576–580. [Google Scholar] [CrossRef]
- Li, L.; Kang, D.G.; Cha, H.J. Functional display of foreign protein on surface of Escherichia coli using N-terminal domain of ice nucleation protein. Biotechnol. Bioeng. 2004, 85, 214–221. [Google Scholar] [CrossRef]
- Liu, M.; Lu, X.; Khan, A.; Ling, Z.; Wang, P.; Tang, Y.; Liu, P.; Li, X. Reducing methylmercury accumulation in fish using Escherichia coli with surface-displayed methylmercury-binding peptides. J. Hazard. Mater. 2019, 367, 35–42. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Y.; Gao, Y.; Zhang, C.; Wang, Y.; Xu, B.; Yang, Y.; Wu, Q.; Huang, Z. Biodegradation of λ-cyhalothrin through cell surface display of bacterial carboxylesterase. Chemosphere 2022, 289, 133130. [Google Scholar] [CrossRef]
- Baiyoumy, A.; Vallapurackal, J.; Schwizer, F.; Heinisch, T.; Kardashliev, T.; Held, M.; Panke, S.; Ward, T.R. Directed Evolution of a Surface-Displayed Artificial Allylic Deallylase Relying on a GFP Reporter Protein. Acs Catal. 2021, 11, 10705–10712. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.S.; Leiss-Maier, F.; Mühlhofer, R.; Boesen, B.; Mustafa, G.; Kugler, H.; Zeymer, C. A De Novo Metalloenzyme for Cerium Photoredox Catalysis. J. Am. Chem. Soc. 2024, 146, 25976–25985. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.A.; Son, R.G.; Ki, M.R.; Pack, S.P. Biosilica-coated carbonic anhydrase displayed on Escherichia coli: A novel design approach for efficient and stable biocatalyst for CO2 sequestration. Int. J. Biol. Macromol. 2024, 277, 134058. [Google Scholar] [CrossRef]
- Kumaravel, A.; Selvamani, V.; Sengupta, T.; Gu Kang, S.; Ho Hong, S. Surface engineered recombinant Escherichia coli for the potential application of the cobalt contaminated wastewater treatment and the photocatalytic dye degradation. Bioresour. Technol. 2024, 403, 130796. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.A.; Earhart, C.F.; Georgiou, G. Transport and anchoring of beta-lactamase to the external surface of Escherichia coli. Proc. Natl. Acad. Sci. USA 1992, 89, 2713–2717. [Google Scholar] [CrossRef]
- Francisco, J.A.; Stathopoulos, C.; Warren, R.A.; Kilburn, D.G.; Georgiou, G. Specific adhesion and hydrolysis of cellulose by intact Escherichia coli expressing surface anchored cellulase or cellulose binding domains. Nat. Biotechnol. 1993, 11, 491–495. [Google Scholar] [CrossRef]
- Gallus, S.; Mittmann, E.; Rabe, K.S. A Modular System for the Rapid Comparison of Different Membrane Anchors for Surface Display on Escherichia coli. ChemBioChem 2021, 23, e202100472. [Google Scholar] [CrossRef]
- Gallus, S.; Peschke, T.; Paulsen, M.; Burgahn, T.; Niemeyer, C.M.; Rabe, K.S. Surface Display of Complex Enzymes by in Situ SpyCatcher-SpyTag Interaction. ChemBioChem 2020, 21, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, G.; Stephens, D.L.; Stathopoulos, C.; Poetschke, H.L.; Mendenhall, J.; Earhart, C.F. Display of beta-lactamase on the Escherichia coli surface: Outer membrane phenotypes conferred by Lpp-OmpA-beta-lactamase fusions. Protein Eng. Des. Sel. 1996, 9, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiao, H.; Krajcikova, D.; Zhang, Z.; Wang, H.; Barak, I.; Tang, J. Physical interaction and assembly of Bacillus subtilis spore coat proteins CotE and CotZ studied by atomic force microscopy. J. Struct. Biol. 2016, 195, 245–251. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2012, 11, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Warth, A.D.; Ohye, D.F.; Murrell, W.G. The composition and structure of bacterial spores. J. Cell Biol. 1963, 16, 579–592. [Google Scholar] [CrossRef]
- Potot, S.; Henriques, A.O.; Schyns, G. Bacillus subtilis spores to display functional enzymes. New Biotechnol. 2009, 25, S97. [Google Scholar] [CrossRef]
- Tian, H.; Furtmann, C.; Lenz, F.; Srinivasamurthy, V.; Bornscheuer, U.T.; Jose, J. Enzyme cascade converting cyclohexanol into ε-caprolactone coupled with NADPH recycling using surface displayed alcohol dehydrogenase and cyclohexanone monooxygenase on E. coli. Microb. Biotechnol. 2022, 15, 2235–2249. [Google Scholar] [CrossRef]
- Jose, J.; Maas, R.M.; Teese, M.G. Autodisplay of enzymes—Molecular basis and perspectives. J. Biotechnol. 2012, 161, 92–103. [Google Scholar] [CrossRef]
- Kodama, T.; Matsubayashi, T.; Yanagihara, T.; Komoto, H.; Ara, K.; Ozaki, K.; Kuwana, R.; Imamura, D.; Takamatsu, H.; Watabe, K.; et al. A Novel Small Protein of Bacillus subtilis Involved in Spore Germination and Spore Coat Assembly. Biosci. Biotechnol. Biochem. 2014, 75, 1119–1128. [Google Scholar] [CrossRef]
- Zilhāo, R.; Serrano, M.N.; Isticato, R.; Ricca, E.; Moran, C.P.; Henriques, A.O. Interactions among CotB, CotG, and CotH during Assembly of the Bacillus subtilis Spore Coat. J. Bacteriol. 2004, 186, 1110–1119. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Sreelatha, A.; Durrant, E.S.; Lopez-Garrido, J.; Muszewska, A.; Dudkiewicz, M.; Grynberg, M.; Yee, S.; Pogliano, K.; Tomchick, D.R.; et al. Phosphorylation of spore coat proteins by a family of atypical protein kinases. Proc. Natl. Acad. Sci. USA 2016, 113, E3482–E3491. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Pelosi, A.; De Felice, M.; Ricca, E. CotE Binds to CotC and CotU and Mediates Their Interaction during Spore Coat Formation in Bacillus subtilis. J. Bacteriol. 2010, 192, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Esposito, G.; Zilhāo, R.; Nolasco, S.; Cangiano, G.; De Felice, M.; Henriques, A.O.; Ricca, E. Assembly of Multiple CotC Forms into the Bacillus subtilis Spore Coat. J. Bacteriol. 2004, 186, 1129–1135. [Google Scholar] [CrossRef]
- Cangiano, G.; Mazzone, A.; Baccigalupi, L.; Isticato, R.; Eichenberger, P.; De Felice, M.; Ricca, E. Direct and Indirect Control of Late Sporulation Genes by GerR of Bacillus subtilis. J. Bacteriol. 2010, 192, 3406–3413. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Kapteyn, J.C.; Van Den Ende, H.; Klis, F.M. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1426, 373–383. [Google Scholar] [CrossRef]
- Lu, C.F.; Montijn, R.C.; Brown, J.L.; Klis, F.; Kurjan, J.; Bussey, H.; Lipke, P.N. Glycosyl phosphatidylinositol-dependent cross-linking of alpha-agglutinin and beta-1,6-glucan in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 1995, 128, 333–340. [Google Scholar] [CrossRef]
- Wojciechowicz, D.; Lu, C.F.; Kurjan, J.; Lipke, P.N. Cell surface anchorage and ligand-binding domains of the Saccharomyces cerevisiae cell adhesion protein alpha-agglutinin, a member of the immunoglobulin superfamily. Mol. Cell. Biol. 1993, 13, 2554–2563. [Google Scholar] [CrossRef]
- de Nobel, H.; Lipke, P.N.; Kurjan, J. Identification of a ligand-binding site in an immunoglobulin fold domain of the Saccharomyces cerevisiae adhesion protein alpha-agglutinin. Mol. Biol. Cell. 1996, 7, 143–153. [Google Scholar] [CrossRef]
- Cappellaro, C.; Baldermann, C.; Rachel, R.; Tanner, W. Mating type-specific cell-cell recognition of Saccharomyces cerevisiae: Cell wall attachment and active sites of a- and alpha-agglutinin. EMBO J. 1994, 13, 4737–4744. [Google Scholar] [CrossRef]
- Martinić Cezar, T.; Lozančić, M.; Novačić, A.; Matičević, A.; Matijević, D.; Vallée, B.; Mrša, V.; Teparić, R.; Žunar, B. Streamlining N-terminally anchored yeast surface display via structural insights into S. cerevisiae Pir proteins. Microb. Cell Factories 2023, 22, 174. [Google Scholar] [CrossRef]
- Ye, M.; Ye, Y.; Du, Z.; Chen, G. Cell-surface engineering of yeasts for whole-cell biocatalysts. Bioprocess Biosyst. Eng. 2021, 44, 1003–1019. [Google Scholar] [CrossRef]
- Castillo, L.; Martinez, A.I.; Garcerá, A.; Elorza, M.V.; Valentín, E.; Sentandreu, R. Functional analysis of the cysteine residues and the repetitive sequence of Saccharomyces cerevisiae Pir4/Cis3: The repetitive sequence is needed for binding to the cell wall beta-1,3-glucan. Yeast 2003, 20, 973–983. [Google Scholar] [CrossRef]
- Ecker, M.; Deutzmann, R.; Lehle, L.; Mrsa, V.; Tanner, W. Pir proteins of Saccharomyces cerevisiae are attached to beta-1,3-glucan by a new protein-carbohydrate linkage. J. Biol. Chem. 2006, 281, 11523–11529. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.V.Y.; Stassen, C.; Stals, I.; Donohue, D.S.; Devreese, B.; De Greve, H.; Willaert, R.G. The N-terminal domain of the Flo1 flocculation protein from Saccharomyces cerevisiae binds specifically to mannose carbohydrates. Eukaryot. Cell 2010, 10, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Willaert, R. Flocculation protein structure and cell-cell adhesion mechanism in Saccharomyces cerevisiae. Biotechnol. Lett. 2010, 32, 1571–1585. [Google Scholar] [CrossRef]
- Inokuma, K.; Kitada, Y.; Bamba, T.; Kobayashi, Y.; Yukawa, T.; den Haan, R.; van Zyl, W.H.; Kondo, A.; Hasunuma, T. Improving the functionality of surface-engineered yeast cells by altering the cell wall morphology of the host strain. Appl. Microbiol. Biotechnol. 2021, 105, 5895–5904. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, S.; You, D.; Shen, J.; Ruan, B.; Wu, M.; Zhang, J.; Luo, X.; Tang, H. Systematic genetic modifications of cell wall biosynthesis enhanced the secretion and surface-display of polysaccharide degrading enzymes in Saccharomyces cerevisiae. Metab. Eng. 2023, 77, 273–282. [Google Scholar] [CrossRef]
- Yang, S.; Shen, J.; Deng, J.; Li, H.; Zhao, J.; Tang, H.; Bao, X. Engineering Cell Polarization Improves Protein Production in Saccharomyces cerevisiae. Microorganisms 2022, 10, 2005. [Google Scholar] [CrossRef]
- Besada-Lombana, P.B.; Chen, W.L.; Da Silva, N.A. An extracellular glucose sensor for substrate-dependent secretion and display of cellulose-degrading enzymes. Biotechnol. Bioeng. 2024, 121, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Miao, Y.L.; Yang, J.M.; Zhao, F.G.; Lin, Y.; Han, S.Y. Efficient improvement of surface displayed lipase from Rhizomucor miehei in PichiaPink™ protease-deficient system. Protein Expr. Purif. 2021, 180, 105804. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.; Liu, N.; Yu, M.R.; Yang, S.T.; Chen, H.L. Cell surface display of carbonic anhydrase on Escherichia coli using ice nucleation protein for CO2 sequestration. Biotechnol. Bioeng. 2011, 108, 2853–2864. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-J.; Lee, S.H. An efficient bacterial surface display system based on a novel outer membrane anchoring element from the Escherichia coli protein YiaT. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef]
- Han, M.J. Novel Bacterial Surface Display System Based on the Escherichia coli Protein MipA. J. Microbiol. Biotechnol. 2020, 30, 1097–1103. [Google Scholar] [CrossRef]
- Yang, X.; Tang, H.; Song, M.; Shen, Y.; Hou, J.; Bao, X. Development of novel surface display platforms for anchoring heterologous proteins in Saccharomyces cerevisiae. Microb. Cell Factories 2019, 18, 85. [Google Scholar] [CrossRef]
- Phienluphon, A.; Mhuantong, W.; Boonyapakron, K.; Deenarn, P.; Champreda, V.; Wichadakul, D.; Suwannarangsee, S. Identification and evaluation of novel anchoring proteins for cell surface display on Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2019, 103, 3085–3097. [Google Scholar] [CrossRef]
- Jia, X.; Li, Y.; Xu, T.; Wu, K. Display of lead-binding proteins on Escherichia coli surface for lead bioremediation. Biotechnol. Bioeng. 2020, 117, 3820–3834. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, M.; Saeed, M.; Li, K.; Chen, Y.; Okoye, C.O.; Fang, Z.; Ni, Z.; Chen, H. The flexible linker and CotG were more effective for the spore surface display of keratinase KERQ7. World J. Microbiol. Biotechnol. 2023, 40, 35. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, W.; Xuan, Q.; Zhou, Y.; Zhou, S.; Huang, J.; Wang, P. Binding Peptide-Guided Immobilization of Lipases with Significantly Improved Catalytic Performance Using Escherichia coli BL21(DE3) Biofilms as a Platform. ACS Appl. Mater. Interfaces 2021, 13, 6168–6179. [Google Scholar] [CrossRef]
- Dong, C.; Qiao, J.; Wang, X.; Sun, W.; Chen, L.; Li, S.; Wu, K.; Ma, L.; Liu, Y. Engineering Pichia pastoris with surface-display minicellulosomes for carboxymethyl cellulose hydrolysis and ethanol production. Biotechnol. Biofuels 2020, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, H.; Wang, Z.; Qi, Q.; Du, J.; Tian, S. A cellulosomal yeast reaction system of lignin-degrading enzymes for cellulosic ethanol fermentation. Biotechnol. Lett. 2024, 46, 531–543. [Google Scholar] [CrossRef]
- Aer, L.; Jiang, Q.; Zhong, L.; Si, Q.; Liu, X.; Pan, Y.; Feng, J.; Zeng, H.; Tang, L. Optimization of polyethylene terephthalate biodegradation using a self-assembled multi-enzyme cascade strategy. J. Hazard. Mater. 2024, 476, 134887. [Google Scholar] [CrossRef]
- Hörnström, D.; Larsson, G.; van Maris, A.J.A.; Gustavsson, M. Molecular optimization of autotransporter-based tyrosinase surface display. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Gätjen, D.; Tomszak, F.; Dettmann, J.-C.; Droste, M.; Nölle, V.; Wieczorek, M. Design of a novel switchable antibody display system in Pichia pastoris. Appl. Microbiol. Biotechnol. 2022, 106, 6209–6224. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, K.; Chi, X.; Zhou, L.; Li, J.; Liu, L.; Zheng, Q.; Wang, Y.; Yu, H.; Gu, Y.; et al. Construction of a bacterial surface display system based on outer membrane protein F. Microb. Cell Factories 2019, 18, 70. [Google Scholar] [CrossRef]
- Koh, D.W.-S.; Tay, J.-H.; Gan, S.K.-E. Engineering Ag43 Signal Peptides with Bacterial Display and Selection. Methods Protoc. 2022, 6, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, Z.; Qin, Y.; Ye, D.-Q.; Song, Y.-Y.; Liu, Y.-L. Efficient Display of Aspergillus niger β-Glucosidase on Saccharomyces cerevisiae Cell Wall for Aroma Enhancement in Wine. J. Agric. Food Chem. 2019, 67, 5169–5176. [Google Scholar] [CrossRef]
- Lengers, I.; Herrmann, F.; Le Borgne, M.; Jose, J. Improved Surface Display of Human Hyal1 and Identification of Testosterone Propionate and Chicoric Acid as New Inhibitors. Pharmaceuticals 2020, 13, 54. [Google Scholar] [CrossRef]
- O’Riordan, N.M.; Jurić, V.; O’Neill, S.K.; Roche, A.P.; Young, P.W. A Yeast Modular Cloning (MoClo) Toolkit Expansion for Optimization of Heterologous Protein Secretion and Surface Display in Saccharomyces cerevisiae. ACS Synth. Biol. 2024, 13, 1246–1258. [Google Scholar] [CrossRef]
- Kajiwara, K.; Aoki, W.; Ueda, M. Evaluation of the yeast surface display system for screening of functional nanobodies. AMB Express 2020, 10, 51. [Google Scholar] [CrossRef]
- Inokuma, K.; Miyamoto, S.; Morinaga, K.; Kobayashi, Y.; Kumokita, R.; Bamba, T.; Ito, Y.; Kondo, A.; Hasunuma, T. Direct production of 4-hydroxybenzoic acid from cellulose using cellulase-displaying Pichia pastoris. Biotechnol. Bioeng. 2023, 120, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Feng, F.; Chen, L.; Yao, Q.; Chen, K. Surface display of Acetobacter pasteurianus AdhA on Bacillus subtilis spores to enhance ethanol tolerance for liquor industrial potential. Eur. Food Res. Technol. 2013, 238, 285–293. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, F.; Zhou, H.; Yu, H.; Shen, L.; Jiang, J.; Qin, Y.; Liu, Y.; Song, Y. Characterization of Trichoderma reesei endoglucanase displayed on the Saccharomyces cerevisiae cell surface and its effect on wine flavor in combination with β-glucosidase. Process Biochem. 2023, 124, 140–149. [Google Scholar] [CrossRef]
- Deng, H.; Gu, Q.; Yu, X.; Zhou, J.; Liu, X. Surface-displayed phenolic acid decarboxylase for increased vinylphenolic pyranoanthocyanins in blueberry wine. Curr. Res. Food Sci. 2024, 8, 100730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Fan, J.; Wang, Z.; Li, L. Detection of catechol using an electrochemical biosensor based on engineered Escherichia coli cells that surface-display laccase. Anal. Chim. Acta 2018, 1009, 65–72. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of Galactooligosaccharides in Milk and Whey: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 678–697. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, X.; Wang, F.; He, Y.; Yang, X.; Hu, J.; Feng, Z.; Yan, Y. Surface-Displayed Thermostable Candida rugosa Lipase 1 for Docosahexaenoic Acid Enrichment. Appl. Biochem. Biotechnol. 2019, 190, 218–231. [Google Scholar] [CrossRef]
- Sun, B.; Li, Z.; Peng, Y.; Wang, F.; Cheng, Y.; Liu, Y.; Ma, L. Whole-Cell Display of Phospholipase D in Escherichia coli for High-Efficiency Extracellular Phosphatidylserine Production. Biomolecules 2024, 14, 430. [Google Scholar] [CrossRef]
- He, Z.; Yang, X.; Tian, X.; Li, L.; Liu, M. Yeast Cell Surface Engineering of a Nicotinamide Riboside Kinase for the Production of β-Nicotinamide Mononucleotide via Whole-Cell Catalysis. ACS Synth. Biol. 2022, 11, 3451–3459. [Google Scholar] [CrossRef]
- Somasundaram, S.; Jeong, J.; Kumaravel, A.; Hong, S.H. Whole-cell display of Pyrococcus horikoshii glutamate decarboxylase in Escherichia coli for high-titer extracellular gamma-aminobutyric acid production. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab039. [Google Scholar] [CrossRef] [PubMed]
- Guirimand, G.G.Y.; Bamba, T.; Matsuda, M.; Inokuma, K.; Morita, K.; Kitada, Y.; Kobayashi, Y.; Yukawa, T.; Sasaki, K.; Ogino, C.; et al. Combined Cell Surface Display of β-D-Glucosidase (BGL), Maltose Transporter (MAL11), and Overexpression of Cytosolic Xylose Reductase (XR) in Saccharomyces cerevisiae Enhance Cellobiose/Xylose Coutilization for Xylitol Bioproduction from Lignocellulosic Biomass. Biotechnol. J. 2019, 14, e1800704. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Chen, P.; Zeng, Y.; Yang, J.; Sun, Y. Efficient production of isomaltulose using engineered Yarrowia lipolytica strain facilitated by non-yeast signal peptide-mediated cell surface display. J. Sci. Food Agric. 2024, 104, 5999–6007. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhu, P.; Liang, J.; Xu, Z.; Feng, X.; Liu, Y.; Xu, H.; Li, S. Economical production of isomaltulose from agricultural residues in a system with sucrose isomerase displayed on Bacillus subtilis spores. Bioprocess Biosyst. Eng. 2019, 43, 75–84. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.P.; Ji, X.F.; Sheng, J. Display of a sucrose isomerase on the cell surface of Yarrowia lipolytica for synthesis of isomaltulose from sugar cane by-products. 3 Biotech 2019, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jiang, B.; Mu, W.; Zhang, T. Production of D-Allulose with D-Psicose 3-Epimerase Expressed and Displayed on the Surface of Bacillus subtilis Spores. J. Agric. Food Chem. 2016, 64, 7201–7207. [Google Scholar] [CrossRef]
- Zhou, R.; Dong, S.; Feng, Y.; Cui, Q.; Xuan, J. Development of highly efficient whole-cell catalysts of cis-epoxysuccinic acid hydrolase by surface display. Bioresour. Bioprocess. 2022, 9, 92. [Google Scholar] [CrossRef]
- Aso, Y.; Tsubaki, M.; Dang Long, B.H.; Murakami, R.; Nagata, K.; Okano, H.; Phuong Dung, N.T.; Ohara, H. Continuous production of D-lactic acid from cellobiose in cell recycle fermentation using β-glucosidase-displaying Escherichia coli. J. Biosci. Bioeng. 2019, 127, 441–446. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Chi, Z.; Liu, G.-L.; Wang, F.; Madzak, C.; Chi, Z.-M. Inulin hydrolysis and citric acid production from inulin using the surface-engineered Yarrowia lipolytica displaying inulinase. Metab. Eng. 2010, 12, 469–476. [Google Scholar] [CrossRef]
- Jia, L.G.; Wang, C.; Wu, Y.F.; Ma, X.Y.; Zhang, X.; Chu, X.X.; Lu, F.P.; Liu, Y.H. Production of L-α-Glycerylphosphorylcholine from Oil Refining Waste Using a Novel Cold-Active Phospholipase B from Bacillus velezensis. ACS Sustain. Chem. Eng. 2021, 9, 13337–13346. [Google Scholar] [CrossRef]
- Luo, B.; Jin, M.M.; Li, X.; Makunga, N.P.; Hu, X. Yeast Surface Display for In Vitro Biosynthetic Pathway Reconstruction. ACS Synth. Biol. 2021, 10, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.A.; Nguyen, T.T.M.; Nguyen, N.H.; Nguyen, T.N.; Dang, T.T.P. Application of yeast surface display system in expression of recombinant pediocin PA-1 in Saccharomyces cerevisiae. Folia Microbiol. 2020, 65, 955–961. [Google Scholar] [CrossRef]
- Chun, J.; Bai, J.; Ryu, S. Yeast Surface Display System for Facilitated Production and Application of Phage Endolysin. ACS Synth. Biol. 2020, 9, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Jun, J.S.; Moon, J.A.; Hong, K.W. Surface display of p75, a Lactobacillus rhamnosus GG derived protein, on Bacillus subtilis spores and its antibacterial activity against Listeria monocytogenes. AMB Express 2020, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, T.; Ye, Y.; Guan, S.; Cai, B.; Zhang, S.; Rong, S. A temperature-induced chitosanase bacterial cell-surface display system for the efficient production of chitooligosaccharides. Biotechnol. Lett. 2021, 43, 1625–1635. [Google Scholar] [CrossRef]
- Li, J.; Xie, X.; Cai, J.; Wang, H.; Yang, J. Enhanced Secretory Expression and Surface Display Level of Bombyx mori Acetylcholinesterase 2 by Pichia pastoris Based on Codon Optimization Strategy for Pesticides Setection. Appl. Biochem. Biotechnol. 2021, 193, 3321–3335. [Google Scholar] [CrossRef]
- GB/T 5009.199-2003; Determination of Organophosphate and Carbamate Pesticide Residues in Foods. Ministry of Health of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2003.
- GB 2763-2019; Maximum Residue Limits for Pesticides in Foods. Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2019.
- Yang, H.; Du, L.; Geng, L.; Liu, X.; Xu, Z.; Liu, R.; Liu, W.; Zuo, H.; Chen, Z.; Wang, X.; et al. A novel yeast-based biosensor for the quick determination of Deoxynivalenol. Anal. Chim. Acta 2024, 1315, 342760. [Google Scholar] [CrossRef]
- Popovic, M.; Gavrovic-Jankulovic, M. Yeast Surface Display Methodology for the Characterization of Food Allergens In Situ. Methods Mol. Biol. 2024, 2717, 41–63. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, P.L.A.; Terán-Ramírez, C.; Mares-Alejandre, R.E.; Márquez-González, A.B.; Madero-Ayala, P.A.; Meléndez-López, S.G.; Ramos-Ibarra, M.A. Surface Engineering of Escherichia coli to Display Its Phytase (AppA) and Functional Analysis of Enzyme Activities. Curr. Issues Mol. Biol. 2024, 46, 3424–3437. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Li, Y.; Pan, K.; OuYang, K.; Song, X.; Xiong, X.; Zang, Y.; Wang, L.; Qu, M.; et al. Characterization of yeast cell surface displayed Lentinula edodes xylanase and its effects on the hydrolysis of wheat. Int. J. Biol. Macromol. 2022, 199, 341–347. [Google Scholar] [CrossRef]
- Han, W.; Zhang, J.; Chen, Q.; Xie, Y.; Zhang, M.; Qu, J.; Tan, Y.; Diao, Y.; Wang, Y.; Zhang, Y. Biodegradation of poly(ethylene terephthalate) through PETase surface-display: From function to structure. J. Hazard. Mater. 2024, 461, 132632. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Svensson, C.-M.; Maneira, C.; Sporniak, M.; Figge, M.T.; Lackner, G. Engineering Adhesion of the Probiotic Strain Escherichia coli Nissle to the Fungal Pathogen Candida albicans. ACS Synth. Biol. 2024, 13, 4027–4039. [Google Scholar] [CrossRef]
- Chung, M.E.; Goroncy, K.; Kolesnikova, A.; Schönauer, D.; Schwaneberg, U. Display of functional nucleic acid polymerase on Escherichia coli surface and its application in directed polymerase evolution. Biotechnol. Bioeng. 2020, 117, 3699–3711. [Google Scholar] [CrossRef] [PubMed]
- Apitius, L.; Rübsam, K.; Jakesch, C.; Jakob, F.; Schwaneberg, U. Ultrahigh-throughput screening system for directed polymer binding peptide evolution. Biotechnol. Bioeng. 2019, 116, 1856–1867. [Google Scholar] [CrossRef]
- Zhang, A.; Hou, Y.; Wang, Y.; Wang, Q.; Shan, X.; Liu, J. Highly efficient low-temperature biodegradation of polyethylene microplastics by using cold-active laccase cell-surface display system. Bioresour. Technol. 2023, 382. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Feng, P.; Kakade, A.; Yang, L.; Chen, G.; Yan, X.; Ni, H.; Liu, P.; Kulshreshtha, S.; Abomohra, A.E.-F.; et al. Reducing residual antibiotic levels in animal feces using intestinal Escherichia coli with surface-displayed erythromycin esterase. J. Hazard. Mater. 2020, 388. [Google Scholar] [CrossRef]
- Yamashita, T.; Matsumoto, T.; Yamada, R.; Ogino, H. Display of PETase on the Cell Surface of Escherichia coli Using the Anchor Protein PgsA. Appl. Biochem. Biotechnol. 2024, 196, 5471–5483. [Google Scholar] [CrossRef]
- Zhong, X.; Yang, S.; Su, X.; Shen, X.; Zhao, W.; Chan, Z. Production of Cyanocarboxylic Acid by Acidovorax facilis 72W Nitrilase Displayed on the Spore Surface of Bacillus subtilis. J. Microbiol. Biotechnol. 2019, 29, 749–757. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, W.; Lee, Y.-S.; Kim, J.-H. Decolorization of Acid Green 25 by Surface Display of CotA laccase on Bacillus subtilis spores. J. Microbiol. Biotechnol. 2019, 29, 1383–1390. [Google Scholar] [CrossRef]
- Kang, S.-J.; Jun, J.-S.; Hong, K.-W. Transcriptome Analysis Reveals Immunomodulatory Effect of Spore-Displayed p75 on Human Intestinal Epithelial Caco-2 Cells. Int. J. Mol. Sci. 2022, 23, 14519. [Google Scholar] [CrossRef]
- Xing, H.; Zhu, L.; Wang, P.; Zhao, G.; Zhou, Z.; Yang, Y.; Zou, H.; Yan, X. Display of receptor-binding domain of SARS-CoV-2 Spike protein variants on the Saccharomyces cerevisiae cell surface. Front. Immunol. 2022, 13, 935573. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, J.; Ma, Y.; Hao, L.; Liang, Z.; Ma, J.; Ke, H.; Li, Y.; Cao, J. Protective immunity against CyHV-3 infection via different prime-boost vaccination regimens using CyHV-3 ORF131-based DNA/protein subunit vaccines in carp Cyprinus carpio var. Jian. Fish Shellfish Immunol. 2020, 98, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.P.; Zhao, L.; Li, Z.Y.; Chen, L.; Li, J.W.; Lu, F.P.; Wang, F.H.; Lu, K.; Liu, Y.H. Biodegradation of bisphenol A by a Pichia pastoris whole-cell biocatalyst with overexpression of laccase from Bacillus pumilus and investigation of its potential degradation pathways. J. Hazard. Mater. 2024, 474. [Google Scholar] [CrossRef] [PubMed]
- Sena, R.O.; Carneiro, C.; Moura, M.V.H.; Brêda, G.C.; Pinto, M.C.C.; Fé, L.X.S.G.M.; Fernandez-Lafuente, R.; Manoel, E.A.; Almeida, R.V.; Freire, D.M.G.; et al. Application of Rhizomucor miehei lipase-displaying Pichia pastoris whole cell for biodiesel production using agro-industrial residuals as substrate. Int. J. Biol. Macromol. 2021, 189, 734–743. [Google Scholar] [CrossRef]
- Inokuma, K.; Kurono, H.; den Haan, R.; van Zyl, W.H.; Hasunuma, T.; Kondo, A. Novel strategy for anchorage position control of GPI-attached proteins in the yeast cell wall using different GPI-anchoring domains. Metab. Eng. 2020, 57, 110–117. [Google Scholar] [CrossRef]

| Optimization Strategies | Strains | Anchor Proteins | Optimization Methods | Results | References |
|---|---|---|---|---|---|
| Truncation of existing anchor proteins | E. coil BL21(DE3) | INP | Construction of INP-N (22 KDa) and INP-NC (33 KDa) by truncating INP (114 KDa) | INP-N showed the highest expression level and enzymatic activity. | [73] |
| E. coli XL10-Gold | YiaT | Truncations at R181 and R232 in the fourth and fifth extracellular loops | The lipase activities of YiaTR181 and YiaTR232 were approximately 10-fold and 20-fold higher, respectively, compared to FadL and OprF. | [74] | |
| E. coli XL10-Gold | MipA | Truncating six C-terminal sites V140, V176, K179, V226, V232, and K234 | MV140 variant had the highest lipase activity, comparable to that of YiaTR232. | [75] | |
| S. cerevisiae strain CEN.PK102-5B | Aga1p-Aga2p | Replacing the Aga1p-Aga2p complex with one subunit (Aga1p) | BGL enzyme activity increased by 39%. | [76] | |
| S. cerevisiae BY 4741 | Pir protein family | Designing 14 S. cerevisiae Hsp150 (Pir2)-based fusion proteins by machine-learning strategies | The display efficiency of Hsp150-derived constructs was 2.5-fold higher than that of full-length Hsp150. | [62] | |
| Discovery of novel anchor proteins | S. cerevisiae BY4743 | GPI-CWPs | Designing 37 GPI-CWPs through prediction of GPI-CWPs by GPIPlus and specific feature extraction using Biopython (www.biopython.org) | Among them, five GPI-CWPs outperformed the conventional α-agglutinin anchor. | [77] |
| Amount of Enzyme | Strains | Optimization Methods | Results | Advantages | Limitations | References |
|---|---|---|---|---|---|---|
| Single-enzyme | E. coli BL21 (DE3) | Using four direct fusion methods:(no-connecting peptide (NL), flexible linker peptide (FL, GGGGS), rigid linker peptide (RL, PAPAP), and rigid helical linker peptide (HL, AEAAAKEAAAKA)) | The flexible linker peptide FL yielded the highest Pb2+ adsorption capacities. | Direct fusion of single passenger proteins: simple and low cost of operation. | Difficult to demonstrate large molecular weight proteins as well as multiple proteins. | [78] |
| B. subtilis DB 403 | Using three direct fusion methods: L1 (GGGGS), L2 (GGGEAAAKGGG), L3 (GGGGSGGGGS) | The extended flexible linker peptide L3 achieved the highest activity. | [79] | |||
| E. coli BL21 (DE3) | Using SpyTag/SpyCatcher technology | The most successfully displayed larger passenger protein BM3 (119 KDa). | Indirect fusion of single passenger proteins: large molecular weight proteins can be demonstrated. | Requires in vitro supplementation of purified proteins, which is costly; low assembly efficiency; difficult to demonstrate multiple proteins. | [40] | |
| E. coli BL21 (DE3) | Constructing LBP2-functionalized biofilm material | This approach achieved higher enzymatic activity compared to the SpyTag/SpyCatcher strategy. | [80] | |||
| Multi-enzyme | P. pastoris GS115 | Harnessing an ultra-high-affinity IM7/CL7 protein pair | This way displayed three recombinant cellulases EG, exoglucanase CBH, and BGL to produce bioethanol (the maximum ethanol titer of 5.1 g/L). | Indirect fusion of multiple passenger proteins: a complex catalytic process that can be synergized with multiple enzymes. | Requires in vitro supplementation of purified proteins, which is costly; low assembly efficiency. | [81] |
| S. cerevisiae EBY100 | Utilizing protein scaffolds | S. cerevisiae achieved a world-record ethanol titer of 8.68 g/L. | [82] | |||
| E. coli BL21 (DE3) | Utilizing protein scaffolds | Degradation reached 11.56 ± 0.64 mM after 7 days. | [83] |
| Strains | Passenger Proteins | Optimization of Promoters | Optimization of Signal Peptides | Results | References |
|---|---|---|---|---|---|
| S. cerevisiae | α-Amylase and eGFP | TPI1 and TDH3p | - | The strong promoter TDH3p increased surface display activity by 23% and 142% when driving α-amylase expression and eGFP, respectively | [70] |
| S. cerevisiae | BGL | GPD and SED1 | - | GPD promoter drove BGL with twice the enzyme activity of the SED1 promoter | [88] |
| E. coli | Hyaluronidase Hyal1 | Rhamnose-dependent promoter (Prha) and constitutive promoter | - | Replacement of the constitutive promoter by a Prha and optimization of reaction conditions resulted in a 100-fold increase in Hyal1 activity | [89] |
| E. coli | Hepatitis B virus (HBV) S antigen and human papilloma virus (HPV) L2 protein | Mutants of the OmpF promoter | - | Under the OmpF promoter mutation, the proportion of positive cells reached 99.1% and 91.6% for HBV S antigen cell and HPV L2 protein cell, respectively, which was significantly higher than that of the control group | [86] |
| S. cerevisiae | mRuby2 | - | 9 pre-signal peptides | Among the 9 pre-signal peptides, the AGA2 pre-signal peptides showed the best effect on mRuby2 secretion and surface display | [90] |
| E. coli | sfGFP | - | 29 mutants of the Ag43 signal peptide | These mutants increased the level of surface presentation 1.4- to 3-fold | [87] |
| S. cerevisiae | Anti-hen egg-white lysozyme nanobody | GAP and GAL1 | α Pre-pro sequence derived from S. cerevisiae and the glucoamylase secretion signal derived from Rhizopus oryzae | GAP promoter drove more nanobody display than GAL1 promoter; α pre-pro sequence more suitable for nanobody display | [91] |
| P. pastoris | Multiple antibodies | ADH1, AOD, AOX1, ENO1, and FLD1 five endogenous P. pastoris promoters | α-Mating factor (α-MF), α-MF: Δ57-70 and SUC2 | It was determined that the combination of the FLD1 promoter, and SUC2 signal peptide resulted in up to 25% antibody fragment presentation, and that antibody presentation was at least twice as high with AOX1 and FLD1 (methanol-inducible promoter) than with ADH1 (glycerol-inducible promoter), AOD, and ENO1 (constitutive promoter); the three signal peptides were similar in their effects | [85] |
| P. pastoris | BGL and EG | GAP and SPI1 | S. cerevisiae alpha-factor and SPI1 secretion signal | The SPI1 promoter and SPI1 secretion signal were approximately 1.3-fold and 2.4-fold higher in cell surface BGL and EG activity than the conventional GAP promoter and secretion signal of S. cerevisiae alpha-factor | [92] |
| E. coli | Tyrosinase | Placlvs and PrhaB | Signal peptide of AIDA-I and signal peptide region from the AT Hemoglobin-binding protease | The construct corresponding to the AIDA-I signal peptide had a higher tyrosinase-specific activity; the PrhaB regulated tyrosinase-specific activity was 50% higher than that of Placlvs | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Gao, X.; Zhou, Y.; You, S.; Qi, W.; Wang, M. Surface Display Technologies for Whole-Cell Biocatalysts: Advances in Optimization Strategies, Food Applications, and Future Perspectives. Foods 2025, 14, 1803. https://doi.org/10.3390/foods14101803
Zhang B, Gao X, Zhou Y, You S, Qi W, Wang M. Surface Display Technologies for Whole-Cell Biocatalysts: Advances in Optimization Strategies, Food Applications, and Future Perspectives. Foods. 2025; 14(10):1803. https://doi.org/10.3390/foods14101803
Chicago/Turabian StyleZhang, Baoyu, Xing Gao, Yu Zhou, Shengping You, Wei Qi, and Mengfan Wang. 2025. "Surface Display Technologies for Whole-Cell Biocatalysts: Advances in Optimization Strategies, Food Applications, and Future Perspectives" Foods 14, no. 10: 1803. https://doi.org/10.3390/foods14101803
APA StyleZhang, B., Gao, X., Zhou, Y., You, S., Qi, W., & Wang, M. (2025). Surface Display Technologies for Whole-Cell Biocatalysts: Advances in Optimization Strategies, Food Applications, and Future Perspectives. Foods, 14(10), 1803. https://doi.org/10.3390/foods14101803






