Loop-Mediated Isothermal Amplification Assay for Visual Detection of Salmonella enterica Serovar Typhimurium in Food Animal Meat Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Meat Sample Collection
2.2. Conventional Culture of Salmonella in Raw Meat Samples
2.3. DNA Extraction via Boiling Lysis Method
2.4. Conventional PCR Assay for Salmonella Detection
2.5. Optimization of PCR Assay for Salmonella Serovar Typhimurium Detection
2.6. Optimization of LAMP Assay for S. Typhimurium Detection
2.7. Specificity and Sensitivity of PCR and LAMP Assays for S. Typhimurium Detection
2.8. Detection of S. Typhimurium from Raw Meat Samples
2.9. Gel Electrophoresis and Visualization
2.10. Data Analysis
3. Results
3.1. Optimized PCR and LAMP Detection of S. Typhimurium Using the STM4497 Gene
3.2. In Silico Analysis of STM4497 Gene PCR and Sequencing Confirmation
3.3. Specificity of the Optimized STM4497 Gene PCR and LAMP Assays
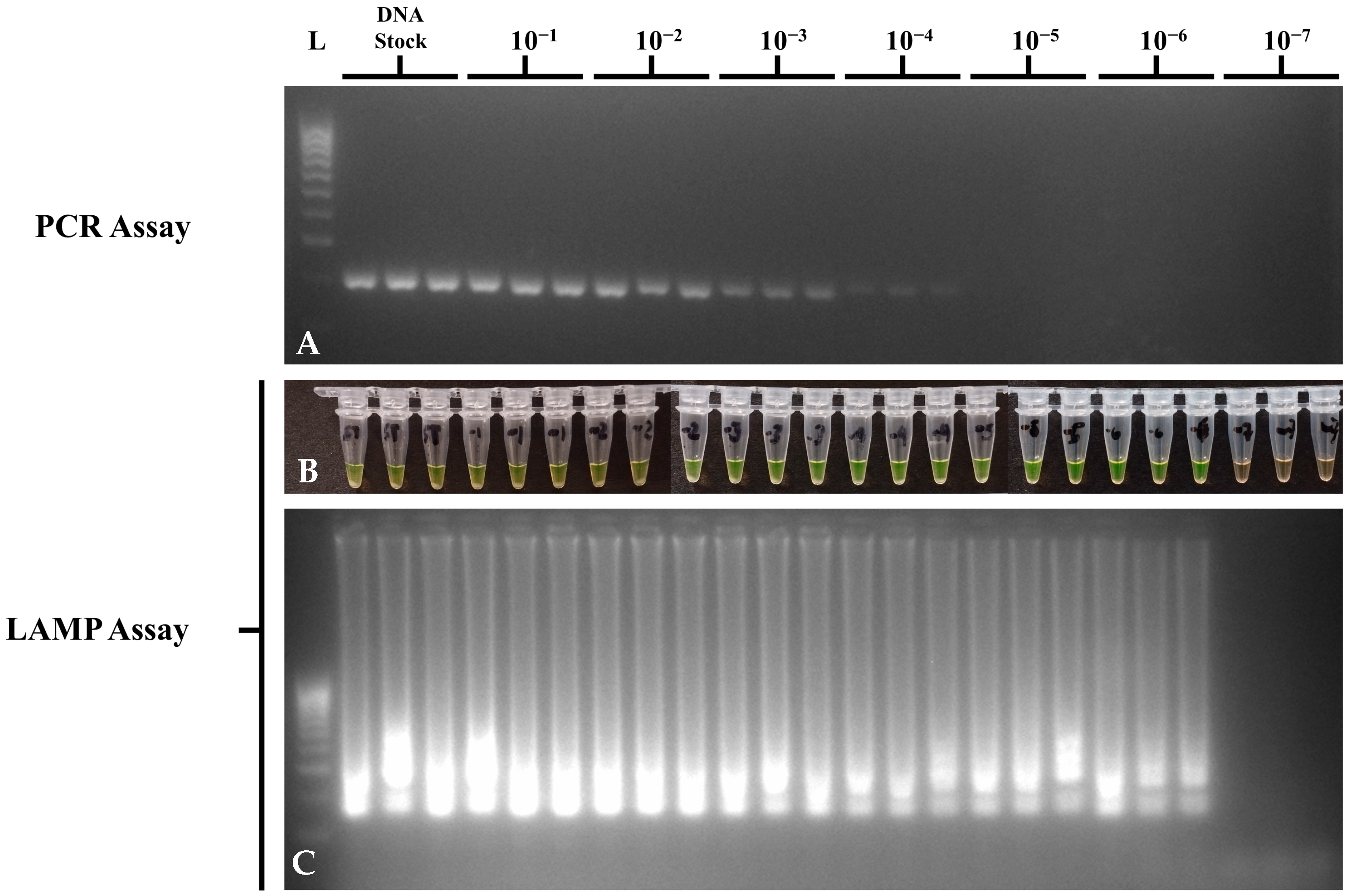
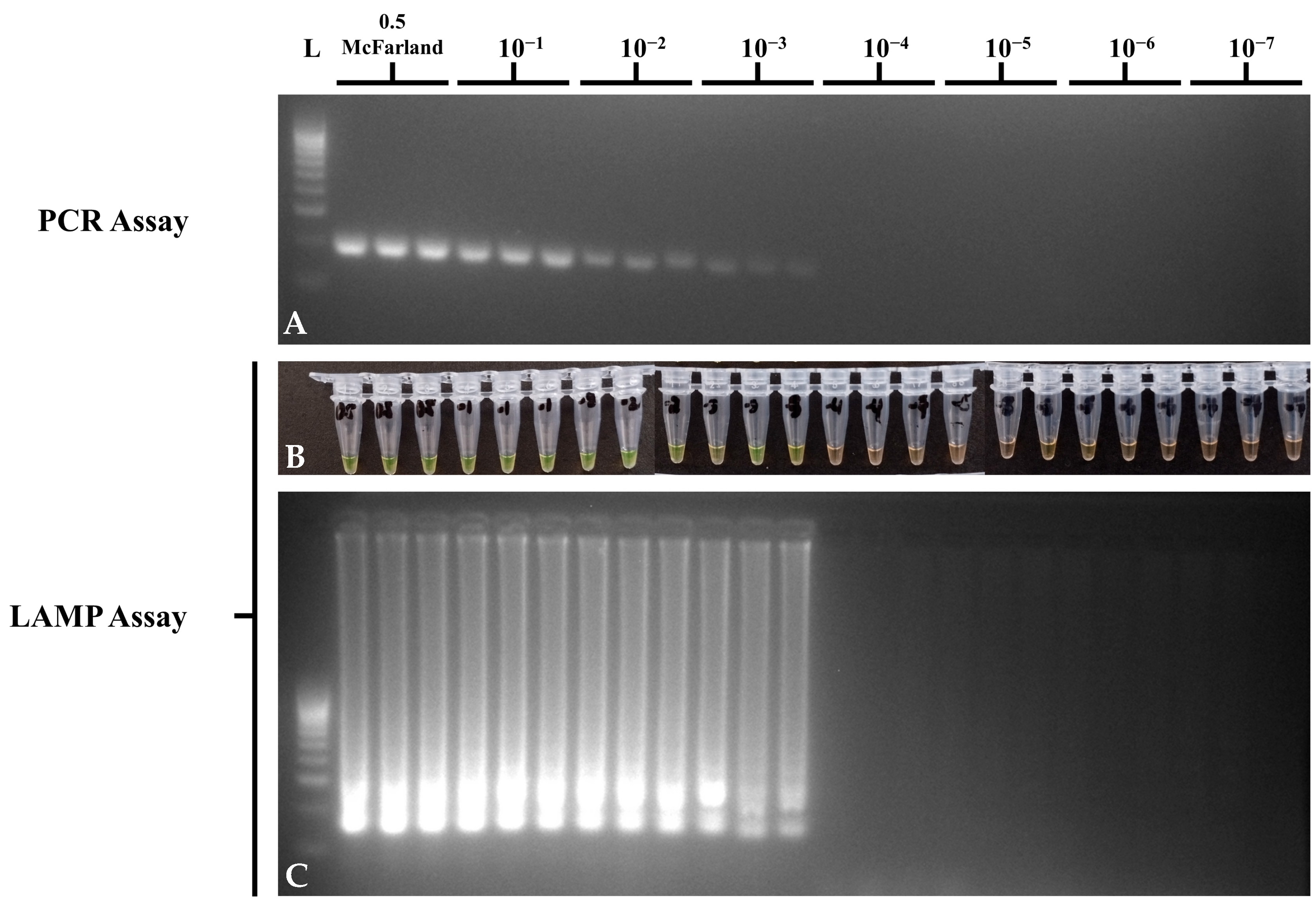
3.4. Sensitivity of the Optimized STM4497 Gene PCR and LAMP Assays
3.5. Prevalence of Salmonella spp. in Retail Meat Samples
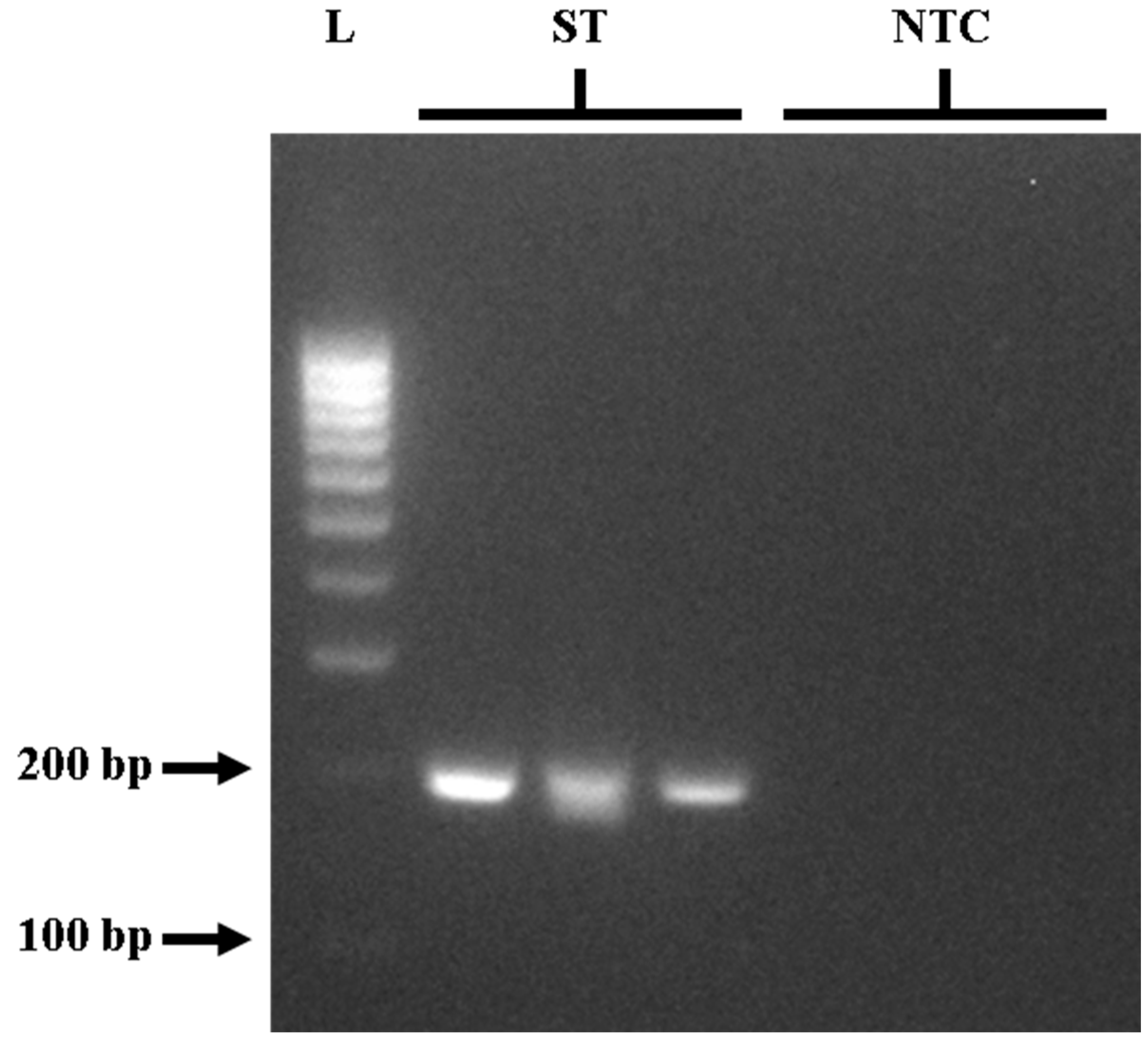
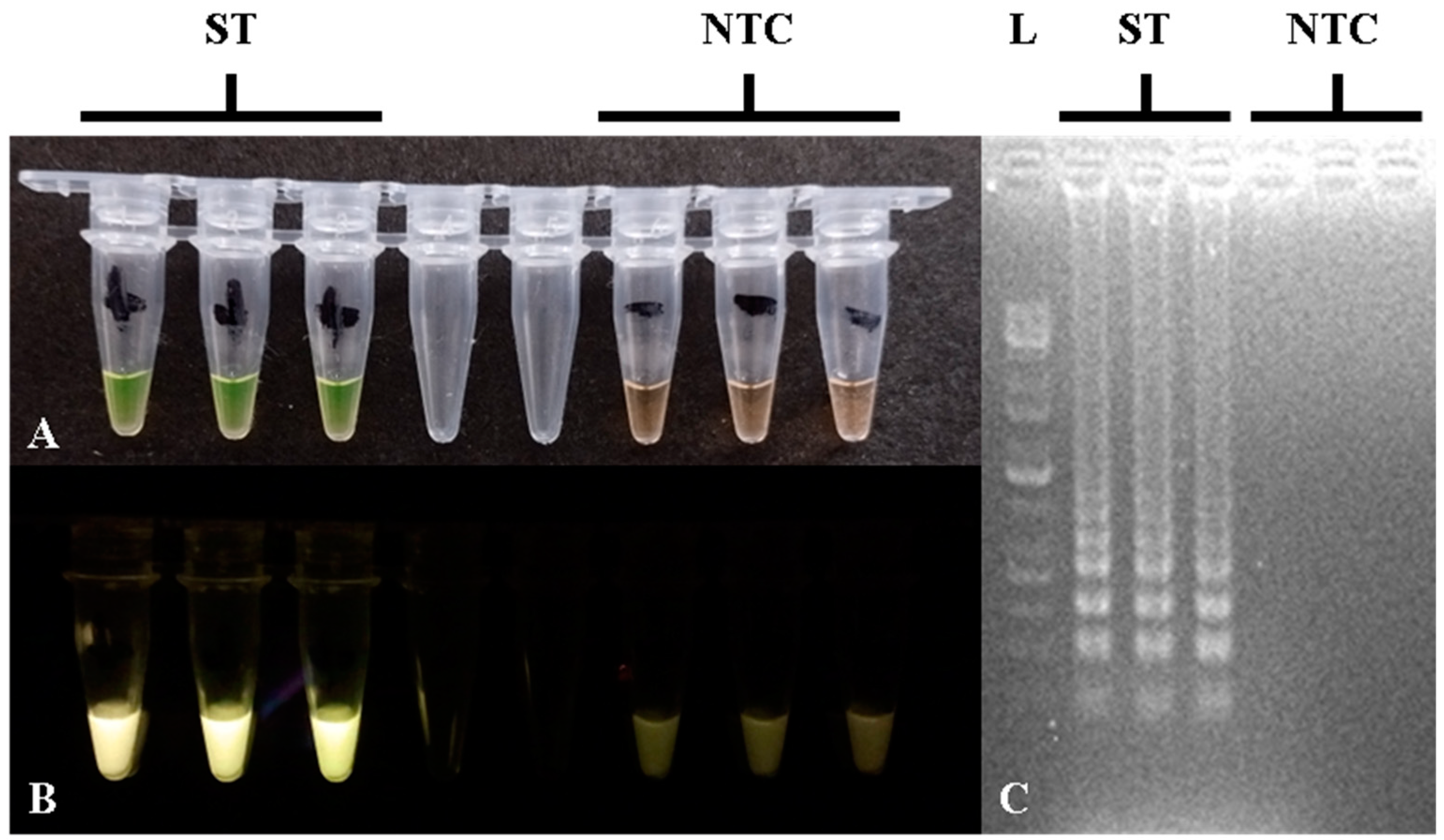
3.6. Performance of the Optimized PCR and LAMP Assays in Meat Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamber, S.; Dougherty, B.; Nguy, K. Salmonella enterica serovars associated with bacteremia in Canada, 2006–2019. J. Food Prot. 2021, 47, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Porwollik, S.; Boyd, E.F.; Choy, C.; Cheng, P.; Florea, L.; Proctor, E.; McClelland, M. Characterization of Salmonella enterica subspecies I genovars by use of microarrays. J. Bacteriol. 2004, 186, 5883–5898. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Li, H.; Feng, Y.; Zeng, S.; Zhuo, Z.; Luo, J.; Chen, X.; Li, X. Prevalence, serotype distribution and antimicrobial resistance of non-typhoidal Salmonella in hospitalized patients in Conghua district of Guangzhou, China. Front. Cell. Infect. Microbiol. 2022, 12, 805384. [Google Scholar] [CrossRef]
- Pavan Kumar, P.; Agarwal, R.K.; Thomas, P.; Sailo, B.; Prasannavadhana, A.; Kumar, A. Rapid detection of Salmonella enterica subspecies enterica serovar Typhimurium by loop mediated isothermal amplification (LAMP) test from field chicken meat samples. Food Biotechnol. 2014, 28, 50–62. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, K.; Yin, H.; Li, Q.; Wang, L.; Liu, Z. Detection of Salmonella and several common Salmonella serotypes in food by loop-mediated isothermal amplification method. Food Sci. Hum. Wellness 2015, 4, 75–79. [Google Scholar] [CrossRef]
- Garrido-Maestu, A.; Fuciños, P.; Azinheiro, S.; Carvalho, J.; Prado, M. Systematic loop-mediated isothermal amplification assays for rapid detection and characterization of Salmonella spp., Enteritidis and Typhimurium in food samples. Food Control 2017, 80, 297–306. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention: Reports of Selected Salmonella Outbreak Investigations. Available online: https://www.cdc.gov/salmonella/outbreaks/index.html (accessed on 10 October 2024).
- Fàbrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef]
- Azanza, M.P.V.; Membrebe, B.N.Q.; Sanchez, R.G.R.; Estilo, E.E.C.; Dollete, U.G.M.; Feliciano, R.J.; Garcia, N.K.A. Foodborne disease outbreaks in the Philippines (2005–2018). Philipp. J. Sci. 2019, 148, 323–342. [Google Scholar]
- Ng, K.C.S.; Rivera, W.L. Multiplex PCR-based serogrouping and serotyping of Salmonella enterica from tonsil and jejunum with jejunal lymph nodes of slaughtered swine in Metro Manila, Philippines. J. Food Prot. 2015, 78, 873–880. [Google Scholar] [CrossRef]
- Soguilon-del Rosario, S.; Rivera, W.L. Incidence and molecular detection of Salmonella enterica serogroups and spvC virulence gene in raw and processed meats from selected wet markets in Metro Manila, Philippines. Int. J. Philipp. Sci. Technol. 2015, 8, 52–55. [Google Scholar] [CrossRef]
- Santos, P.D.M.; Widmer, K.W.; Rivera, W.L. PCR-based detection and serovar identification of Salmonella in retail meat collected from wet markets in Metro Manila, Philippines. PLoS ONE 2020, 15, e0239457. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.F.B.; Meclat, V.Y.B.; Calayag, A.M.B.; Campino, S.; Hafalla, J.C.R.; Hibberd, M.L.; Phelan, J.E.; Clark, T.G.; Rivera, W.L. Genomic analysis of Salmonella enterica from Metropolitan Manila abattoirs and markets reveals insights into circulating virulence and antimicrobial resistance genotypes. Front. Microbiol. 2024, 14, 1304283. [Google Scholar] [CrossRef]
- International Organization for Standardization: Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. (ISO 6579-1:2017). Available online: https://www.iso.org/standard/56712.html (accessed on 10 October 2024).
- Graziani, C.; Losasso, C.; Luzzi, I.; Ricci, A.; Scavia, G.; Pasquali, P. Salmonella . In Foodborne Diseases, 3rd ed.; Dodd, C.E.R., Aldsworth, T., Stein, R.A., Cliver, D.O., Riemann, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 133–169. [Google Scholar] [CrossRef]
- Diep, B.; Barretto, C.; Portmann, A.C.; Fournier, C.; Karczmarek, A.; Voets, G.; Li, S.; Deng, X.; Klijn, A. Salmonella serotyping; Comparison of the traditional method to a microarray-based method and an in silico platform using whole genome sequencing data. Front. Microbiol. 2019, 10, 2554. [Google Scholar] [CrossRef]
- Awang, M.S.; Bustami, Y.; Hamzah, H.H.; Zambry, N.S.; Najib, M.A.; Khalid, M.F.; Ismail, A.; Manaf, A.A. Advancement in Salmonella detection methods: From conventional to electrochemical-based sensing detection. Biosensors 2021, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Hara-Kudo, Y.; Yoshino, M.; Kojima, T.; Ikedo, M. Loop-mediated isothermal amplification for the rapid detection of Salmonella. FEMS Microbiol. Lett. 2005, 253, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ma, L.M.; Zheng, S.; He, X.; Hammack, T.S.; Brown, E.W.; Zhang, G. Development of a novel loop-mediated isothermal amplification (LAMP) assay for the detection of Salmonella ser. Enteritidis from egg products. Food Control 2018, 88, 190–197. [Google Scholar] [CrossRef]
- Justo, C.A.C.; Mapile, M.R.F.; Santos, P.D.M.; Rivera, W.L. Determination of the optimal pre-enrichment period for the detection of Salmonella enterica in artificially inoculated meat products using culture, PCR and LAMP assays. Philipp. Sci. Lett. 2018, 11, 43–50. [Google Scholar]
- Quoc, N.B.; Phuong, N.D.N.; Chau, N.N.B.; Linh, D.T.P. Closed tube loop-mediated isothermal amplification assay for rapid detection of hepatitis B virus in human blood. Heliyon 2018, 4, e00561. [Google Scholar] [CrossRef]
- Yang, Q.; Domesle, K.J.; Ge, B. Loop-mediated isothermal amplification for Salmonella detection in food and feed: Current applications and future directions. Foodborne Pathog. Dis. 2018, 15, 309–331. [Google Scholar] [CrossRef]
- Fischbach, J.; Xander, N.C.; Frohme, M.; Glökler, J.F. Shining a light on LAMP assays--a comparison of LAMP visualization methods including the novel use of berberine. BioTechniques 2015, 58, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Yao, S.; Xu, K.; Wang, J.; Song, X.; Mu, Y.; Zhao, C.; Li, J. A novel visual-mixed-dye for LAMP and its application in the detection of foodborne pathogens. Anal. Biochem. 2019, 574, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, Z.; Yan, S.; Yin, F.; Feng, X.; Liu, B. Identifying multiple bacterial pathogens by loop-mediated isothermal amplification on a rotate & react slip chip. Sens. Actuators B Chem. 2016, 228, 491–499. [Google Scholar] [CrossRef]
- Fang, J.; Wu, Y.; Qu, D.; Ma, B.; Yu, X.; Zhang, M.; Han, J. Propidium monoazide real-time loop-mediated isothermal amplification for specific visualization of viable Salmonella in food. Lett. Appl. Microbiol. 2018, 67, 79–88. [Google Scholar] [CrossRef]
- Sayad, A.; Ibrahim, F.; Mukim Uddin, S.; Cho, J.; Madou, M.; Thong, K.L. A microdevice for rapid, monoplex and colorimetric detection of foodborne pathogens using a centrifugal microfluidic platform. Biosens. Bioelectron. 2018, 100, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.A.; Ziros, P.G.; Bellou, M.; Vantarakis, A. Loop-mediated isothermal amplification (LAMP) for the detection of Salmonella in food. Food Anal. Methods 2014, 7, 512–526. [Google Scholar] [CrossRef]
- Pavon, R.D.N.; Mendoza, P.D.G.; Flores, C.A.R.; Calayag, A.M.B.; Rivera, W.L. Genotypic virulence profiles and associations in Salmonella isolated from meat samples in wet markets and abattoirs of Metro Manila, Philippines. BMC Microbiol. 2022, 22, 292. [Google Scholar] [CrossRef]
- Chiu, C.H.; Ou, J.T. Rapid identification of Salmonella serovars in feces by specific detection of virulence genes, invA and spvC, by an enrichment broth culture-multiplex PCR combination assay. J. Clin. Microbiol. 1996, 34, 2619–2622. [Google Scholar] [CrossRef]
- Azinheiro, S.; Carvalho, J.; Prado, M.; Garrido-Maestu, A. Evaluation of different genetic targets for Salmonella enterica serovar Enteriditis and Typhimurium, using Loop-mediated isothermal AMPlification for detection in food samples. Front. Sustain. Food Syst. 2018, 2, 5. [Google Scholar] [CrossRef]
- Bikandi, J.; San Millán, R.; Rementeria, A.; Garaizar, J. In silico analysis of complete bacterial genomes: PCR, AFLP-PCR and endonuclease restriction. Bioinform 2004, 20, 798–799. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Balaga, K.B.; Pavon, R.D.N.; Calayag, A.M.B.; Justo, C.A.C.; Adao, D.E.V.; Rivera, W.L. Development of a closed-tube, calcein-based loop-mediated isothermal amplification assay to detect Salmonella spp. in raw meat samples. Microbiol. Methods 2024, 220, 106922. [Google Scholar] [CrossRef]
- Henke, W.; Herdel, K.; Jung, K.; Schnorr, D.; Loening, S.A. Betaine improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Res. 1997, 25, 3957–3958. [Google Scholar] [CrossRef]
- Calayag, A.M.B.; Paclibare, P.A.P.; Santos, P.D.; Bautista, C.A.C.; Rivera, W.L. Molecular characterization and antimicrobial resistance of Salmonella enterica from swine slaughtered in two different types of Philippine abattoir. Food Microbiol. 2017, 65, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Anoushirvani, A.A.; Kheiri, Z.; Rahbari, A.; Jadidi, A. The importance of evaluating serum levels of tumor markers M2-PK and inhibin A in patients undergoing colonoscopy. Technol. Cancer Res. Treat. 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.L.; Quave, K. Quantitative Anthropology: A Workbook; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Chan, K.; Baker, S.; Kim, C.C.; Detweiler, C.S.; Dougan, G.; Falkow, S. Genomic comparison of Salmonella enterica serovars and Salmonella bongori by use of an S. enterica serovar Typhimurium DNA microarray. J. Bacteriol. 2003, 185, 553–563. [Google Scholar] [CrossRef]
- Bishop, A.L.; Baker, S.; Jenks, S.; Fookes, M.; Gaora, P.O.; Pickard, D.; Anjum, M.; Farrar, J.; Hien, T.T.; Ivens, A.; et al. Analysis of the hypervariable region of the Salmonella enterica genome associated with tRNA leuX. J. Bacteriol. 2005, 187, 2469–2482. [Google Scholar] [CrossRef]
- Zaworski, J.; Dagva, O.; Brandt, J.; Baum, C.; Ettwiller, L.; Fomenkov, A.; Raleigh, E.A. Reassembling a cannon in the DNA defense arsenal: Genetics of StySA, a BREX phage exclusion system in Salmonella lab strains. PLoS Genet. 2022, 18, e1009943. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.H.; Lee, T.H.; Nahm, B.H.; Chung, Y.H.; Seo, K.H.; Kim, H.Y. Identification of Salmonella enterica serovar Typhimurium using specific PCR primers obtained by comparative genomics in Salmonella serovars. J. Food Prot. 2006, 69, 1653–1661. [Google Scholar] [CrossRef]
- Saeki, E.K.; Alves, J.; Bonfante, R.C.; Hirooka, E.Y.; De Oliveira, T.C.R.M. Multiplex PCR (mPCR) for the Detection of Salmonella spp. and the differentiation of the Typhimurium and Enteritidis serovars in chicken meat. J. Food Saf. 2012, 33, 25–29. [Google Scholar] [CrossRef]
- Ogunremi, D.; Nadin-Davis, S.; Dupras, A.A.; Márquez, I.G.; Omidi, K.; Pope, L.; Devenish, J.; Burke, T.; Allain, R.; Leclair, D. Evaluation of a multiplex PCR assay for the identification of Salmonella serovars Enteritidis and Typhimurium using retail and abattoir samples. J. Food Prot. 2017, 80, 295–301. [Google Scholar] [CrossRef]
- Ren, J.; Man, Y.; Li, A.; Liang, G.; Jin, X.; Pan, L. Detection of Salmonella Enteritidis and Salmonella Typhimurium in foods using a rapid, multiplex real-time recombinase polymerase amplification assay. J. Food Saf. 2020, 40, e12784. [Google Scholar] [CrossRef]
- Nurjayadi, M.; Efrianti, U.R.; Azizah, N.; Kurniadewi, F.; Saamia, V.; Wiranatha, M.; Nastassya, L.; El-Enshasy, H.A. Detection of Salmonella Typhimurium on artificially contaminated milk by real time PCR using STM4497 and fljB primers. AIP Conf. Proc. 2021, 2331, 040028. [Google Scholar] [CrossRef]
- Das, P.K.; Mandal, A.; Rahman, M.M.; Sarkar, S.L.; Jahid, I.K.; Hossain, M.A.; Alam, A.S.M.R.U.; Roy, P.C. Salmonella enterica serovar Typhimurium and Enteritidis isolated from raw shrimp in Bangladesh: An investigation based on molecular characteristics, survival, virulence, antibiotic resistance, and biofilm formation attributes. J. Food Qual. 2022, 2022, 3420364. [Google Scholar] [CrossRef]
- Aziz, A.A.T.A.; Abdul-Lateef, L.A. Genetic and biochemical detection of Salmonella enterica isolated from patients suffering watery diarrhea and typhoid fever in Babylon Province. Med. J. Babylon 2023, 20, 383–387. [Google Scholar] [CrossRef]
- Johnson, T.J.; Flores-Figueroa, C.; Munoz-Aguayo, J.; Pinho, G.; Miller, E. Persistence of vaccine origin Salmonella Typhimurium through the poultry production continuum, and development of a rapid typing scheme for their differentiation from wild type field isolates. Poult. Sci. 2024, 103, 103707. [Google Scholar] [CrossRef]
- Oliveira, S.; Santos, L.R.; Schuch, D.M.; Silva, A.B.; Salle, C.T.; Canal, C.W. Detection and identification of salmonellas from poultry-related samples by PCR. Vet. Microbiol. 2002, 87, 25–35. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Zhou, X.; Xu, L.; Zhang, L.; Shi, X. Comprehensive analysis reveals two distinct evolution patterns of Salmonella flagellin gene clusters. Front. Microbiol. 2017, 8, 2604. [Google Scholar] [CrossRef]
- Shanmugasundaram, M.; Radhika, M.; Murali, H.S.; Batra, H.V. Detection of Salmonella enterica serovar Typhimurium by selective amplification of fliC, fljB, iroB, invA, rfbJ, STM2755, STM4497 genes by polymerase chain reaction in a monoplex and multiplex format. World J. Microbiol. Biotechnol. 2009, 25, 1385–1394. [Google Scholar] [CrossRef]
- Boland, C.; Van Hessche, M.; Mahillon, J.; Wattiau, P. A liquid bead array for the identification and characterization of fljB -positive and fljB -negative monophasic variants of Salmonella Typhimurium. Food Microbiol. 2017, 71, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Aabo, S.; Rasmussen, O.; Rossen, L. Oligonucleotide probes specific for the genus Salmonella and for Salm. typhimurium. Lett. Appl. Microbiol. 1995, 20, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, U.M.; Loske, A.M.; Castaño-Tostado, E.; Prieto, F.E. Inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes by underwater shock waves. Innov. Food Sci. Emerg. Technol. 2004, 5, 459–463. [Google Scholar] [CrossRef]
- Salih, W.; Yousif, A.A. Molecular detection of Salmonella Typhimurium isolated from canine feces by PCR. Adv. Anim. Vet. Sci. 2018, 6, 542–547. [Google Scholar] [CrossRef]
- Takahashi-Íñiguez, T.; Aburto-Rodríguez, N.; Vilchis-González, A.L.; Flores, M.E. Function, kinetic properties, crystallization, and regulation of microbial malate dehydrogenase. J. Zhejiang Univ. Sci. B 2016, 17, 247–261. [Google Scholar] [CrossRef]
- Boyd, E.F.; Nelson, K.; Wang, F.S.; Whittam, T.S.; Selander, R.K. Molecular genetic basis of allelic polymorphism in malate dehydrogenase (mdh) in natural populations of Escherichia coli and Salmonella enterica. Proc. Natl. Acad. Sci. USA 1994, 91, 1280–1284. [Google Scholar] [CrossRef]
- McQuiston, J.R.; Herrera-Leon, S.; Wertheim, B.C.; Doyle, J.; Fields, P.I.; Tauxe, R.V.; Logsdon, J.M., Jr. Molecular phylogeny of the salmonellae: Relationships among Salmonella species and subspecies determined from four housekeeping genes and evidence of lateral gene transfer events. J. Bacteriol. 2008, 190, 7060–7067. [Google Scholar] [CrossRef]
- Al-Habsi, K.; Yang, R.; Abraham, S.; Ryan, U.; Miller, D.; Jacobson, C. Molecular characterisation of Salmonella enterica serovar Typhimurium and Campylobacter jejuni faecal carriage by captured rangeland goats. Small Rumin. Res. 2017, 158, 48–53. [Google Scholar] [CrossRef]
- Suzuki, R.; Ihira, M.; Enomoto, Y.; Yano, H.; Maruyama, F.; Emi, N.; Asano, Y.; Yoshikawa, T. Heat denaturation increases the sensitivity of the cytomegalovirus loop-mediated isothermal amplification method. Microbiol. Immunol. 2010, 54, 466–470. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Neshani, A.; Zare, H.; Sadeghian, H.; Safdari, H.; Riahi-Zanjani, B.; Aryan, E. A comparative study on visual detection of Mycobacterium tuberculosis by closed tube loop-mediated isothermal amplification: Shedding light on the use of eriochrome black T. Diagnostics 2023, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.B. Development of a loop-mediated isothermal amplification (LAMP) assay for detection of miRNAs. In VCU Master of Science in Forensic Science Directed Research Projects; Virginia Commonwealth University: Richmond, VA, USA, 2022. [Google Scholar]
- Foo, P.C.; Najian, A.B.N.; Muhamad, N.A.; Ahamad, M.; Mohamed, M.; Yean, C.Y.; Lim, B.H. Loop-mediated isothermal amplification (LAMP) reaction as viable PCR substitute for diagnostic applications: A comparative analysis study of LAMP, conventional PCR, nested PCR (nPCR) and real-time PCR (qPCR) based on Entamoeba histolytica DNA derived from faecal sample. BMC Biotechnol. 2020, 20, 34. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, J.; Zhang, J.; An, Z.; Quan, F.; Zhang, L.; Cai, X.; Pu, S.W.J. Codeposition of DNTPs detection for rapid LAMP-based sexing of bovine embryos. Reprod. Domest. Anim. 2009, 44, 116–121. [Google Scholar] [CrossRef]
- Qiu, X.; Li, T.; Zhang, G.; Cao, J.; Jin, Y.; Xing, G.; Liao, M.; Zhou, J. Development of a loop-mediated isothermal amplification method to rapidly detect porcine circovirus genotypes 2a and 2b. Virol. J. 2012, 9, 318. [Google Scholar] [CrossRef]
- Zhan, L.; Song, D.; Gu, Q.; Yan, T.; Ma, C. Reverse transcription—Loop-mediated isothermal amplification assay for the rapid detection of pathogenic Listeria monocytogenes in meat products. Can. J. Microbiol. 2019, 65, 913–921. [Google Scholar] [CrossRef]
- Chen, D.; Tian, F.; Liu, W.; Yu, J.; Song, D. Reverse transcription loop-mediated isothermal amplification assay with high sensitivity to rapid detection of viable Salmonella in foods. Jundishapur J. Microbiol. 2022, 14, e117938. [Google Scholar] [CrossRef]
- Vittal, R.; Mohanraj, J.R.; Chakraborty, G. Rapid detection of Salmonella spp. from meat: Loop mediated isothermal amplification (LAMP). J. Pure Appl. Microbiol. 2022, 16, 929–936. [Google Scholar] [CrossRef]
- Lee, P.L.M. DNA amplification in the field: Move over PCR, here comes LAMP. Mol. Ecol. Resour. 2017, 17, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Rahn, K.; De Grandis, S.; Clarke, R.; McEwen, S.; Galán, J.; Ginocchio, C.; Curtiss, R.; Gyles, C. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol. Cell. Probes. 1992, 6, 271–279. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Beaulieu, J.C.; Stein, R.E.; Ge, B. Rapid detection of viable salmonellae in produce by coupling propidium monoazide with loop-mediated isothermal amplification. Appl. Environ. Microbiol. 2011, 77, 4008–4016. [Google Scholar] [CrossRef]
- Fallahi, S.; Tabaei, S.J.S.; Pournia, Y.; Zebardast, N.; Kazemi, B. Comparison of loop-mediated isothermal amplification (LAMP) and nested-PCR assay targeting the RE and B1 gene for detection of Toxoplasma gondii in blood samples of children with leukaemia. Diagn. Microbiol. Infect. Dis. 2014, 79, 347–354. [Google Scholar] [CrossRef] [PubMed]
- De Lira Nunes, M.; Mendes-Marques, C.L.; De Almeida, A.M.P.; Leal, N.C. The development of a loop-mediated isothermal amplification (LAMP) procedure for plague diagnostic. Am. J. Anal. Chem. 2014, 5, 1069–1077. [Google Scholar] [CrossRef]
- Zhuang, L.; Gong, J.; Li, Q.; Zhu, C.; Yu, Y.; Dou, X.; Liu, X.; Xu, B.; Wang, C. Detection of Salmonella spp. by a loop-mediated isothermal amplification (LAMP) method targeting bcfD gene. Lett. Appl. Microbiol. 2014, 59, 658–664. [Google Scholar] [CrossRef]
- Mei, X.; Zhai, X.; Lei, C.; Ye, X.; Kang, Z.; Wu, X.; Xiang, R.; Wang, Y.; Wang, H. Development and application of a visual loop-mediated isothermal amplification combined with lateral flow dipstick (LAMP-LFD) method for rapid detection of Salmonella strains in food samples. Food Control 2019, 104, 9–19. [Google Scholar] [CrossRef]
- Vichaibun, V.; Kanchanaphum, P. Quantitative LAMP and PCR detection of Salmonella in chicken samples collected from local markets around Pathum Thani province, Thailand. Int. J. Food Sci. 2020, 2020, 8833173. [Google Scholar] [CrossRef]
- Kalendar, R.; Khassenov, B.; Ramankulov, Y.; Samuilova, O.; Ivanov, K.I. FastPCR: An in silico tool for fast primer and probe design and advanced sequence analysis. Genomics 2017, 109, 312–319. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, P.; Zhuang, L.; Zhang, D.; Qi, K.; Dou, X.; Wang, C.; Gong, J. Multiplex polymerase chain reaction to detect Salmonella serovars Indiana, Enteritidis, and Typhimurium in raw meat. J. Food Saf. 2019, 39, e12674. [Google Scholar] [CrossRef]
- Ravan, H.; Amandadi, M. A rapid and specific detection of pathogenic serovar Salmonella Typhimurium by loop-mediated isothermal amplification method (LAMP). J. Microb. Biol. 2017, 6, 85–94. [Google Scholar] [CrossRef]
- Gong, J.; Jiang, Y.; Zhang, D.; Li, T.; Fu, L.; Dou, X. One-tube detection of Salmonella Typhimurium using LAMP and CRISPR-Cas12b. Microbiol. Spectr. 2024, 12, e0127124. [Google Scholar] [CrossRef]
- Edel, B.; Glöckner, S.; Stoll, S.; Lindig, N.; Boden, K.; Wassill, L.; Simon, S.; Löffler, B.; Rödel, J. Development of a rapid diagnostic test based on loop-mediated isothermal amplification to identify the most frequent non-typhoidal Salmonella serovars from culture. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 461–470. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.; Kim, H. Direct triplex loop-mediated isothermal amplification assay for the point-of-care molecular detection of Salmonella genus, subspecies I, and serovar Typhimurium. Food Control 2020, 120, 107504. [Google Scholar] [CrossRef]
- Lapierre, S.G.; Drancourt, M. RPOB Targeted Loop-Mediated Isothermal Amplification (LAMP) assay for consensus detection of mycobacteria associated with pulmonary infections. Front. Med. 2018, 5, 332. [Google Scholar] [CrossRef]
- Zhang, G.; Brown, E.W.; González-Escalona, N. Comparison of real-time PCR, reverse transcriptase real-time PCR, loop-mediated isothermal amplification, and the FDA conventional microbiological method for the detection of Salmonella spp. in produce. Appl. Environ. Microbiol. 2011, 77, 6495–6501. [Google Scholar] [CrossRef]
- Hsieh, K.; Mage, P.L.; Csordas, A.T.; Eisenstein, M.; Soh, H.T. Simultaneous elimination of carryover contamination and detection of DNA with uracil-DNA-glycosylase-supplemented loop-mediated isothermal amplification (UDG-LAMP). Chem. Commun. 2014, 50, 3747. [Google Scholar] [CrossRef]
- Techathuvanan, C.; Draughon, F.A.; D’Souza, D.H. Comparison of reverse transcriptase PCR, reverse transcriptase loop-mediated isothermal amplification, and culture-based assays for Salmonella detection from pork processing environments have been done. J. Food Prot. 2012, 74, 294–301. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Diez-Valcarce, M.; Robles, S.; Losilla-Garcia, B.; Cook, N. A Loop-mediated isothermal amplification-based method for analysing animal feed for the presence of Salmonella. Food Anal. Methods. 2015, 8, 2409–2416. [Google Scholar] [CrossRef]
- Li, J.; Zhai, L.; Bie, X.; Lu, Z.; Kong, X.; Yu, Q.; Lv, F.; Zhang, C.; Zhao, H. A novel visual loop-mediated isothermal amplification assay targeting gene62181533 for the detection of Salmonella spp. in foods. Food Control 2016, 60, 230–236. [Google Scholar] [CrossRef]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques 2009, 46, 167–172. [Google Scholar] [CrossRef]
- Oliveira, S.; Rodenbusch, C.; Cé, M.; Rocha, S.; Canal, C. Evaluation of selective and non-selective enrichment PCR procedures for Salmonella detection. Lett. Appl. Microbiol. 2003, 36, 217–221. [Google Scholar] [CrossRef]
- Goodman, L.B.; McDonough, P.L.; Anderson, R.R.; Franklin-Guild, R.J.; Ryan, J.R.; Perkins, G.A.; Thachil, A.J.; Glaser, A.L.; Thompson, B.S. Detection of Salmonella spp. in veterinary samples by combining selective enrichment and real-time PCR. J. Vet. Diagn. Invest. 2017, 29, 844–851. [Google Scholar] [CrossRef]
- Freschi, C.R.; De Oliveira E Silva Carvalho, L.F.; De Oliveira, C.J.B. Comparison of DNA-extraction methods and selective enrichment broths on the detection of Salmonella Typhimurium in swine feces by polymerase chain reaction (PCR). Braz. J. Microbiol. 2005, 36, 363–367. [Google Scholar] [CrossRef]
- Chan, S.H.; Liau, S.H.; Low, Y.J.; Chng, K.R.; Wu, Y.; Chan, J.S.H.; Tan, L.K. A Real-Time PCR Approach for Rapid Detection of Viable Salmonella Enteritidis in Shell Eggs. Microorganisms 2023, 11, 844. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, Z.; Yang, S.; Li, Y.; Li, M.; Wang, B.; Hu, Y.; Xia, X.; Jin, X.; Yu, N.; et al. A mismatch-tolerant reverse transcription loop-mediated isothermal amplification method and its application on simultaneous detection of all four serotype of dengue viruses. Front. Microbiol. 2019, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Lei, Y. Mini review: Recent progress in RT-LAMP enabled COVID-19 detection. Sens. Actuator Rep. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Jang, M.; Kim, S. Inhibition of non-specific amplification in loop-mediated isothermal amplification via tetramethylammonium chloride. Biochip J. 2022, 16, 326–333. [Google Scholar] [CrossRef]
- Shirshikov, F.V.; Bespyatykh, J.A. Loop-mediated isothermal amplification: From theory to practice. Russ. J. Bioorg. Chem. 2022, 48, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Oscorbin, I.; Filipenko, M. Bst polymerase—A humble relative of Taq polymerase. Comput. Struct. Biotechnol. J. 2023, 21, 4519–4535. [Google Scholar] [CrossRef]
- Fan, F.; Du, P.; Kan, B.; Yan, M. The development and evaluation of a loop-mediated isothermal amplification method for the rapid detection of Salmonella enterica serovar Typhi. PLoS ONE 2015, 10, e0124507. [Google Scholar] [CrossRef]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef]
- Nwe, M.K.; Jangpromma, N.; Taemaitree, L. Evaluation of molecular inhibitors of loop-mediated isothermal amplification (LAMP). Sci. Rep. 2024, 14, 5916. [Google Scholar] [CrossRef]
- Ge, B.; Domesle, K.J.; Yang, Q.; Hammack, T.S.; Wang, S.S.; Deng, X.; Hu, L.; Zhang, G.; Hu, Y.; Lai, X.; et al. Multi-laboratory validation of a loop-mediated isothermal amplification method for screening Salmonella in animal food. Front. Microbiol. 2019, 10, 562. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, X.; Shi, L.; Lu, X.; Tang, S.; Wang, Y.; Li, Y.; Alam, M.; Yan, H. Development of a fimY-based loop-mediated isothermal amplification assay for detection of Salmonella in food. Int. Food Res. 2012, 45, 1011–1015. [Google Scholar] [CrossRef]
- Stone, G.G.; Oberst, R.D.; Hays, M.P.; McVey, S.; Chengappa, M.M. Detection of Salmonella serovars from clinical samples by enrichment broth cultivation-PCR procedure. J. Clin. Microbiol. 1994, 32, 1742–1749. [Google Scholar] [CrossRef]
- Hyeon, J.Y.; Hwang, I.G.; Kwak, H.S.; Park, C.; Choi, I.S.; Seo, K.H. Evaluation of PCR inhibitory effect of enrichment broths and comparison of DNA extraction methods for detection of Salmonella Enteritidis using real-time PCR assay. J. Vet. Sci. 2010, 11, 143–149. [Google Scholar] [CrossRef]
- Suleman, E.; Mtshali, M.S.; Lane, E. Investigation of false positives associated with loop-mediated isothermal amplification assays for detection of Toxoplasma gondii in archived tissue samples of captive felids. J. Vet. Diagn. Investig. 2016, 28, 536–542. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Kim, U.; Oh, S. Diverse methods of reducing and confirming false-positive results of loop-mediated isothermal amplification assays: A review. Anal. Chim. Acta 2023, 1280, 341693. [Google Scholar] [CrossRef]
- Dong, M.; Kshirsagar, A.; Politza, A.J.; Guan, W. High fidelity machine-learning-assisted false positive discrimination in loop-mediated isothermal amplification using Nanopore-Based sizing and counting. ACS Nano 2024, 18, 7170–7179. [Google Scholar] [CrossRef]
- Alhamid, G.; Tombuloglu, H.; Al-Suhaimi, E. Development of loop-mediated isothermal amplification (LAMP) assays using five primers reduces the false-positive rate in COVID-19 diagnosis. Sci. Rep. 2023, 13, 5066. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Primer | Sequence (5′-3′) | Reference |
|---|---|---|---|
| Salmonella spp. PCR Primers | |||
| invA | invAF | ACAGTGCTCGTTTACGACCTGAAT | [31] |
| invAR | AGACGACTGGTACTGATCTAT | ||
| Salmonella enterica serovar Typhimurium LAMP Primers | |||
| STM4497 | STMFIP | ACCTGCAGCTCATTCTGAGCAGTCAAAAACAACGGCTCCGG | [32] |
| STMBIP | GAAAAGGACCACAAGTTCGCGCTCAGTGAGCATGTCGACGAT | ||
| STMF3 | AGCCGCATTAGCGAAGAG | ||
| STMB3 | GCGGTCAAATAACCCACGT | ||
| STMLoopF | TCAAAAATCCAGAACCCAATCTCA | ||
| PCR ASSAY (STM4497 Gene) | ||
| Components | Concentration Tested | Optimized |
| GoTaq®G2 | 1x | 1x |
| F3 | 0.4 µM | 0.4 µM |
| B3 | 0.4 µM | 0.4 µM |
| Nuclease-Free H2O | Make up 12.5 µL | Make up 12.5 µL |
| DNA Template | 1 µL | 1 µL |
| LAMP ASSAY (STM4497 Gene) | ||
| Components | Concentration Tested | Optimized |
| Calcein | 64 µM | 64 µM |
| MnCl2 | 1 mM | 1 mM |
| MgSO4 (Added) | 3 mM | 3 mM |
| Isothermal Amplification Buffer | 1x | 1x |
| dNTPs | 1.4 mM | 1.4 mM |
| FIP/BIP Primers | 1.6 µM | 1.6 µM |
| F3/B3 Primers | 0.2 µM | 0.2 µM |
| Loop F Primers | 0.4 µM | 0.4 µM |
| Nuclease-Free H2O | Make up 25 µL reaction | Make up 25 µL reaction |
| Betaine | 1–1.5 M | 1.5 M |
| Bst Polymerase 2.0 | 320 U/mL | 320 U/mL |
| DNA Template | 1 µL | 1 µL |
| ATCC Strain | PCR | LAMP |
|---|---|---|
| Non-Salmonella strains (n = 9) | ||
| Acinetobacter baumanii BAA 1605 | − | − |
| Escherichia coli 25922 | − | − |
| Escherichia coli O157:H7 43888 | − | − |
| Escherichia coli O78:H11 35401 | − | − |
| Enterococcus faecalis 14506 | − | − |
| Klebsiella pneumoniae 13883 | − | − |
| Klebsiella pneumoniae 700603 | − | − |
| Pseudomonas aeruginosa 10145 | − | − |
| Vibrio parahaemolyticus 13204 | − | − |
| Salmonella enterica subsp. enterica serovar strains (n = 9) | ||
| Anatum 9270 | − | − |
| Choleraesuis 7001 | − | − |
| Choleraesuis 10708 | − | − |
| Enteritidis 13076 | − | − |
| Enteritidis 49223 | − | − |
| Heidelberg 8386 | − | − |
| Newport 6962 | − | − |
| Typhimurium 14028 | + | + |
| Typhimurium 25241 | + | + |
| 10-Fold Dilution | Concentration | PCR | LAMP |
|---|---|---|---|
| Stock | 220 ng/μL | + | + |
| 1 | 28.2 ng/μL | + | + |
| 2 | 2.59 ng/μL | + | + |
| 3 | * 220 pg/μL | + | + |
| 4 | * 22 pg/μL | + | + |
| 5 | * 2.2 pg/μL | − | + |
| 6 | * 220 fg/μL | − | + |
| 7 | * 22 fg/μL | − | − |
| NTC | − | − | − |
| 10-Fold Dilution | LOD (CFU/mL) | PCR | LAMP |
|---|---|---|---|
| 0.5 McFarland | 4.98 × 107 | + | + |
| 1 | 4.98 × 106 | + | + |
| 2 | 4.98 × 105 | + | + |
| 3 | 4.98 × 104 | + | + |
| 4 | 4.98 × 103 | − | − |
| 5 | 4.98 × 102 | − | − |
| 6 | 49.8 | − | − |
| 7 | 4.98 | − | − |
| NTC | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavon, R.D.N.; Rivera, W.L. Loop-Mediated Isothermal Amplification Assay for Visual Detection of Salmonella enterica Serovar Typhimurium in Food Animal Meat Products. Foods 2025, 14, 1731. https://doi.org/10.3390/foods14101731
Pavon RDN, Rivera WL. Loop-Mediated Isothermal Amplification Assay for Visual Detection of Salmonella enterica Serovar Typhimurium in Food Animal Meat Products. Foods. 2025; 14(10):1731. https://doi.org/10.3390/foods14101731
Chicago/Turabian StylePavon, Rance Derrick N., and Windell L. Rivera. 2025. "Loop-Mediated Isothermal Amplification Assay for Visual Detection of Salmonella enterica Serovar Typhimurium in Food Animal Meat Products" Foods 14, no. 10: 1731. https://doi.org/10.3390/foods14101731
APA StylePavon, R. D. N., & Rivera, W. L. (2025). Loop-Mediated Isothermal Amplification Assay for Visual Detection of Salmonella enterica Serovar Typhimurium in Food Animal Meat Products. Foods, 14(10), 1731. https://doi.org/10.3390/foods14101731







