Effect of Harvest Seasons on Biochemical Components and Volatile Compounds in White Teas from Two Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Tea Sample Preparation
2.3. Sensory Evaluation
2.4. Detection of Biochemical Components
2.5. Extraction and Determination of Volatile Compounds
2.6. Calculation of Odor Activity Values (OAVs)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Tea Sample Sensory Evaluation
3.2. Analysis of Main Biochemical Components
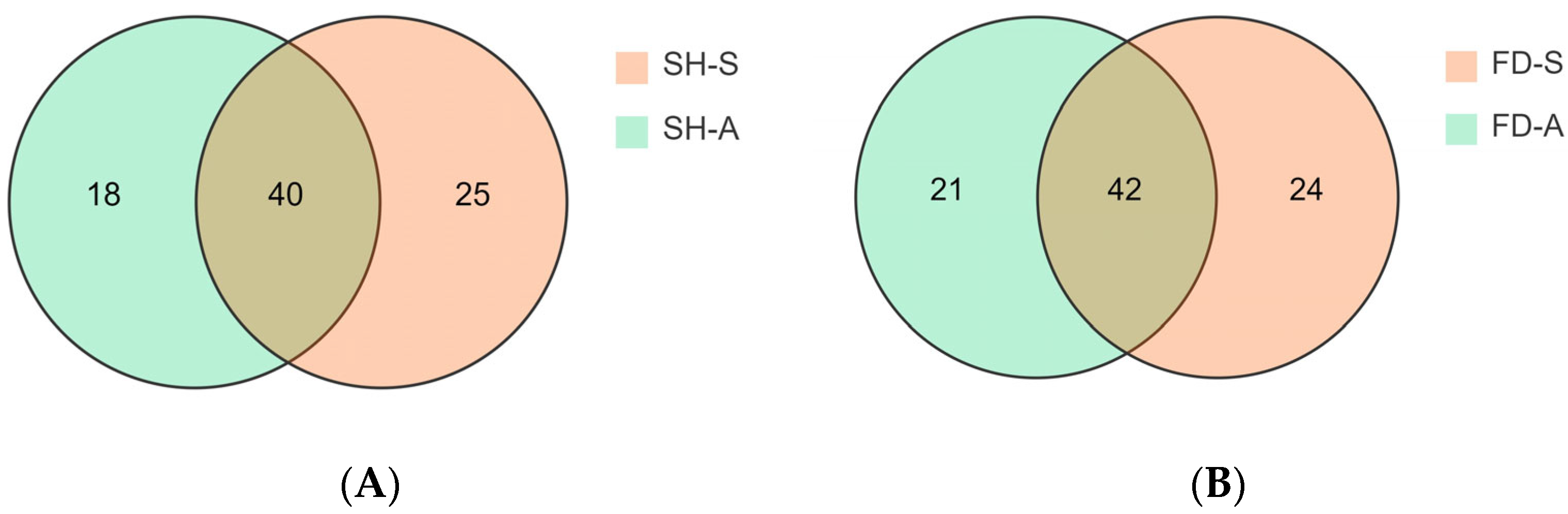
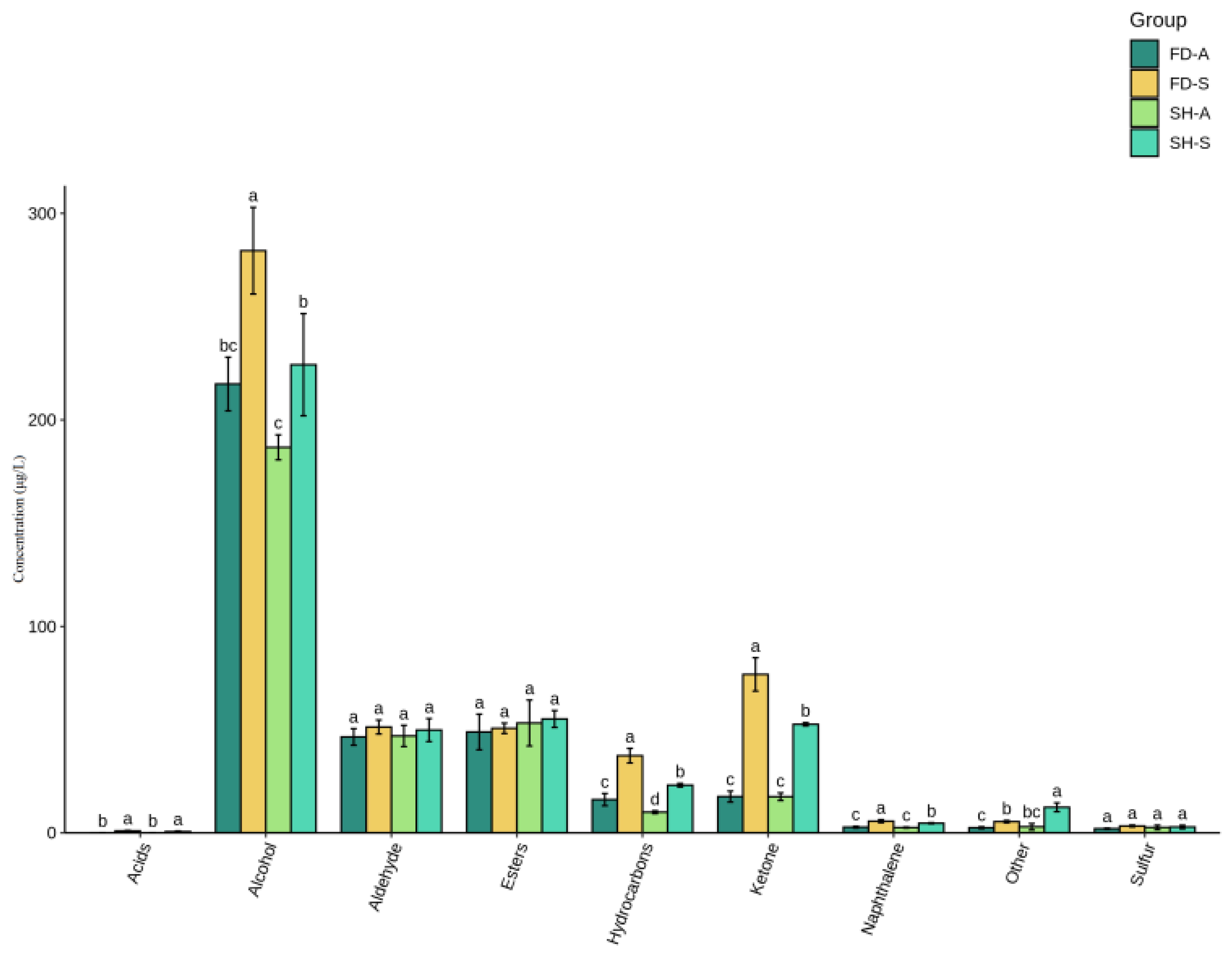
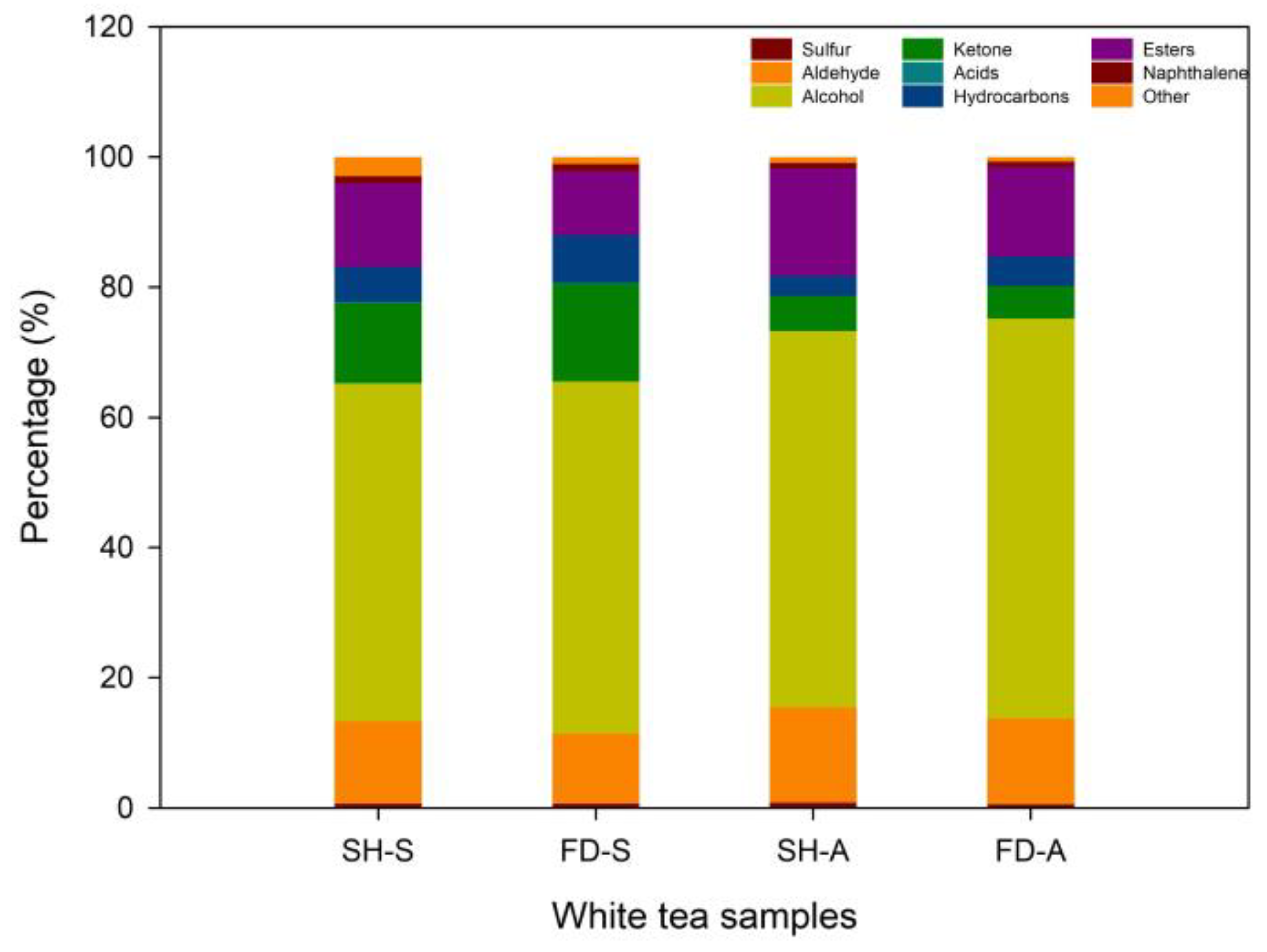
3.3. Overall Determination of Volatile Compounds
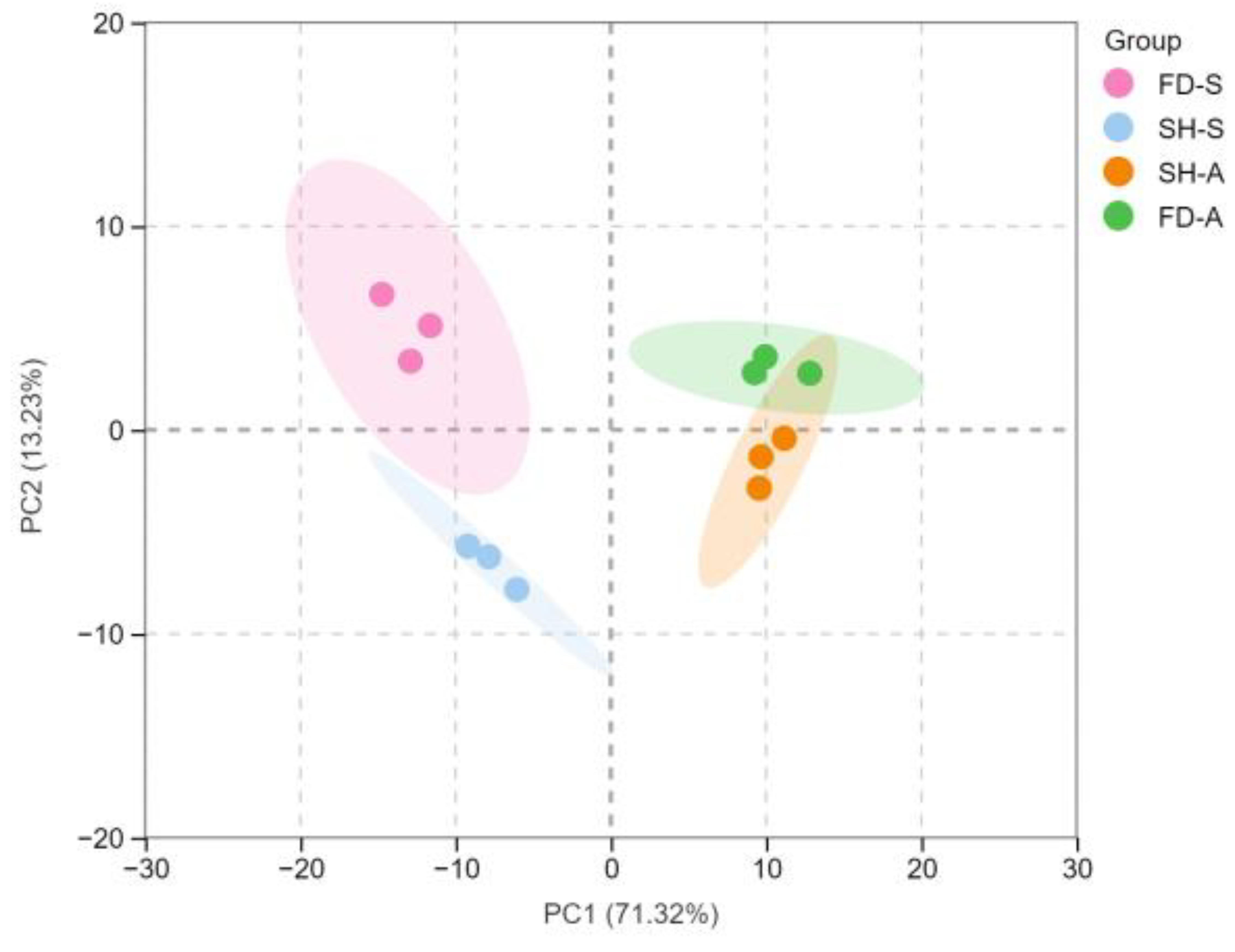
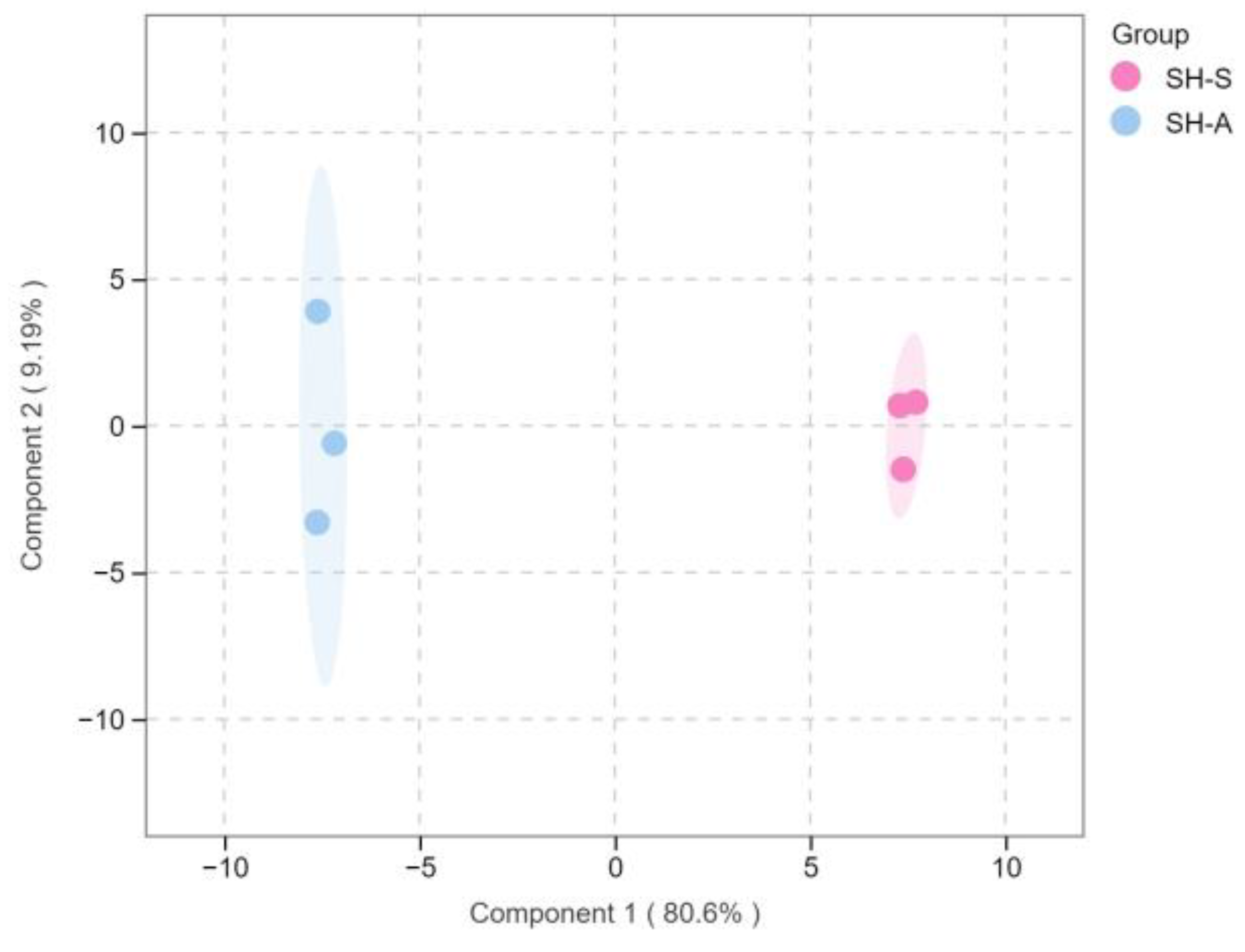
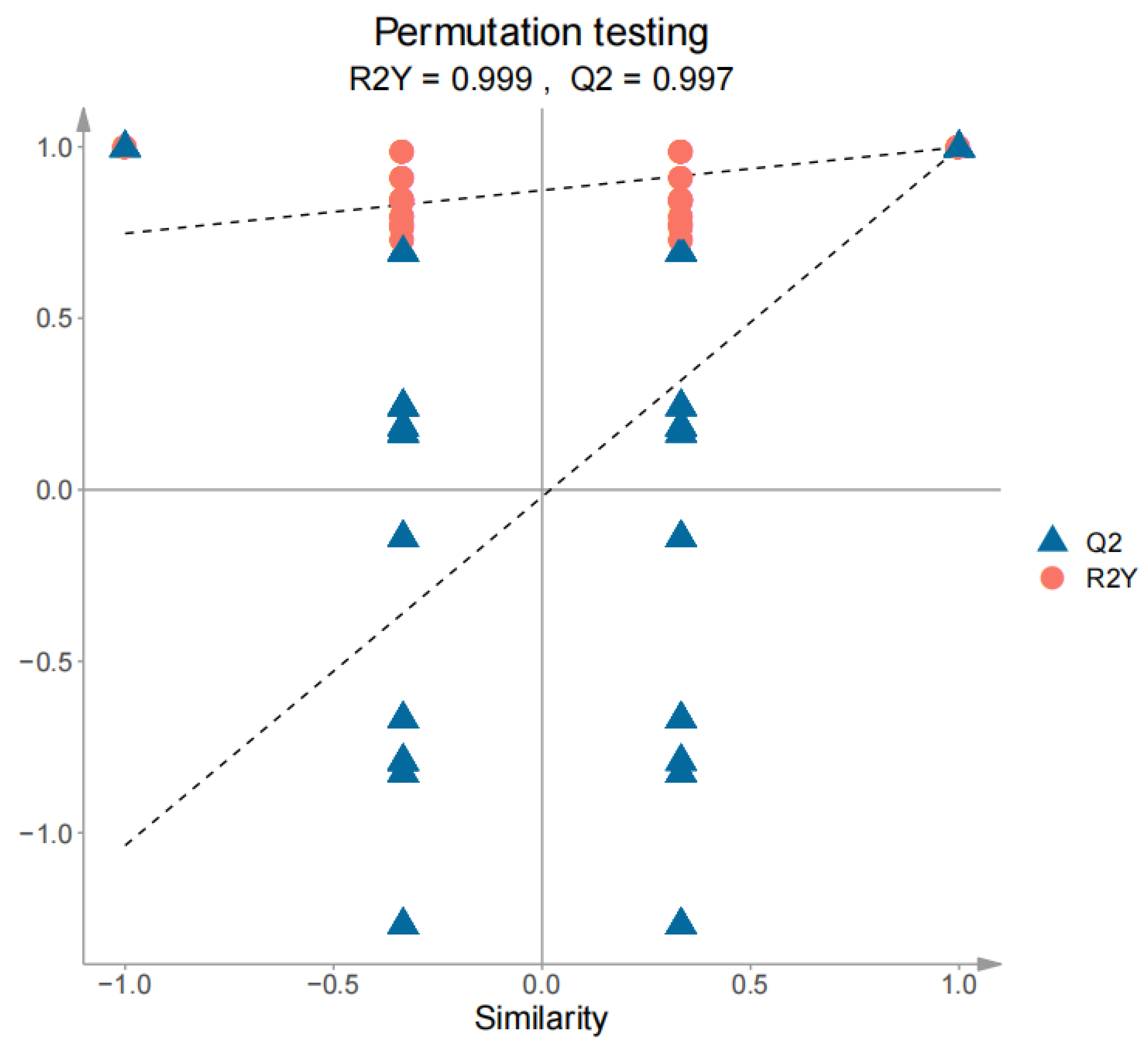
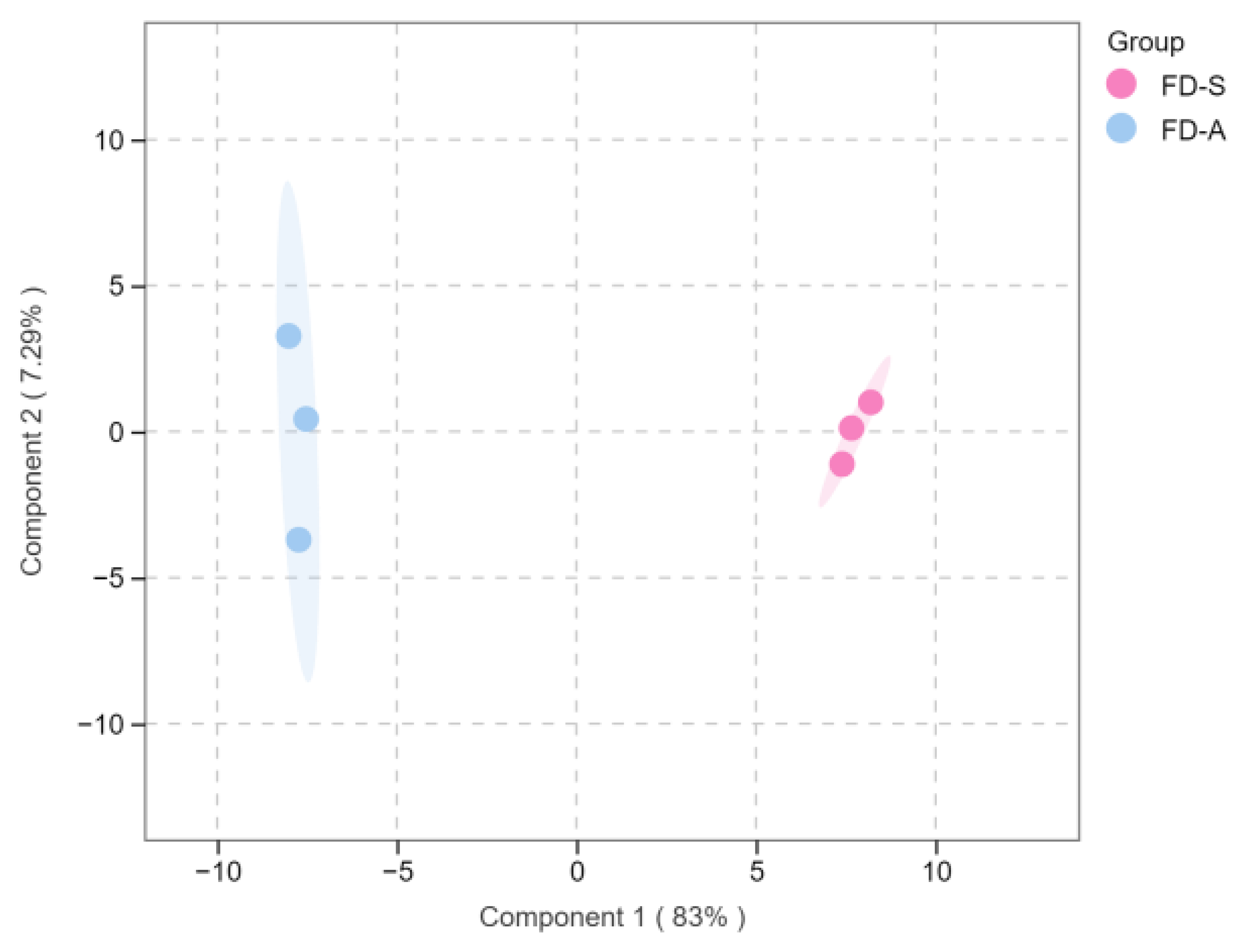
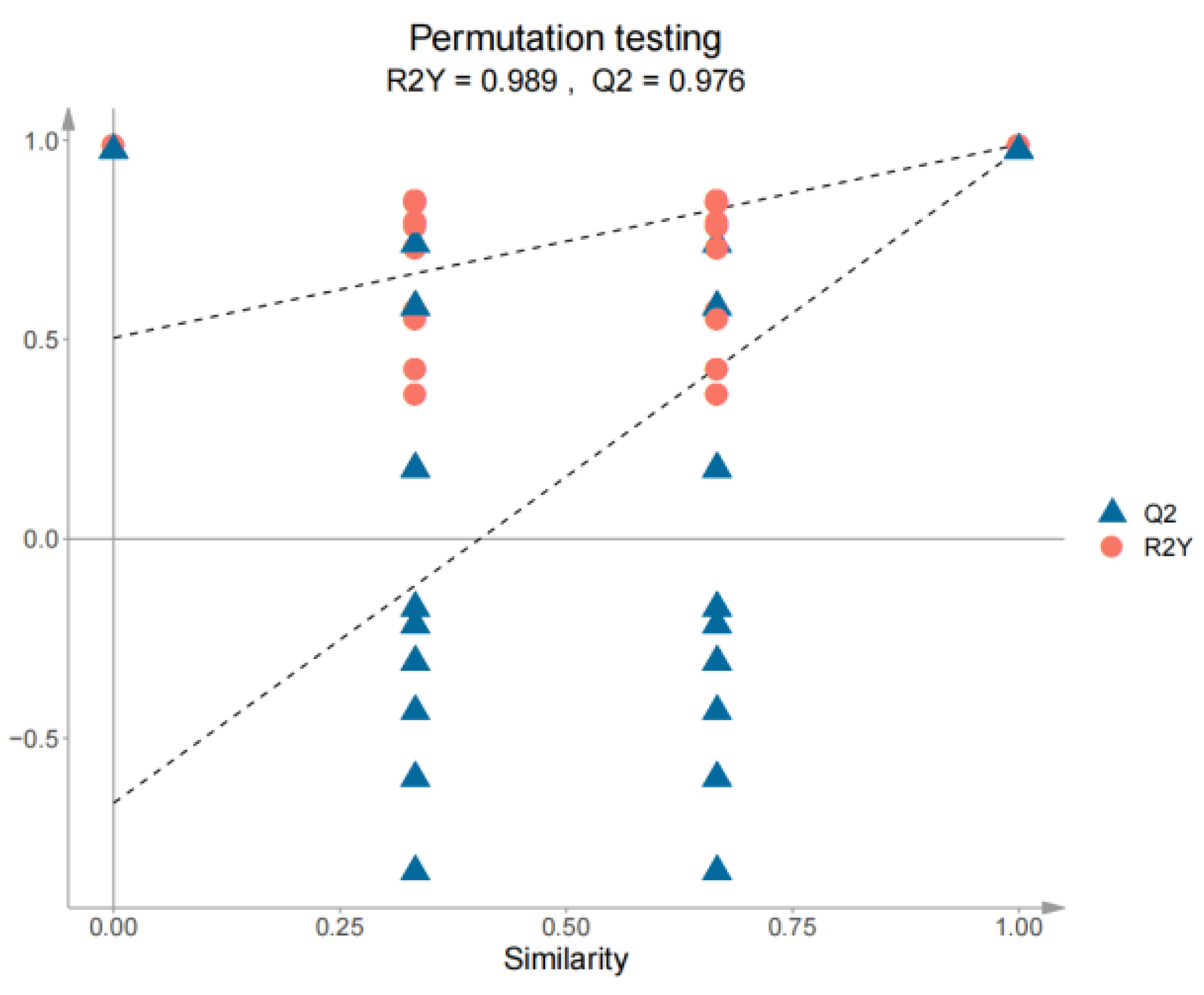
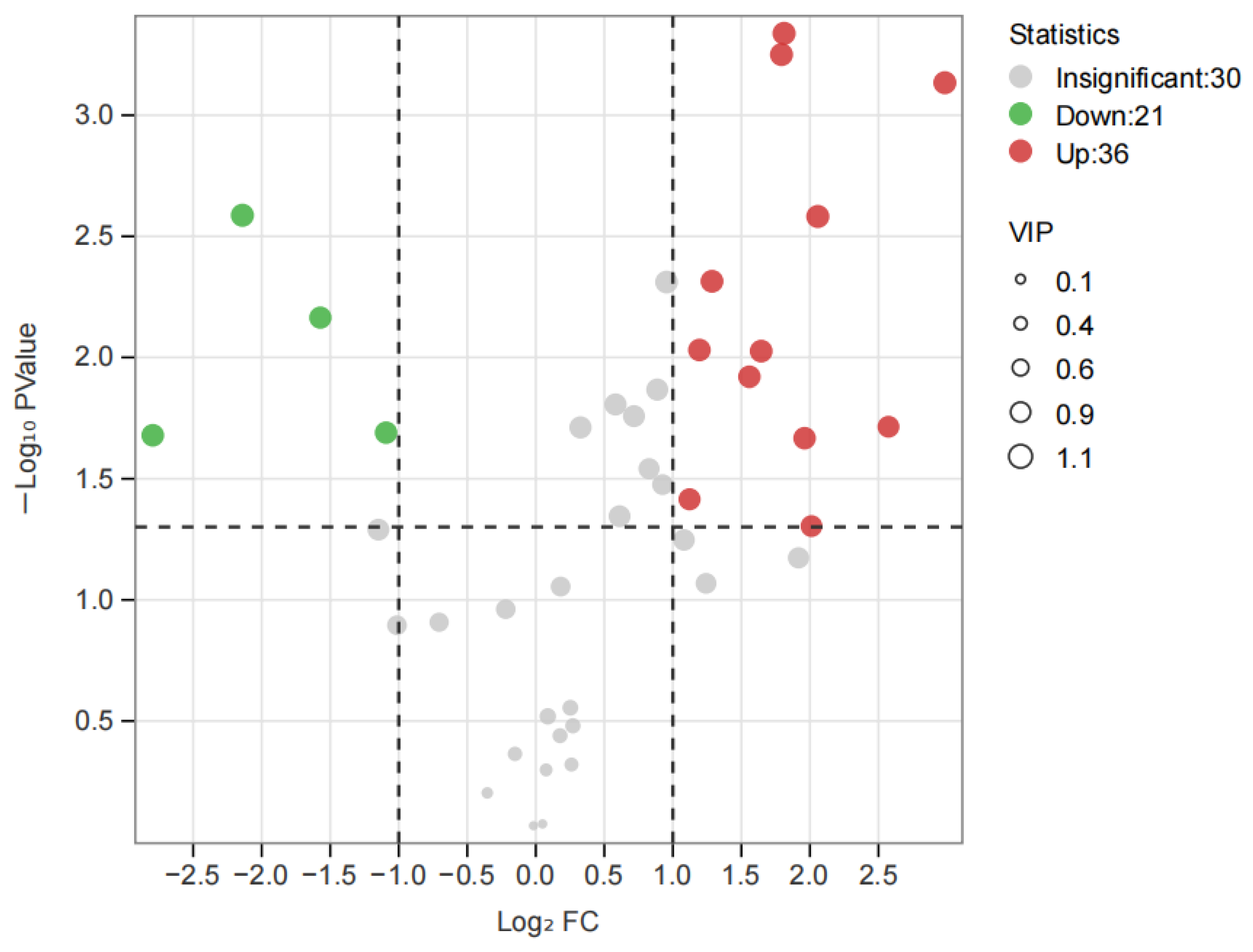
3.4. PCA and OPLS-DA Statistical Analyses of the Volatile Compounds
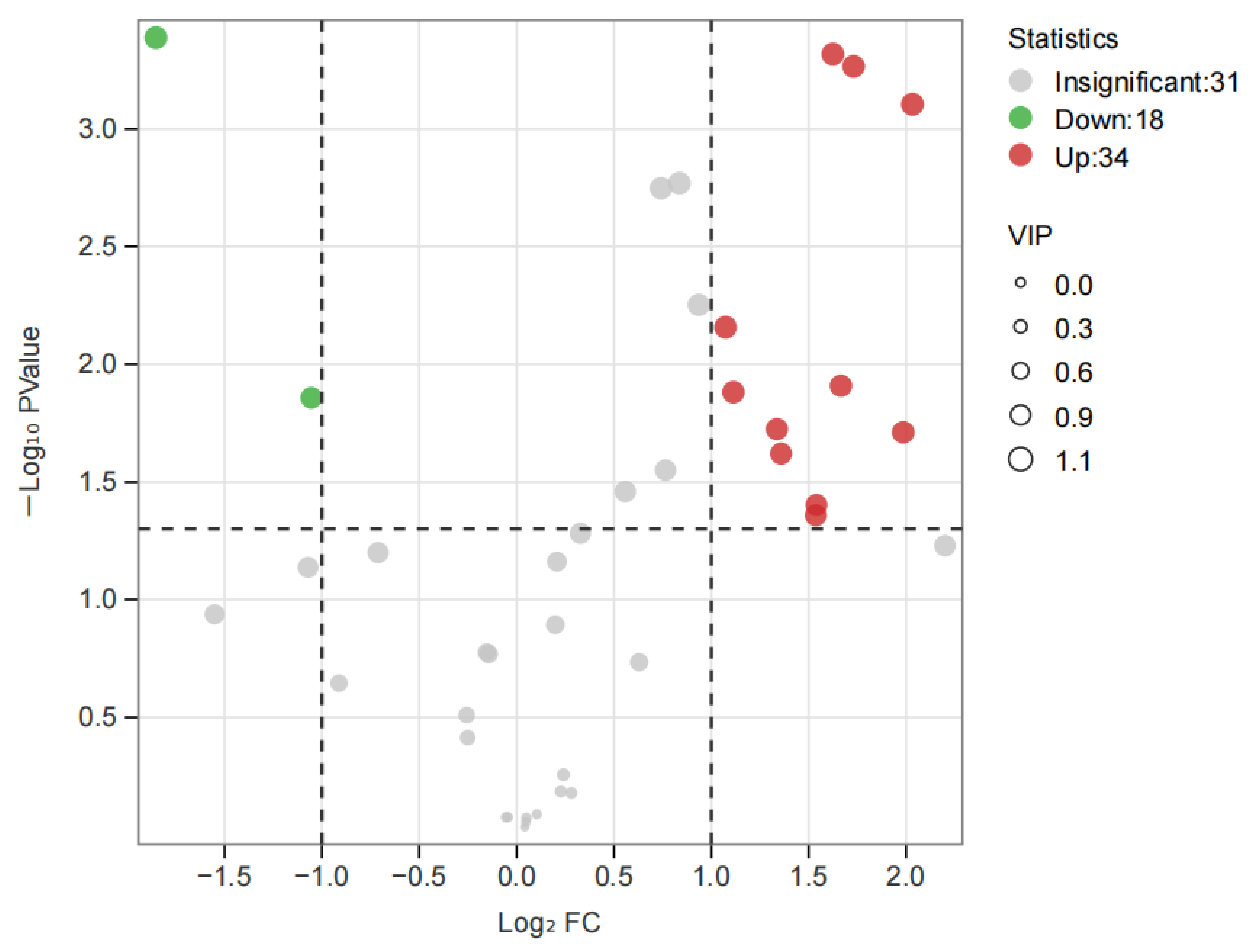
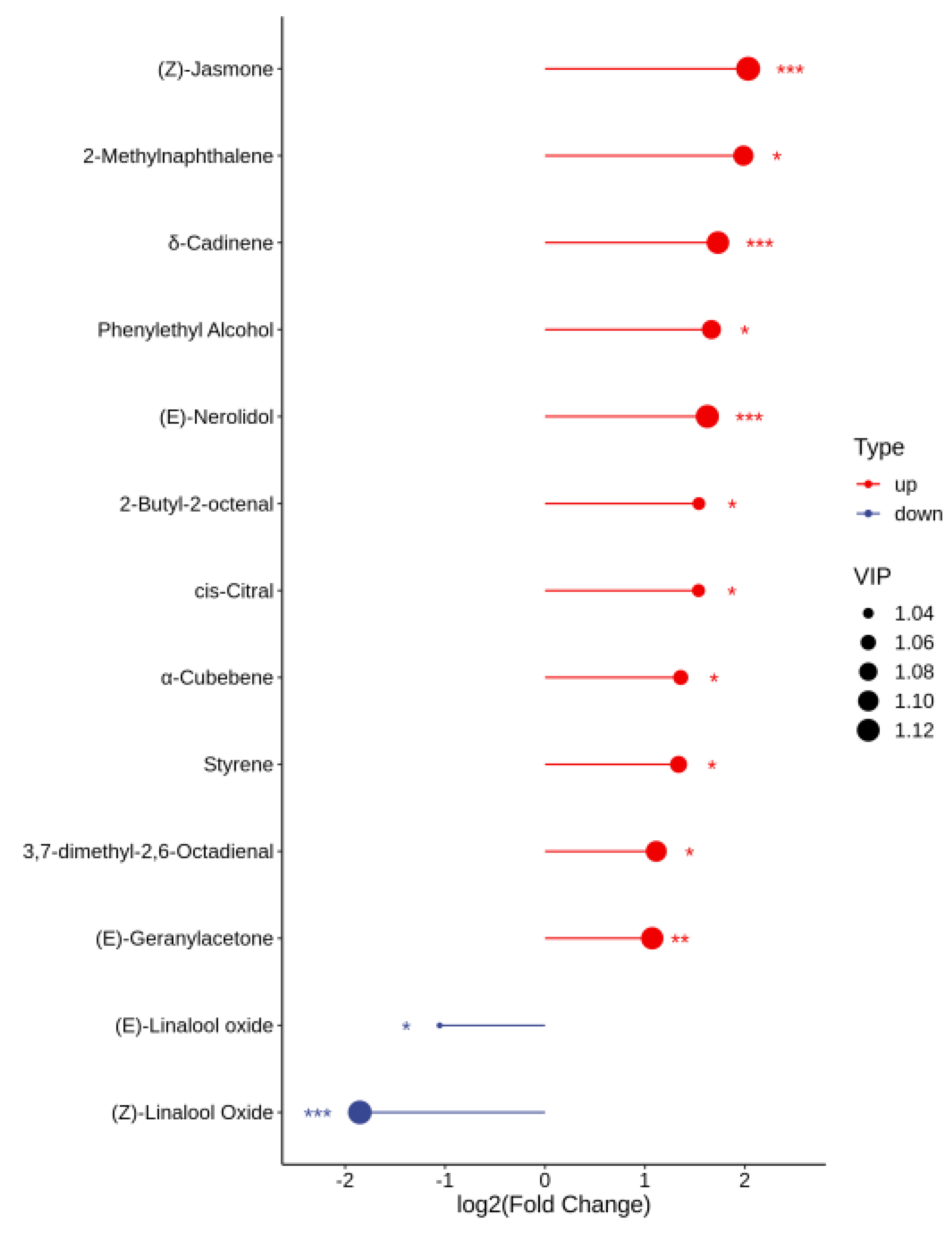
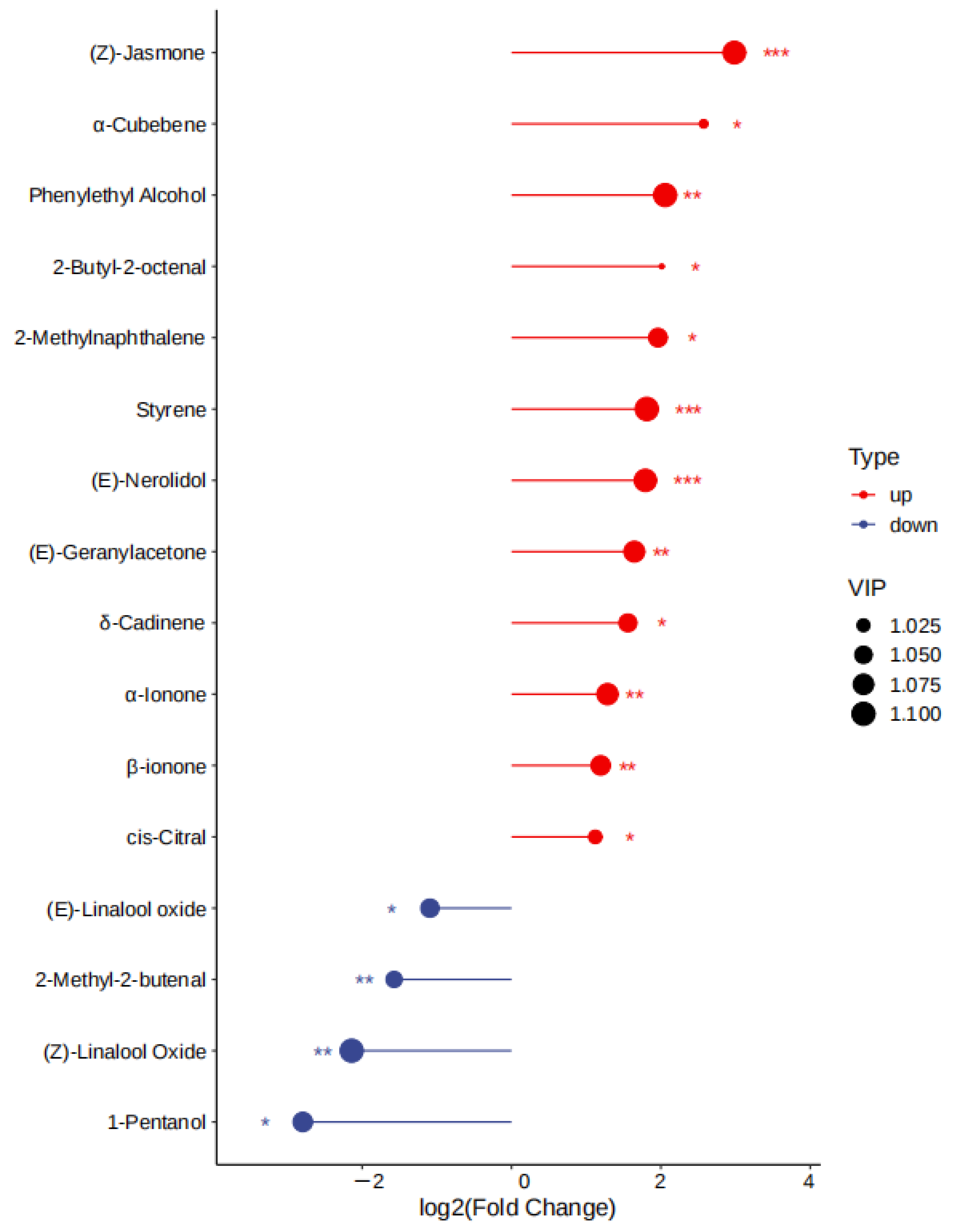
3.5. OAV Analysis of the Differential Aroma Substances
| No. | Index | Odor Description a | Threshold μg/L | OAV | |||
|---|---|---|---|---|---|---|---|
| SH-S | SH-A | FD-S | FD-A | ||||
| 1 | Dimethyl sulfide | green, fresh | 1.1 [61] | 2.587 ab | 2.408 ab | 3.039 a | 1.850 b |
| 2 | Hexanal | fresh, green, grass, fruity, apple | 4.5 [62] | 0.624 a | 1.310 a | 0.583 a | 1.292 a |
| 3 | Nonanal | fresh, fruity, floral, | 1 [62] | 3.747 a | 7.05 a | 2.937 a | 4.789 a |
| 4 | (E)-2-Nonenal | cucumber flavor | 0.4 [62] | 3.509 a | 3.645 a | 3.806 a | 3.613 a |
| 5 | Decanal | soap, orange peel, tallow | 0.1 [62] | 16.894 a | 18.663 a | 16.601 a | 19.326 a |
| 6 | (E,E)-2,4-Nonadienal | fresh, cucumber-like | 0.062 [62] | 0.000 b | 14.396 a | 0.000 b | 14.836 a |
| 7 | (E,E)-2,4-Heptadienal | floral | 0.03 [62] | 85.000 a | 50.000 b | 62.500 ab | 63.130 ab |
| 8 | (E)-2-Decenal | fat | 0.3 [62] | 5.412 a | 0.000 b | 6.732 a | 0.000 b |
| 9 | 2-Undecen-1-al | green, fresh | 0.78 [63] | 1.498 a | 0.000 b | 1.977 a | 0.000 b |
| 10 | Oct-1-en-3-ol | grass, oily, earthy, fungal, vegetative-like, fresh, mushroom-like | 1 [62] | 0.881 a | 0.909 a | 1.199 a | 1.158 a |
| 11 | (E)-Linalool oxide | fresh, citrus, fruity | 6 [53] | 0.976 d | 2.028 b | 2.014 c | 4.294 a |
| 12 | Linalool | floral, sweet,grape-like, woody | 0.22 [62] | 348.887 b | 387.966 ab | 464.531 a | 409.690 a |
| 13 | Geraniol | rose-like, sweet, waxy | 40 [62] | 2.491 a | 1.302 b | 2.702 a | 1.395 b |
| 14 | (Z)-Jasmone | floral, Jasmin, woody, herbal | 1.9 [62] | 4.583 b | 1.120 c | 6.359 a | 0.804 c |
| 15 | α-Ionone | sweet, fruity, woody, floral | 0.4 [62] | 8.494 a | 4.759 b | 11.499 a | 4.717 b |
| 16 | β-ionone | sweet, fruity, woody, floral | 0.007 [62] | 2406.334 a | 1439.131 b | 3375.147 a | 1475.371 b |
| 17 | β-Pinene | pine,turpentine | 6 [62] | 0.000 b | 0.000 b | 0.000 b | 1.054 a |
| 18 | Ocimene | herbal, sweet | 0.02 [62] | 108.301 a | 128.897 a | 152.647 a | 126.525 a |
| 19 | δ-Cadinene | herbal, woody | 1.5 [45] | 3.734 a | 1.125 b | 4.206 a | 1.428 b |
| 20 | Methyl salicylate | mint-like | 40 [62] | 1.098 a | 1.311 a | 1.038 a | 1.153 a |
| 21 | Naphthalene | pungent | 0.44 [64] | 4.194 a | 2.849 b | 4.997 a | 2.704 b |
| 22 | 2-Pentylfuran | floral,milk-like, beany, earthy | 6 [62] | 1.253 a | 0.000 b | 0.000 b | 0.000 b |
| 23 | Undecylic aldehyde | fresh, orange peel, fat | 0.014 [62] | 45.072 a | 0.000 b | 59.197 a | 0.000 b |
| 24 | α-Cedrol | woody | 0.5 [63] | 0.000 b | 3.500 a | 0.000 b | 3.312 a |
| 25 | 3,5-Octadien-2-one | fruity, green, grassy | 0.15 [65] | 60.986 b | 0.000 c | 100.4 a | 0.000 c |
| 26 | 3-Tridecanone | waxy | 0.5 [63] | 0.000 b | 1.539 a | 0.000 b | 1.423 a |
| 27 | 5,6-Epoxy-β-ionone | sweet, fruity, woody, floral | 0.007 [66] | 670.916 a | 0 b | 1118.136 a | 0 b |
| 28 | 4-Nonanolide | fruity | 0.28 [62] | 3.348 a | 0.000 b | 3.110 a | 0.000 b |
| 29 | β-Cyclocitral | fresh, sweet | 3 [62] | 1.070 ab | 0.940 ab | 1.140 a | 0.750 b |
| 30 | Ethyl nonylate | fruity, floral | 1.2 [21] | 1.210 a | 0.207 a | 1.230 a | 0.330 a |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SH-S | Sanhua1951 harvested in March |
| FD-S | Fudingdabai harvested in March |
| SH-A | Sanhua1951 harvested in August |
| FD-A | Fudingdabai harvested in August |
| PCA | Principal component analysis |
| OPLS-DA | orthogonal projections to latent structures–discriminant analysis |
| VIP | Variable importance for projection |
| OAV | Odor activity values |
References
- Fang, J.; Sureda, A.; Silva, A.S.; Khan, F.; Xu, S.W.; Nabavi, S.M. Trends of tea in cardiovascular health and disease: A critical review. Trends Food Sci. Technol. 2019, 88, 385–396. [Google Scholar] [CrossRef]
- Karakas, S.M.; Yilmaz, A.; Mercantepe, T.; Topcu, A.; Pinarbas, E. Positive effects of white tea on breast cancer: N-methyl-N-nitrozourea intraductal induced breast carcinoma model. J. Funct. Foods 2024, 122. [Google Scholar]
- Berilli, P.; Fanaro, G.B.; Santos, J.P.; Reyes Reyes, F.G.; Iglesias, A.H.; Reis, M.; Cazarin, C.B.B.; Maróstica Junior, M.R. White tea modulates antioxidant defense of endurance-trained rats. Curr. Res. Physiol. 2022, 5, 256–264. [Google Scholar] [CrossRef]
- Ma, B.; Wang, J.; Zhou, B.; Wang, Z.; Huang, Y.; Ma, C.; Li, X. Impact of harvest season on bioactive compounds, amino acids and in vitro antioxidant capacity of white tea through multivariate statistical analysis. LWT-Food Sci. Technol. 2022, 164, 113655. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Zang, E.; Shi, R.; Liu, Q.; Zhang, M.; Zhang, K.; Li, M. Review on herbal tea as a functional food: Classification, active compounds, biological activity, and industrial status. J. Future Foods 2023, 3, 206–219. [Google Scholar] [CrossRef]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Lin, Y.P.; Huang, Y.B.; Zhou, S.; Li, X.L.; Tao, Y.K.; Pan, Y.N.; Feng, X.Y.; Guo, H.W.; Chen, P.; Chu, Q. A newly-discovered tea population variety processed Bai Mu Dan white tea: Flavor characteristics and chemical basis. Food Chem. 2024, 446, 138851. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.X.; Zhu, Y.L.; Lin, Z.Y.; Chen, D.; Zhang, Y.; Lv, H.P.; Dai, W.D.; Dai, D.J.; Lin, Z.; et al. Comprehensive comparison of aroma profiles and chiral free and glycosidically bound volatiles in Fujian and Yunnan white teas. Food Chem. 2024, 448, 139067. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhang, X.M.; Jiang, R.G.; Ouyang, J.; Liu, Q.; Li, J.; Wen, H.T.; Li, Q.; Chen, J.H.; Xiong, L.G.; et al. Characterization of aroma differences on three drying treatments in Rucheng Baimao (Camellia pubescens) white tea. LWT-Food Sci. Technol. 2023, 179, 114659. [Google Scholar] [CrossRef]
- Chen, Q.C.; Zhu, Y.; Dai, W.D.; Lv, H.P.; Mu, B.; Li, P.L.; Tan, J.F.; Ni, D.J.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef]
- Mu, B.; Zhu, Y.; Lv, H.P.; Yan, H.; Peng, Q.H.; Lin, Z. The enantiomeric distributions of volatile constituents in different tea cultivars. Food Chem. 2018, 265, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.C.V.; Ye, L.; Baek, N.; Teixeira, G.H.A.; O’Keefe, S.F. Vine tea (Ampelopsis grossedentata): A review of chemical composition, functional properties, and potential food applications. J. Funct. Foods 2021, 76, 104317. [Google Scholar] [CrossRef]
- Xu, P.F.; Yu, J.B.; Ma, R.H.; Ji, Y.Y.; Hu, Q.; Mao, Y.H.; Ding, C.Q.; Li, Z.Z.; Ge, S.B.; Deng, W.W.; et al. Chlorophyll and Carotenoid Metabolism Varies with Growth Temperatures among Tea Genotypes with Different Leaf Colors in (Camellia sinensis). Int. J. Mol. Sci. 2024, 25, 10772. [Google Scholar] [CrossRef]
- Mi, S.S.; Han, S.J.; Wang, M.X.; Han, B.Y. Comprehensive analysis of the effect of etiolated tea cultivars and harvest seasons on volatile compounds and in vitro antioxidant capacity in steamed green teas. Food Chem.-X 2024, 22, 101279. [Google Scholar] [CrossRef]
- Ma, W.; Guo, A.Q.; Zhang, Y.L.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Guo, X.Y.; Ho, C.T.; Schwab, W.; Wan, X.C. Aroma profiles of green tea made with fresh tea leaves plucked in summer. Food Chem. 2021, 363, 130328. [Google Scholar] [CrossRef]
- Liu, C.S.; Li, J.L.; Li, H.X.; Xue, J.H.; Wang, M.; Jian, G.T.; Zhu, C.; Zeng, L.T. Differences in the quality of black tea (Camellia sinensis var. Yinghong No. 9) in different seasons and the underlying factors. Food Chem.-X 2023, 20, 100998. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Feng, Y.N.; Jiang, Y.W.; Yuan, H.B.; Shan, X.J.; Zhang, Q.T.; Niu, L.C.; Wang, S.N.; Zhou, Q.H.; et al. Seasonal variation in non-volatile flavor substances of fresh tea leaves (Camellia sinensis) by integrated lipidomics and metabolomics using UHPLC-Q-Exactive mass spectrometry. Food Chem. 2025, 462, 140986. [Google Scholar] [CrossRef]
- Zhou, J.L.; Gao, S.L.; Du, Z.H.; Jin, S.; Yang, Z.B.; Xu, T.D.; Zheng, C.; Liu, Y. Seasonal variations and sensory profiles of oolong tea: Insights from metabolic analysis of Tieguanyin cultivar. Food Chem. 2025, 462, 140977. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.Z.; Xu, K.; Tian, C.Y.; Zhang, M.C.; Lu, L.; Zhu, C.; Lai, Z.X.; Guo, Y.Q. A Comprehensive Investigation of Macro-Composition and Volatile Compounds in Spring-Picked and Autumn-Picked White Tea. Foods 2022, 11, 3628. [Google Scholar] [CrossRef]
- Yin, Y. (Ed.) Tea Standards and Regulations; China Standards Press: Beijing, China, 2020. [Google Scholar]
- Xu, H.; Pan, S.; Wang, J.; Ye, T.; Yan, M.; Liang, X.; Qian, G.; Yan, T.; Xin, G. Comparative characterization of volatile organic compounds and aroma profiles in 10 Actinidia arguta cultivars by gas chromatography-mass spectrometry (GC–MS), sensory analysis, and odor activity value (OAV) combined with chemometrics. J. Food Compost. Anal. 2024, 133, 106450. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, L.; Zhou, S.; Hong, Y.; Zhang, X.; Chen, H. Analysis of differences in the accumulation of tea compounds under various processing techniques, geographical origins, and harvesting seasons. Food Chem. 2024, 430, 137000. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Xie, D.; Tan, J.; et al. Nontargeted Analysis Using Ultraperformance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry Uncovers the Effects of Harvest Season on the Metabolites and Taste Quality of Tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Metabolomics analysis of Camellia sinensis with respect to harvesting time. Food Res. Int. 2020, 128, 108814. [Google Scholar] [CrossRef]
- Yao, L.; Caffin, N.; D’Arcy, B.; Jiang, Y.; Shi, J.; Singanusong, R.; Liu, X.; Datta, N.; Kakuda, Y.; Xu, Y. Seasonal Variations of Phenolic Compounds in Australia-Grown Tea (Camellia sinensis). J. Agric. Food Chem. 2005, 53, 6477–6483. [Google Scholar] [CrossRef]
- Wang HsuehFang, W.H.; Tsai YungSheng, T.Y.; Lin MuLien, L.M.; ShauMei, O. Effects of different variety, production area and season on the major chemical constituents in Taiwan GABA tea. 2006, 44, 90–99.
- He, Y.; Li, J.; Mei, H.; Zhuang, J.; Zhao, Z.; Jeyaraj, A.; Wang, Y.; Chen, X.; Li, X.; Liu, Z. Effects of leaf-spreading on the volatile aroma components of green tea under red light of different intensities. Food Res. Int. 2023, 168, 112759. [Google Scholar] [CrossRef]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Yang, P.; Liu, Z.h.; Huang, J.a.; Xiong, L.; Li, J. Relationship between theanine, catechins and related genes reveals accumulation mechanism during spring and summer in tea plant (Camellia sinensis L.). Sci. Hortic. 2022, 302, 111142. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, X.; Zhu, Q.; Yuan, Y.; Chen, R.; Wang, W.; Liu, A.; Wu, C.; Ma, C.; Li, J.; et al. Metabonomics analysis of the flavor characteristics of Wuyi Rock Tea (Rougui) with “rock flavor” and microbial contributions to the flavor. Food Chem. 2024, 450, 139376. [Google Scholar] [CrossRef]
- Shen, S.; Huang, J.; Li, T.; Wei, Y.; Xu, S.; Wang, Y.; Ning, J. Untargeted and targeted metabolomics reveals potential marker compounds of an tea during storage. LWT 2022, 154, 112791. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Li, D.; Song, C. Dynamic change in amino acids, catechins, alkaloids, and gallic acid in six types of tea processed from the same batch of fresh tea (Camellia sinensis L.) leaves. J. Food Compost. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Bengtsson, M.; Bäckman, A.-C.; Liblikas, I.; Ramirez, M.I.; Borg-Karlson, A.-K.; Ansebo, L.; Anderson, P.; Löfqvist, J.; Witzgall, P. Plant Odor Analysis of Apple: Antennal Response of Codling Moth Females to Apple Volatiles during Phenological Development. J. Agric. Food Chem. 2001, 49, 3736–3741. [Google Scholar] [CrossRef] [PubMed]
- Zawirska-Wojtasiak, R. Chirality and the Nature of Food Authenticity of Aroma. Acta Sci. Pol. Technol. Aliment. 2006, 5, 21–36. [Google Scholar]
- Yu, P.; Huang, Y.; Li, Z.; Zhao, X.; Huang, H.; Zhong, N.; Zheng, H.; Chen, Q. Difference in Aroma Components of Black Teas Processed on Different Dates in the Spring Season. Foods 2023, 12, 4368. [Google Scholar] [CrossRef]
- Peng, Y.; Li, G.; Liu, X.; Lv, C.; Xiong, C.Y. SPME/GC-MS analysis of aroma quality of black tea from different producing areas. Sci. Technol. Food Ind. 2021, 42, 237–244. [Google Scholar]
- Lehner, T.B.; Siegmund, B. The impact of ventilation during postharvest ripening on the development of flavour compounds and sensory quality of mangoes (Mangifera indica L.) cv. Kent. Food Chem. 2020, 320, 126608. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Zhang, L.; Huang, W.; Lin, F.; Xiao, C.; Zheng, Z.; Huang, Y.; Sun, W. Underlying characteristic aroma of white tea from diverse geographical origins and its prediction. J. Sci. Food Agric. 2025, 105, 4558–4568. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Feng, W.; Shen, S.; Wei, Y.; Jia, H.; Wang, Y.; Deng, W.; Ning, J. Effects of three different withering treatments on the aroma of white tea. Foods 2022, 11, 2502. [Google Scholar] [CrossRef]
- Yin, P.; Kong, Y.-S.; Liu, P.-P.; Wang, J.-J.; Zhu, Y.; Wang, G.-M.; Sun, M.-F.; Chen, Y.; Guo, G.-Y.; Liu, Z.-H. A critical review of key odorants in green tea: Identification and biochemical formation pathway. Trends Food Sci. Technol. 2022, 129, 221–232. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.-T.; Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef]
- Ni, H.; Jiang, Q.; Lin, Q.; Ma, Q.; Wang, L.; Weng, S.; Huang, G.; Li, L.; Chen, F. Enzymatic hydrolysis and auto-isomerization during β-glucosidase treatment improve the aroma of instant white tea infusion. Food Chem. 2021, 342, 128565. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Tian, C.; Zhou, C.; Zhu, C.; Weng, J.; Sun, Y.; Lin, Y.; Lai, Z.; Guo, Y. Non-Targeted Metabolomics Analysis Revealed the Characteristic Non-Volatile and Volatile Metabolites in the Rougui Wuyi Rock Tea (Camellia sinensis) from Different Culturing Regions. Foods 2022, 11, 1694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Dai, H.; Wu, S.; Song, B.; Lin, F.; Huang, Y.; Lin, X.; Sun, W. Identification of characteristic aroma and bacteria related to aroma evolution during long-term storage of compressed white tea. Front. Nutr. 2022, 9, 1092048. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sheng, C.; Lu, M.; Ke, H.; Li, T.; Wei, Y.; Shen, S.; Yin, X.; Lu, C.; Wang, Y.; et al. Identification of the causes of aroma differences in white tea under different withering methods by targeted metabolomics. Food Biosci. 2024, 59, 104020. [Google Scholar] [CrossRef]
- Shi, J.; Wu, W.; Zhang, Y.; Baldermann, S.; Peng, Q.; Wang, J.; Xu, L.; Yang, G.; Fu, J.; Lv, H.; et al. Comprehensive analysis of carotenoids constituents in purple-coloured leaves and carotenoid-derived aroma differences after processing into green, black, and white tea. LWT 2023, 173, 114286. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wu, Y.; Qu, F.; Liu, L.; Wang, B.; Wang, P.; Zhang, X. HS−SPME/GC−MS Reveals the Season Effects on Volatile Compounds of Green Tea in High−Latitude Region. Foods 2022, 11, 3016. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Z.; Zhang, L.; Dai, H.; Wu, W.; Zheng, Z.; Lin, F.; Xu, J.; Huang, Y.; Sun, W. Characterization of volatile compounds and identification of key aroma compounds in different aroma types of Rougui Wuyi rock tea. Food Chem. 2024, 455, 139931. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Wen, S.; Chen, R.; Sun, S.; Lai, X.; Li, Q.; Zhang, Z.; Lai, Z.; Li, Z.; et al. Analysis of aroma quality changes of large-leaf black tea in different storage years based on HS-SPME and GC–MS. Food Chem. X 2023, 20, 100991. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Rothenberg, D.O.N.; Zhang, K.; Lin, Y.; Jin, K.; Li, J.; Zhou, H.; Su, H.; Ji, R.; et al. Multiomics correlation analysis on the mechanism of flavor substance formation during the processing of “Huanong 53″ black tea. LWT 2024, 214, 117086. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wu, J.; Wen, J.; Yu, Y.; An, K.; Zou, B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021, 150, 110784. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, P.; Li, Y.; Wang, X.; Liu, H.; Wang, S.; Han, W.; Wu, R.; Li, X.; Guan, Y.; et al. New Insights into Stress-Induced β-Ocimene Biosynthesis in Tea (Camellia sinensis) Leaves during Oolong Tea Processing. J. Agric. Food Chem. 2021, 69, 11656–11664. [Google Scholar] [CrossRef]
- Yu, J.; Ho, C.-T.; Lin, Z.; Zhu, Y.; Feng, Z.; Ni, D.; Zeng, S.; Zeng, X.; Wang, Y.; Ning, J.; et al. Sensomics-Assisted Characterization of Key Flowery Aroma Compounds in Lu’an Guapian Green Tea Infusion (Camellia sinensis). J. Agric. Food Chem. 2023, 71, 6120–6132. [Google Scholar] [CrossRef]
- Flaig, M.; Qi, S.; Wei, G.; Yang, X.; Schieberle, P. Characterization of the Key Odorants in a High-Grade Chinese Green Tea Beverage (Camellia sinensis; Jingshan cha) by Means of the Sensomics Approach and Elucidation of Odorant Changes in Tea Leaves Caused by the Tea Manufacturing Process. J. Agric. Food Chem. 2020, 68, 5168–5179. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhang, X.; Chen, H.; Ruan, D.; Bai, S.; Huang, J.; Liu, Z. Characterization of Volatile Substances in Pu-erh Tea (Raw Tea) at Different Storage Times. Foods 2025, 14, 840. [Google Scholar] [CrossRef]
- Salum, P.; Guclu, G.; Selli, S. Comparative Evaluation of Key Aroma-Active Compounds in Raw and Cooked Red Mullet (Mullus barbatus) by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2017, 65, 8402–8408. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Let, M.B.; Meyer, A.S.; Jacobsen, C. Modeling the Sensory Impact of Defined Combinations of Volatile Lipid Oxidation Products on Fishy and Metallic Off-Flavors. J. Agric. Food Chem. 2004, 52, 1635–1641. [Google Scholar] [CrossRef]
- Cho, S.; Nuijten, E.; Shewfelt, R.L.; Kays, S.J. Aroma chemistry of African Oryza glaberrima and Oryza sativa rice and their interspecific hybrids. J. Sci. Food Agric. 2014, 94, 727–735. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of Aroma-Active Volatiles in Oolong Tea Infusions Using GC–Olfactometry, GC–FPD, and GC–MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Leffingwell, D. GRAS flavor chemicals-detection thresholds. Perfum. Flavorist 1991, 16, 1–19. [Google Scholar]
- Van Gemert, L. Compilations of Odour Threshold Values in Air, Water and Other Media; Boelens Aroma Chemical Information Service: Huissen, The Netherlands, 2003. [Google Scholar]
- Cui, J.; Zhai, X.; Guo, D.; Du, W.; Gao, T.; Zhou, J.; Schwab, W.G.; Song, C. Characterization of key odorants in Xinyang Maojian green tea and their changes during the manufacturing process. J. Agric. Food Chem. 2021, 70, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Torchio, F.; Segade, S.R.; Gerbi, V.; Cagnasso, E.; Giordano, M.; Giacosa, S.; Rolle, L. Changes in varietal volatile composition during shelf-life of two types of aromatic red sweet Brachetto sparkling wines. Food Res. Int. 2012, 48, 491–498. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Bennett, J.L.; Raymer, J.H.J.A.; Meteorology, F. Quantification of odors and odorants from swine operations in North Carolina. Agric. For. Meteorol. 2001, 108, 213–240. [Google Scholar] [CrossRef]
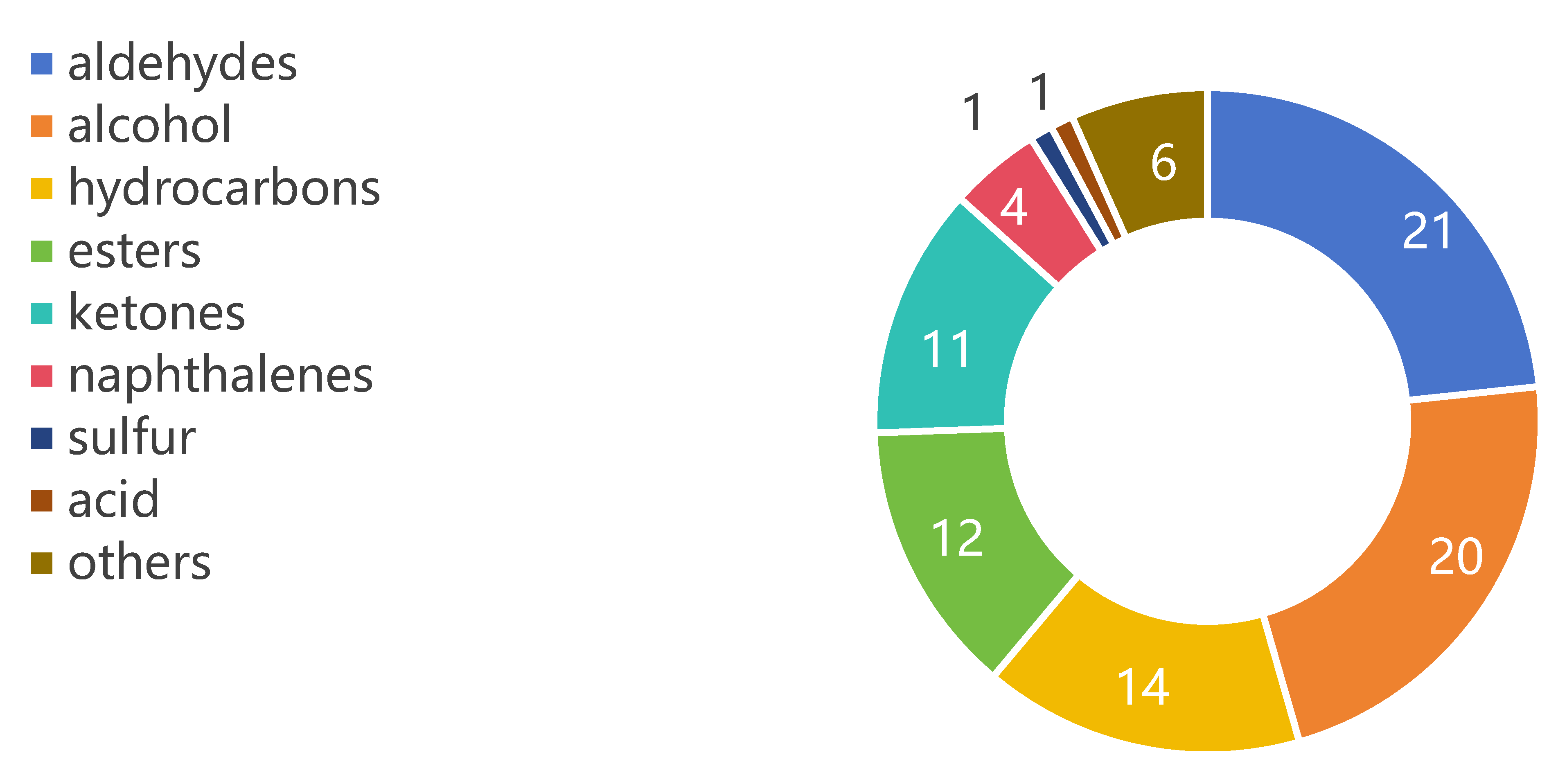
| Tea Cultivars | Tea Cultivars | Appearance | Aroma | Taste | |||
|---|---|---|---|---|---|---|---|
| Comment | Score | Comment | Score | Comment | Score | ||
| SH-S |  | more thickset buds, fat and bold, more tippy | 98 ± 1.07 a | more pekoe aroma, floral, a little fresh and woody | 92.94 ± 1.32 a | fresh and brisk taste, sweet, mellow | 92.06 ± 1.05 b |
| FD-S |  | thickset buds, fat and bold, more tippy | 94.87 ± 1.03 b | more pekoe aroma, lasting floral, a little fresh and woody | 94.81 ± 1.16 a | more fresh and brisk taste, sweeter, more mellow | 94.94 ± 0.90 a |
| SH-A |  | thin buds; fewer trichomes | 92.94 ± 1.02 c | a little pekoe, floral, and fruity | 88.00 ± 1.53 b | astringency, more bitterness | 84.88 ± 1.03 c |
| FD-A |  | thin buds; fewer trichomes | 89.63 ± 1.53 d | a little pekoe, floral, and fruity | 89.00 ± 1.44 b | a little bitterness | 86 ± 1.44 c |
| Biochemical Components (mg/g) | SH-S | FD-S | SH-A | FD-A |
|---|---|---|---|---|
| EC | 0.26 ± 0.03 c | 1.66 ± 0.02 b | 1.61 ± 0.2 b | 2.85 ± 0.05 a |
| ECG | 0.66 ± 0.01 b | 0.3 ± 0.01 b | 24.37 ± 1.58 a | 20.44 ± 15.17 a |
| EGC | 3.76 ± 0.03 a | 3.9 ± 0.09 a | 1.67 ± 0.35 c | 2.47 ± 0.25 b |
| EGCG | 68.48 ± 0.49 b | 80.36 ± 2.66 a | 67.07 ± 2.1 b | 68.73 ± 0.64 b |
| C | 15.58 ± 0.37 a | 14.47 ± 0.86 a | 18.63 ± 0.6 a | 17.73 ± 0.06 a |
| Caffeine | 35.52 ± 0.5 c | 34.26 ± 0.35 c | 47.87 ± 1.6 a | 45.33 ± 0.58 b |
| Free amino acid | 56.23 ± 0.4 b | 62.37 ± 2.05 a | 47 ± 1.0 d | 41.67 ± 1.15 c |
| Tea polyphenol | 159.34 ± 4.34 b | 149.59 ± 5.53 c | 204.33 ± 3.06 a | 195.33 ± 1.53 a |
| Soluble sugar | 31.86 ± 1.89 a | 33.86 ± 1.19 a | 22.85 ± 1.73 c | 27.43 ± 1.71 b |
| Water extracts content | 475.60 ± 1.44 a | 476.47 ± 2.37 a | 470.33 ± 3.51 b | 471.67 ± 1.53 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Wu, H.; Luo, F.; Wang, Y.; Ye, Y.; Gong, Y.; Ye, X. Effect of Harvest Seasons on Biochemical Components and Volatile Compounds in White Teas from Two Cultivars. Foods 2025, 14, 1795. https://doi.org/10.3390/foods14101795
Huang F, Wu H, Luo F, Wang Y, Ye Y, Gong Y, Ye X. Effect of Harvest Seasons on Biochemical Components and Volatile Compounds in White Teas from Two Cultivars. Foods. 2025; 14(10):1795. https://doi.org/10.3390/foods14101795
Chicago/Turabian StyleHuang, Fan, Haijun Wu, Fan Luo, Yingchun Wang, Yulong Ye, Yiyun Gong, and Xianlin Ye. 2025. "Effect of Harvest Seasons on Biochemical Components and Volatile Compounds in White Teas from Two Cultivars" Foods 14, no. 10: 1795. https://doi.org/10.3390/foods14101795
APA StyleHuang, F., Wu, H., Luo, F., Wang, Y., Ye, Y., Gong, Y., & Ye, X. (2025). Effect of Harvest Seasons on Biochemical Components and Volatile Compounds in White Teas from Two Cultivars. Foods, 14(10), 1795. https://doi.org/10.3390/foods14101795




