Utilization of Native CRISPR-Cas9 System for Expression of Glucagon-like Peptide-1 in Lacticaseibacillus paracasei
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmid, and Culture Conditions
2.2. Analyses of the CRISPR-Cas System
2.3. Construction of CRISPR-Cas9 Editing Plasmids
2.4. Preparation of L. paracasei Competent Cells
2.5. Electrotransformation of L. paracasei
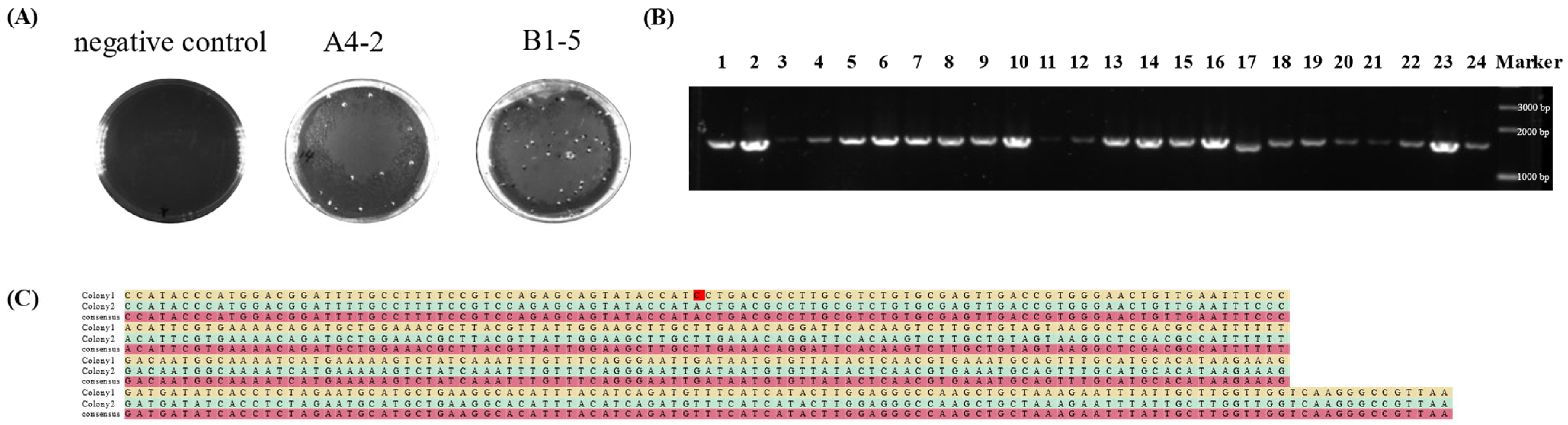
2.6. Colony PCR Verification
2.7. Plasmid Curing
2.8. Stability Testing of Gene-Edited Strains
2.9. RT-qPCR
2.10. NanoLC-MS
2.11. Statistical Analyses
3. Results
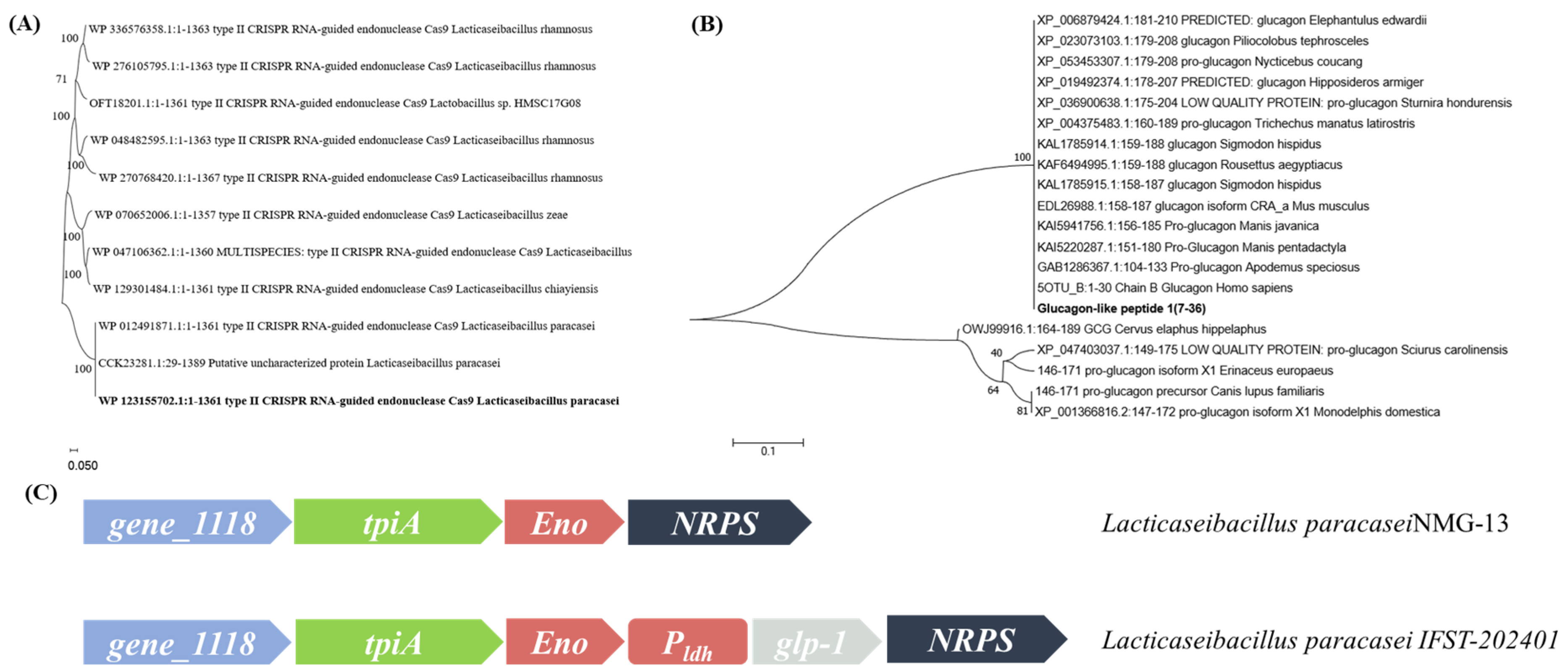
3.1. Determination of Elements of the Gene-Editing Plasmid
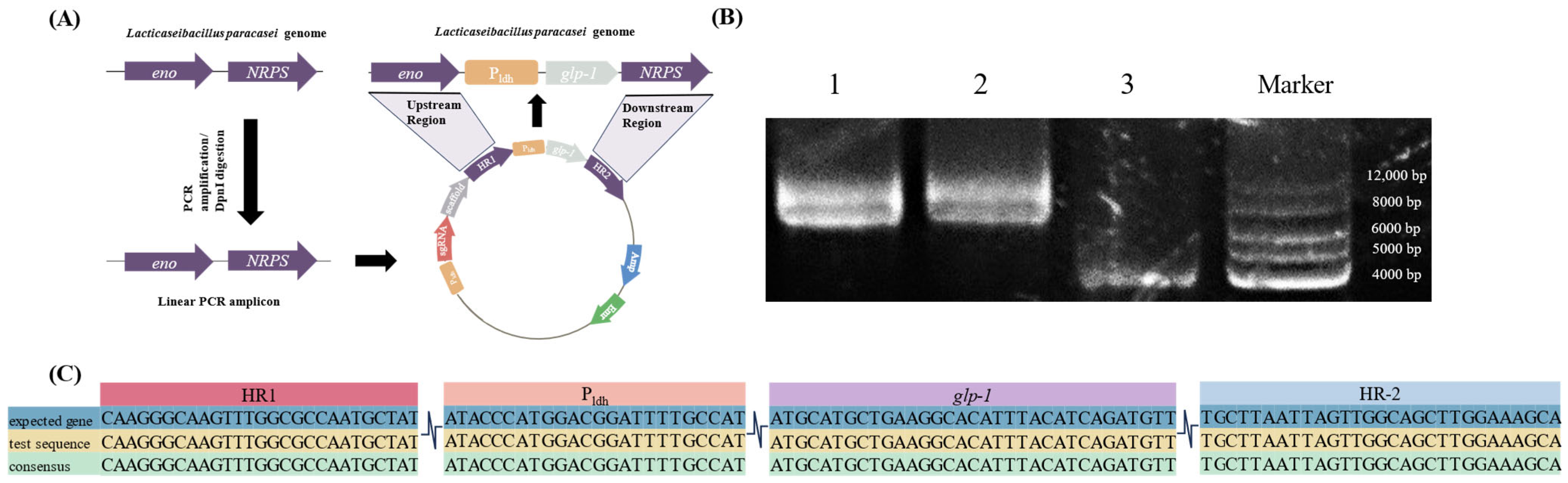
3.2. Construction of Gene-Editing Plasmids
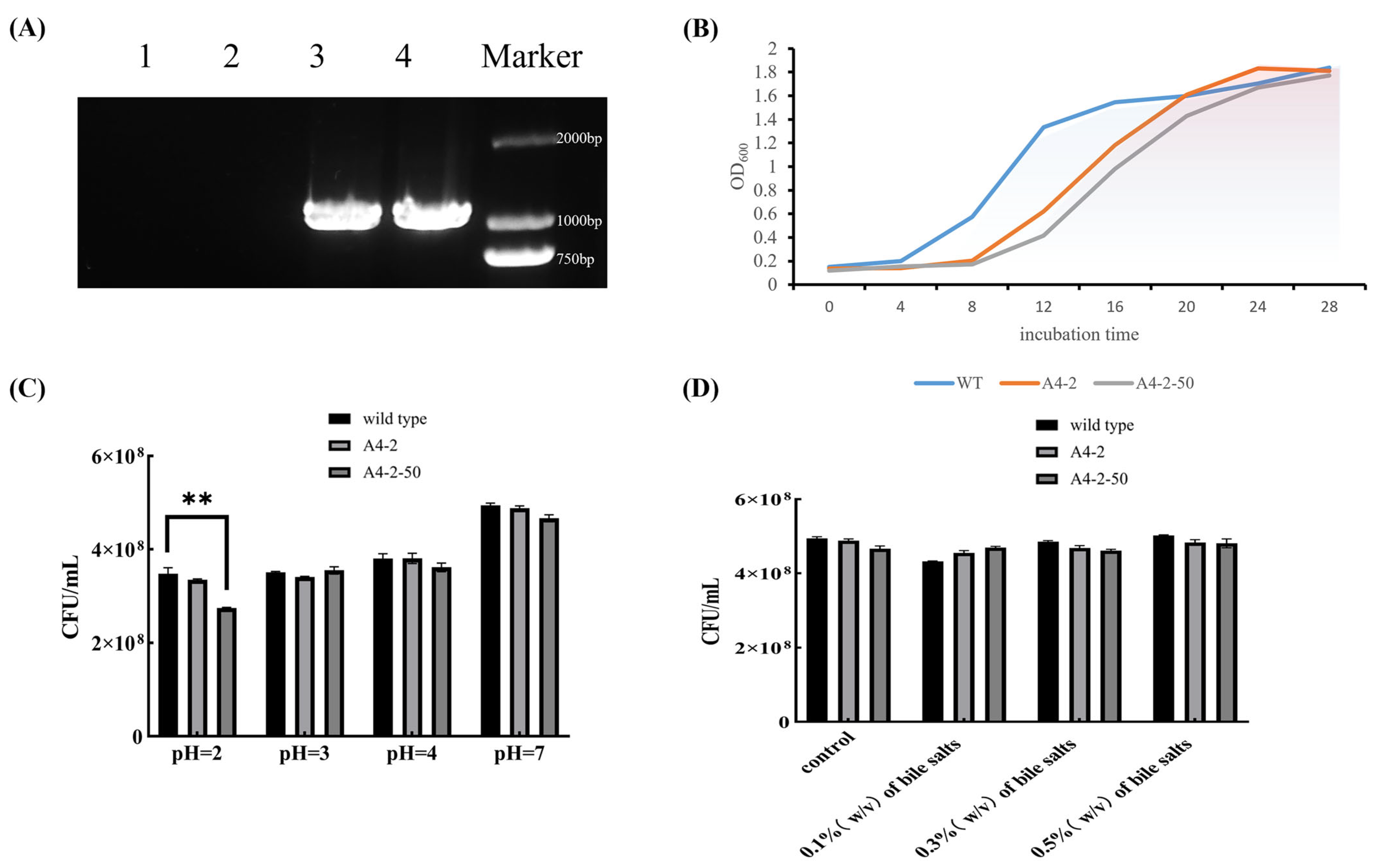
3.3. glp-1 Insertion into L. paracasei NMG-13
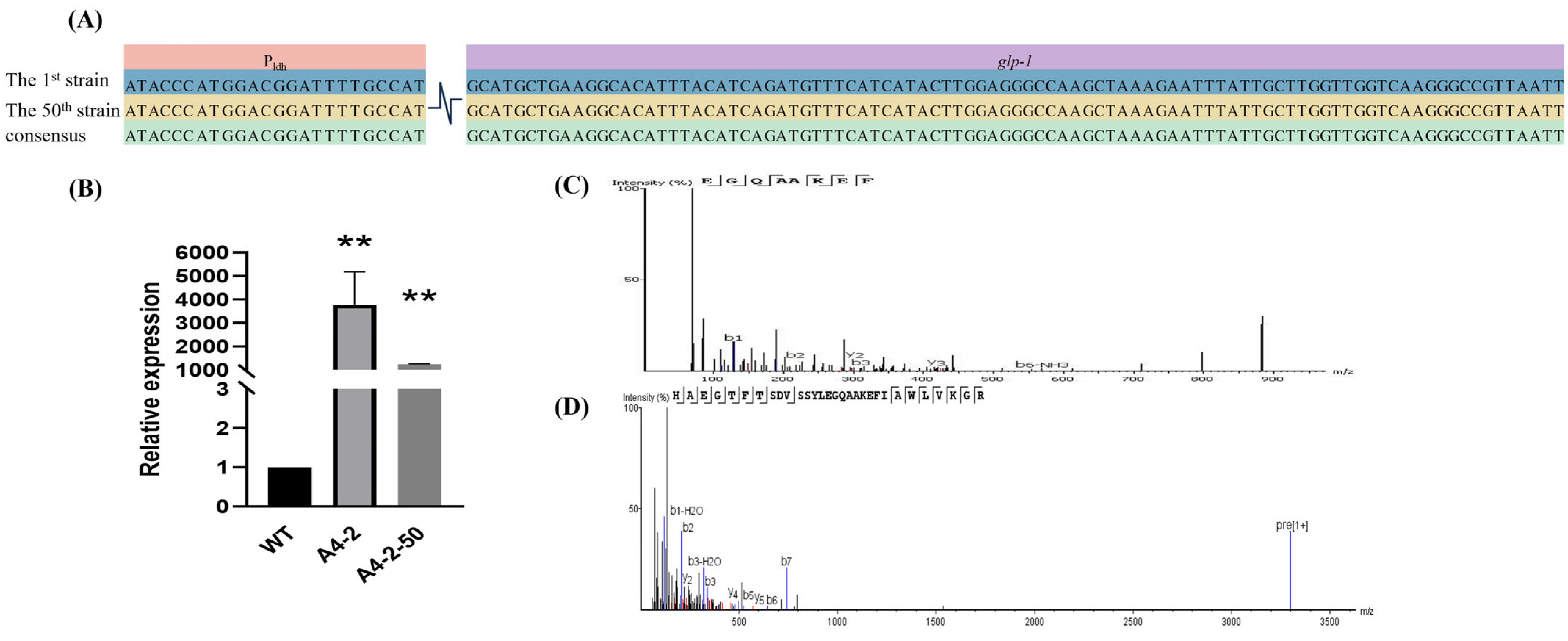
3.4. The Expression of GLP-1 in L. paracasei
3.5. Removal of Gene-Editing Plasmids
3.6. Basic Physiological Properties of Colonies
3.7. Stable Expression of GLP-1 After Successive Passage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwandt, P. On the Occasion of the World Diabetes Day: Diabetes Mellitus—A Globally Increasing Health Problem. Int. J. Prev. Med. 2012, 3, 747–748. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Defronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.I.; Sanchez-Pescador, R.; Laybourn, P.J.; Najarian, R.C. Exon duplication and divergence in the human preproglucagon gene. Nature 1983, 304, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S98–S110. [Google Scholar] [CrossRef]
- Zaazouee, M.S.; Hamdallah, A.; Helmy, S.K.; Hasabo, E.A.; Sayed, A.K.; Gbreel, M.I.; Elmegeed, A.A.; Aladwan, H.; Elshanbary, A.A.; Abdel-Aziz, W.; et al. Semaglutide for the treatment of type 2 Diabetes Mellitus: A systematic review and network meta-analysis of safety and efficacy outcomes. Diabetes Metab. Syndr. 2022, 16, 102511. [Google Scholar] [CrossRef]
- Min, T.; Bain, S.C. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2021, 12, 143–157. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020, 19, 277–289. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Steidler, L.; Rottiers, P. Therapeutic Drug Delivery by Genetically Modified Lactococcus lactis. Ann. N. Y. Acad. Sci. 2006, 1072, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Mobergslien, A.; Vasovic, V.; Mathiesen, G.; Fredriksen, L.; Westby, P.; Eijsink, V.G.; Peng, Q.; Sioud, M. Recombinant Lactobacillus plantarum induces immune responses to cancer testis antigen NY-ESO-1 and maturation of dendritic cells. Hum. Vaccin. Immunother. 2015, 11, 2664–2673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, W.K.; Gallo, R.L.; Fang, E.F.; Hu, W.; Ling, T.K.; Shen, J.; Chan, R.L.; Lu, L.; Luo, X.M.; et al. Critical Role of Antimicrobial Peptide Cathelicidin for Controlling Helicobacter pylori Survival and Infection. J. Immunol. 2016, 196, 1799–1809. [Google Scholar] [CrossRef]
- Takiishi, T.; Cook, D.P.; Korf, H.; Sebastiani, G.; Mancarella, F.; Cunha, J.P.; Wasserfall, C.; Casares, N.; Lasarte, J.J.; Steidler, L.; et al. Reversal of Diabetes in NOD Mice by Clinical-Grade Proinsulin and IL-10-Secreting Lactococcus lactis in Combination with Low-Dose Anti-CD3 Depends on the Induction of Foxp3-Positive T Cells. Diabetes 2017, 66, 448–459. [Google Scholar] [CrossRef]
- Chen, C.; Ai, L.; Zhou, F.; Wang, L.; Zhang, H.; Chen, W.; Guo, B. Complete genome sequence of the probiotic bacterium Lactobacillus casei LC2W. J. Bacteriol. 2011, 193, 3419–3420. [Google Scholar] [CrossRef]
- McLaughlin, C.B. Readily Prepared Medium for Cultivation of Lactobacilli. J. Bacteriol. 1946, 51, 560–561. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Biswas, A.; Gagnon, J.N.; Brouns, S.J.J.; Fineran, P.C.; Brown, C.M. CRISPRTarget. RNA Biol. 2013, 10, 817–827. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Brubaker, P.L.; Drucker, D.J. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 2004, 145, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Crawley, A.B.; Henriksen, E.D.; Stout, E.; Brandt, K.; Barrangou, R. Characterizing the activity of abundant, diverse and active CRISPR-Cas systems in Lactobacilli. Sci. Rep. 2018, 8, 11544. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Villa-Rodríguez, E.; Ibarra-Gámez, C.; de Los Santos-Villalobos, S. Extraction of high-quality RNA from Bacillus subtilis with a lysozyme pre-treatment followed by the Trizol method. J. Microbiol. Methods 2018, 147, 14–16. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Ujiroghene, O.J.; Yang, Y.; Lu, J.; Zhang, S.; Pang, X.; Lv, J. Occurrence and Diversity of CRISPR Loci in Lactobacillus casei Group. Front. Microbiol. 2020, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zhang, J.; Li, L.; Zhong, J. Repurposing the Endogenous CRISPR-Cas9 System for High-Efficiency Genome Editing in Lacticaseibacillus paracasei. ACS Synth. Biol. 2022, 11, 4031–4042. [Google Scholar] [CrossRef]
- Agarwal, P.; Khatri, P.; Billack, B.; Low, W.K.; Shao, J. Oral delivery of glucagon like peptide-1 by a recombinant Lactococcus lactis. Pharm. Res. 2014, 31, 3404–3414. [Google Scholar] [CrossRef]
- Doench, J.G.; Hartenian, E.; Graham, D.B.; Tothova, Z.; Hegde, M.; Smith, I.; Sullender, M.; Ebert, B.L.; Xavier, R.J.; Root, D.E. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 2014, 32, 1262–1267. [Google Scholar] [CrossRef]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, C.; Li, T.; Jin, F.J.; Sung, Y.J.; Oh, H.M.; Lee, H.G.; Jin, L. Development and Applications of CRISPR/Cas9-Based Genome Editing in Lactobacillus. Int. J. Mol. Sci. 2022, 23, 2852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Dai, C.L.; Evans, E.D.; Lu, Z.; Alm, E.J. Tracking strains predicts personal microbiomes and reveals recent adaptive evolution. bioRxiv 2020. [Google Scholar] [CrossRef]
- Meurman, J.H.; Stamatova, I. Probiotics: Contributions to oral health. Oral Dis. 2007, 13, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
| Title | Features | Source |
|---|---|---|
| Trelief ® 5α Chemically | E. coli clone | Tsing ke Biotechnology Co., Ltd. Beijing, China |
| L.paracaise NMG-13 | Lactobacillus paracasei, strain to be edited | Laboratory storage |
| pTRST | pTRKH2-Amp derived plasmid, backbone plasmid incorporating Pldh-sgRNA and LpCas9 Scaffold element | Laboratory storage |
| pTRST-sgRNA-A | Derived from pTRST with the addition of sgRNA | This experiment |
| pTRST-sgRNA-B | Derived from pTRST with the addition of sgRNA | This experiment |
| pTRST-sgRNA-A1 | Derived from pTRST-sgRNA-A with the addition of the Pldh promoter | This experiment |
| pTRST-sgRNA-B1 | Derived from pTRST-sgRNA-B with the addition of the Pldh promoter | This experiment |
| pTRST- GLP-A | Derived from pTRST-sgRNA-A1 with the addition of glp-1 and homology arms | This experiment |
| pTRST- GLP-B | Derived from pTRST-sgRNA-B1 with the addition of glp-1 and homology arms | This experiment |
| Primers | Sequences |
|---|---|
| SgRNA-A | CTAGAGTTGTTTCACATCGTTCCGGC |
| SgRNA-B | CTAGACCGCAAGTCCTTCTACAATGC |
| pTRST-A-F | CTAGAGTTGTTTCACATCGTTCCGGC |
| pTRST-A-R | AGACGCCGGAACGATGTGAAACAACT |
| KZ-Pldh -F | ATGTGAAAGCAATCGACTAACCATACCCATGGACGGATTTT |
| KZ-Pldh-R | AATGTGCCTTCAGCATGCATAGGTGATATCATCCTTTCTTATGTGC |
| PD-GLP-R | TTAACGGCCCTTGACCAACC |
| YZ-DPD-F1 | CAGGTAGCGAACTACACGT |
| HR1-F | GTTGGGCCATACATTTTTTTCAAGGGCAAGTTTGGCGC |
| HR1-R | CTTCAGCATGCATTTAGTCGATTGCTTTCACATTGTAGA |
| DPD-F1 | GCCCATGTTGGGCCATACATTTTTTTCAAGGGCAAGTTTGGCGC |
| DPD-R1 | CAGTGAATTCCCGGGGATCCATATACCACAGGCCACGATTGC |
| HR2-F | GTCAAGGGCCGTTAATTTGCTTAATTAGTTGGCAGCTTG |
| HR2-R | CAGTGAATTCCCGGGGATCCATATACCACAGGCCACGATTGC |
| pTRST-B-F | CTAGACCGCAAGTCCTTCTACAATGC |
| ZTKZ-F | GGATCCCCGGGAATTCACT |
| ZTKZ-R | AAAAAAATGTATGGCCCAACATG |
| YZ-QR-F | TACAAGCCAGGCGACGACAT |
| YZ-QR-R | TAGCAGCTTGGCCCTCCAAGT |
| pTRST-B-R | AGACGCATTGTAGAAGGACTTGCGGT |
| RNA-A-F | ATGCATGCTGAAGGCACATTT |
| RNA-A-R | TTAACGGCCCTTGACCAACCAA |
| PD-16S-F | CCTCCAACACCTAGCATTCAT |
| PD-16S-R | TGTAACTGACGCTGAGGCT |
| No. | PAM | Cut Position | Strand | Guide Sequence | On-Target Score | Off-Target Score |
|---|---|---|---|---|---|---|
| A | tgaaa | 1113 | + | gttgtttcacatcgttccgg | 42.6 | 100.0 |
| B | tgaaa | 1286 | + | ccgcaagtccttctacaatg | 40.3 | 100.0 |
| C | tgaaa | 1108 | − | tcagtttcaccggaacgatg | 35.6 | 99.5 |
| D | tgaaa | 1532 | + | tttagcgaggtcgcttgggt | 7.1 | 100.0 |
| E | tgaaa | 1615 | + | atactagggataagcacaaa | 16.3 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Zhang, S.; Wang, Y.; Xie, N.; Wang, X.; Lv, J.; Pang, X.; Li, X. Utilization of Native CRISPR-Cas9 System for Expression of Glucagon-like Peptide-1 in Lacticaseibacillus paracasei. Foods 2025, 14, 1785. https://doi.org/10.3390/foods14101785
Zheng M, Zhang S, Wang Y, Xie N, Wang X, Lv J, Pang X, Li X. Utilization of Native CRISPR-Cas9 System for Expression of Glucagon-like Peptide-1 in Lacticaseibacillus paracasei. Foods. 2025; 14(10):1785. https://doi.org/10.3390/foods14101785
Chicago/Turabian StyleZheng, Mumin, Shuwen Zhang, Yunna Wang, Ning Xie, Xiaodan Wang, Jiaping Lv, Xiaoyang Pang, and Xu Li. 2025. "Utilization of Native CRISPR-Cas9 System for Expression of Glucagon-like Peptide-1 in Lacticaseibacillus paracasei" Foods 14, no. 10: 1785. https://doi.org/10.3390/foods14101785
APA StyleZheng, M., Zhang, S., Wang, Y., Xie, N., Wang, X., Lv, J., Pang, X., & Li, X. (2025). Utilization of Native CRISPR-Cas9 System for Expression of Glucagon-like Peptide-1 in Lacticaseibacillus paracasei. Foods, 14(10), 1785. https://doi.org/10.3390/foods14101785








