Effect of Supplementation with Probiotics in Patients with Schizophrenia: Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials
Abstract
1. Introduction
2. Experimental Section
2.1. Protocol and Registration
2.2. Search Strategy and Selection Criteria
2.3. Study Selection and Quality Evaluation
2.3.1. Data Collection Process
2.3.2. Quality Evaluation
2.3.3. Statistical Analysis
3. Results
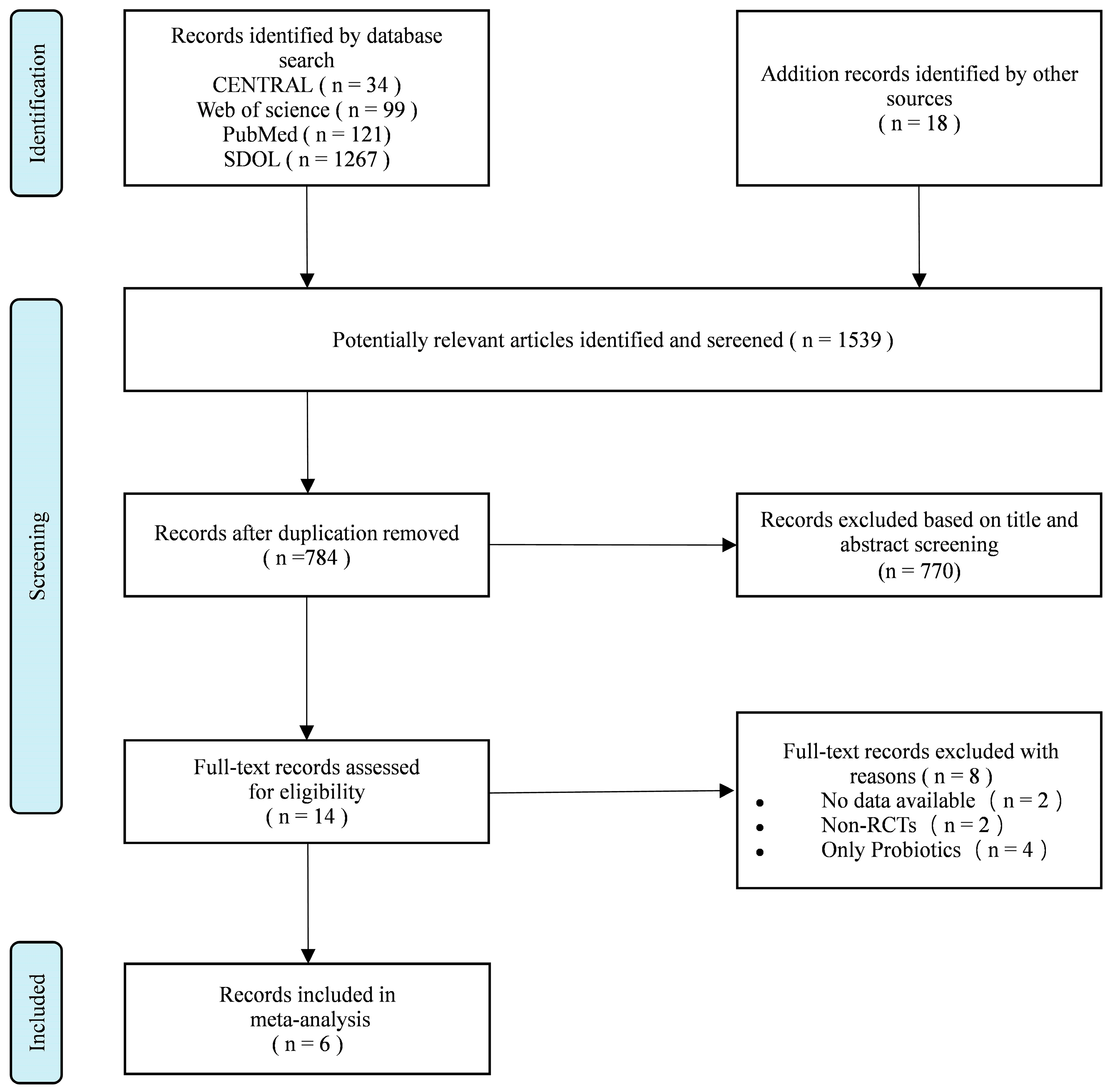
3.1. Study Selection
3.2. Study Characteristics
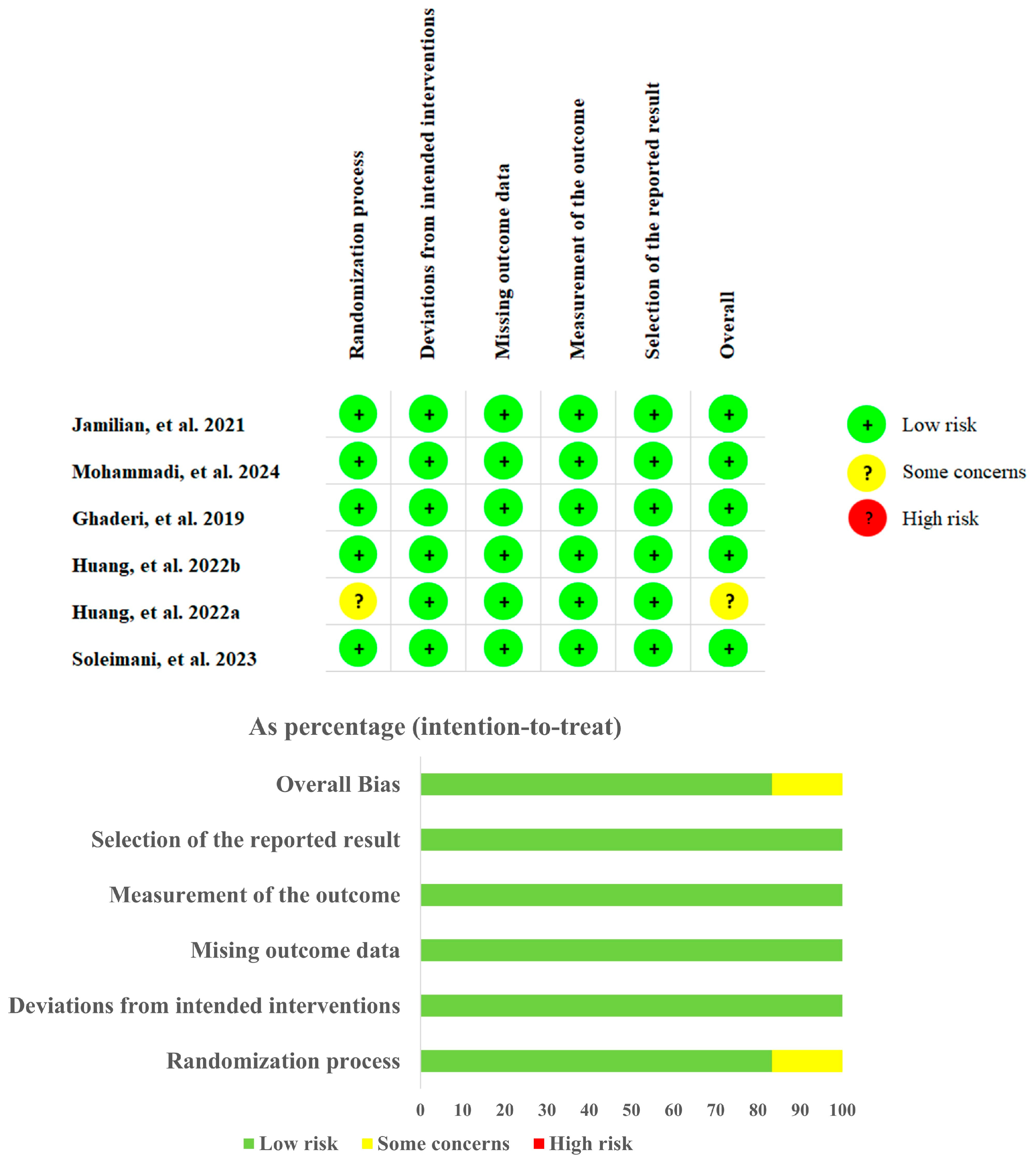
3.3. Quality Evaluation
3.4. Outcomes
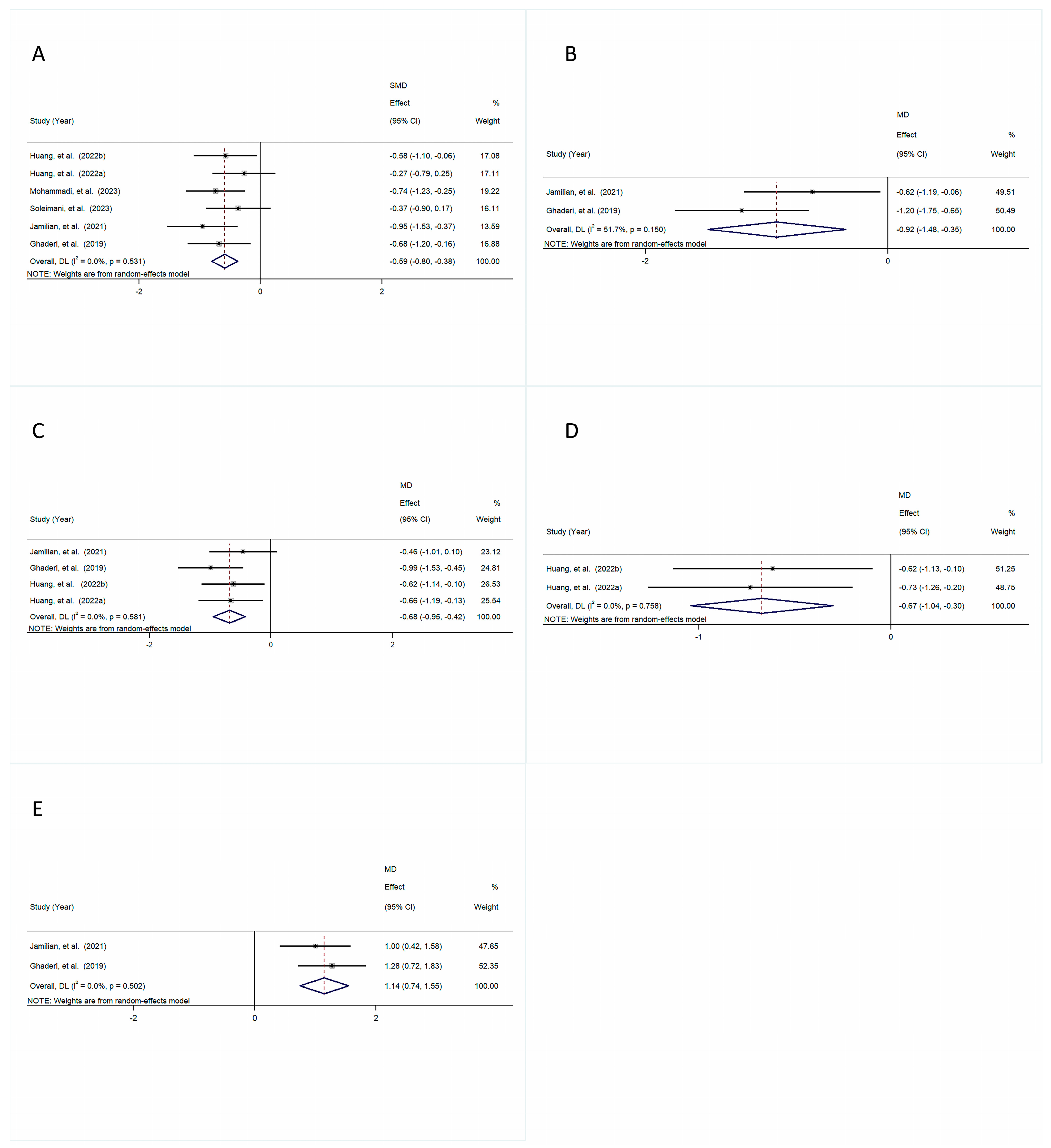
3.4.1. Psychiatric Symptoms
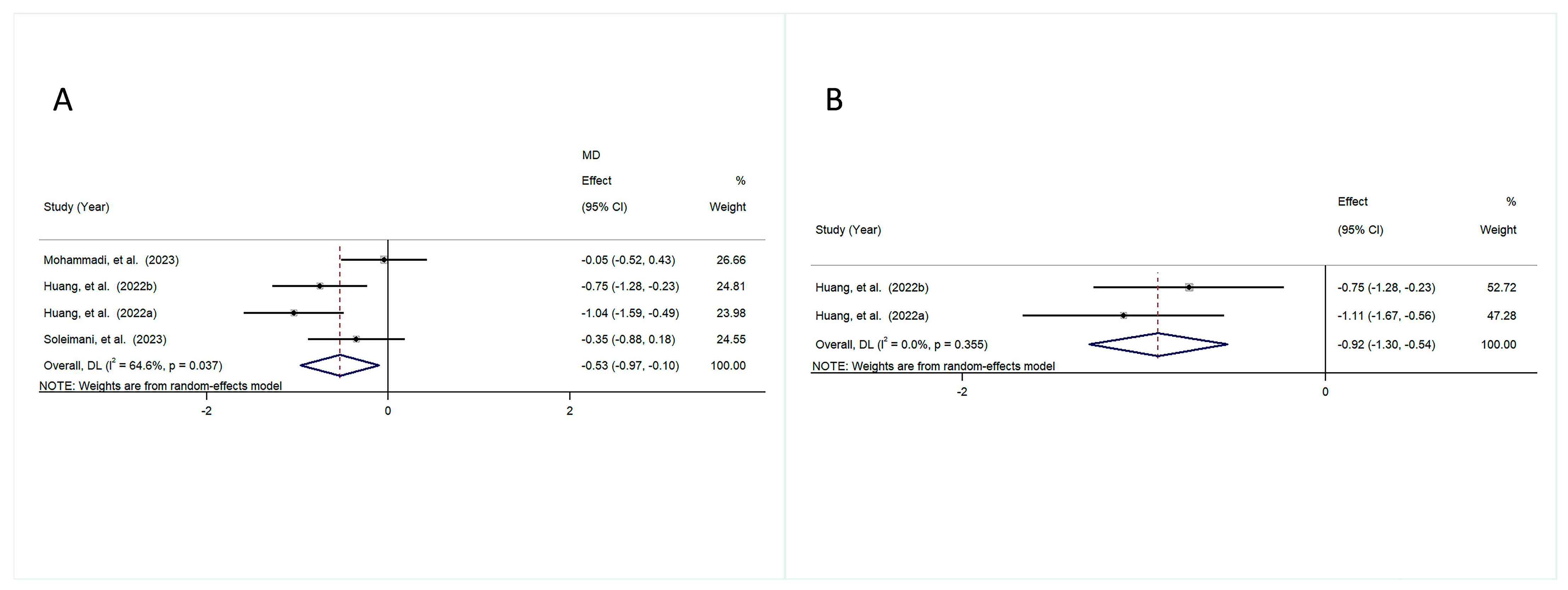
3.4.2. Anthropometric Indicators
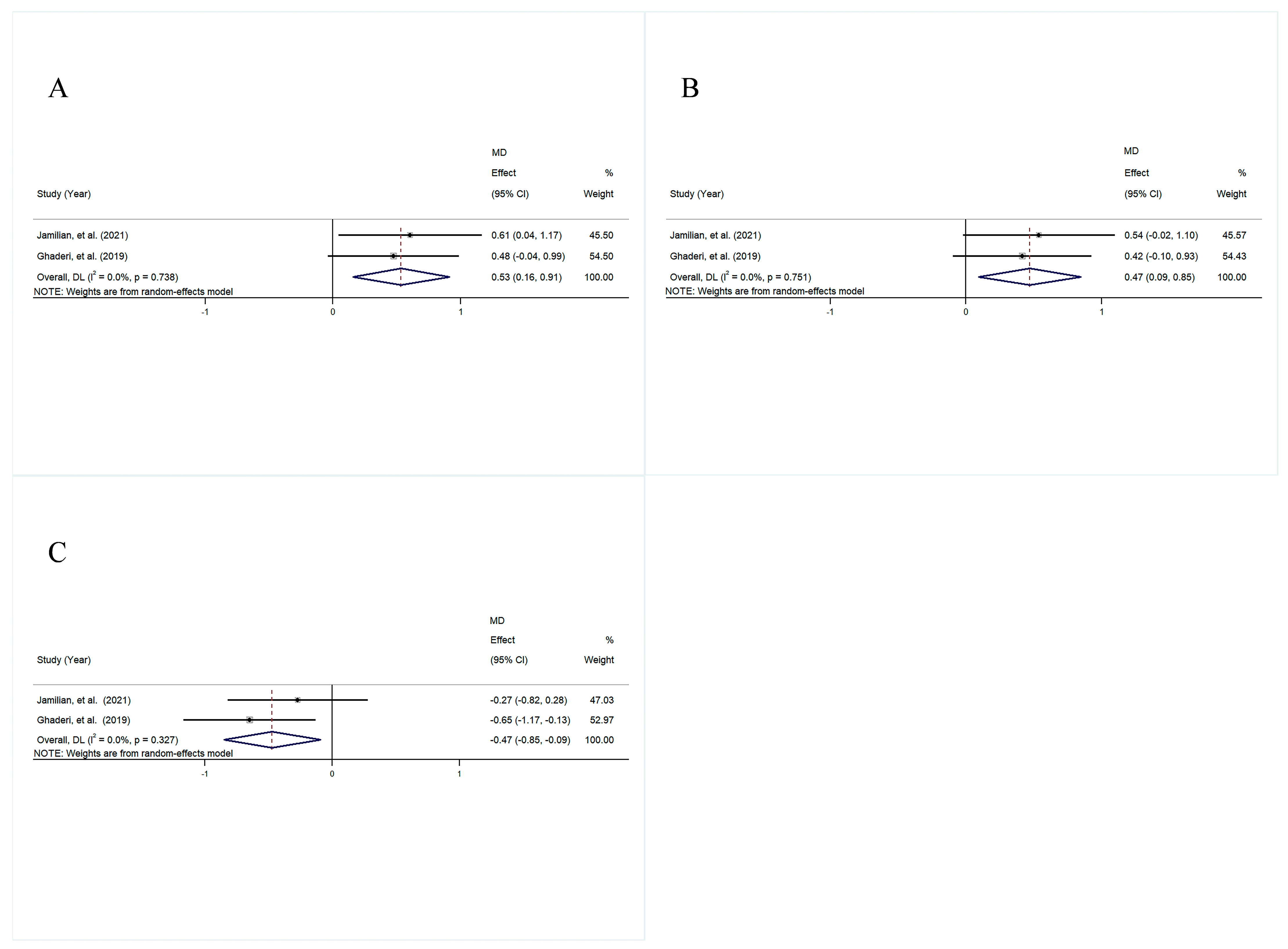
3.4.3. Lipid Profile
3.4.4. Glycemic Indices
3.4.5. Inflammation
3.4.6. Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M.; et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, M.H.; Avramopoulos, D. Gene-Environment Interactions in Schizophrenia: A Literature Review. Genes 2021, 12, 1850. [Google Scholar] [CrossRef]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef]
- Ceraso, A.; Lin, J.J.; Schneider-Thoma, J.; Siafis, S.; Heres, S.; Kissling, W.; Davis, J.M.; Leucht, S. Maintenance Treatment with Antipsychotic Drugs in Schizophrenia: A Cochrane Systematic Review and Meta-analysis. Schizophr. Bull. 2022, 48, 738–740. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Detraux, J.; van Winkel, R.; Yu, W.; Correll, C.U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 2011, 8, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Lis, M.; Stańczykiewicz, B.; Liśkiewicz, P.; Misiak, B. Impaired hormonal regulation of appetite in schizophrenia: A narrative review dissecting intrinsic mechanisms and the effects of antipsychotics. Psychoneuroendocrinology 2020, 119, 104744. [Google Scholar] [CrossRef]
- Dayabandara, M.; Hanwella, R.; Ratnatunga, S.; Seneviratne, S.; Suraweera, C.; de Silva, V.A. Antipsychotic-associated weight gain: Management strategies and impact on treatment adherence. Neuropsychiatr. Dis. Treat. 2017, 13, 2231–2241. [Google Scholar] [CrossRef]
- Jin, H.; Meyer, J.M.; Mudaliar, S.; Jeste, D.V. Impact of atypical antipsychotic therapy on leptin, ghrelin, and adiponectin. Schizophr. Res. 2008, 100, 70–85. [Google Scholar] [CrossRef]
- Tillisch, K. The effects of gut microbiota on CNS function in humans. Gut Microbes 2014, 5, 404–410. [Google Scholar] [CrossRef]
- Bauer, K.C.; Huus, K.E.; Finlay, B.B. Microbes and the mind: Emerging hallmarks of the gut microbiota-brain axis. Cell Microbiol. 2016, 18, 632–644. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Dawidowski, B.; Górniak, A.; Podwalski, P.; Lebiecka, Z.; Misiak, B.; Samochowiec, J. The Role of Cytokines in the Pathogenesis of Schizophrenia. J. Clin. Med. 2021, 10, 3849. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Borrelli, L.; Aceto, S.; Agnisola, C.; De Paolo, S.; Dipineto, L.; Stilling, R.M.; Dinan, T.G.; Cryan, J.F.; Menna, L.F.; Fioretti, A. Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci. Rep. 2016, 6, 30046. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Kim, G.-C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.-E.; Nam, J.H.; Im, S.-H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227. [Google Scholar] [CrossRef]
- Davari, S.; Talaei, S.A.; Alaei, H.; Salami, M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: Behavioral and electrophysiological proofs for microbiome–gut–brain axis. Neuroscience 2013, 240, 287–296. [Google Scholar] [CrossRef]
- Nemani, K.; Hosseini Ghomi, R.; McCormick, B.; Fan, X. Schizophrenia and the gut–brain axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 155–160. [Google Scholar] [CrossRef]
- Kanji, S.; Fonseka, T.M.; Marshe, V.S.; Sriretnakumar, V.; Hahn, M.K.; Müller, D.J. The microbiome-gut-brain axis: Implications for schizophrenia and antipsychotic induced weight gain. European Arch. Psychiatry Clin. Neurosci. 2017, 268, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.W.; Gorbovskaya, I.; Hahn, M.K.; Müller, D.J. The Gut Microbiome in Schizophrenia and the Potential Benefits of Prebiotic and Probiotic Treatment. Nutrients 2021, 13, 1152. [Google Scholar] [CrossRef]
- Ng, Q.X.; Soh, A.Y.S.; Venkatanarayanan, N.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.-S. A Systematic Review of the Effect of Probiotic Supplementation on Schizophrenia Symptoms. Neuropsychobiology 2019, 78, 1–6. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Knüppel, S.; Schwedhelm, C.; Hoffmann, G.; Missbach, B.; Stelmach-Mardas, M.; Dietrich, S.; Eichelmann, F.; Kontopanteils, E.; Iqbal, K.; et al. Perspective: NutriGrade: A Scoring System to Assess and Judge the Meta-Evidence of Randomized Controlled Trials and Cohort Studies in Nutrition Research. Adv. Nutr. 2016, 7, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jamilian, H.; Ghaderi, A. The Effects of Probiotic and Selenium Co-supplementation on Clinical and Metabolic Scales in Chronic Schizophrenia: A Randomized, Double-blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2021, 199, 4430–4438. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sadighi, G.; Nazeri Astaneh, A.; Tajabadi-Ebrahimi, M.; Dejam, T. Co-administration of probiotic and vitamin D significantly improves cognitive function in schizophrenic patients: A double-blinded randomized controlled trial. Neuropsychopharmacol. Rep. 2024, 44, 389–398. [Google Scholar] [CrossRef]
- Ghaderi, A.; Banafshe, H.R.; Mirhosseini, N.; Moradi, M.; Karimi, M.-A.; Mehrzad, F.; Bahmani, F.; Asemi, Z. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry 2019, 19, 7. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.; Yang, Y.; Kang, D.; Xiao, J.; Long, Y.; Lang, B.; Peng, X.; Wang, W.; Wang, X.; et al. The effects of probiotics plus dietary fiber on antipsychotic-induced weight gain: A randomized clinical trial. Transl. Psychiatry 2022, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kang, D.; Zhang, F.; Yang, Y.; Liu, C.; Xiao, J.; Long, Y.; Lang, B.; Peng, X.; Wang, W.; et al. Probiotics Plus Dietary Fiber Supplements Attenuate Olanzapine-Induced Weight Gain in Drug-Naïve First-Episode Schizophrenia Patients: Two Randomized Clinical Trials. Schizophr. Bull. 2022, 48, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, R.; Jalali, M.M.; Bakhtiari, H.; Eslamdoust-Siahestalkhi, F.; Jalali, S.M. Probiotic Add-on Therapy in the First-episode Schizophrenia: A Randomized Controlled Trial. Casp. J. Neurol. Sci. 2023, 9, 229–243. [Google Scholar] [CrossRef]
- Si, T.; Shi, C.; Sun, L.; Zhang, Y.; Zhang, L. Assessment of the Minimum Clinically Important Difference in Symptoms and Functions of Patients with Acute Schizophrenia: A Post hoc Analysis of an Open-Label, Single-Arm Multicenter Study. Front. Psychiatry 2021, 12, 653916. [Google Scholar] [CrossRef]
- Aboraya, A.; Nasrallah, H.A. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, draw-backs, and a new alternative for schizophrenia research. Ann. Clin. Psychiatry 2016, 28, 125–131. [Google Scholar] [PubMed]
- Okubo, R.; Koga, M.; Katsumata, N.; Odamaki, T.; Matsuyama, S.; Oka, M.; Narita, H.; Hashimoto, N.; Kusumi, I.; Xiao, J.; et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: A proof-of-concept study. J. Affect. Disord. 2019, 245, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.B.; Stallings, C.; Origoni, A.; Katsafanas, E.; Savage, C.L.; Schweinfurth, L.A.; Goga, J.; Khushalani, S.; Yolken, R.H. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim. Care Companion CNS Disord. 2014, 16, 26294. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cai, Y.; Xue, X.; Li, X.; Li, Z.; Xu, C.; Xie, G.; Yu, Y. Does Schizophrenia Itself Cause Obesity? Front. Psychiatry 2022, 13, 934384. [Google Scholar] [CrossRef]
- Minami, J.-i.; Kondo, S.; Yanagisawa, N.; Odamaki, T.; Xiao, J.-z.; Abe, F.; Nakajima, S.; Hamamoto, Y.; Saitoh, S.; Shimoda, T. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J. Nutr. Sci. 2015, 4, e17. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Xin, S.-S.; Ding, L.-N.; Ding, W.-Y.; Hou, Y.-L.; Liu, C.-Q.; Zhang, X.-D. The Potential Role of Probiotics in Controlling Overweight/Obesity and Associated Metabolic Parameters in Adults: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat Med. 2019, 2019, 3862971. [Google Scholar] [CrossRef]
- Jung, S.; Lee, Y.J.; Kim, M.; Kim, M.; Kwak, J.H.; Lee, J.-W.; Ahn, Y.-T.; Sim, J.-H.; Lee, J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduced body adiposity and Lp-PLA2 activity in overweight subjects. J. Funct. Foods 2015, 19, 744–752. [Google Scholar] [CrossRef]
- Sanz, Y.; Park, D.-Y.; Ahn, Y.-T.; Park, S.-H.; Huh, C.-S.; Yoo, S.-R.; Yu, R.; Sung, M.-K.; McGregor, R.A.; Choi, M.-S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE 2013, 8, e59470. [Google Scholar]
- Liu, H.; Huang, Z.; Zhang, X.; He, Y.; Gu, S.; Mo, D.; Wang, S.; Yuan, Z.; Huang, Y.; Zhong, Q.; et al. Association between lipid metabolism and cognitive function in patients with schizophrenia. Front. Psychiatry 2022, 13, 1013698. [Google Scholar] [CrossRef]
- de Oliveira, Y.; Cavalcante, R.G.S.; Cavalcanti Neto, M.P.; Magnani, M.; Braga, V.d.A.; de Souza, E.L.; de Brito Alves, J.L. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020, 11, 5581–5594. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Effects of Probiotic Supplementation on Dyslipidemia in Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Foods 2020, 9, 1540. [Google Scholar] [CrossRef]
- Pongpirul, K.; Janchot, K.; Dai, Y. Single strain probiotics for dyslipidemia, fatty liver, and obesity: A systematic review and meta-analysis. World J. Meta—Anal. 2019, 7, 323–338. [Google Scholar] [CrossRef]
- Roosterman, D.; Cottrell, G.S. The two-cell model of glucose metabolism: A hypothesis of schizophrenia. Mol. Psychiatr. 2021, 26, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wu, L.; Xi, Y.; Li, Y.; Xie, X.; Fan, C.; Yang, L.; Yang, S.; Chen, X.; Zhang, J.; et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 61, 1670–1688. [Google Scholar] [CrossRef]
- Li, Y.-K.; Xiao, C.-L.; Ren, H.; Li, W.-R.; Guo, Z.; Luo, J.-Q. Comparison of the effectiveness of probiotic supplementation in glucose metabolism, lipid profile, inflammation and oxidative stress in pregnant women. Food Funct. 2024, 15, 3479–3495. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 50–55. [Google Scholar] [CrossRef]
- Müller, N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. The relationship between inflammatory biomarkers and cognitive dysfunction in patients with schizophrenia: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 121, 110668. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pei, C.; Wang, X.; Wang, Y.; Huang, D.; Shi, S.; Shen, Z.; Li, S.; He, Y.; Wang, Z.; et al. Probiotics ameliorates pulmonary inflammation via modulating gut microbiota and rectifying Th17/Treg imbalance in a rat model of PM2.5 induced lung injury. Ecotox Environ. Safe 2022, 244, 114060. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Mousavi, S.M.; Sadeghi, A.; Salari-Moghaddam, A.; Parohan, M.; Larijani, B.; Esmaillzadeh, A. The effect of probiotics on inflammatory biomarkers: A meta-analysis of randomized clinical trials. Eur. J. Nutr. 2019, 59, 633–649. [Google Scholar] [CrossRef]
- Goh, X.X.; Tang, P.Y.; Tee, S.F. Effects of antipsychotics on antioxidant defence system in patients with schizophrenia: A meta-analysis. Psychiatry Res. 2022, 309, 114429. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as Potential Antioxidants: A Systematic Review. J. Agr. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, F.; Zhao, N.; Zhai, Q.; Zhang, H.; Chen, W. Effects of probiotics on d -galactose-induced oxidative stress in plasma: A meta-analysis of animal models. J. Funct. Foods 2017, 39, 44–49. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A.M. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000. [Google Scholar] [CrossRef]
- Pourrajab, B.; Fatahi, S.; Sohouli, M.H.; Găman, M.-A.; Shidfar, F. The effects of probiotic/synbiotic supplementation compared to placebo on biomarkers of oxidative stress in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 62, 490–507. [Google Scholar] [CrossRef]



| Study | Country | Population | Intervention Group | Control Group | Duration (Weeks) | Main Results | ||
|---|---|---|---|---|---|---|---|---|
| N (IG/CG) | Age | BMI | ||||||
| [28] | Iran | 25/26 | 18–60 | IG: 25.1 ± 4.4 CG: 24.5 ± 3.0 | 200 μg/day selenium plus 8 × 109 CFU/day of probiotic supplements (equal amounts of L. acidophilus, B. lactis, B. bifidum, and B. longum). | Placebo | 12 | ↑: TAC, GSH, QUICKI ↓: hs-CRP, fasting glucose, insulin, HOMA-IR |
| [29] | Iran | 35/35 | 18–65 | IG: 27.26 ± 5.03 CG: 25.50 ± 6.37 | 2 × 109 CFU/day of Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus reuteri, Lactobacillus paracasei, Bifidobacterium longum, and Bacillus coagulans and 400 IU vitamin D. | Placebo | 12 | ↑: MOCA ↓: TC, FBS, CRP |
| [30] | Iran | 30/30 | 25–65 | IG: 23.1 ± 2.8 CG: 24.5 ± 3.7 | 50,000 IU of vitamin D3 every 2 weeks plus 8 × 109 CFU/day of probiotics containing Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum (each 2 × 109 CFU/day). | Placebo | 12 | ↑: TAC, PANSS General, Total PANSS ↓: MDA, hs-CRP, FPG, insulin, HOMA-IR, TGs, TC |
| [31] | China | 32/28 | 18–45 | IG: 5.02–27.48 CG: 26.18–29.05 | 1.7 × 109 CFU/g of Bifidobacterium, 3.8 × 108 CFU/g of Lactobacillus, and 7.8 × 108 CFU/g of Enterococcus, in total (1680 mg/d), plus dietary fiber (60 g/d). | Placebo | 12 | ↓: Weight, BMI, TC |
| [32] | China | 30/28 | 18–50 | IG: 20.04 ± 2.84 CG: 21.12 ± 1.56 | Bifidobacterium, Lactobacillus, and Enterococcus at concentrations ≥5.0 × 107 CFU/g, in total (840 mg twice daily), plus dietary fiber (30 g twice daily). | Placebo | 12 | ↓: Weight, IRI |
| [33] | Iran | 31/31 | 18–60 | IG: 27.3 ± 2.6 CG: 27.5 ± 2.9 | 38.5 mg fructooligosaccharides, 9 × 109 CFU/g of Lactobacilli, 1.25 × 1010 CFU/g of Bifidobacteria plus 1.5 × 1010 CFU/g of Streptococcus Salivarius subsp. Thermophilus, in total (500 mg daily). | Placebo | 12 | ↓: TGs, TC, FBS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Du, F.; Liu, X.; Song, M.; Grosso, G.; Battino, M.; Boesch, C.; Li, H.; Liu, X. Effect of Supplementation with Probiotics in Patients with Schizophrenia: Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Foods 2025, 14, 1773. https://doi.org/10.3390/foods14101773
Li L, Du F, Liu X, Song M, Grosso G, Battino M, Boesch C, Li H, Liu X. Effect of Supplementation with Probiotics in Patients with Schizophrenia: Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Foods. 2025; 14(10):1773. https://doi.org/10.3390/foods14101773
Chicago/Turabian StyleLi, Lu, Fengqi Du, Xilong Liu, Mengyao Song, Giuseppe Grosso, Maurizio Battino, Christine Boesch, He Li, and Xinqi Liu. 2025. "Effect of Supplementation with Probiotics in Patients with Schizophrenia: Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials" Foods 14, no. 10: 1773. https://doi.org/10.3390/foods14101773
APA StyleLi, L., Du, F., Liu, X., Song, M., Grosso, G., Battino, M., Boesch, C., Li, H., & Liu, X. (2025). Effect of Supplementation with Probiotics in Patients with Schizophrenia: Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Foods, 14(10), 1773. https://doi.org/10.3390/foods14101773